SPARC Is Highly Expressed in Young Skin and Promotes Extracellular Matrix Integrity in Fibroblasts via the TGF-β Signaling Pathway
Abstract
1. Introduction
2. Results
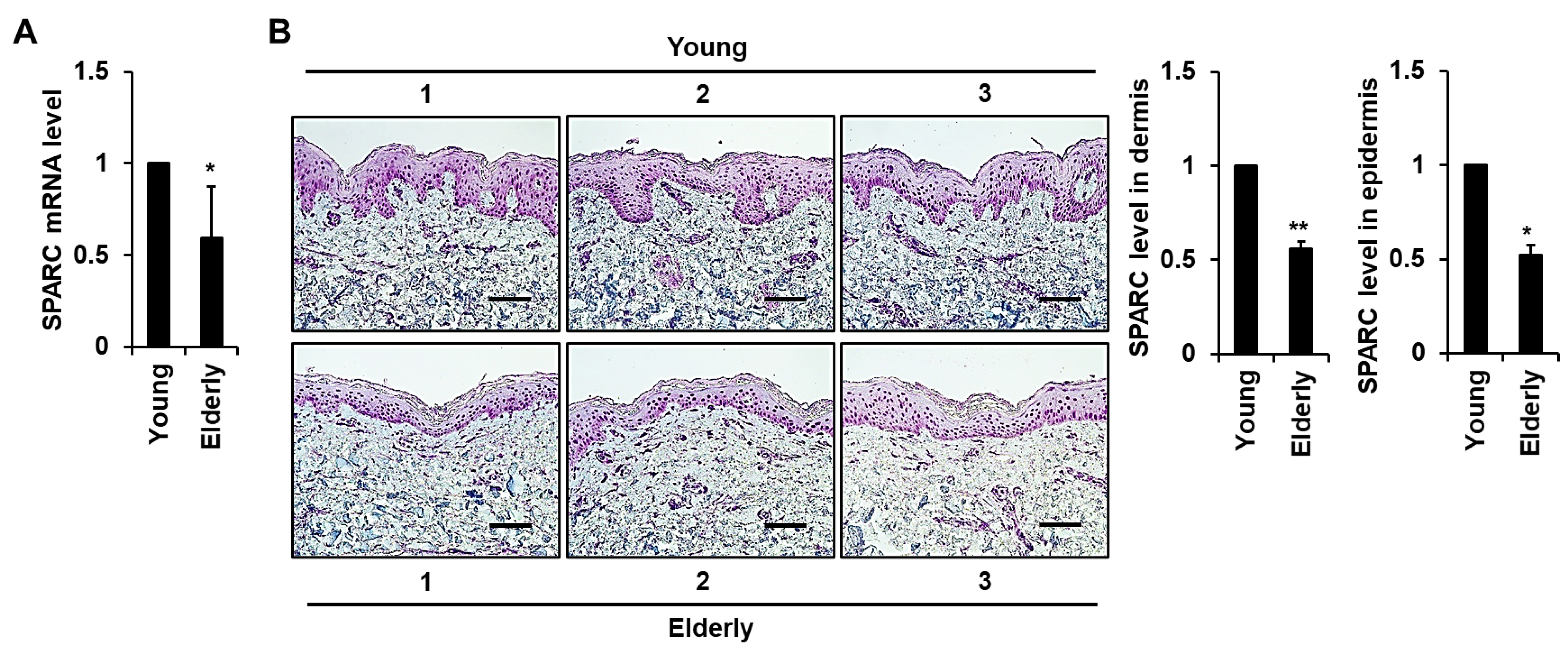
2.1. Expression of SPARC Is Reduced in Elderly Skin Tissues
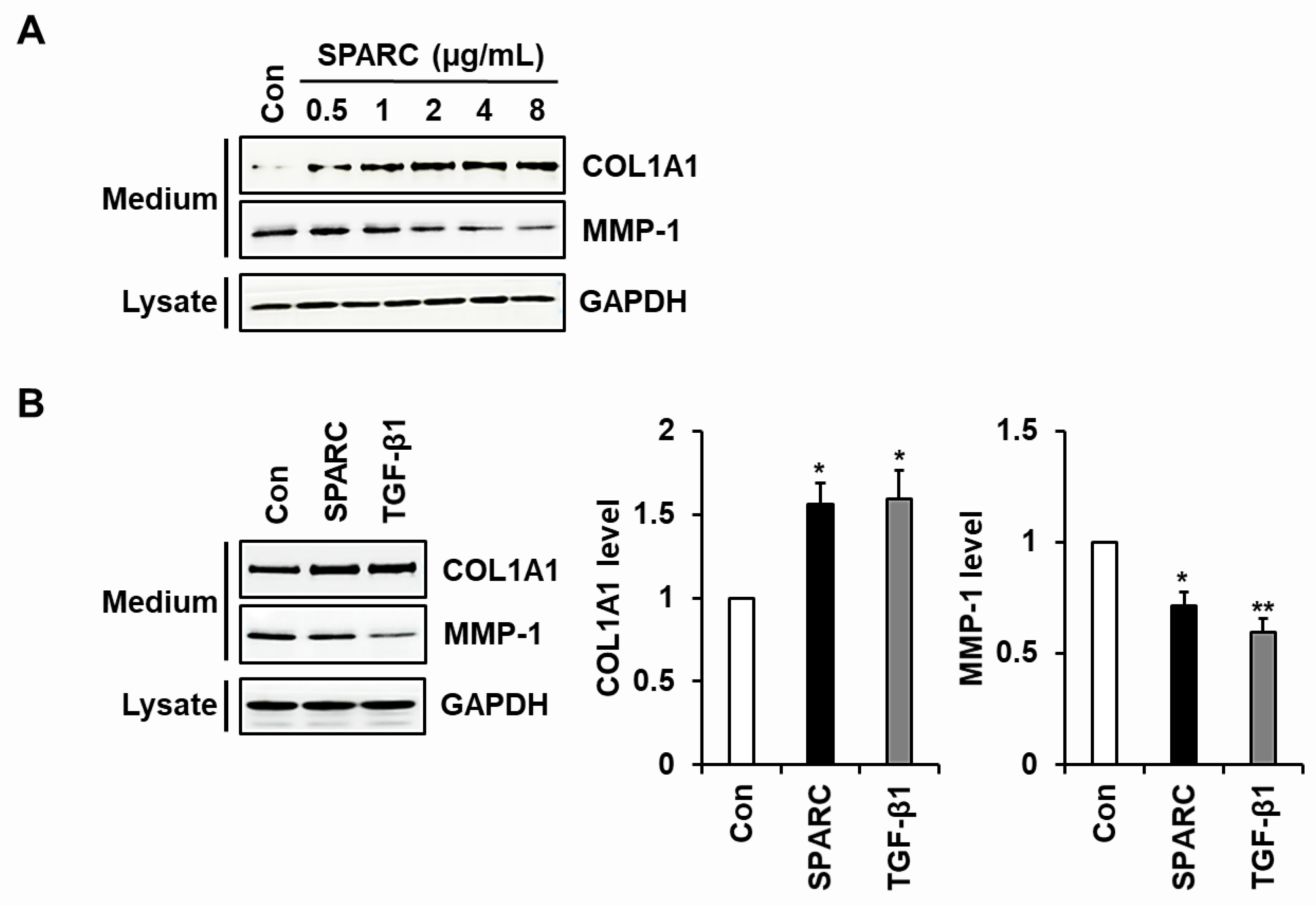
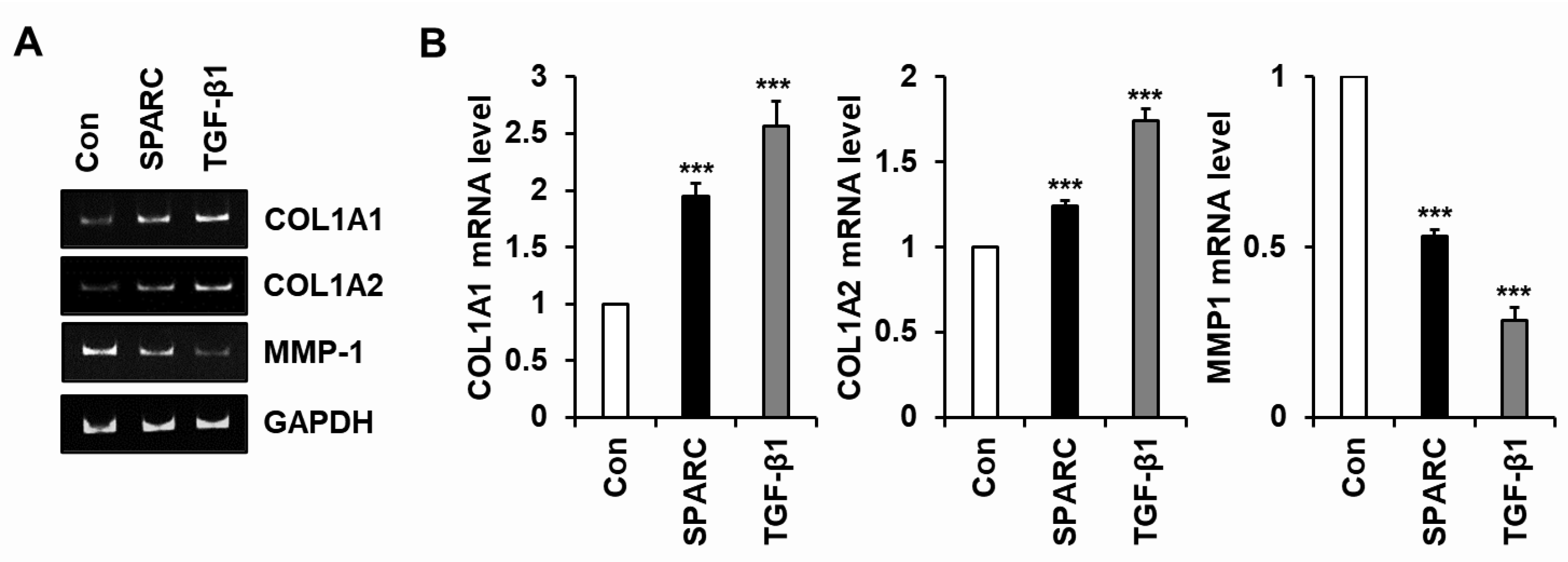
2.2. SPARC Increased Type I Collagen and Reduced MMP-1 in Human Skin Fibroblasts
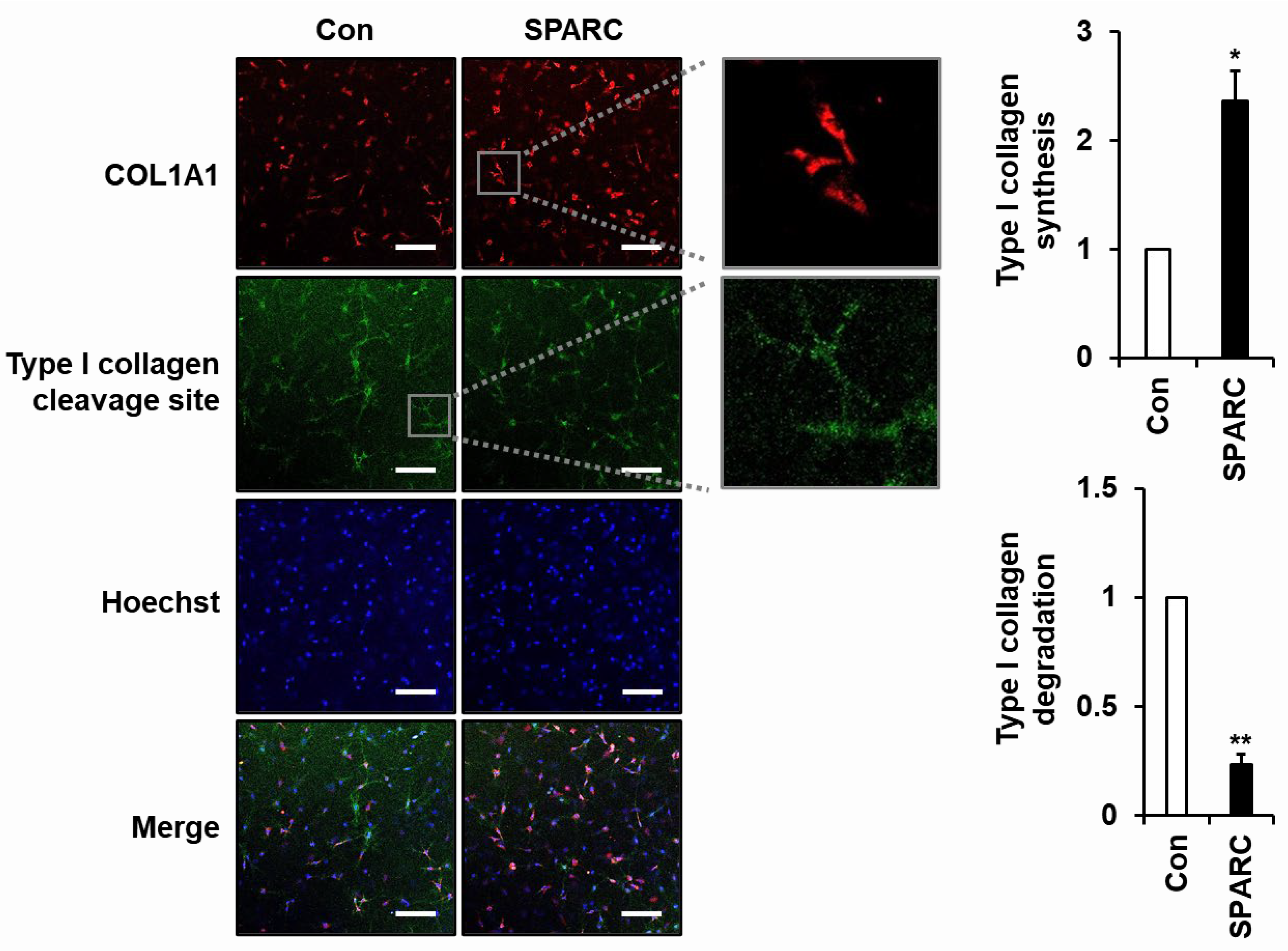
2.3. SPARC Increased Secretion of Type I Collagen and Decreased Its Degradation in 3D Cultured Fibroblasts
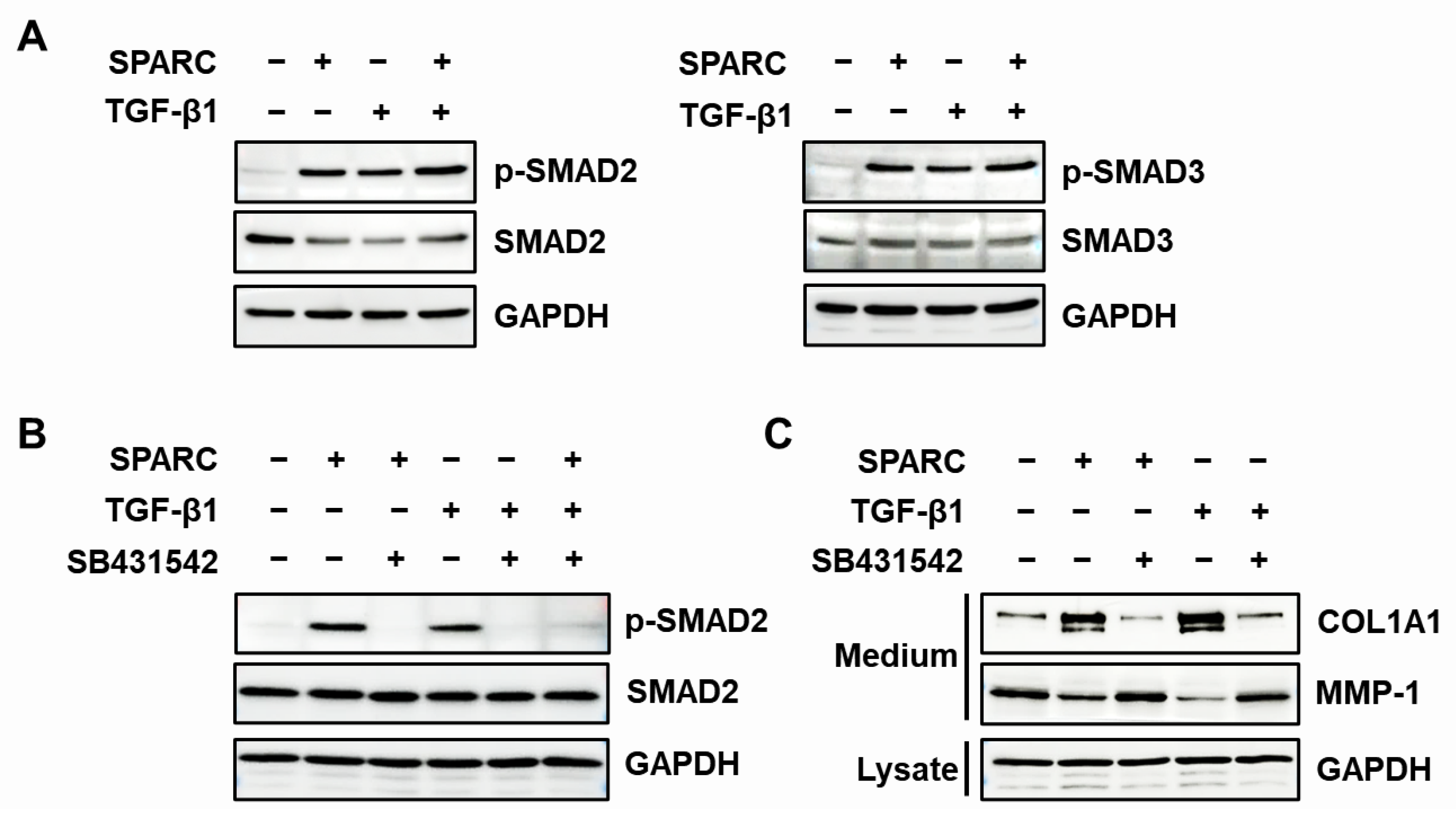
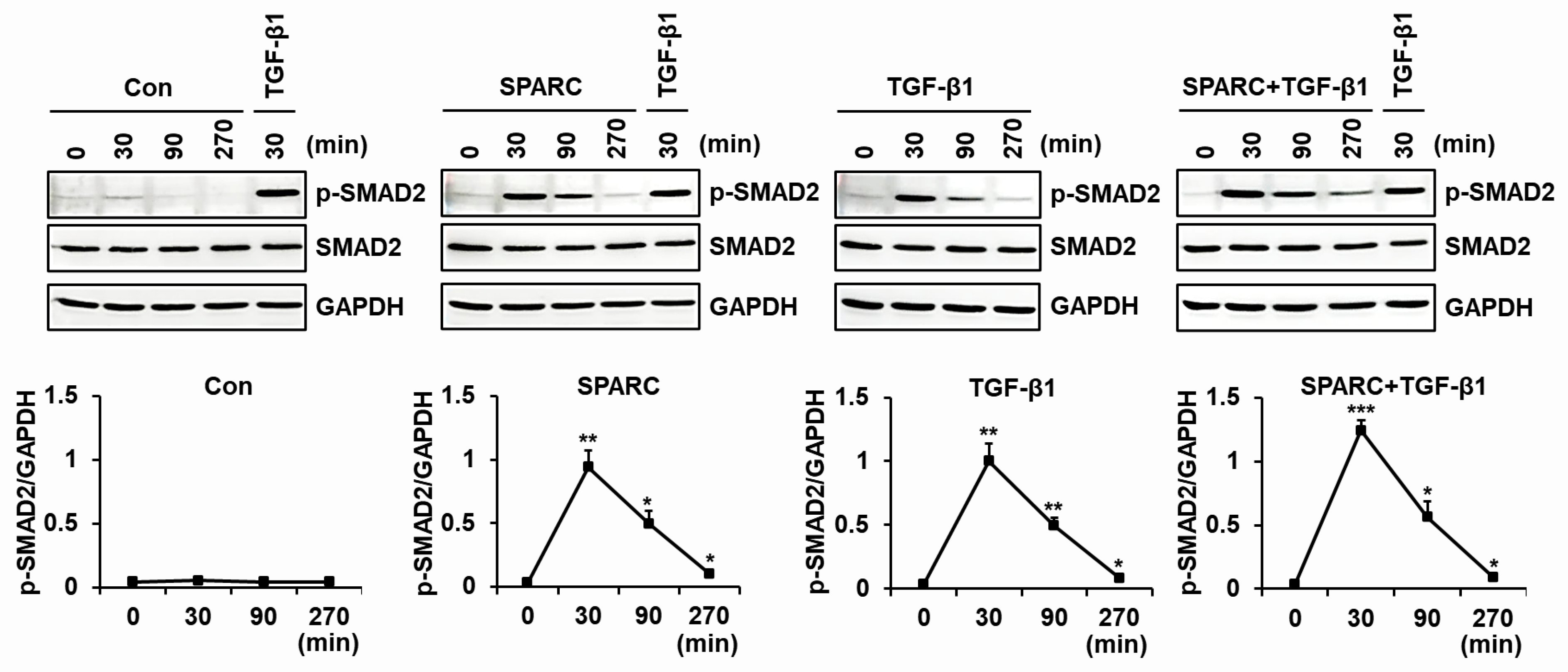
2.4. SPARC Enhances the Activity of R-SMADs and TGF-β Receptors
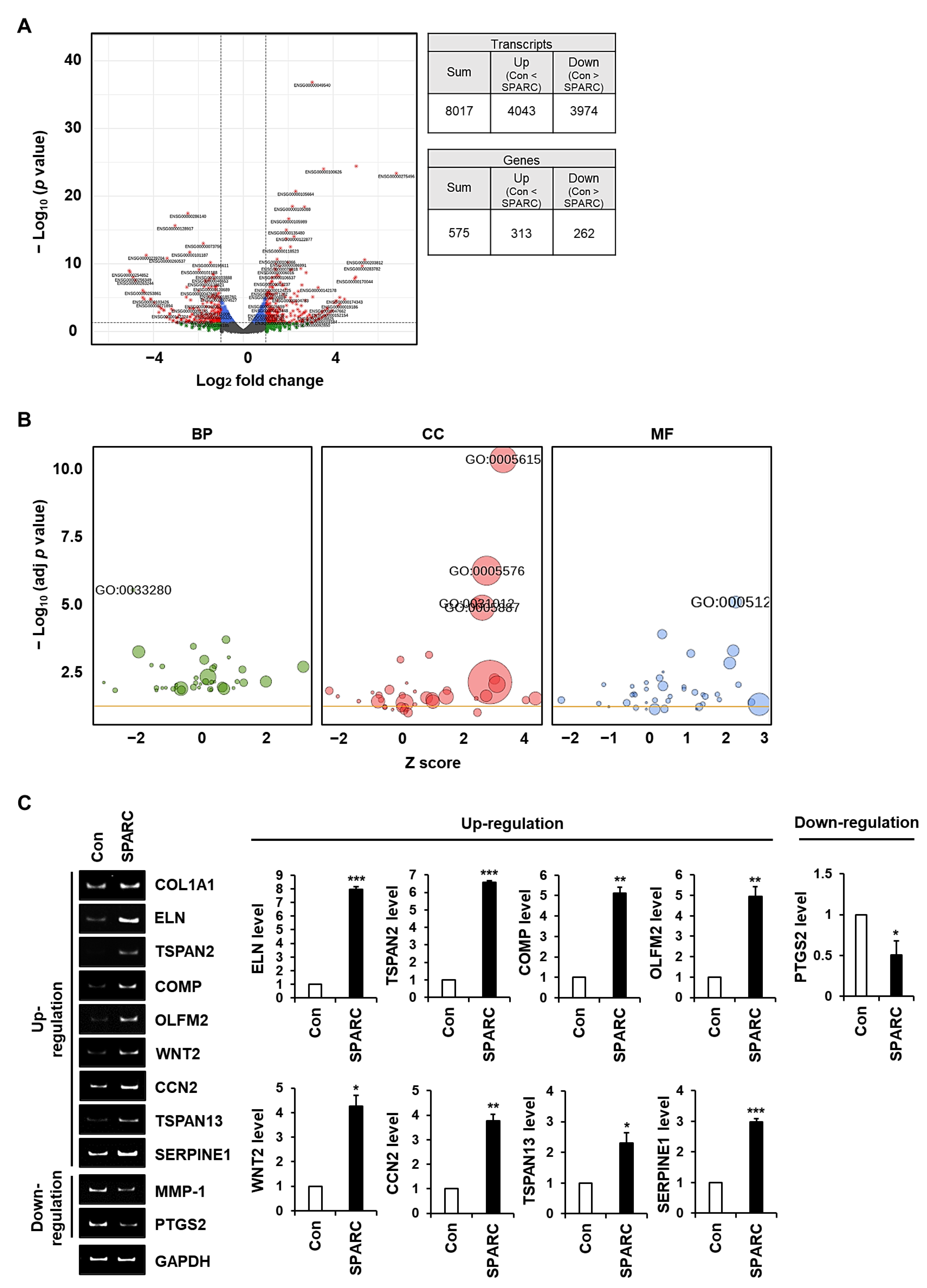
2.5. SPARC Regulates the Expression of Genes Associated with ECM Integrity in Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Antibodies
4.2. Collection of Human Skin Tissues
4.3. IHC Analysis of SPARC
4.4. Cell Culture
4.5. Construction of Human SPARC Expression Vector
4.6. Purification of Recombinant Human SPARC-His
4.7. Western Blotting
4.8. RNA Extraction and RT-PCR
4.9. Synthesis and Degradation of Type I Collagen in 3D-Culture
4.10. Transcriptomic Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scavelli, K.; Chatterjee, A.; Rhee, D.J. Secreted protein acidic and rich in cysteine in ocular tissue. J. Ocul. Pharmacol. Ther. 2015, 31, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Sage, H.; Decker, J.; Funk, S.; Chow, M. SPARC: A Ca2+-binding extracellular protein associated with endothelial cell injury and proliferation. J. Mol. Cell Cardiol. 1989, 21 (Suppl. S1), 13–22. [Google Scholar] [CrossRef] [PubMed]
- Wrana, J.L.; Overall, C.M.; Sodek, J. Regulation of the expression of a secreted acidic protein rich in cysteine (SPARC) in human fibroblasts by transforming growth factor beta. Comparison of transcriptional and post-transcriptional control with fibronectin and type I collagen. Eur. J. Biochem. 1991, 197, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Jundt, G.; Berghäuser, K.H.; Termine, J.D.; Schulz, A. Osteonectin-a differentiation marker of bone cells. Cell Tissue Res. 1987, 248, 409–415. [Google Scholar] [CrossRef]
