Ligand-Dependent Intramolecular Motion of Native Nicotinic Acetylcholine Receptors Determined in Living Myotube Cells via Diffracted X-ray Tracking
Abstract
1. Introduction
2. Results
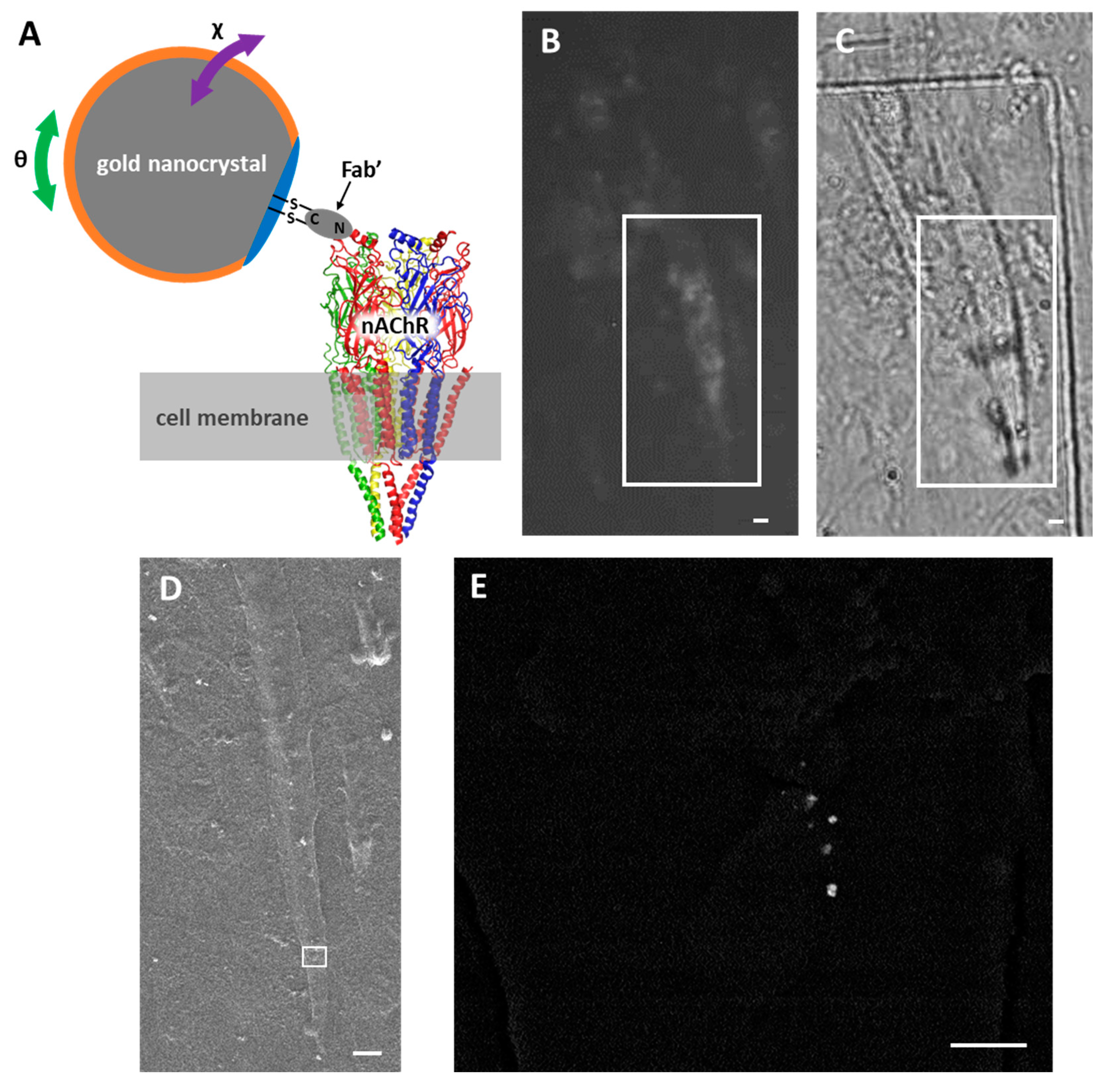
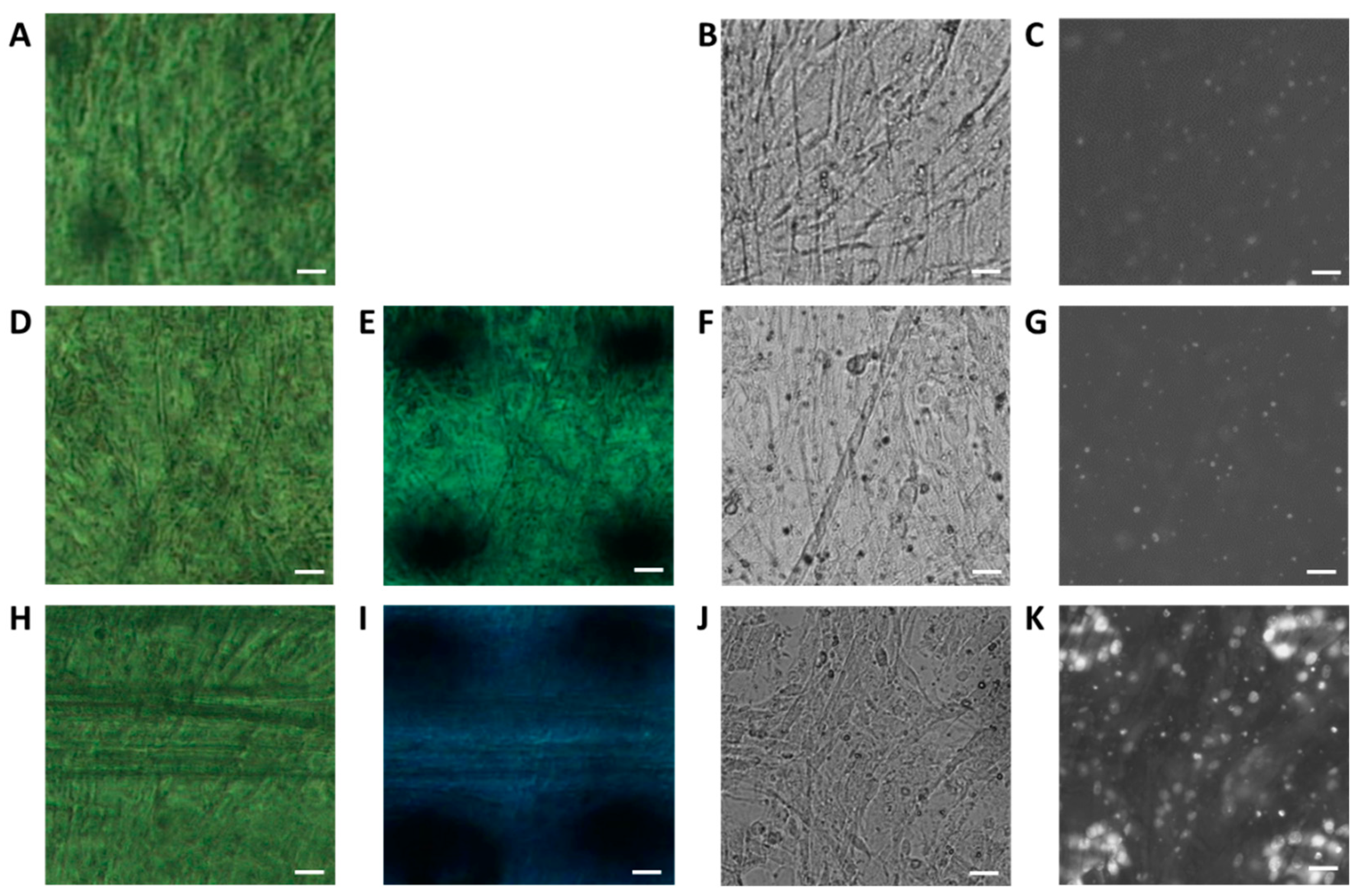
2.1. Labeling of nAChRs with Ab-AuNCs
2.2. Evaluation of X-ray Irradiation Damage to Myotube Cells
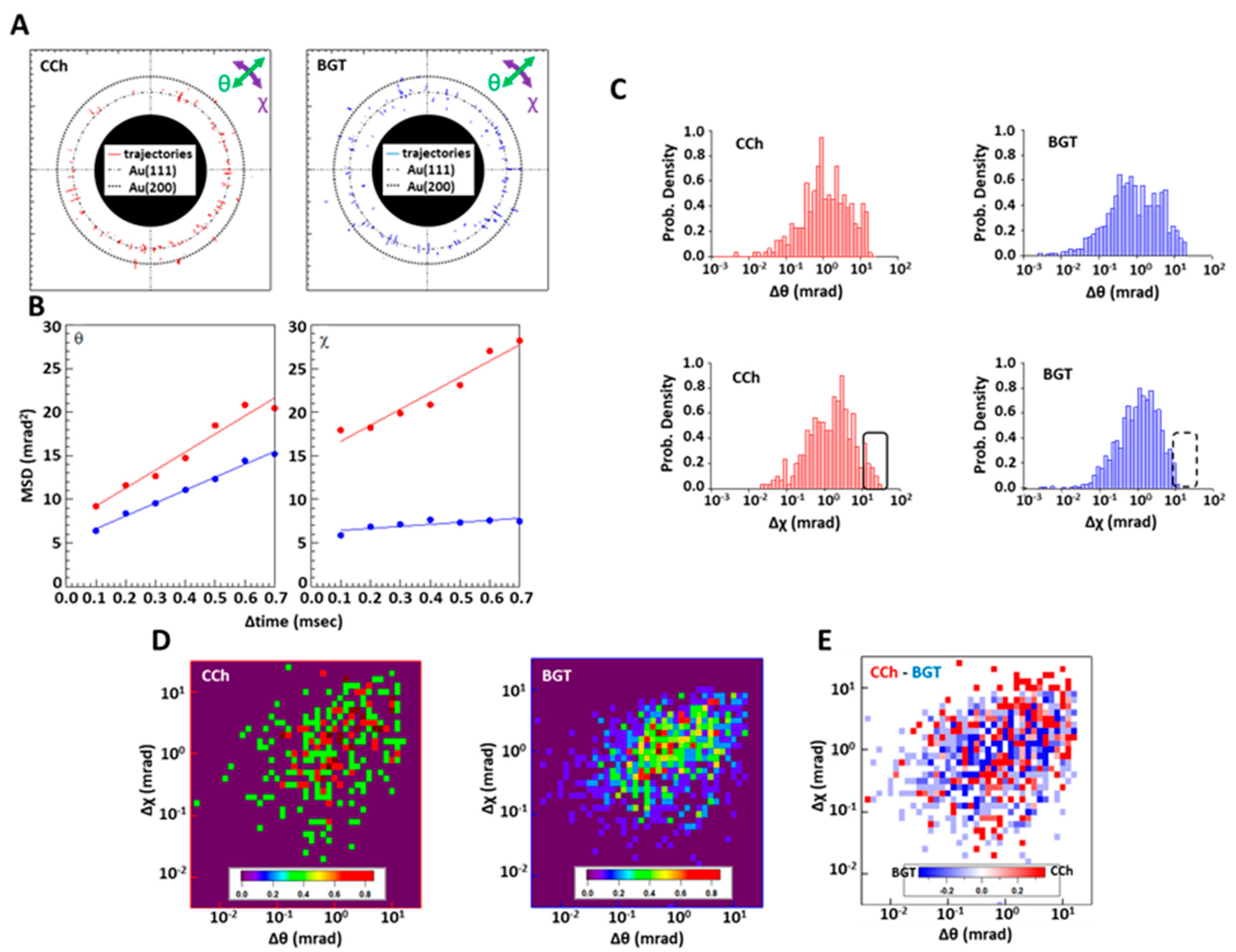
2.3. DXT Analysis of nAChRs on Myotube Surface
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation of Ab-AuNCs
4.3. Gold Nanocrystal Labeling of nAChRs
4.4. Correlative Light and Electron Microscopy
4.5. Assessments of Cell Viability
4.6. DXT
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsetlin, V.; Kuzmin, D.; Kasheverov, I. Assembly of nicotinic and other Cys-loop receptors. J. Neurochem. 2011, 116, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Unwin, N.; Fujiyoshi, Y. Gating movement of acetylcholine receptor caught by plunge-freezing. J. Mol. Biol. 2012, 422, 617–634. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, A.; Fujiyoshi, Y.; Unwin, N. Structure and gating mechanism of the acetylcholine receptor pore. Nature 2003, 423, 949–955. [Google Scholar] [CrossRef]
- Zhang, Y.; Dijkman, P.M.; Zou, R.; Zandl-Lang, M.; Sanchez, R.M.; Eckhardt-Strelau, L.; Köfeler, H.; Vogel, H.; Yuan, S.; Kudryashev, M. Asymmetric opening of the homopentameric 5-HT3A serotonin receptor in lipid bilayers. Nat. Commun. 2021, 12, 1074. [Google Scholar] [CrossRef]
- Kim, J.J.; Gharpure, A.; Teng, J.; Zhuang, Y.; Howard, R.J.; Zhu, S.; Noviello, C.M.; Walsh, R.M., Jr.; Lindahl, E.; Hibbs, R.E. Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature 2020, 585, 303–308. [Google Scholar] [CrossRef]
- Kumar, A.; Basak, S.; Rao, S.; Gicheru, Y.; Mayer, M.L.; Sansom, M.S.P.; Chakrapani, S. Mechanisms of activation and desensitization of full-length glycine receptor in lipid nanodiscs. Nat. Commun. 2020, 11, 3752. [Google Scholar] [CrossRef]
