Modified Lactoperoxidase System as a Promising Anticaries Agent: In Vitro Studies on Streptococcus mutans Biofilms
Abstract
1. Introduction
2. Results
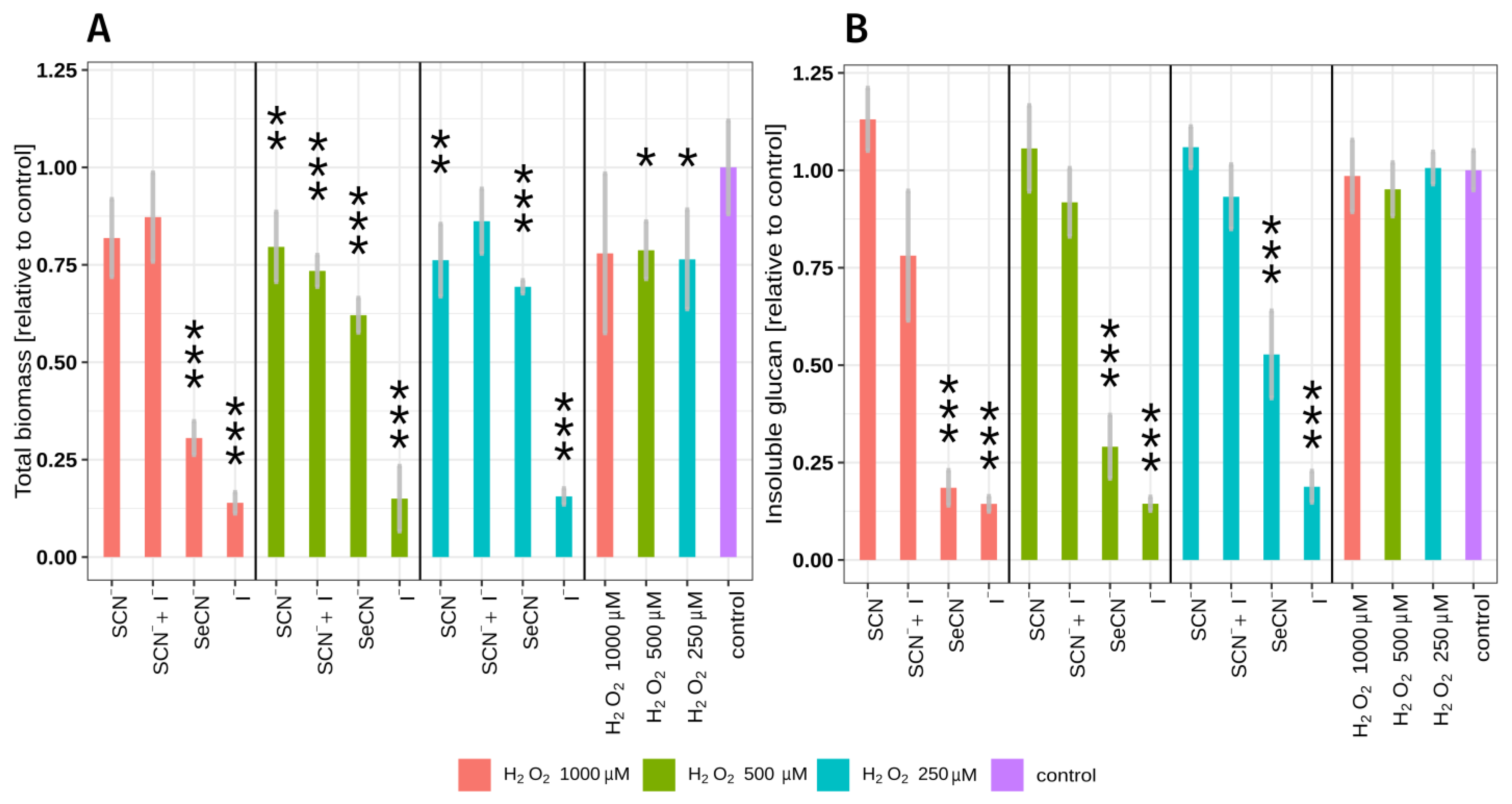
2.1. LPO System Influence on Total Biomass and Insoluble Polysaccharide Mass
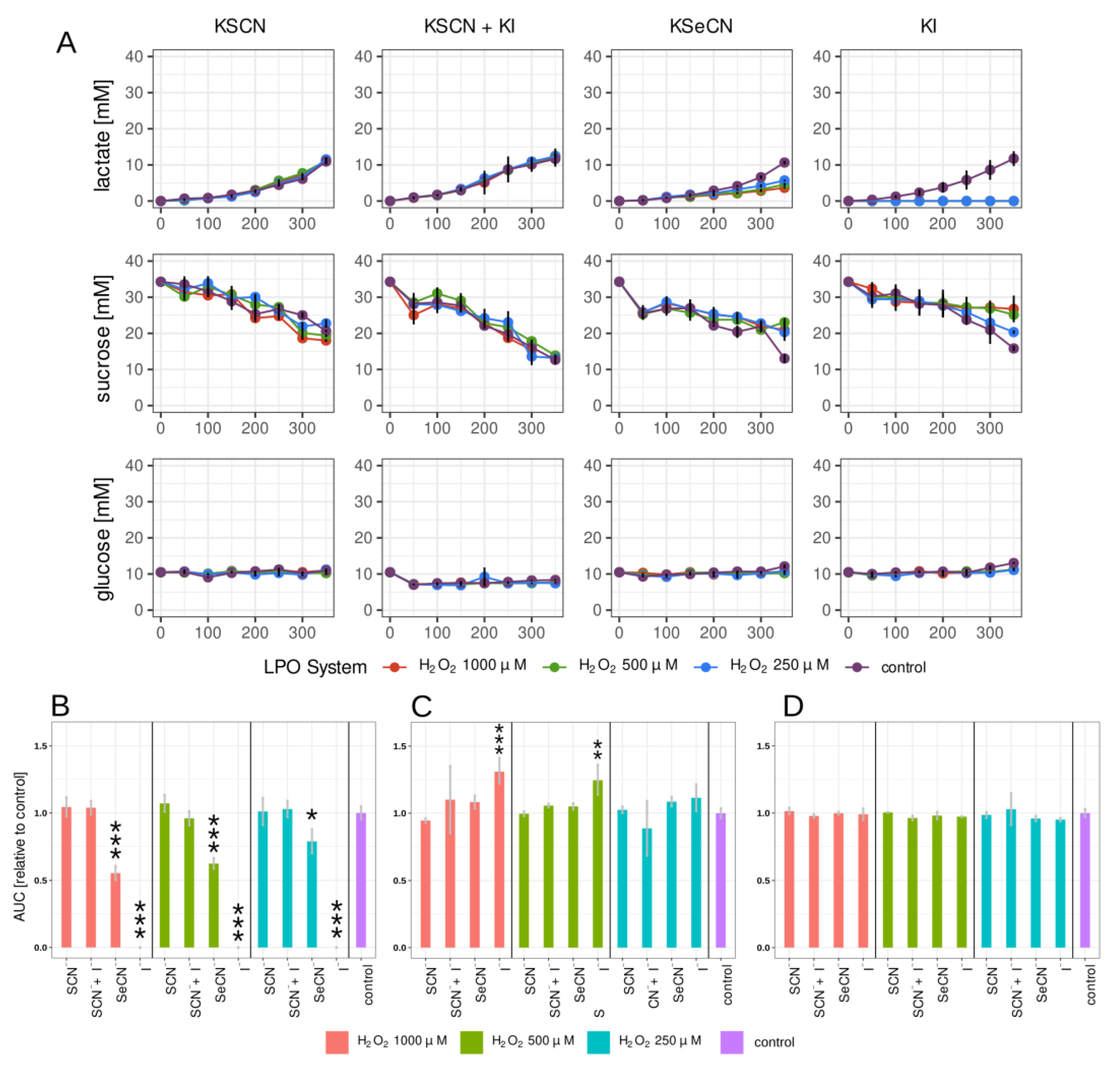
2.2. Lactate, Glucose, and Sucrose Metabolism in Biofilm
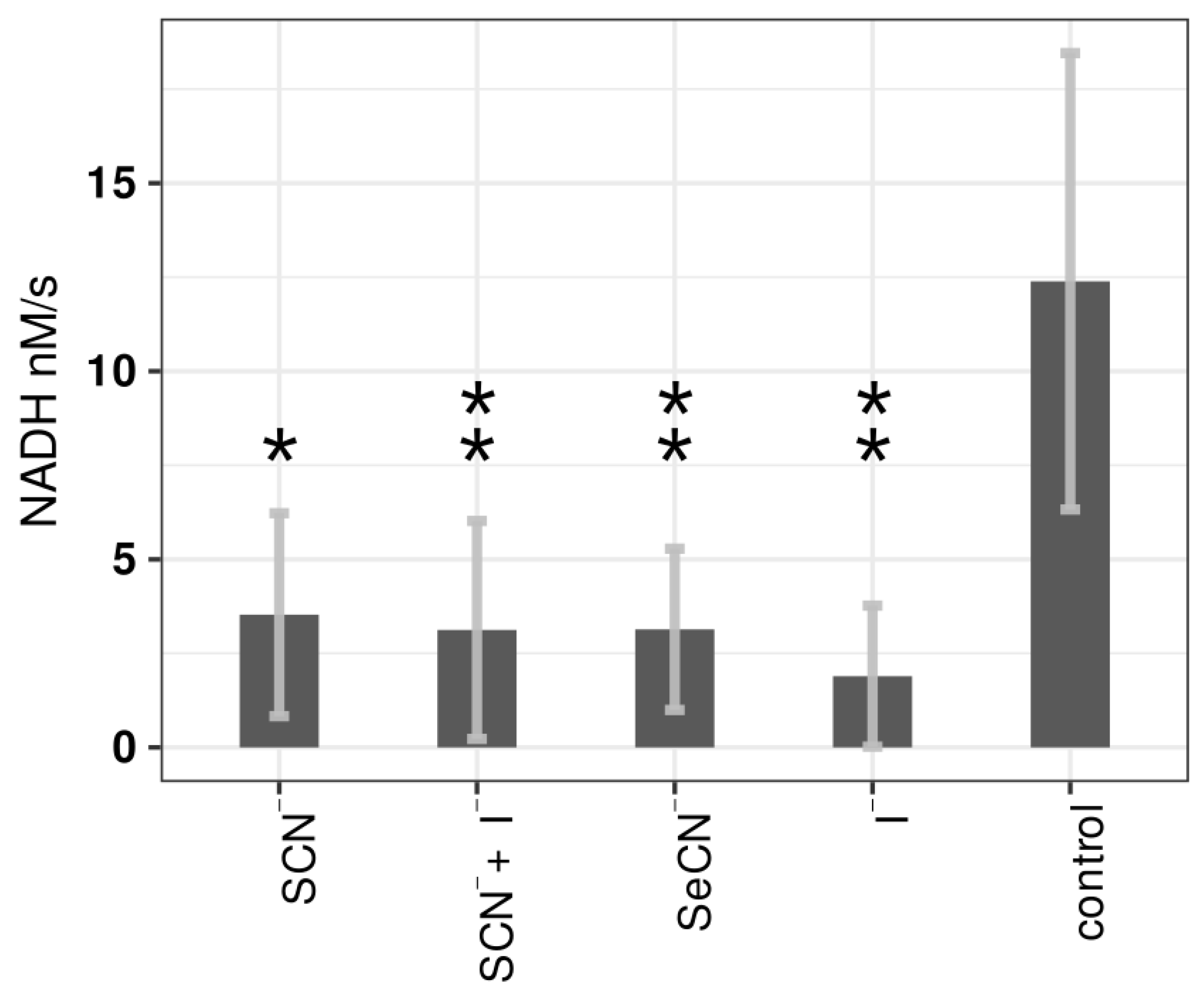
2.3. Influence of LPO Systems on PTS Activity
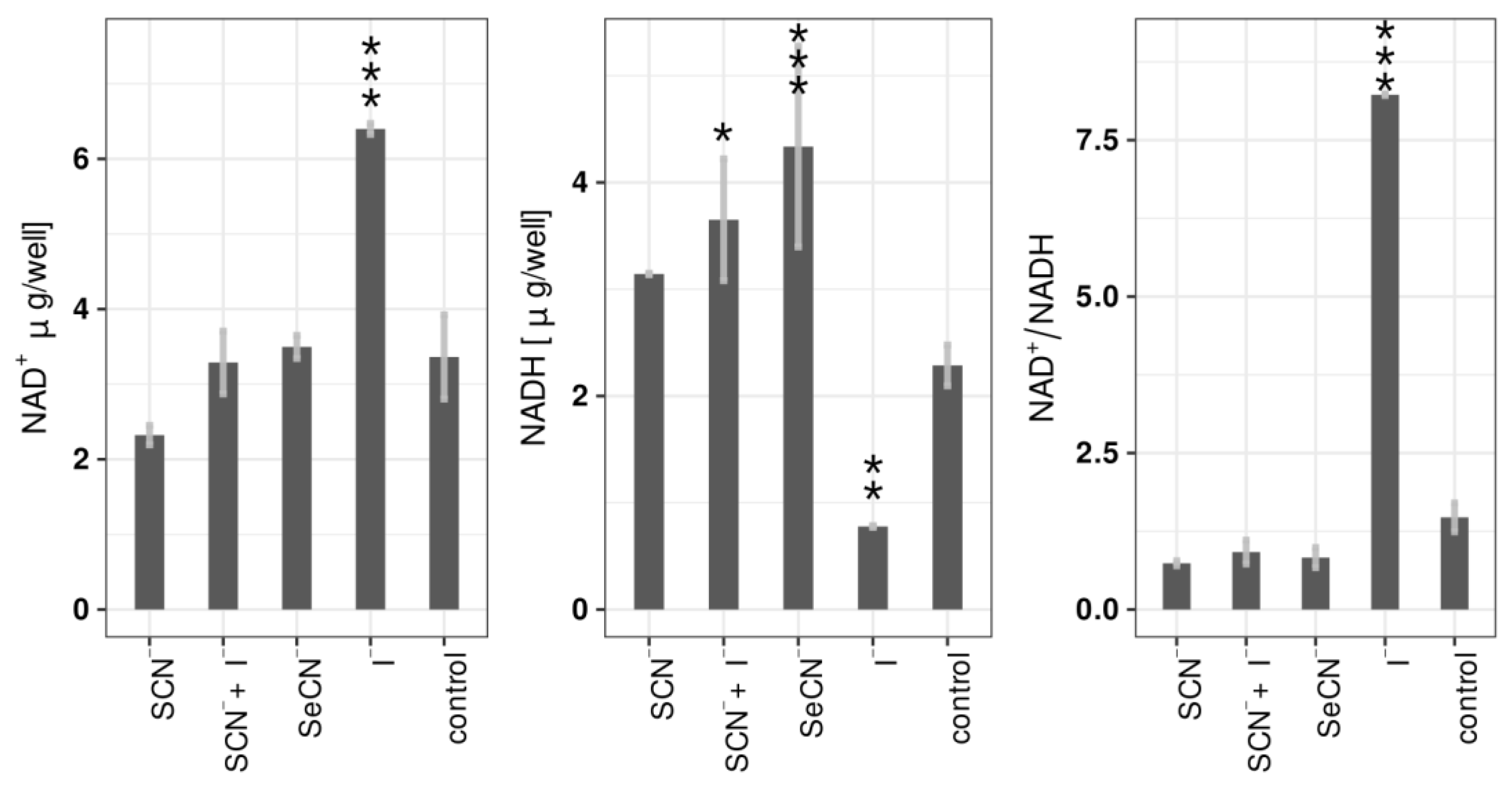
2.4. Influence of LPO Systems on Biofilm NAD+/NADH
3. Discussion
4. Materials and Methods
4.1. LPO System Setup
4.2. Biofilm Growth
4.3. Total Biofilm Biomass Assay
4.4. Insoluble Extracellular Polysaccharide Mass Assay
4.5. Lactate, Glucose, and Sucrose Concentration Assay
4.6. PTS System Activity Assay
4.7. NAD+/NADH EC Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Untreated Caries. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Chenicheri, S.; Usha, R.; Ramachandran, R.; Thomas, V.; Wood, A. Insight into Oral Biofilm: Primary, Secondary and Residual Caries and Phyto-Challenged Solutions. Open Dent. J. 2017, 11, 312–333. [Google Scholar] [CrossRef] [PubMed]
- Kozmos, M.; Virant, P.; Rojko, F.; Abram, A.; Rudolf, R.; Raspor, P.; Zore, A.; Bohinc, K. Bacterial Adhesion of Streptococcus Mutans to Dental Material Surfaces. Molecules 2021, 26, 1152. [Google Scholar] [CrossRef]
- Vieira, A.R. Genetics and Caries: Prospects. Braz. Oral Res. 2012, 26, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xie, T.; Li, S.; Qiao, X.; Lu, Y.; Feng, Y. Analysis of Oral Microbial Dysbiosis Associated with Early Childhood Caries. BMC Oral Health 2021, 21, 181. [Google Scholar] [CrossRef]
- Lee, Y. Diagnosis and Prevention Strategies for Dental Caries. J. Lifestyle Med. 2013, 3, 107–109. [Google Scholar]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The Virulence of Streptococcus Mutans and the Ability to Form Biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef]
