Aerobic Exercise Training Improves Calcium Handling and Cardiac Function in Rats with Heart Failure Resulting from Aortic Stenosis
Abstract
1. Introduction
2. Results
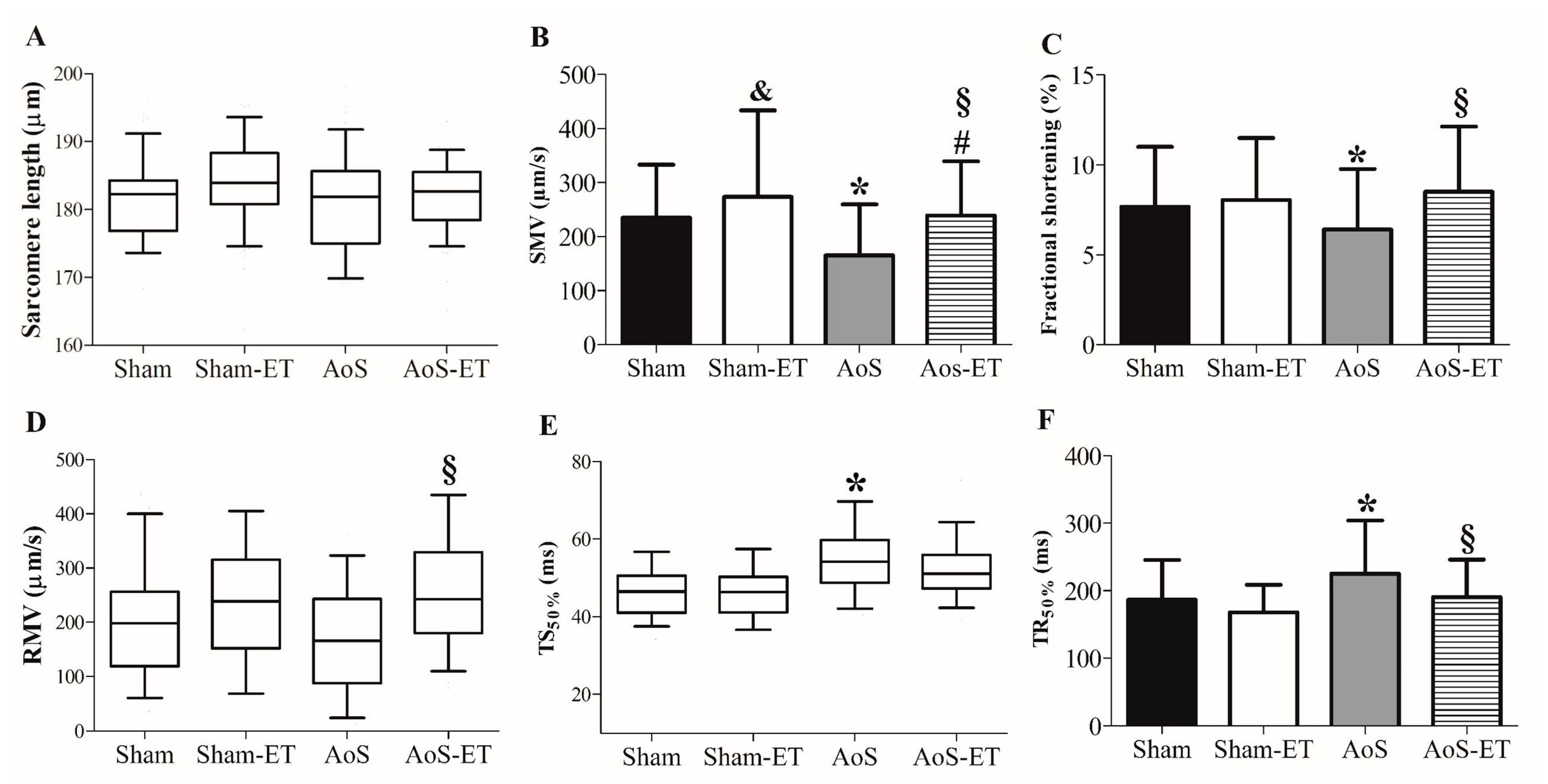
2.1. AET Improves Systolic and Diastolic Cardiac Function, and Signs of Heart Failure in Animals with AoS
2.2. AoS Impairs the Rate of Tension Development and Resting Tension, Whereas AET Is Effective in Recovering Resting Tension in AoS Animals
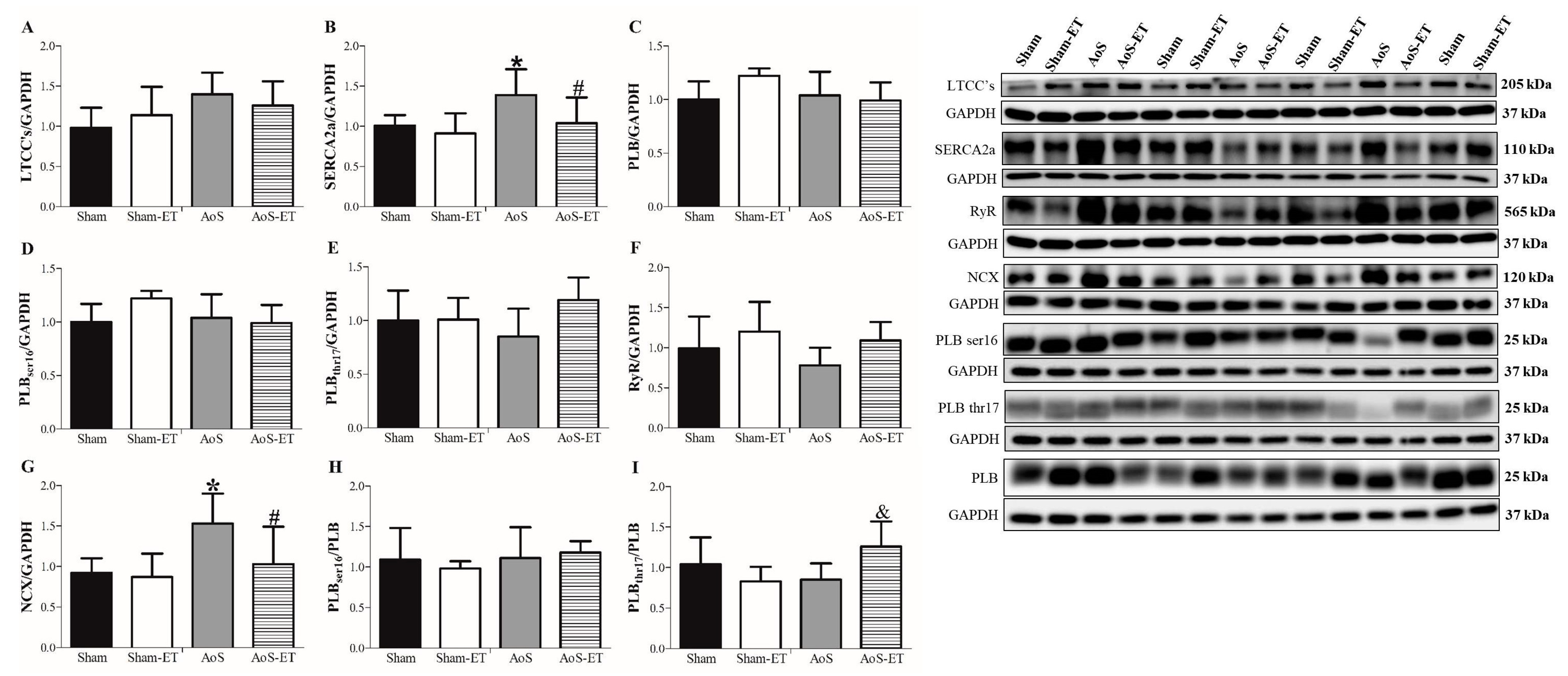
2.3. AoS Deteriorates SERCA2a Function, While AET Attenuates the Loss of SERCA2a Functionality in AoS-ET Animals
2.4. AET Improved the Shortening and Relaxation Function in AoS Animals at a Cellular Level
2.5. AET Improves the Responsiveness of Myofilaments to Ca2+ in the Isolated Cardiomyocyte Experiment
2.6. AoS Causes Changes in Ca2+ Handling Proteins, Which Are Restored in Animals Submitted to AET
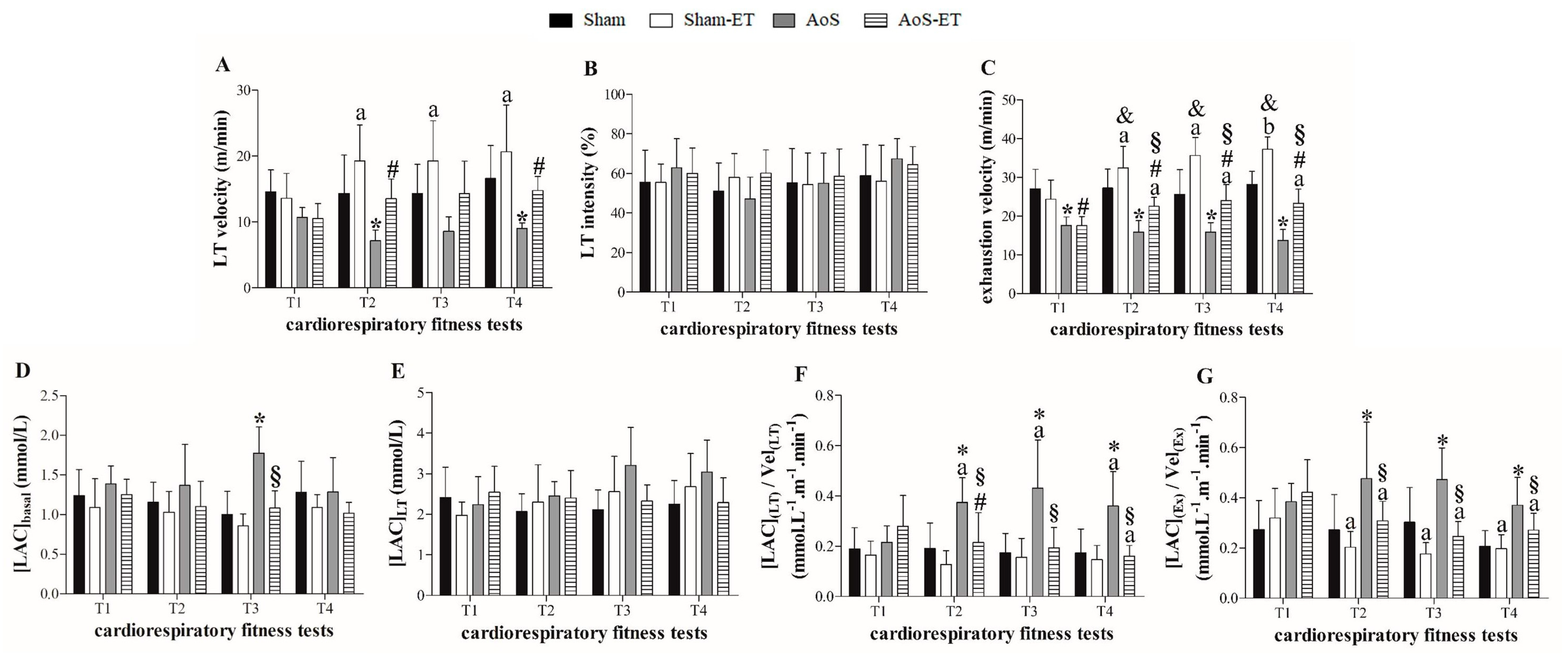
2.7. AET Improves the Functional Capacity and Metabolic Profile of Exercise in AoS Animals
3. Discussion
4. Materials and Methods
4.1. Study Design
- The first experimental line evaluated cardiac function by echocardiogram, isolated papillary muscles, and the expression of Ca2+-handling regulatory protein by Western Blot. SERCA2a and L-type Ca2+ channels activity were analyzed during post-rest contraction and Ca2+ elevation (Sham, n = 22; Sham-ET, n = 20; AoS, n = 16; AoS-ET, n = 16), respectively, and by the cumulative administration of extracellular Ca2+ in the presence of SERCA2a or L-type Ca2+ channels specific blockers in the isolated papillary muscle assay. In addition, to assess functional capacity and prescribe AET, the animals were submitted to cardiorespiratory fitness tests at weeks 18, 22, 25, and 28 of the experimental protocol.
- The second experimental line evaluated cardiac function by echocardiogram and isolated cardiomyocyte assay, which measured mechanical function and Ca2+ handling (Sham, n = 6; Sham-ET, n = 6; AoS, n = 6; AoS-ET, n = 6). The week 28 cardiorespiratory fitness tests were not performed on these animals due to technical issues.
4.2. Animals
4.3. AoS Surgery
4.4. Cardiorespiratory Fitness Test (CFT)
4.5. Aerobic Exercise Training (AET)
4.6. Cardiac Function
4.6.1. Echocardiogram
4.6.2. Isolated Papillary Muscle Assay
4.6.3. Isolated Cardiomyocyte Assay
- Cardiomyocyte Contractility
- Intracellular Ca2+ Measurements
4.7. Expression of Ca2+ Handling Protein
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blair, S.N. Exercício, Envelhecimento Bem-Sucedido e Prevenção das Doenças. In Fisiologia do Exercício: Nutrição, Energia e Desempenho Humano, 7th ed.; Guanabara Koogan: Barueri, SP, Brazil, 2011; pp. 854–957. [Google Scholar]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell. Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Anderson, E.; Durstine, J.L. Physical activity, exercise, and chronic disease: A brief review. Sports Med. Health Sci. 2019, 10, 3–10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 30 May 2023).