- Noviello, C.M.; Gharpure, A.; Mukhtasimova, N.; Cabuco, R.; Baxter, L.; Borek, D.; Sine, S.M.; Hibbs, R.E. Structure and gating mechanism of the α7 nicotinic acetylcholine receptor. Cell 2021, 184, 2121–2134.e13. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Teng, J.; Worrell, B.T.; Noviello, C.M.; Lee, M.; Karlin, A.; Stowell, M.H.B.; Hibbs, R.E. Structure of the native muscle-type nicotinic receptor and inhibition by snake venom toxins. Neuron 2020, 106, 952–962.e5. [Google Scholar] [CrossRef]
- Fabiani, C.; Georgiev, V.; Peñalva, D.; Sigaut, L.; Pietrasanta, L.; Corradi, J.; Dimova, R.; Antollini, S. Membrane lipid organization and nicotinic acetylcholine receptor function: A two-way physiological relationship. Arch. Biochem. Biophys. 2022, 730, 109413. [Google Scholar] [CrossRef] [PubMed]
- Dacosta, C.J.B.; Medaglia, S.A.; Lavigne, N.; Wang, S.; Carswell, C.L.; Baenziger, J.E. Anionic lipids allosterically modulate multiple nicotinic acetylcholine receptor conformational equilibria. J. Biol. Chem. 2009, 284, 33841–33849. [Google Scholar] [CrossRef]
- Asmar-Rovira, G.A.; Asseo-García, A.M.; Quesada, O.; Hanson, M.A.; Cheng, A.; Nogueras, C.; Lasalde-Dominicci, J.A.; Stevens, R.C. Biophysical and ion channel functional characterization of the Torpedo californica nicotinic acetylcholine receptor in varying detergent–lipid environments. J. Membr. Biol. 2008, 223, 13–26. [Google Scholar] [CrossRef][Green Version]
- Baenziger, J.E.; Dacosta, C.J.B. Molecular mechanisms of acetylcholine receptor–lipid interactions: From model membranes to human biology. Biophys. Rev. 2013, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barrantes, F.J. Lipid matters: Nicotinic acetylcholine receptor-lipid interactions (Review). Mol. Membr. Biol. 2002, 19, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Dacosta, C.J.; Baenziger, J.E. A Lipid-dependent uncoupled conformation of the acetylcholine receptor. J. Biol. Chem. 2009, 284, 17819–17825. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, A.K.; Sanghvi, M.; Sauls, D.; Machu, T.K.; Blanton, M.P. Assessing the lipid requirements of the Torpedo californica nicotinic acetylcholine receptor. Biochemistry 2006, 45, 4327–4337. [Google Scholar] [CrossRef]