- Odorici, A.; Colombari, B.; Bellini, P.; Meto, A.; Venturelli, I.; Blasi, E. Novel Options to Counteract Oral Biofilm Formation: In Vitro Evidence. Int. J. Environ. Res. Public Health 2022, 19, 8056. [Google Scholar] [CrossRef]
- Luiz, M.T.; di Filippo, L.D.; Dutra, J.A.P.; Viegas, J.S.R.; Silvestre, A.L.P.; Anselmi, C.; Duarte, J.L.; Calixto, G.M.F.; Chorilli, M. New Technological Approaches for Dental Caries Treatment: From Liquid Crystalline Systems to Nanocarriers. Pharmaceutics 2023, 15, 762. [Google Scholar] [CrossRef]
- Bafort, F.; Parisi, O.; Perraudin, J.-P.; Jijakli, M.H. Mode of Action of Lactoperoxidase as Related to Its Antimicrobial Activity: A Review. Enzym. Res. 2014, 2014, 517164. [Google Scholar] [CrossRef]
- Magacz, M.; Kędziora, K.; Sapa, J.; Krzyściak, W. The Significance of Lactoperoxidase System in Oral Health: Application and Efficacy in Oral Hygiene Products. Int. J. Mol. Sci. 2019, 20, 1443. [Google Scholar] [CrossRef] [PubMed]
- Gothefors, L.; Marklund, S. Lactoperoxidase Activity in Human Milk and in Saliva of Newborn Infants. Infect. Immun. 1975, 11, 1210–1215. [Google Scholar] [CrossRef]
- Luepker, R.V.; Pechacek, T.F.; Murray, D.M.; Johnson, C.A.; Hund, F.; Jacobs, D.R. Saliva Thiocyanate: A Chemical Indicator of Cigarette Smoking in Adolescents. Am. J. Public Health 1981, 71, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Reiter, B. The Lactoperoxidase-Thiocyanate-Hydrogen Peroxide Antibacterium System. In Novartis Foundation Series; Ciba Foundation: London, UK, 2008; pp. 285–294. [Google Scholar]
- Schlorke, D.; Atosuo, J.; Flemmig, J.; Lilius, E.M.; Arnhold, J. Impact of Cyanogen Iodide in Killing of Escherichia Coli by the Lactoperoxidase-Hydrogen Peroxide-(Pseudo)Halide System. Free Radic. Res. 2016, 50, 1287–1295. [Google Scholar] [CrossRef]