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Health benefits of physical activity: The evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [PubMed]
- Leskinen, T.; Stenholm, S.; Aalto, V.; Head, J.; Kivimäki, M.; Vahtera, J. Physical activity level as a predictor of healthy and chronic disease-free life expectancy between ages 50 and 75. Age Ageing 2018, 47, 423–429. [Google Scholar] [CrossRef]
- Zanesco, A.; Antunes, E. Effects of exercise training on the cardiovascular system: Pharmacological approaches. Pharmacol. Ther. 2007, 114, 307–317. [Google Scholar] [CrossRef]
- Myers, J. Exercise and Cardiovascular Health. Circulation 2003, 107, 81. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B.K. Exercise as medicine—Evidence for prescribing exercise as 95 therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef]
- Mota, G.A.F.; de Souza, S.L.B.; da Silva, V.L.; Gatto, M.; de Campos, D.H.S.; Sant’ana, P.G.; Vileigas, D.F.; Padovani, C.R.; Casarini, D.E.; Oliveira, E.M.; et al. Cardioprotection Generated by Aerobic Exercise Training is Not Related to the Proliferation of Cardiomyocytes and Angiotensin-(1-7) Levels in the Hearts of Rats with Supravalvar Aortic Stenosis. Cell. Physiol. Biochem. 2020, 54, 719–735. [Google Scholar] [CrossRef]
- de Souza, S.L.B.; Mota, G.A.F.; da Silva, V.L.; Sant’ana, P.G.; Vileigas, D.F.; de Campos, D.H.S.; Padovani, C.R.; Rodrigues, M.A.M.; Nascimento, A.F.D.; Sugizaki, M.M.; et al. Adjustments in β-Adrenergic Signaling Contribute to the Amelioration of Cardiac Dysfunction by Exercise Training in Supravalvular Aortic Stenosis. Cell. Physiol. Biochem. 2020, 54, 665–681. [Google Scholar] [CrossRef]
- Kaminsky, L.A.; Arena, R.; Ellingsen, Ø.; Harber, M.P.; Myers, J.; Ozemek, C.; Ross, R. Cardiorespiratory fitness and cardiovascular disease—The past, present, and future. Prog. Cardiovasc. Dis. 2019, 62, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, J.A.; Veteto, A.B.; Lambert, M.D.; Olver, T.D.; Ferguson, B.S.; McDonald, K.S.; Emter, C.A.; Domeier, T.L. Chronic low-intensity exercise attenuates cardiomyocyte contractile dysfunction and impaired adrenergic responsiveness in aortic-banded mini-swine. J. Appl. Physiol. 2018, 124, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.; Rolim, N.P.L.; Oliveira, R.S.F.; Rosa, K.T.; Mattos, K.C.; Casarini, D.E.; Irigoyen, M.C.; Krieger, E.M.; Krieger, J.E.; Negrão, C.E.; et al. Exercise training delays cardiac dysfunction and prevents calcium handling abnormalities in sympathetic hyperactivity-induced heart failure mice. J. Appl. Physiol. 2008, 104, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, B.C.; Ooi, J.Y.Y.; Weeks, K.L.; Patterson, N.L.; McMullen, J.R. Understanding Key Mechanisms of Exercise-Induced Cardiac Protection to Mitigate Disease: Current Knowledge and Emerging Concepts. Physiol. Rev. 2018, 98, 419–475. [Google Scholar] [CrossRef]