- Zarkadas, E.; Pebay-Peyroula, E.; Thompson, M.J.; Schoehn, G.; Uchański, T.; Steyaert, J.; Chipot, C.; Dehez, F.; Baenziger, J.E.; Nury, H. Conformational transitions and ligand-binding to a muscle-type nicotinic acetylcholine receptor. Neuron 2022, 110, 1358–1370.e5. [Google Scholar] [CrossRef]
- Rahman, M.; Basta, T.; Teng, J.; Lee, M.; Worrell, B.T.; Stowell, M.H.B.; Hibbs, R.E. Structural mechanism of muscle nicotinic receptor desensitization and block by curare. Nat. Struct. Mol. Biol. 2022, 29, 386–394. [Google Scholar] [CrossRef]
- Adler, E.M. Stretching and setting boundaries. J. Gen. Physiol. 2012, 140, 341–342. [Google Scholar] [CrossRef]
- Unwin, N. Protein–lipid interplay at the neuromuscular junction. Microscopy 2022, 71, i66–i71. [Google Scholar] [CrossRef]
- Mio, K.; Ishihara, M.; Fujimura, S.; Sasaki, D.; Nozawa, S.; Ichiyanagi, K.; Fukaya, R.; Adachi, S.-I.; Kuramochi, M.; Sekiguchi, H.; et al. X-ray-based living-cell motion analysis of individual serotonin receptors. Biochem. Biophys. Res. Commun. 2020, 529, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Mio, K.; Fujimura, S.; Ishihara, M.; Kuramochi, M.; Sekiguchi, H.; Kubo, T.; Sasaki, Y.C. Living-cell diffracted X-ray tracking analysis confirmed internal salt bridge is critical for ligand-induced twisting motion of serotonin receptors. Int. J. Mol. Sci. 2021, 22, 5285. [Google Scholar] [CrossRef] [PubMed]
- Ichiyanagi, K.; Sekiguchi, H.; Hoshino, M.; Kajiwara, K.; Hoshisashi, K.; Jae-Won, C.; Tokue, M.; Matsushita, Y.; Nishijima, M.; Inoue, Y.; et al. Diffracted X-ray tracking for monitoring intramolecular motion in individual protein molecules using broad band X-ray. Rev. Sci. Instrum. 2013, 84, 103701. [Google Scholar] [CrossRef]
- Shimizu, H. Diffracted X-ray tracking method for recording single-molecule protein motions. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2020, 1864, 129361. [Google Scholar] [CrossRef]