- Day, B.J.; Bratcher, P.E.; Chandler, J.D.; Kilgore, M.B.; Min, E.; LiPuma, J.J.; Hondal, R.J.; Nichols, D.P. The Thiocyanate Analog Selenocyanate Is a More Potent Antimicrobial Pro-Drug That Also Is Selectively Detoxified by the Host. Free Radic. Biol. Med. 2020, 146, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Geiszt, M.; Witta, J.; Baffi, J.; Lekstrom, K.; Leto, T.L. Dual Oxidases Represent Novel Hydrogen Peroxide Sources Supporting Mucosal Surface Host Defense. FASEB J. 2003, 17, 1502–1504. [Google Scholar] [CrossRef]
- Cawley, A.; Golding, S.; Goulsbra, A.; Hoptroff, M.; Kumaran, S.; Marriott, R. Microbiology Insights into Boosting Salivary Defences through the Use of Enzymes and Proteins. J. Dent. 2019, 80, S19–S25. [Google Scholar] [CrossRef] [PubMed]
- Herrero, E.R.; Boon, N.; Bernaerts, K.; Slomka, V.; Verspecht, T.; Quirynen, M.; Teughels, W. Clinical Concentrations of Peroxidases Cause Dysbiosis in In Vitro Oral Biofilms. J. Periodontal Res. 2018, 53, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L.; Sharma, S.; Singh, T.P. Iodide Supplementation of the Anti-Viral Duox-Lactoperoxidase Activity May Prevent Some SARS-CoV-2 Infections. Eur. J. Clin. Nutr. 2022, 76, 629–630. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Nematollahi, A.; Shadnoush, M.; Mortazavian, A.M.; Khorshidian, N. Antimicrobial Activity of Films and Coatings Containing Lactoperoxidase System: A Review. Front. Nutr. 2022, 9, 828065. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.L.; Aune, T.M. Susceptibility of Escherichia Coli to Bactericidal Action of Lactoperoxidase, Peroxide, and Iodide or Thiocyanate. Antimicrob. Agents Chemother. 1978, 13, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Schlorke, D.; Flemmig, J.; Birkemeyer, C.; Arnhold, J. Formation of Cyanogen Iodide by Lactoperoxidase. J. Inorg. Biochem. 