- Melo, S.F.S.; Barauna, V.G.; Neves, V.J.; Fernandes, T.; Lara, L.S.; Mazzotti, D.R.; Oliveira, E.M. Exercise training restores the cardiac microRNA-1 and -214 levels regulating Ca2+ handling after myocardial infarction. BMC Cardiovasc. Disord. 2015, 15, 166. [Google Scholar] [CrossRef]
- Garciarena, C.D.; Pinilla, A.O.; Nolly, M.B.; Laguens, R.P.; Escudero, E.M.; Cingolani, H.E.; Weeks, K.L.; Patterson, N.L. Endurance training in the spontaneously hypertensive rat: Conversion of pathological into physiological cardiac hypertrophy. Hypertension 2009, 53, 708–714. [Google Scholar] [CrossRef]
- Lu, L.; Mei, D.F.; Gu, A.G.; Wang, S.; Lentzner, B.; Gutstein, D.E.; Zwas, D.; Homma, S.; Yi, G.-H.; Wang, J. Exercise training normalizes altered calcium-handling proteins during development of heart failure. J. Appl. Physiol. 2002, 92, 1524–1530. [Google Scholar] [CrossRef]
- Høydal, M.A.; Stølen, T.O.; Kettlewell, S.; Maier, L.S.; Brown, J.H.; Sowa, T.; Hoydal, M.A.; Ellingsen, O. Exercise training reverses myocardial dysfunction induced by CaMKII overexpression by restoring Ca2+ homeostasis. J. Appl. Physiol. 2016, 121, 212–220. [Google Scholar] [CrossRef]
- Carneiro-Júnior, M.A.; Quintão-Júnior, J.F.; Drummond, L.R.; Lavorato, V.N.; Drummond, F.R.; Amadeu, M.A.; Oliveira, E.; Felix, L.; Cruz, J.; Mill, J.; et al. Effect of exercise training on Ca2+ release units of left ventricular myocytes of spontaneously hypertensive rats. Braz. J. Med. Biol. Res. 2014, 47, 960–965. [Google Scholar] [CrossRef][Green Version]
- Kemi, O.J.; MacQuaide, N.; Hoydal, M.A.; Ellingsen, O.; Smith, G.L.; Wisløff, U. Exercise training corrects control of spontaneous calcium waves in hearts from myocardial infarction heart failure rats. J. Cell. Physiol. 2012, 227, 20–26. [Google Scholar] [CrossRef]
- Bito, V.; de Waard, M.C.; Biesmans, L.; Lenaerts, I.; Ozdemir, S.; van Deel, E.; Abdel-Mottaleb, Y.; Driesen, R.; Holemans, P.; Duncker, D.J.; et al. Early exercise training after myocardial infarction prevents contractile but not electrical remodeling or hypertrophy. Cardiovasc. Res. 2010, 86, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Rolim, N.P.; Medeiros, A.; Rosa, K.T.; Mattos, K.C.; Irigoyen, M.C.; Krieger, E.M.; Krieger, J.E.; Negrão, C.E.; Brum, P.C. Exercise training improves the net balance of cardiac Ca2+ handling protein expression in heart failure. Physiol. Genom. 2007, 29, 246–252. [Google Scholar] [CrossRef]
- Wisløff, U.; Loennechen, J.P.; Currie, S.; Smith, G.L.; Ellingsen, O. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA2a in rat after myocardial infarction. Cardiovasc. Res. 2002, 54, 162–174. [Google Scholar] [CrossRef] [PubMed]
