New Evidence for Artemisia absinthium as an Alternative to Classical Antibiotics: Chemical Analysis of Phenolic Compounds, Screening for Antimicrobial Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Antimicrobial Activity of MEAA
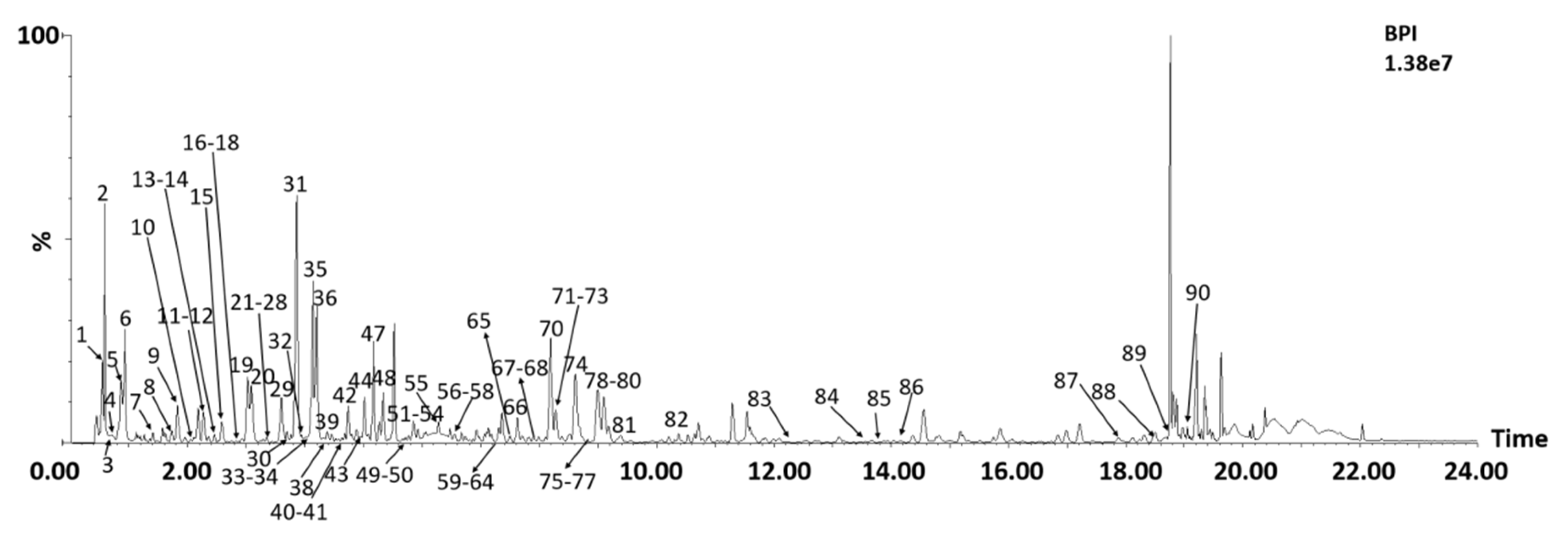
2.2. Chemical Profile of A. absinthium
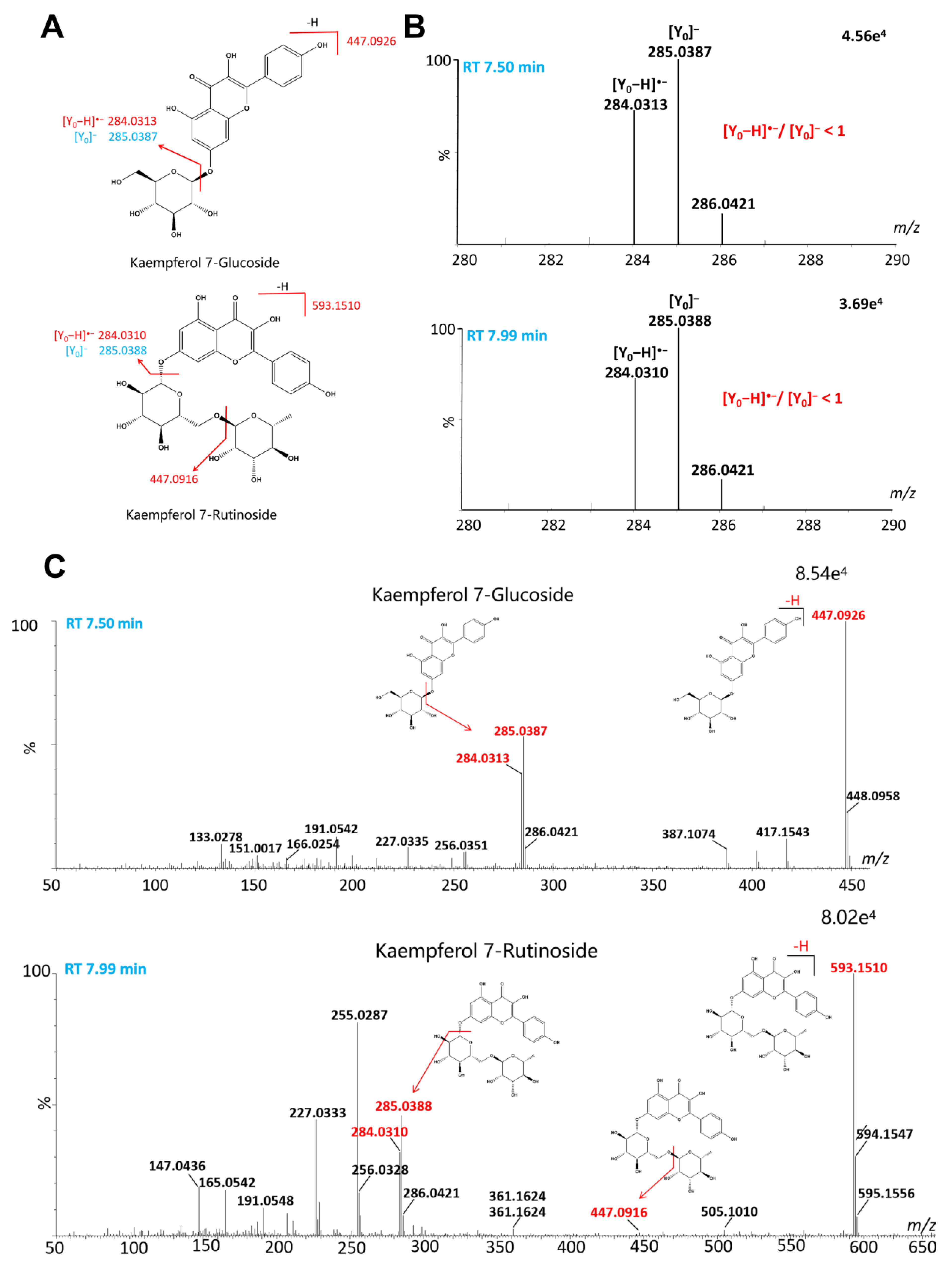
2.2.1. Flavonoids
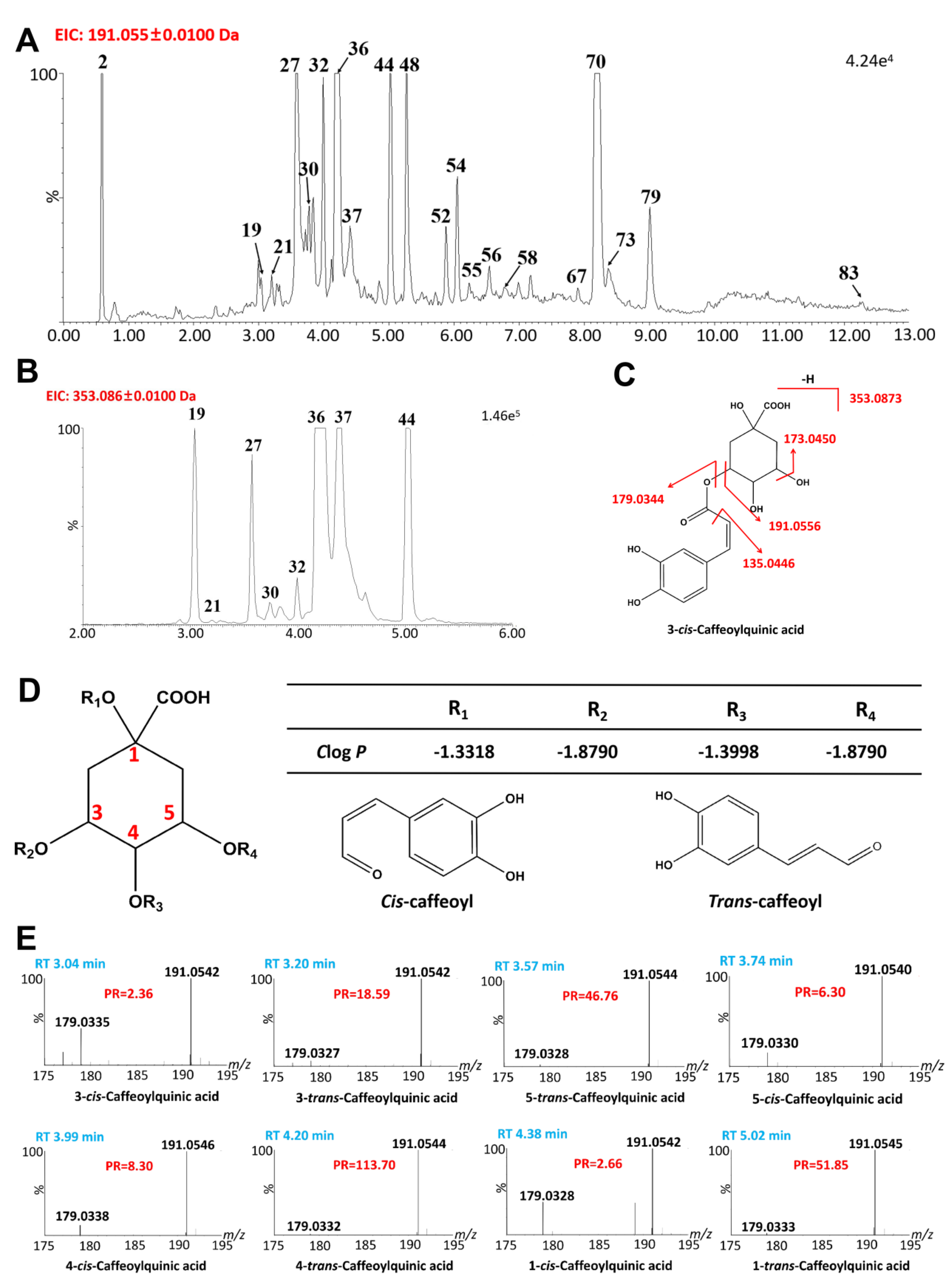
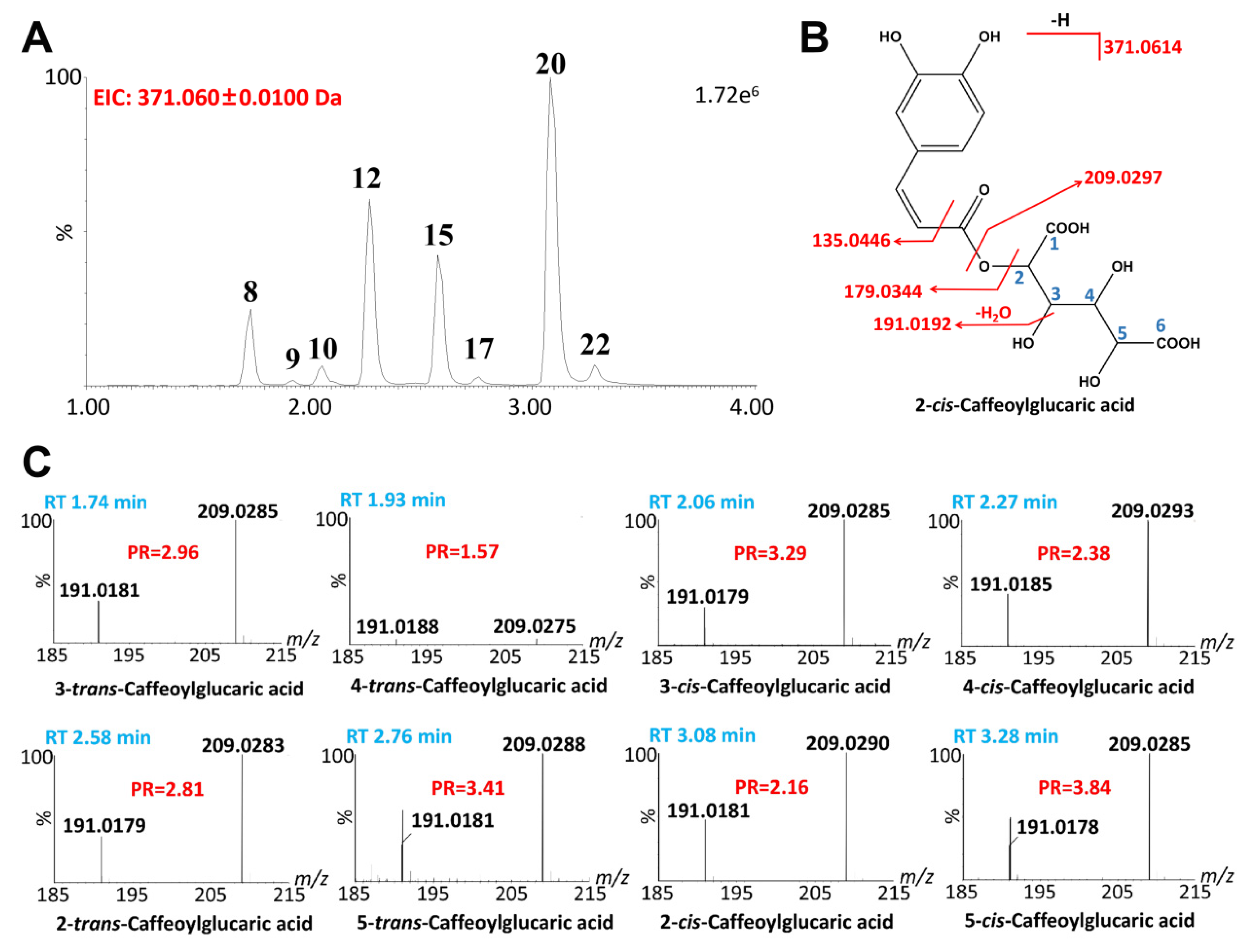
2.2.2. Quinic Acids
2.2.3. Glucaric Acids
| No. | RT | Formula | [M-H]− Measured | (m/z) Predicted | Δ (ppm) | ESI-MS/MS (m/z) | Identification | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.55 | C6H12O7 | 195.0496 | 195.0505 | −4.6 | 195.0496, 177.0385, 129.0175 | Gluconic acid | [38] |
| 2 | 0.59 | C7H12O6 | 191.0545 | 191.0556 | −5.8 | 383.1183 ([2M-H]−), 191.0545, 179.0544, 173.0440, 127.0384, 85.0280 | Quinic acid | [32] |