- Fujimura, S.; Mio, K.; Ohkubo, T.; Arai, T.; Kuramochi, M.; Sekiguchi, H.; Sasaki, Y.C. Diffracted X-ray tracking method for measuring intramolecular dynamics of membrane proteins. Int. J. Mol. Sci. 2022, 23, 2343. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, H.; Suzuki, Y.; Nishino, Y.; Kobayashi, S.; Shimoyama, Y.; Cai, W.; Nagata, K.; Okada, M.; Ichiyanagi, K.; Ohta, N.; et al. Real time ligand-induced motion mappings of AChBP and nAChR using X-ray single molecule tracking. Sci. Rep. 2014, 4, 6384. [Google Scholar] [CrossRef]
- Tank, D.W.; Huganir, R.L.; Greengard, P.; Webb, W.W. Patch-recorded single-channel currents of the purified and reconstituted Torpedo acetylcholine receptor. Proc. Natl. Acad. Sci. USA 1983, 80, 5129–5133. [Google Scholar] [CrossRef]
- Pęziński, M.; Daszczuk, P.; Pradhan, B.S.; Lochmüller, H.; Prószyński, T.J. An improved method for culturing myotubes on laminins for the robust clustering of postsynaptic machinery. Sci. Rep. 2020, 10, 4524. [Google Scholar] [CrossRef]
- Chang, Y.-F.; Chou, H.-J.; Yen, Y.-C.; Chang, H.-W.; Hong, Y.-R.; Huang, H.-W.; Tseng, C.-N. Agrin induces association of Chrna1 mRNA and nicotinic acetylcholine receptor in C2C12 myotubes. FEBS Lett. 2012, 586, 3111–3116. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-S.; Hsieh, T.-L.; Liou, G.-G.; Li, T.-N.; Lin, H.-C.; Chang, C.-W.; Wu, H.-Y.; Yao, C.-K.; Liu, Y.-W. Dynamin-2 regulates postsynaptic cytoskeleton organization and neuromuscular junction development. Cell Rep. 2020, 33, 108310. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-J.; Valenzuela, I.M.-P.Y.; Aittaleb, M.; Akaaboune, M. AChRs are essential for the targeting of rapsyn to the postsynaptic membrane of nmjs in living mice. J. Neurosci. 2016, 36, 5680–5685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Luo, S.; Wang, Q.; Suzuki, T.; Xiong, W.C.; Mei, L. LRP4 serves as a coreceptor of agrin. Neuron 2008, 60, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Cruz, P.M.R.; Cossins, J.; Cheung, J.; Maxwell, S.; Jayawant, S.; Herbst, R.; Waithe, D.; Kornev, A.P.; Palace, J.; Beeson, D. Congenital myasthenic syndrome due to mutations in MUSK suggests that the level of MuSK phosphorylation is crucial for governing synaptic structure. Hum. Mutat. 