- van Deel, E.D.; de Boer, M.; Kuster, D.W.; Boontje, N.M.; Holemans, P.; Sipido, K.R.; van der Velden, J.; Duncker, D.J. Exercise training does not improve cardiac function in compensated or decompensated left ventricular hypertrophy induced by aortic stenosis. J. Mol. Cell. Cardiol. 2011, 50, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.L.; de Souza, S.L.B.; Mota, G.A.F.; de Campos, D.H.S.; Melo, A.B.; Vileigas, D.F.; Sant’ana, P.G.; Coelho, P.M.; Bazan, S.G.Z.; Leopoldo, A.S.; et al. The Dysfunctional Scenario of the Major Components Responsible for Myocardial Calcium Balance in Heart Failure Induced by Aortic Stenosis. Arq. Bras. Cardiol. 2022, 118, 463–475. [Google Scholar]
- de Tomasi, L.C.; Campos, D.H.S.; Sant’Ana, P.G.; Okoshi, K.; Padovani, C.R.; Murata, G.M.; Nguyen, S.; Kolwicz, S.C., Jr.; Cicogna, A.C. Pathological hypertrophy and cardiac dysfunction are linked to aberrant endogenous unsaturated fatty acid metabolism. PLoS ONE 2018, 13, e0193553. [Google Scholar]
- Pacagnelli, F.L.; Okoshi, K.; Campos, D.H.S.; de Souza, R.W.A.; Padovani, C.R.; Carvalho, R.F.; Aguiar, A.F.; Dal Pai, M.; Cicogna, A.C. Physical training attenuates cardiac remodeling in rats with supra-aortic stenosis. Exp. Clin. Cardiol. 2014, 20, 1–17. [Google Scholar]
- Gomes, M.J.; Martinez, P.F.; Campos, D.H.; Pagan, L.U.; Bonomo, C.; Lima, A.R.; Damatto, R.L.; Cezar, M.D.M.; Damatto, F.C.; Rosa, C.M.; et al. Beneficial Effects of Physical Exercise on Functional Capacity and Skeletal Muscle Oxidative Stress in Rats with Aortic Stenosis-Induced Heart Failure. Oxid Med. Cell Longev. 2016, 2016, 5716. [Google Scholar] [CrossRef]
- Souza, R.W.A.; Fernandez, G.J.; Cunha, J.P.Q.; Piedade, W.P.; Soares, L.C.; Souza, P.A.T.; de Campos, D.H.S.; Okoshi, K.; Cicogna, A.C.; Dal-Pai-Silva, M.; et al. Regulation of cardiac microRNAs induced by aerobic exercise training during heart failure. Am. J. Physiol. Heart. Circ. Physiol. 2015, 309, H1629–H1641. [Google Scholar] [CrossRef]
- de Souza, P.A.T.; de Souza, R.W.A.; Soares, L.C.; Piedade, W.P.; Campos, D.H.S.; Carvalho, R.F.; Padovani, C.R.; Okoshi, K.; Cicogna, A.C.; Matheus, S.M.M.; et al. Aerobic training attenuates nicotinic acethylcholine receptor changes in the diaphragm muscle during heart failure. Histol. Histopathol. 2015, 30, 801–811. [Google Scholar]
- Souza, R.W.A.; Piedade, W.P.; Soares, L.C.; Souza, P.A.T.; Aguiar, A.F.; Vechetti-Júnior, I.J.; Campos, D.H.S.; Fernandes, A.A.H.; Okoshi, K.; Carvalho, R.F.; et al. Aerobic exercise training prevents heart failure-induced skeletal muscle atrophy by anti-catabolic, but not anabolic actions. PLoS ONE 2014, 9, e110020. [Google Scholar] [CrossRef]
- Mann, D.L. Pathophysiology of Heart Failure. In Braunwald’s Heart Disease—A Textbook of Cardiovascular Medicine, 11th ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 487–504. [Google Scholar]