- Hunzelmann, N.; Hafner, M.; Anders, S.; Krieg, T.; Nischt, R. BM-40 (osteonectin, SPARC) is expressed both in the epidermal and in the dermal compartment of adult human skin. J. Invest. Dermatol. 1998, 110, 122–126. [Google Scholar] [CrossRef][Green Version]
- Trombetta-Esilva, J.; Bradshaw, A.D. The function of SPARC as a mediator of fibrosis. Open Rheumatol. J. 2012, 6, 146–155. [Google Scholar] [CrossRef]
- Carvalheiro, T.; Malvar Fernández, B.; Ottria, A.; Giovannone, B.; Marut, W.; Reedquist, K.A.; Garcia, S.; Radstake, T.R. Extracellular SPARC cooperates with TGF-β signalling to induce pro-fibrotic activation of systemic sclerosis patient dermal fibroblasts. Rheumatology 2020, 59, 2258–2263. [Google Scholar] [CrossRef]
- Pichler, R.H.; Hugo, C.; Shankland, S.J.; Reed, M.J.; Bassuk, J.A.; Andoh, T.F.; Lombardi, D.M.; Schwartz, S.M.; Bennett, W.M.; Alpers, C.E.; et al. SPARC is expressed in renal interstitial fibrosis and in renal vascular injury. Kidney Int. 1996, 50, 1978–1989. [Google Scholar] [CrossRef]
- Kuhn, C.; Mason, R.J. Immunolocalization of SPARC, tenascin, and thrombospondin in pulmonary fibrosis. Am. J. Pathol. 1995, 147, 1759–1769. [Google Scholar]
- Fan, J.; Zhang, X.; Jiang, Y.; Chen, L.; Sheng, M.; Chen, Y. SPARC knockdown attenuated TGF-β1-induced fibrotic effects through Smad2/3 pathways in human pterygium fibroblasts. Arch. Biochem. Biophys. 2021, 713, 109049. [Google Scholar] [CrossRef]
- Zhou, X.; Tan, F.K.; Guo, X.; Arnett, F.C. Attenuation of collagen production with small interfering RNA of SPARC in cultured fibroblasts from the skin of patients with scleroderma. Arthritis Rheum. 2006, 54, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tan, F.K.; Guo, X.; Wallis, D.; Milewicz, D.Z.; Xue, S.; Arnett, F.C. Small interfering RNA inhibition of SPARC attenuates the profibrotic effect of transforming growth factor beta1 in cultured normal human fibroblasts. Arthritis Rheum. 