2020, 41, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, A.; Grassi, F.; Limatola, C.; Mattei, E.; Ragozzino, D.; Eusebi, F. Acetylcholine-activated inward current induces cytosolic Ca2+ mobilization in mouse C2C12 myotubes. Cell Calcium 1995, 18, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hicks, M.R.; Cao, T.V.; Standley, P.R. Biomechanical strain vehicles for fibroblast-directed skeletal myoblast differentiation and myotube functionality in a novel coculture. Am. J. Physiol.-Cell Physiol. 2014, 307, C671–C683. [Google Scholar] [CrossRef][Green Version]
- Walker, C.M.; Saldaña, D.A.; Gladish, G.W.; Dicks, D.L.; Kicska, G.; Mitsumori, L.M.; Reddy, G.P. Cardiac complications of oncologic therapy. Radiographics 2013, 33, 1801–1815. [Google Scholar] [CrossRef]
- Meents, A.; Gutmann, S.; Wagner, A.; Schulze-Briese, C. Origin and temperature dependence of radiation damage in biological samples at cryogenic temperatures. Proc. Natl. Acad. Sci. USA 2010, 107, 1094–1099. [Google Scholar] [CrossRef]
- Kempner, E.S.; Haigler, H.T. The influence of low temperature on the radiation sensitivity of enzymes. J. Biol. Chem. 1982, 257, 13297–13299. [Google Scholar] [CrossRef]
- Sommers, C.H.; Niemira, B.A. Effect of temperature on the radiation resistance of Yersinia pestis suspended in raw ground pork. J. Food Saf. 2007, 27, 317–325. [Google Scholar] [CrossRef]
- Jackson, M.; Lecar, H.; Askanas, V.; Engel, W. Single cholinergic receptor channel currents in cultured human muscle. J. Neurosci. 1982, 2, 1465–1473. [Google Scholar] [CrossRef]