| 3 | 0.62 | C4H6O5 | 133.0128 | 133.0137 | −6.8 | 133.0128, 115.0018, 85.0276, 71.0127 | Malic acid | [13] |
| 4 | 0.71 | C4H6O5 | 133.0126 | 133.0137 | −9.8 | 133.0128, 115.0018, 85.0276, 71.0127 | L-(−)-malic acid | [13] |
| 5 | 0.87 | C6H8O7 | 191.0184 | 191.0192 | −4.2 | 383.0455 ([2M-H]−), 191.0181, 111.0070, 87.0071 | Citric acid | [13] |
| 6 | 0.93 | C6H8O7 | 191.0179 | 191.0192 | −6.8 | 383.0465 ([2M-H]−), 191.0179, 111.0073, 87.0075 | Isocitric acid | [13] |
| 7 | 1.47 | C7H6O5 | 169.0124 | 169.0137 | −7.7 | 169.0124, 125.0241 | 3,4,5-Trihydroxybenzoic acid | [34] |
| 8 | 1.74 | C15H16O11 | 371.0605 | 371.0614 | −2.7 | 743.1323 ([2M-H]−), 371.0605, 209.0285, 191.0181, 135.0430, 85.0277 | 3-trans-Caffeoylglucaric acid | [29,30] |