2016, 154, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus Biofilm: An Emerging Battleground in Microbial Communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ren, Z.; Zhou, X.; Zeng, J.; Zou, J.; Li, Y. Inhibition of Streptococcus Mutans Biofilm Formation, Extracellular Polysaccharide Production, and Virulence by an Oxazole Derivative. Appl. Microbiol. Biotechnol. 2016, 100, 857–867. [Google Scholar] [CrossRef]
- Krzyściak, W.; Kościelniak, D.; Papież, M.; Vyhouskaya, P.; Zagórska-Świeży, K.; Kołodziej, I.; Bystrowska, B.; Jurczak, A. Effect of a Lactobacillus Salivarius Probiotic on a Double-Species Streptococcus Mutans and Candida Albicans Caries Biofilm. Nutrients 2017, 9, 1242. [Google Scholar] [CrossRef]
- Magacz, M.; Papież, M.; Kościelniak, D.; Jurczak, A.; Kędziora, K.; Pamuła, E.; Krzyściak, W. Safety Assessment of the Modified Lactoperoxidase System—In Vitro Studies on Human Gingival Fibroblasts. Int. J. Mol. Sci. 2023, 24, 2640. [Google Scholar] [CrossRef] [PubMed]
- Al-Baarri, A.N.; Damayanti, N.T.; Legowo, A.M.; Tekiner, İ.H.; Hayakawa, S. Enhanced Antibacterial Activity of Lactoperoxidase–Thiocyanate–Hydrogen Peroxide System in Reduced-Lactose Milk Whey. Int. J. Food Sci. 2019, 2019, 8013402. [Google Scholar] [CrossRef]
- Furtmüller, P.G.; Jantschko, W.; Regelsberger, G.; Jakopitsch, C.; Arnhold, J.; Obinger, C. Reaction of Lactoperoxidase Compound I with Halides and Thiocyanate. Biochemistry 2002, 41, 11895–11900. [Google Scholar] [CrossRef]
- Lumikari, M.; Soukka, T.; Nurmio, S.; Tenovuo, J. Inhibition of the Growth of Streptococcus Mutans, Streptococcus Sobrinus and Lactobacillus Casei by Oral Peroxidase Systems in Human Saliva. Arch. Oral Biol. 1991, 36, 155–160. [Google Scholar] [CrossRef]