2005, 52, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Termine, J.D.; Kleinman, H.K.; Whitson, S.W.; Conn, K.M.; McGarvey, M.L.; Martin, G.R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell 1981, 26, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Tremble, P.M.; Lane, T.F.; Sage, E.H.; Werb, Z. SPARC, a secreted protein associated with morphogenesis and tissue remodeling, induces expression of metalloproteinases in fibroblasts through a novel extracellular matrix-dependent pathway. J. Cell Biol. 1993, 121, 1433–1444. [Google Scholar] [CrossRef]
- Bradshaw, A.D.; Sage, E.H. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J. Clin. Investig. 2001, 107, 1049–1054. [Google Scholar] [CrossRef]
- Brekken, R.A.; Sage, E.H. SPARC, a matricellular protein: At the crossroads of cell-matrix. Matrix Biol. 2000, 19, 569–580. [Google Scholar] [CrossRef]
- Tai, I.T.; Tang, M.J. SPARC in cancer biology: Its role in cancer progression and potential for therapy. Drug Resist. Updates 2008, 11, 231–246. [Google Scholar] [CrossRef]
- Pepper, M.S. Transforming growth factor-beta: Vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor. Rev. 1997, 8, 21–43. [Google Scholar] [CrossRef]
- Roberts, A.B.; Flanders, K.C.; Heine, U.I.; Jakowlew, S.; Kondaiah, P.; Kim, S.J.; Sporn, M.B. Transforming growth factor-beta, multifunctional regulator of differentiation and development. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1990, 327, 145–154. [Google Scholar]
- Nakao, A.; Imamura, T.; Souchelnytskyi, S.; Kawabata, M.; Ishisaki, A.; Oeda, E.; Tamaki, K.; Hanai, J.; Heldin, C.H.; Miyazono, K.; et al. TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. Embo J. 1997, 16, 5353–5362. [Google Scholar] [CrossRef]
- Poncelet, A.C.; Schnaper, H.W. Regulation of human mesangial cell collagen expression by transforming growth factor-beta1. Am. J. Physiol. 1998, 275, F458–F466. [Google Scholar]
- Francki, A.; McClure, T.D.; Brekken, R.A.; Motamed, K.; Murri, C.; Wang, T.; Sage, E.H. SPARC regulates TGF-beta1-dependent signaling in primary glomerular mesangial cells. J. Cell Biochem. 2004, 91, 915–925. [Google Scholar] [CrossRef]