| 9 | 1.93 | C15H16O11 | 371.0600 | 371.0614 | 2.4 | 743.1322 ([2M-H]−), 371.0623, 209.0275, 191.0188, 135.048, 85.0283 | 4-trans-Caffeoylglucaric acid | [29,30] |
| 10 | 2.06 | C15H16O11 | 371.0604 | 371.0614 | −2.4 | 743.1312 ([2M-H]−), 371.0605, 209.0285, 191.0179, 135.0426, 85.0281 | 3-cis-Caffeoylglucaric acid | [29,30] |
| 11 | 2.18 | C13H16O9 | 315.0708 | 315.0716 | −1.3 | 631.1515 ([2M-H]−), 315.0708, 153.0171, 152.0098, 109.0270, 108.0199 | Protocatechuic acid 3-glucoside | [13] |
| 12 | 2.27 | C15H16O11 | 371.0607 | 371.0614 | 0.5 | 743.1342 ([2M-H]−), 371.0616, 209.0293, 191.0185, 135.0437, 85.0281 | 4-cis-Caffeoylglucaric acid | [29,30] |
| 13 | 2.46 | C14H18O9 | 329.0872 | 329.0871 | −0.6 | 659.1819 ([2M-H]−), 329.0872, 167.0332 | Vanillic acid glucoside | [13] |