- Maillet, M.; van Berlo, J.H.; Molkentin, J.D. Molecular basis of physiological heart growth: Fundamental concepts and new players. Nat. Rev. Mol. Cell. Biol. 2013, 14, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Schirone, L.; Forte, M.; Palmerio, S.; Yee, D.; Nocella, C.; Angelini, F.; Pagano, F.; Schiavon, S.; Bordin, A.; Carrizzo, A.; et al. A Review of the Molecular Mechanisms Underlying the Development and Progression of Cardiac Remodeling. Oxid. Med. Cell. Longev. 2017, 2017, 195. [Google Scholar] [CrossRef]
- Diwan, A.; Hill, J.A.; Force, T.L. Molecular Basis for Heart Failure. In Heart Failure: A Companion to Braunwald’s Heart Disease, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–27. [Google Scholar]
- Katz, A.M. Heart Failure. In Physiology of the Heart, 5th ed.; Wolters Kluwer: Riverwoods, IL, USA, 2011; pp. 510–547. [Google Scholar]
- Plitt, G.D.; Spring, J.T.; Moulton, M.J.; Agrawal, D.K. Mechanisms, diagnosis, and treatment of heart failure with preserved ejection fraction and diastolic dysfunction. Expert. Rev. Cardiovasc. Ther. 2018, 16, 579–589. [Google Scholar] [CrossRef] [PubMed]
- van Heerebeek, L.; Paulus, W.J. Alterations in Ventricular Function: Diastolic Heart Failure. In Heart Failure: A Companion to Braunwald’s Heart Disease, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 156–176. [Google Scholar]
- Gu, J.; Zhao, F.; Wang, Y.; Gao, J.; Wang, X.; Xue, J.; Zhou, H. The Molecular Mechanism of Diastolic Heart Failure. Integr. Med. Int. 2015, 2, 143–148. [Google Scholar] [CrossRef]
- Souders, C.A.; Borg, T.K.; Banerjee, I.; Baudino, T.A. Pressure overload induces early morphological changes in the heart. Am. J. Pathol. 2012, 181, 1226–1235. [Google Scholar] [CrossRef]
- Silveira, C.F.S.M.P.; Campos, D.H.S.; Freire, P.P.; Deus, A.F.; Okoshi, K.; Padovani, C.R.; Cicogna, A.C. Importance of SERCA2a on early isolated diastolic dysfunction induced by supravalvular aortic stenosis in rats. Braz. J. Med. Biol. Res. 2017, 50, e5742. [Google Scholar] [CrossRef]
- Kim, G.H.; Uriel, N.; Burkhoff, D. Reverse remodeling and myocardial recovery in heart failure. Nat. Rev. Cardiol. 2018, 15, 83–96. [Google Scholar] [CrossRef]
- Wikman-Coffelt, J.; Parmley, W.W.; Mason, D.T. The Cardiac Hypertrophy Process. Analyses of Factors Determining Pathological Vs. Physiological Development. Circ. Res. 1979, 45, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Scheuer, J.; Malhotra, A.; Hirsch, C.; Capasso, J. Physiologic Cardiac Hypertrophy Corrects Contratile Protein Abnormalities Associated with Pathologic Hypertrophy in Rats. J. Clin. Investig. 1982, 70, 1300–1305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Agarwal, S.K. Cardiovascular benefits of exercise. Int. J. Gen. Med. 2012, 5, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Gielen, S.; Schuler, G.; Adams, V. Cardiovascular effects of exercise training: Molecular mechanisms. Circulation 2010, 122, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Brito Vieira, W.H.; Halsberghe, M.J.; Schwantes, M.L.; Perez, S.E.; Baldissera, V.; Prestes, J.; Farias, D.; Parizotto, N. Increased lactate threshold after five weeks of treadmill aerobic training in rats. Braz. J. Biol. 2014, 74, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A. Anaerobic threshold: Its concept and role in endurance sport. Malays J. Med. Sci. 2004, 11, 24–36. [Google Scholar]