- Schiemann, B.J.; Neil, J.R.; Schiemann, W.P. SPARC inhibits epithelial cell proliferation in part through stimulation of the transforming growth factor-beta-signaling system. Mol. Biol. Cell 2003, 14, 3977–3988. [Google Scholar] [CrossRef] [PubMed]
- Toba, H.; de Castro Brás, L.E.; Baicu, C.F.; Zile, M.R.; Lindsey, M.L.; Bradshaw, A.D. Increased ADAMTS1 mediates SPARC-dependent collagen deposition in the aging myocardium. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E1027–E1035. [Google Scholar] [CrossRef] [PubMed]
- Ford, R.; Wang, G.; Jannati, P.; Adler, D.; Racanelli, P.; Higgins, P.J.; Staiano-Coico, L. Modulation of SPARC expression during butyrate-induced terminal differentiation of cultured human keratinocytes, regulation via a TGF-beta-dependent pathway. Exp. Cell Res. 1993, 206, 261–275. [Google Scholar] [CrossRef]
- Reed, M.J.; Vernon, R.B.; Abrass, I.B.; Sage, E.H. TGF-beta 1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J. Cell Physiol. 1994, 158, 169–179. [Google Scholar] [CrossRef]
- Shiba, H.; Fujita, T.; Doi, N.; Nakamura, S.; Nakanishi, K.; Takemoto, T.; Hino, T.; Noshiro, M.; Kawamoto, T.; Kurihara, H.; et al. Differential effects of various growth factors and cytokines on the syntheses of DNA, type I collagen, laminin, fibronectin, osteonectin/secreted protein, acidic and rich in cysteine (SPARC), and alkaline phosphatase by human pulp cells in culture. J. Cell Physiol. 1998, 174, 194–205. [Google Scholar] [CrossRef]
- Shanker, G.; Olson, D.; Bone, R.; Sawhney, R. Regulation of extracellular matrix proteins by transforming growth factor beta1 in cultured pulmonary endothelial cells. Cell Biol. Int. 1999, 23, 61–72. [Google Scholar] [CrossRef]
- Bradshaw, A.D. The role of SPARC in extracellular matrix assembly. J. Cell Commun. Signal 2009, 3, 239–246. [Google Scholar] [CrossRef]
- Reed, M.J.; Puolakkainen, P.; Lane, T.F.; Dickerson, D.; Bornstein, P.; Sage, E.H. Differential expression of SPARC and thrombospondin 1 in wound repair, immunolocalization and in situ hybridization. J. Histochem. Cytochem. 1993, 41, 1467–1477. [Google Scholar] [CrossRef]