- Criado, M.; Eibl, H.; Barrantes, F.J. Functional properties of the acetylcholine receptor incorporated in model lipid membranes. Differential effects of chain length and head group of phospholipids on receptor affinity states and receptor-mediated ion translocation. J. Biol. Chem. 1984, 259, 9188–9198. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, N.; Nury, H.; Baaden, M.; Le Poupon, C.; Changeux, J.-P.; Delarue, M.; Corringer, P.-J. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 2009, 457, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Hilf, R.J.C.; Dutzler, R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 2008, 452, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Hilf, R.J.C.; Dutzler, R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 2009, 457, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Mowrey, D.; Cheng, M.H.; Liu, L.T.; Willenbring, D.; Lu, X.; Wymore, T.; Xu, Y.; Tang, P. Asymmetric ligand binding facilitates conformational transitions in pentameric ligand-gated ion channels. J. Am. Chem. Soc. 2013, 135, 2172–2180. [Google Scholar] [CrossRef]
- Bouzat, C.; Sine, S.M. Nicotinic acetylcholine receptors at the single-channel level. Br. J. Pharmacol. 2018, 175, 1789–1804. [Google Scholar] [CrossRef]
- Auerbach, A. Agonist activation of a nicotinic acetylcholine receptor. Neuropharmacology 2015, 96 Pt B, 150–156. [Google Scholar] [CrossRef]
- Schmauder, R.; Kosanic, D.; Hovius, R.; Vogel, H. Correlated optical and electrical single-molecule measurements reveal conformational diffusion from ligand binding to channel gating in the nicotinic acetylcholine receptor. ChemBioChem 2011, 12, 2431–2434. [Google Scholar] [CrossRef]
- Corradi, J.; Gumilar, F.; Bouzat, C. Single-channel kinetic analysis for activation and desensitization of homomeric 5-HT3A receptors. Biophys. J. 2009, 97, 1335–1345. [Google Scholar] [CrossRef]
- Dacosta, C.J.B.; Dey, L.; Therien, J.P.D.; Baenziger, J.E. A distinct mechanism for activating uncoupled nicotinic acetylcholine receptors. Nat. Chem. Biol. 2013, 9, 701–707. [Google Scholar] [CrossRef]
- Cetin, H.; Liu, W.; Cheung, J.; Cossins, J.; Vanhaesebrouck, A.; Maxwell, S.; Vincent, A.; Beeson, D.; Webster, R. Rapsyn facilitates recovery from desensitization in fetal and adult acetylcholine receptors expressed in a muscle cell line. J. Physiol. 2019, 597, 3713–3725. [Google Scholar] [CrossRef]