- van Deel, E.D.; Octavia, Y.; Waard, M.C.; Boer, M.; Duncker, D.J. Exercise training has contrasting effects in myocardial infarction and pressure overload due to divergent endothelial nitric oxide synthase regulation. Int. J. Mol. Sci. 2018, 19, 1968. [Google Scholar] [CrossRef]
- Kemi, O.J.; Ceci, M.; Condorelli, G.; Smith, G.L.; Wisløff, U. Myocardial sarcoplasmic reticulum Ca2+ ATPase function is increased by aerobic interval training. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 145–148. [Google Scholar] [CrossRef]
- Wisløff, U.; Loennechen, J.P.; Falck, G.; Beisvag, V.; Currie, S.; Smith, G.; Ellingsen, D. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats. Cardiovasc. Res. 2001, 50, 495–508. [Google Scholar] [CrossRef]
- Kemi, O.J.; Haram, P.M.; Loennechen, J.P.; Osnes, J.B.; Skomedal, T.; Wisløff, U.; Ellingsen, D. Moderate vs. high exercise intensity: Differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc. Res. 2005, 67, 161–172. [Google Scholar] [CrossRef]
- Morán, M.; Saborido, A.; Megías, A. Ca2+ regulatory systems in rat myocardium are altered by 24 weeks treadmill training. Pflugers Arch. 2003, 446, 161–168. [Google Scholar] [CrossRef]
- Katz, A.M. Excitation-Contraction Coupling: Extracellular and Intracellular Calcium Cycles. In Physiology of the Heart, 5th ed.; Wolters Kluwer: Riverwoods, IL, USA, 2011; pp. 143–176. [Google Scholar]
- Wang, K.; Xu, Y.; Sun, Q.; Long, J.; Liu, J.; Ding, J. Mitochondria regulate cardiac contraction through ATP-dependent and independent mechanisms. Free Radic. Res. 2018, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.C.; Queliconi, B.B.; Bozi, L.H.M.; Bechara, L.R.G.; Dourado, P.M.M.; Andres, A.M. Exercise reestablishes autophagic flux and mitochondrial quality control in heart failure. Autophagy 2017, 13, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.D., Jr.; Jara, Z.P.; Peres, R.; Lima, L.S.; Scavone, C.; Montezano, A.C. Temporal changes in cardiac oxidative stress, inflammation and remodeling induced by exercise in hypertension: Role for local angiotensin II reduction. PLoS ONE 2017, 12, e0189535. [Google Scholar] [CrossRef] [PubMed]
- Mokelke, E.A.; Palmer, B.M.; Cheung, J.Y.; Moore, R.L. Endurance training does not affect intrinsic calcium current characteristics in rat myocardium. Am. J. Physiol.—Heart Circ. Physiol. 1997, 42, H1193–H1197. [Google Scholar] [CrossRef]
- Delgado, J.; Saborido, A.; Morán, M.; Megías, A. Chronic and acute exercise do not alter Ca2+ regulatory systems and ectonucleotidase activities in rat heart. J. Appl. Physiol. 1999, 87, 152–156. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Zheng, J.; Shu, M.; Zhu, S.; Jie, D. Swimming training can affect intrinsic calcium current characteristics in rat myocardium. Eur. J. Appl. Physiol. 2008, 104, 549–555. [Google Scholar] [CrossRef]