- Brekken, R.A. and E.H. Sage, SPARC, a matricellular protein, at the crossroads of cell-matrix communication. Matrix Biol. 2001, 19, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, A.D.; Puolakkainen, P.; Dasgupta, J.; Davidson, J.M.; Wight, T.N.; Helene Sage, E. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J. Investig. Dermatol. 2003, 120, 949–955. [Google Scholar] [CrossRef]
- McCabe, M.C.; Hill, R.C.; Calderone, K.; Cui, Y.; Yan, Y.; Quan, T.; Fisher, G.J.; Hansen, K.C. Alterations in extracellular matrix composition during aging and photoaging of the skin. Matrix Biol. Plus 2020, 8, 100041. [Google Scholar] [CrossRef] [PubMed]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef]
- Fligiel, S.E.; Varani, J.; Datta, S.C.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J. Investig. Dermatol. 2003, 120, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Li, Q.; Liu, Y.; Zhang, R.; Chen, H. SPARC promotes fibroblast proliferation, migration, and collagen production in keloids by inactivation of p53. J. Dermatol. Sci. 2023, 109, 2–11. [Google Scholar] [CrossRef]
- Lane, T.F.; Sage, E.H. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 1994, 8, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, P.; Sage, E.H. Matricellular proteins, extracellular modulators of cell function. Curr. Opin. Cell Biol. 2002, 14, 608–616. [Google Scholar] [CrossRef]
- Nakamura, T.; Yoshida, H.; Ota, Y.; Endo, Y.; Sayo, T.; Hanai, U.; Imagawa, K.; Sasaki, M.; Takahashi, Y. SPARC promotes production of type IV and VII collagen and their skin basement membrane accumulation. J. Dermatol. Sci. 2022, 107, 109–112. [Google Scholar] [CrossRef]
- Francki, A.; Bradshaw, A.D.; Bassuk, J.A.; Howe, C.C.; Couser, W.G.; Sage, E.H. SPARC regulates the expression of collagen type I and transforming growth factor-beta1 in mesangial cells. J. Biol. Chem. 1999, 274, 32145–32152. [Google Scholar] [CrossRef]
- Atorrasagasti, C.; Aquino, J.B.; Hofman, L.; Alaniz, L.; Malvicini, M.; Garcia, M.; Benedetti, L.; Friedman, S.L.; Podhajcer, O.; Mazzolini, G. SPARC downregulation attenuates the profibrogenic response of hepatic stellate cells induced by TGF-β1 and PDGF. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G739–G748. [Google Scholar] [CrossRef] [PubMed]