- Suokka, T.; Lumikari, M.; Tenovuo, J. Combined Inhibitory Effect of Lactoferrin and Lactoperoxidase System on the Viability of Streptococcus Mutans, Serotype c. Eur. J. Oral Sci. 1991, 99, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.L.; Pera, K.A.; Smith, K.W.; Chwang, A.K. Inhibition of Streptococcus Mutans by the Lactoperoxidase Antimicrobial System. Infect. Immun. 1983, 39, 767–778. [Google Scholar] [CrossRef]
- Thomas, E.L. Lactoperoxidase-Catalyzed Oxidation of Thiocyanate: Equilibriums between Oxidized Forms of Thiocyanate. Biochemistry 1981, 20, 3273–3280. [Google Scholar] [CrossRef]
- Kalmár, J.; Woldegiorgis, K.L.; Biri, B.; Ashby, M.T. Mechanism of Decomposition of the Human Defense Factor Hypothiocyanite Near Physiological PH. J. Am. Chem. Soc. 2011, 133, 19911–19921. [Google Scholar] [CrossRef] [PubMed]
- Lenander-Lumikari, M.; Tenovuo, J.; Mikola, H. Effects of a Lactoperoxidase System-Containing Toothpaste on Levels of Hypothiocyanite and Bacteria in Saliva. Caries Res. 1993, 27, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, E.; Kobayashi, T.; Wakabayashi, H.; Yamauchi, K.; Iwatsuki, K.; Yoshie, H. Effects of Orally Administered Lactoferrin and Lactoperoxidase-Containing Tablets on Clinical and Bacteriological Profiles in Chronic Periodontitis Patients. Int. J. Dent. 2011, 2011, 405139. [Google Scholar] [CrossRef]
- Gudipaneni, R.K. Short Term Comparative Evaluation of Antimicrobial Efficacy of Tooth Paste Containing Lactoferrin, Lysozyme, Lactoperoxidase in Children with Severe Early Childhood Caries: A Clinical Study. J. Clin. Diagn. Res. 2014, 8, ZC18. [Google Scholar] [CrossRef]
- Dirix, P.; Nuyts, S.; Vander Poorten, V.; Delaere, P.; Van den Bogaert, W. Efficacy of the BioXtra Dry Mouth Care System in the Treatment of Radiotherapy-Induced Xerostomia. Support. Care Cancer 2007, 15, 1429–1436. [Google Scholar] [CrossRef]
- Shin, K.; Yaegaki, K.; Murata, T.; Ii, H.; Tanaka, T.; Aoyama, I.; Yamauchi, K.; Toida, T.; Iwatsuki, K. Effects of a Composition Containing Lactoferrin and Lactoperoxidase on Oral Malodor and Salivary Bacteria: A Randomized, Double-Blind, Crossover, Placebo-Controlled Clinical Trial. Clin. Oral Investig. 2011, 15, 485–493. [Google Scholar] [CrossRef]