- Bregagnollo, E.A.; Zornoff, L.A.M.; Okoshi, K.; Sugizaki, M.; Mestrinel, M.A.; Padovani, C.R.; Cicogna, A.C. Myocardial contractile dysfunction contributes to the development of heart failure in rats with aortic stenosis. Int. J. Cardiol. 2007, 117, 109–114. [Google Scholar] [CrossRef]
- Bregagnollo, E.A.; Mestrinel, M.A.; Okoshi, K.; Carvalho, F.C.; Bregagnollo, I.F.; Padovani, C.R.; Angelini, F.; Pagano, F. Relative role of left ventricular geometric remodeling and of morphological and functional myocardial remodeling in the transition from compensated hypertrophy to heart failure in rats with supravalvar aortic stenosis. Arq. Bras. Cardiol. 2007, 88, 225–233. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, B.; Figueroa, D.M.; Mostarda, C.T.; Heeren, M.V.; Irigoyen, M.C.; De Angelis, K. Maximal exercise test is a useful method for physical capacity and oxygen consumption determination in streptozotocin-diabetic rats. Cardiovasc. Diabetol. 2007, 6, 38. [Google Scholar] [CrossRef]



| Before AET | After AET | |||||||
|---|---|---|---|---|---|---|---|---|
| Sham | Sham-ET | AoS | AoS-ET | Sham | Sham-ET | AoS | AoS-ET | |
| BW (g) | 469 ± 38 | 472 ± 55 | 450 ± 54 | 452 ± 59 | 519 ± 45 | 494 ± 60 | 494 ± 70 | 487 ± 59 |
| HR (bpm) | 285 (280; 301) | 290 (269; 301) | 290 (262; 312) | 290 (280; 301) | 298 ± 42 | 292 ± 37 | 293 ± 43 | 305 ± 36 |
| LVDD (mm) | 7.41 (6.90; 7.66) | 7.30 (6.90; 7.59) | 7.88 (7.21; 8.46) | 7.41 (6.32; 7.95) | 7.41 (7.15; 7.66) | 7.41 (7.09; 7.92) | 8.43 (7.27; 9.13) ** | 7.92 (7.34; 8.49) |
| LVSD (mm) | 3.07 (2.81; 3.32) | 2.81 (2.55; 3.07) | 3.58 (3.24; 4.02) | 3.32 (2.74; 3.58) | 3.07 (2.81; 3.32) | 3.07 (2.81; 3.38) | 3.65 (3.32; 5.40) ** | 3.58 (2.81; 4.52) |
| PWDT (mm) | 1.53 (1.53; 1.53) | 1.53 (1.53; 1.57) | 3.07 (2.81; 3.07) * | 2.92 (2.81; 3.07) # | 1.53 (1.53; 1.65) | 1.53 (1.53; 1.61) | 2.81 (2.55; 3.03) ** | 2.30 (2.55; 2.81) ## |
| ISDT (mm) | 1.56 (1.53; 1.65) | 1.55 (1.53; 1.65) | 3.07 (2.98; 3.24) * | 3.07 (3.07; 3.32) # | 1.62 (1.53; 1.70) | 1.60 (1.53; 1.65) | 2.95 (2.81; 3.07) ** | 2.81 (2.55; 3.07) ## |
| RWT | 0.43 ± 0.03 | 0.43 ± 0.04 | 0.76 ± 0.13 * | 0.81 ± 0.11 # | 0.43 ± 0.03 | 0.42 ± 0.03 | 0.68 ± 0.14 ** | 0.68 ± 0.11 ## |
| LA/Ao | 1.21 (1.19; 1.30) | 1.23 (1.19; 1.29) | 1.93 (1.67; 2.00) * | 1.87 (1.52; 2.11) # | 1.23 ± 0.09 | 1.24 ± 0.09 | 1.93 ± 0.19 ** | 1.79 ± 0.19 ##§ |
| E wave (cm/s) | 81 ± 7 | 83 ± 8 | 124 ± 24 * | 127 ± 22 # | 85 ± 7 | 82 ± 9 | 130 ± 20 ** | 120 ± 25 ##§ |
| E/A | 1.64 ± 0.22 | 1.64 ± 0.25 | 3.55 ± 1.67 * | 3.82 ± 2.00 # | 1.50 ± 0.20 | 1.54 ± 0.18 | 5.22 ± 1.42 ** | 3.82 ± 1.85 ##§ |
| E′ (cm/s) | 5.60 (5.30; 5.80) | 5.60 (3.20; 6.10) | 5.40 (3.70; 6.25) | 5.40 (3.70; 6.12) | 6.13 ± 0.77 | 6.20 ± 0.73 | 5.19 ± 0.99 ** | 5.40 ± 1.18 ## |