- Pan, N.C.; Ma, J.J.; Peng, H.B. Mechanosensitivity of nicotinic receptors. Pflügers Arch. 2012, 464, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.B. Dependence of acetylcholine receptor channel kinetics on agonist concentration in cultured mouse muscle fibres. J. Physiol. 1988, 397, 555–583. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.; Wong, B.; Jackson, M.; Lecar, H. Single-channel currents activated by curare in cultured embryonic rat muscle. J. Neurosci. 1983, 3, 2525–2531. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oishi, K.; Nagamori, M.; Kashino, Y.; Sekiguchi, H.; Sasaki, Y.C.; Miyazawa, A.; Nishino, Y. Ligand-Dependent Intramolecular Motion of Native Nicotinic Acetylcholine Receptors Determined in Living Myotube Cells via Diffracted X-ray Tracking. Int. J. Mol. Sci. 2023, 24, 12069. https://doi.org/10.3390/ijms241512069
Oishi K, Nagamori M, Kashino Y, Sekiguchi H, Sasaki YC, Miyazawa A, Nishino Y. Ligand-Dependent Intramolecular Motion of Native Nicotinic Acetylcholine Receptors Determined in Living Myotube Cells via Diffracted X-ray Tracking. International Journal of Molecular Sciences. 2023; 24(15):12069. https://doi.org/10.3390/ijms241512069
Chicago/Turabian StyleOishi, Koichiro, Mayu Nagamori, Yasuhiro Kashino, Hiroshi Sekiguchi, Yuji C. Sasaki, Atsuo Miyazawa, and Yuri Nishino. 2023. "Ligand-Dependent Intramolecular Motion of Native Nicotinic Acetylcholine Receptors Determined in Living Myotube Cells via Diffracted X-ray Tracking" International Journal of Molecular Sciences 24, no. 15: 12069. https://doi.org/10.3390/ijms241512069
APA StyleOishi, K., Nagamori, M., Kashino, Y., Sekiguchi, H., Sasaki, Y. C., Miyazawa, A., & Nishino, Y. (2023). Ligand-Dependent Intramolecular Motion of Native Nicotinic Acetylcholine Receptors Determined in Living Myotube Cells via Diffracted X-ray Tracking. International Journal of Molecular Sciences, 24(15), 12069. https://doi.org/10.3390/ijms241512069