| 14 | 2.52 | C7H6O4 | 153.0177 | 153.0188 | −7.2 | 153.0180, 109.0268 | Protocatechuic acid | [40] |
| 15 | 2.58 | C15H16O11 | 371.0603 | 371.0614 | 0.3 | 743.1326 ([2M-H]−), 371.0615, 209.0287, 191.0181, 135.0436, 85.0280 | 2-trans-Caffeoylglucaric acid | [29,30] |
| 16 | 2.67 | C13H16O9 | 315.0715 | 315.0716 | −1.0 | 315.0713, 153.0175, 152.0112, 109.0275, 108.0197 | Protocatechuic acid 4-glucoside | [13] |
| 17 | 2.76 | C15H16O11 | 371.0611 | 371.0614 | −0.8 | 743.1360 ([2M-H]−), 371.0615, 209.0288, 191.0195, 135.0440, 85.0278 | 5-trans-Caffeoylglucaric acid | [29,30] |
| 18 | 3.00 | C15H20O10 | 359.0974 | 359.0978 | −1.1 | 719.2036 ([2M-H]−), 359.0976, 197.0440, 153.539, 135.0437 | Glucosyringic acid | [13] |
| 19 + | 3.04 | C16H18O9 | 353.0867 | 353.0873 | −1.4 | 353.0867, 191.0542, 179.0335, 135.0441 | 3-cis-Caffeoylquinic acid | [31] |