| A′ (cm/s) | 3.75 (3.70; 3.90) | 3.70 (3.35; 4.20) | 3.30 (3.03; 5.35) | 3.20 (3.20; 3.75) | 4.24 ± 0.72 | 4.10 ± 0.60 | 3.08 ± 1.20 ** | 3.95 ± 1.50 § |
| E/E′ | 14.3 ± 1.58 | 14.9 ± 1.98 | 25.9 ± 6.78 * | 26.3 ± 7.82 # | 14.1 ± 2.24 | 13.4 ± 1.6 | 25.6 ± 4.85 ** | 22.5 ± 3.66 ##§ |
| EDT (ms) | 48 ± 7 | 48 ± 5 | 34 ± 11 * | 36 ± 13 # | 49 ± 6 | 50 ± 4 | 28 ± 8 ** | 34 ± 11 ##§ |
| PWSV (cm/s) | 67 ± 9 | 71 ± 7 | 53 ± 12 * | 55 ± 8 # | 68 ± 9 | 70 ± 9 | 37 ± 10 ** | 49 ± 9 ##§ |
| FS (%) | 26 ± 3 | 28 ± 4 | 25 ± 4 | 25 ± 4 # | 26 ± 3 | 25 ± 4 | 23 ± 5 ** | 24 ± 4 |
| EF (%) | 93 (91; 94) | 94 (92; 95) | 90 (87; 92) | 89 (79; 92) # | 93 ± 2 | 92 ± 3 | 86 ± 9 ** | 90 ± 4 § |
| Sham | Sham-ET | AoS | AoS-ET | |
|---|---|---|---|---|
| CSA (mm2) | 1.14 ± 0.13 | 1.15 ± 0.14 | 1.16 ± 0.16 | 1.20 ± 0.19 |
| DT (g/mm2) | 6.23 ± 1.43 | 6.12 ± 1.61 | 5.20 ± 1.11 | 5.23 ± 1.96 |
| RT (g/mm2) | 0.57 ± 0.19 | 0.61 ± 0.18 | 0.82 ± 0.22 * | 0.66 ± 0.17 § |
| +dT/dt (g/mm2/s) | 66.6 ± 17.2 | 65.9 ± 18.1 | 46.9 ± 10.3 * | 47.9 ± 20.0 # |
| −dT/dt (g/mm2/s) | 21.8 ± 5.50 | 22.1 ± 5.39 | 24.2 ± 5.57 | 23.0 ± 7.61 |
| TPT (ms) | 180 (180; 200) | 180 (160; 180) | 210 (182; 220) | 200 (180; 210) # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, V.L.; Mota, G.A.F.; de Souza, S.L.B.; de Campos, D.H.S.; Melo, A.B.; Vileigas, D.F.; Coelho, P.M.; Sant’Ana, P.G.; Padovani, C.; Lima-Leopoldo, A.P.; et al. Aerobic Exercise Training Improves Calcium Handling and Cardiac Function in Rats with Heart Failure Resulting from Aortic Stenosis. Int. J. Mol. Sci. 2023, 24, 12306. https://doi.org/10.3390/ijms241512306
da Silva VL, Mota GAF, de Souza SLB, de Campos DHS, Melo AB, Vileigas DF, Coelho PM, Sant’Ana PG, Padovani C, Lima-Leopoldo AP, et al. Aerobic Exercise Training Improves Calcium Handling and Cardiac Function in Rats with Heart Failure Resulting from Aortic Stenosis. International Journal of Molecular Sciences. 2023; 24(15):12306. https://doi.org/10.3390/ijms241512306
Chicago/Turabian Styleda Silva, Vítor Loureiro, Gustavo Augusto Ferreira Mota, Sérgio Luiz Borges de Souza, Dijon Henrique Salomé de Campos, Alexandre Barroso Melo, Danielle Fernandes Vileigas, Priscila Murucci Coelho, Paula Grippa Sant’Ana, Carlos Padovani, Ana Paula Lima-Leopoldo, and et al. 2023. "Aerobic Exercise Training Improves Calcium Handling and Cardiac Function in Rats with Heart Failure Resulting from Aortic Stenosis" International Journal of Molecular Sciences 24, no. 15: 12306. https://doi.org/10.3390/ijms241512306
APA Styleda Silva, V. L., Mota, G. A. F., de Souza, S. L. B., de Campos, D. H. S., Melo, A. B., Vileigas, D. F., Coelho, P. M., Sant’Ana, P. G., Padovani, C., Lima-Leopoldo, A. P., Bazan, S. G. Z., Leopoldo, A. S., & Cicogna, A. C. (2023). Aerobic Exercise Training Improves Calcium Handling and Cardiac Function in Rats with Heart Failure Resulting from Aortic Stenosis. International Journal of Molecular Sciences, 24(15), 12306. https://doi.org/10.3390/ijms241512306