- Seet, L.F.; Su, R.; Toh, L.Z.; Wong, T.T. In vitro analyses of the anti-fibrotic effect of SPARC silencing in human Tenon’s fibroblasts, comparisons with mitomycin C. J. Cell Mol. Med. 2012, 16, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Trombetta-eSilva, J.; Eadie, E.P.; Zhang, Y.; Norris, R.A.; Borg, T.K.; Bradshaw, A.D. The effects of age and the expression of SPARC on extracellular matrix production by cardiac fibroblasts in 3-D cultures. PLoS ONE 2013, 8, e79715. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yan, Y.; Bai, S.; Zhou, Y.; Wang, X.; Feng, Z.; Li, G.; Zhou, S.; Zhang, J.; Ren, J. SPARC expression in tumor microenvironment induces partial epithelial-to-mesenchymal transition of esophageal adenocarcinoma cells via cooperating with TGF-β signaling. Cell Biol. Int. 2023, 47, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Sonnylal, S.; Shi-Wen, X.; Leoni, P.; Naff, K.; Van Pelt, C.S.; Nakamura, H.; Leask, A.; Abraham, D.; Bou-Gharios, G.; de Crombrugghe, B. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 2010, 62, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Guo, X.; Chen, S.Y. Olfactomedin 2, a novel regulator for transforming growth factor-β-induced smooth muscle differentiation of human embryonic stem cell-derived mesenchymal cells. Mol. Biol. Cell 2014, 25, 4106–4114. [Google Scholar] [CrossRef]
- Bayle, J.; Fitch, J.; Jacobsen, K.; Kumar, R.; Lafyatis, R.; Lemaire, R. Increased expression of Wnt2 and SFRP4 in Tsk mouse skin, role of Wnt signaling in altered dermal fibrillin deposition and systemic sclerosis. J. Investig. Dermatol. 2008, 128, 871–881. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Vaughan, D.E. PAI-1 in tissue fibrosis. J. Cell Physiol. 2012, 227, 493–507. [Google Scholar] [CrossRef]
- Arencibia, J.M.; Martín, S.; Pérez-Rodríguez, F.J.; Bonnin, A. Gene expression profiling reveals overexpression of TSPAN13 in prostate cancer. Int. J. Oncol. 2009, 34, 457–463. [Google Scholar] [CrossRef][Green Version]