- Nakano, M.; Shimizu, E.; Wakabayashi, H.; Yamauchi, K.; Abe, F. A Randomized, Double-Blind, Crossover, Placebo-Controlled Clinical Trial to Assess Effects of the Single Ingestion of a Tablet Containing Lactoferrin, Lactoperoxidase, and Glucose Oxidase on Oral Malodor. BMC Oral Health 2016, 16, 37. [Google Scholar] [CrossRef]
- Pinheiro, S.L.; Azenha, G.R.; Araujo, G.S.A.; Puppin Rontani, R.M. Effectiveness of Casein Phosphopeptide-Amorphous Calcium Phosphate and Lysozyme, Lactoferrin, and Lactoperoxidase in Reducing Streptococcus Mutans Counts in Dentinal Caries. Gen. Dent. 2017, 65, 47–50. [Google Scholar]
- Rageh, A.H.; Kaltz, A.; Pyell, U. Determination of Urinary Nucleosides via Borate Complexation Capillary Electrophoresis Combined with Dynamic PH Junction-Sweeping-Large Volume Sample Stacking as Three Sequential Steps for Their on-Line Enrichment. Anal. Bioanal. Chem. 2014, 406, 5877–5895. [Google Scholar] [CrossRef]
- Hogg, D.M.; Jago, G.R. The Oxidation of Reduced Nicotinamide Nucleotides by Hydrogen Peroxide in the Presence of Lactoperoxidase and Thiocyanate, Iodide or Bromide. Biochem. J. 1970, 117, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Pan, Y.; Chen, Y.; Yu, S.; Huang, J.; Liu, Y.; Gong, T.; Zhang, Q.; Sun, Q.; Zou, J.; et al. Acetylation of Lactate Dehydrogenase Negatively Regulates the Acidogenicity of Streptococcus Mutans. mBio 2022, 13, e02013-22. [Google Scholar] [CrossRef] [PubMed]
- Prütz, W.A.; Kissner, R.; Koppenol, W.H.; Rüegger, H. On the Irreversible Destruction of Reduced Nicotinamide Nucleotides by Hypohalous Acids. Arch. Biochem. Biophys. 2000, 380, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.; Iwami, Y.; Yamada, T. Hydrogen Peroxide Excretion by Oral Streptococci and Effect of Lactoperoxidase-Thiocyanate-Hydrogen Peroxide. Infect. Immun. 1983, 40, 70–80. [Google Scholar] [CrossRef]