| 20 | 3.08 | C15H16O11 | 371.0610 | 371.0614 | −0.3 | 743.1329 ([2M-H]−), 371.0605, 209.0290, 191.0181, 85.0281 | 2-cis-Caffeoylglucaric acid | [29,30] |
| 21 | 3.20 | C16H18O10 | 353.0853 | 353.0873 | 3.7 | 707.1833 ([2M-H]−), 353.0886, 191.0542, 179.0327, 173.0440, 135.0439, 85.0284 | 3-trans-Caffeoylquinic acid | [31] |
| 22 | 3.28 | C15H16O11 | 371.0609 | 371.0614 | −1.3 | 371.0609, 209.0285, 191.0547, 135.0437, 85.0276 | 5-cis-Caffeoylglucaric acid | [29,30] |
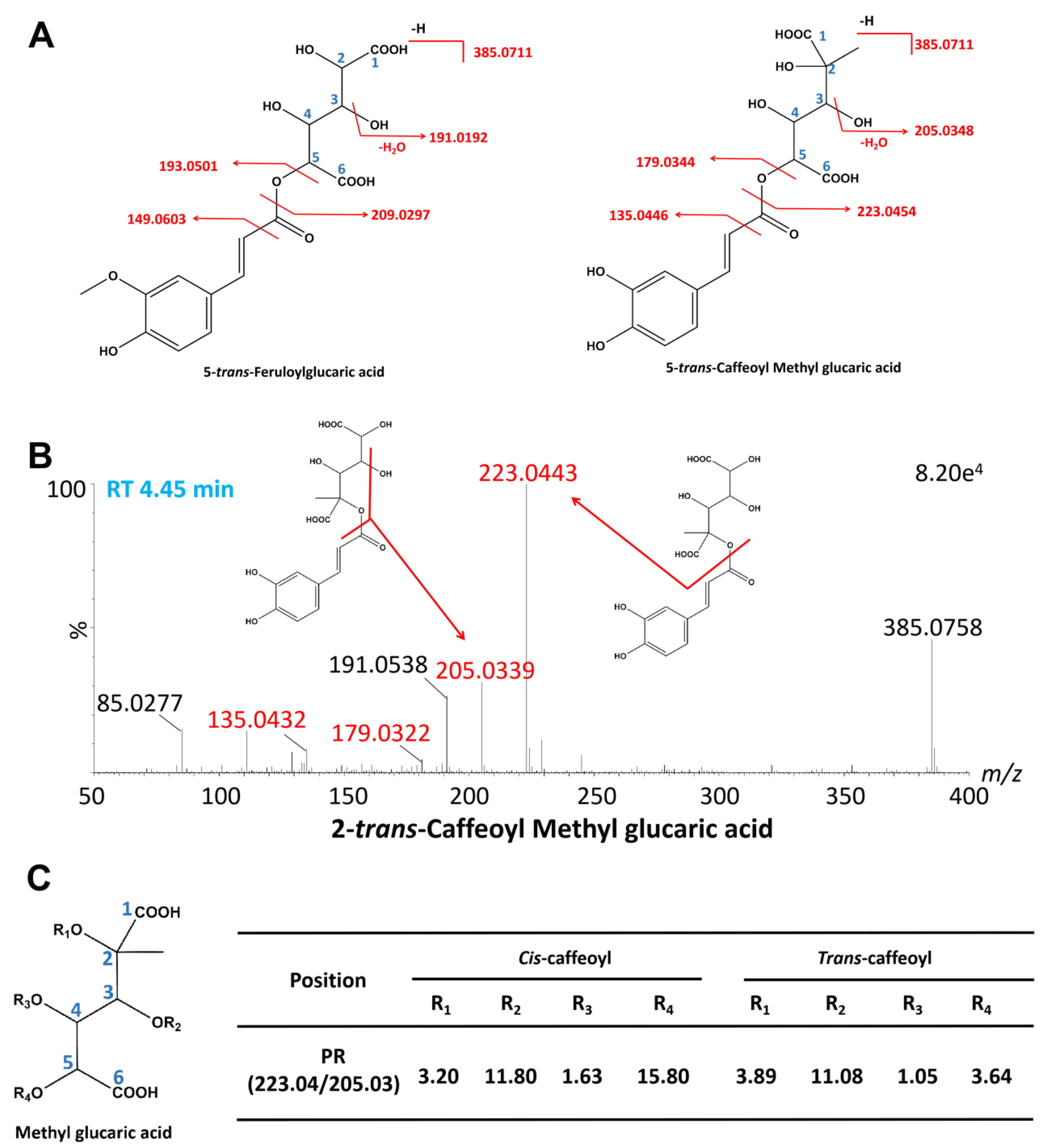
| 23 * | 3.31 | C16H18O11 | 385.0757 | 385.0771 | −3.6 | 385.0757, 223.0437, 191.0174 | 3-trans-Caffeoyl methyl glucaric acid | [37] |
| 24 | 3.35 | C7H12O5 | 175.0596 | 175.0606 | −5.7 | 175.0596, 157.0489, 113.0591 | 3-Isopropylmalic acid | [34] |
| 25 + | 3.45 | C7H6O3 | 137.0227 | 137.0239 | −4.4 | 137.0233, 93.0321 | Salicylic acid | [34] |