- Zhao, J.; Wu, W.; Zhang, W.; Lu, Y.W.; Tou, E.; Ye, J.; Gao, P.; Jourd’heuil, D.; Singer, H.A.; Wu, M.; et al. Selective expression of TSPAN2 in vascular smooth muscle is independently regulated by TGF-β1/SMAD and myocardin/serum response factor. FASEB J. 2017, 31, 2576–2591. [Google Scholar] [CrossRef]
- Surowiak, P.; Gansukh, T.; Donizy, P.; Halon, A.; Rybak, Z. Increase in cyclooxygenase-2 (COX-2) expression in keratinocytes and dermal fibroblasts in photoaged skin. J. Cosmet. Dermatol. 2014, 13, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, Y.K.; Kim, M.K.; Kim, S.; Kim, J.Y.; Lee, D.H.; Chung, J.H. UV-induced inhibition of adipokine production in subcutaneous fat aggravates dermal matrix degradation in human skin. Sci. Rep. 2016, 6, 25616. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jeong, H.D.; Song, M.J.; Lee, D.H.; Chung, J.H.; Lee, S.-T. SOD3 suppresses the expression of MMP-1 and increases the integrity of extracellular matrix in fibroblasts. Antioxidants 2022, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.E.; Song, M.J.; Hara, M.; Imanaka-Yoshida, K.; Lee, D.H.; Chung, J.H.; Lee, S.-T. Effects of tenascin C on the integrity of extracellular matrix and skin aging. Int. J. Mol. Sci. 2020, 21, 8693. [Google Scholar] [CrossRef]
- Oh, S.W.; Shin, W.S.; Lee, S.-T. Anti-PTK7 monoclonal antibodies inhibit angiogenesis by suppressing PTK7 function. Cancers 2022, 14, 4463. [Google Scholar] [CrossRef]
- Shin, W.S.; Maeng, Y.S.; Jung, J.W.; Min, J.K.; Kwon, Y.G.; Lee, S.-T. Soluble PTK7 inhibits tube formation, migration, and invasion of endothelial cells and angiogenesis. Biochem. Biophys. Res. Commun. 2008, 371, 793–835. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, W.S.; Lee, S.R.; Kim, S.; Choi, S.Y.; Lee, S.-T. Anti-PTK7 monoclonal antibodies exhibit anti-tumor activity at the cellular level and in mouse xenograft models of esophageal squamous cell carcinoma. Int. J. Mol. Sci. 2022, 23, 12195. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.-T. Polyamine oxidase expression is downregulated by 17β-estradiol via estrogen receptor 2 in human MCF-7 breast cancer cells. Int. J. Mol. Sci. 2022, 23, 7521. [Google Scholar] [CrossRef]