- Baker, J.L.; Derr, A.M.; Faustoferri, R.C.; Quivey, R.G. Loss of NADH Oxidase Activity in Streptococcus Mutans Leads to Rex-Mediated Overcompensation in NAD+ Regeneration by Lactate Dehydrogenase. J. Bacteriol. 2015, 197, 3645–3657. [Google Scholar] [CrossRef]
- Watanabe, S. Salivary Clearance from Different Regions of the Mouth in Children. Caries Res. 1992, 26, 423–427. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Balsandorj, Z.; Hao, Z.; Pan, L. High-Precision Measurement of PH in the Full Toothpaste Using NMR Chemical Shift. J. Magn. Reson. 2020, 317, 106771. [Google Scholar] [CrossRef]
- Virion, A.; Michot, J.L.; Deme, D.; Pommier, J. NADPH Oxidation Catalyzed by the Peroxidase/H2O2 System. Iodide-Mediated Oxidation of NADPH to Iodinated NADP. Eur. J. Biochem. 1985, 148, 239–243. [Google Scholar] [CrossRef]
- Staszczyk, M.; Jurczak, A.; Magacz, M.; Kościelniak, D.; Gregorczyk-Maga, I.; Jamka-Kasprzyk, M.; Kępisty, M.; Kołodziej, I.; Kukurba-Setkowicz, M.; Krzyściak, W. Effect of Polyols and Selected Dental Materials on the Ability to Create a Cariogenic Biofilm–On Children Caries-Associated Streptococcus Mutans Isolates. Int. J. Environ. Res. Public Health 2020, 17, 3720. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, M.M.; Emam, T.; Raafat, M.M. Hindering of Cariogenic Streptococcus Mutans Biofilm by Fatty Acid Array Derived from an Endophytic Arthrographis Kalrae Strain. Biomolecules 2020, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, D.J.; Crow, V.L.; Lee, L.N.; Garon, C.F. Influence of the Lactose Plasmid on the Metabolism of Galactose by Streptococcus Lactis. J. Bacteriol. 1979, 137, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. Capillary Electrophoretic Separation of Mono- and Dinucleotides of Adenosine Using Cyclodextrin Solutions with MgCl2 Additive. J. Chromatogr. A 1998, 802, 167–177. [Google Scholar] [CrossRef]