- Jeong, H.D.; Kim, J.H.; Kwon, G.E.; Lee, S.-T. Expression of polyamine oxidase in fibroblasts induces MMP-1 and decreases the integrity of extracellular matrix. Int. J. Mol. Sci. 2022, 23, 10487. [Google Scholar] [CrossRef]
- Lee, Y.H.; Seo, E.K.; Lee, S.-T. Skullcap flavone II inhibits degradation of type I collagen by suppressing MMP-1 transcription in human skin fibroblasts. Int. J. Mol. Sci. 2019, 20, 2734. [Google Scholar] [CrossRef]
- Kim, J.E.; Song, D.; Kim, J.; Choi, J.; Kim, J.R.; Yoon, H.S.; Bae, J.S.; Han, M.; Lee, S.; Hong, J.S.; et al. Oral Supplementation with Cocoa Extract Reduces UVB-Induced Wrinkles in Hairless Mouse Skin. J. Invest. Dermatol. 2016, 136, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Q.; Xu, C.B. A convenient method for quantifying collagen fibers in atherosclerotic lesions by ImageJ software. Int. J. Clin. Exp. Med. 2017, 10, 14904–14910. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ham, S.M.; Song, M.J.; Yoon, H.-S.; Lee, D.H.; Chung, J.H.; Lee, S.-T. SPARC Is Highly Expressed in Young Skin and Promotes Extracellular Matrix Integrity in Fibroblasts via the TGF-β Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 12179. https://doi.org/10.3390/ijms241512179
Ham SM, Song MJ, Yoon H-S, Lee DH, Chung JH, Lee S-T. SPARC Is Highly Expressed in Young Skin and Promotes Extracellular Matrix Integrity in Fibroblasts via the TGF-β Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(15):12179. https://doi.org/10.3390/ijms241512179
Chicago/Turabian StyleHam, Seung Min, Min Ji Song, Hyun-Sun Yoon, Dong Hun Lee, Jin Ho Chung, and Seung-Taek Lee. 2023. "SPARC Is Highly Expressed in Young Skin and Promotes Extracellular Matrix Integrity in Fibroblasts via the TGF-β Signaling Pathway" International Journal of Molecular Sciences 24, no. 15: 12179. https://doi.org/10.3390/ijms241512179
APA StyleHam, S. M., Song, M. J., Yoon, H.-S., Lee, D. H., Chung, J. H., & Lee, S.-T. (2023). SPARC Is Highly Expressed in Young Skin and Promotes Extracellular Matrix Integrity in Fibroblasts via the TGF-β Signaling Pathway. International Journal of Molecular Sciences, 24(15), 12179. https://doi.org/10.3390/ijms241512179