- Markuszewski, M.J.; Britz-McKibbin, P.; Terabe, S.; Matsuda, K.; Nishioka, T. Determination of Pyridine and Adenine Nucleotide Metabolites in Bacillus Subtilis Cell Extract by Sweeping Borate Complexation Capillary Electrophoresis. J. Chromatogr. A 2003, 989, 293–301. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magacz, M.; Alatorre-Santamaría, S.; Kędziora, K.; Klasa, K.; Mamica, P.; Pepasińska, W.; Lebiecka, M.; Kościelniak, D.; Pamuła, E.; Krzyściak, W. Modified Lactoperoxidase System as a Promising Anticaries Agent: In Vitro Studies on Streptococcus mutans Biofilms. Int. J. Mol. Sci. 2023, 24, 12136. https://doi.org/10.3390/ijms241512136
Magacz M, Alatorre-Santamaría S, Kędziora K, Klasa K, Mamica P, Pepasińska W, Lebiecka M, Kościelniak D, Pamuła E, Krzyściak W. Modified Lactoperoxidase System as a Promising Anticaries Agent: In Vitro Studies on Streptococcus mutans Biofilms. International Journal of Molecular Sciences. 2023; 24(15):12136. https://doi.org/10.3390/ijms241512136
Chicago/Turabian StyleMagacz, Marcin, Sergio Alatorre-Santamaría, Karolina Kędziora, Kacper Klasa, Paweł Mamica, Wiktoria Pepasińska, Magdalena Lebiecka, Dorota Kościelniak, Elżbieta Pamuła, and Wirginia Krzyściak. 2023. "Modified Lactoperoxidase System as a Promising Anticaries Agent: In Vitro Studies on Streptococcus mutans Biofilms" International Journal of Molecular Sciences 24, no. 15: 12136. https://doi.org/10.3390/ijms241512136
APA StyleMagacz, M., Alatorre-Santamaría, S., Kędziora, K., Klasa, K., Mamica, P., Pepasińska, W., Lebiecka, M., Kościelniak, D., Pamuła, E., & Krzyściak, W. (2023). Modified Lactoperoxidase System as a Promising Anticaries Agent: In Vitro Studies on Streptococcus mutans Biofilms. International Journal of Molecular Sciences, 24(15), 12136. https://doi.org/10.3390/ijms241512136