| 26 | 3.50 | C15H16O9 | 339.0705 | 339.0716 | −3.2 | 339.0707, 177.0173, 133.0274 | Esculin | [34] |
| 27 + | 3.57 | C16H18O11 | 353.0867 | 353.0873 | 1.0 | 707.1833 ([2M-H]−), 353.0857, 191.0544, 179.0328, 173.0432, 135.0433, 85.0277 | 5-trans-Caffeoylquinic acid | [31] |
| 28 * | 3.59 | C16H18O11 | 385.0761 | 385.0771 | −2.6 | 385.0761, 223.0433, 191.0182 | 4-trans-Caffeoyl methyl glucaric acid | [37] |
| 29 | 3.60 | C7H12O5 | 175.0595 | 175.0606 | −6.3 | 175.0590, 157.0486, 113.0590 | 2-Isopropylmalic acid | [13] |
| 30 | 3.74 | C16H18O12 | 353.0868 | 353.0873 | 1.4 | 707.1833 ([2M-H]−), 353.0857, 191.0540, 179.0330, 173.0433, 135.0436, 85.0283 | 5-cis-Caffeoylquinic acid | [31] |
| 31 | 3.86 | C8H14O5 | 189.0753 | 189.0763 | −5.3 | 189.0755, 127.0742, 99.0799 | 3-Hydroxyoctanedioic acid | [13] |
| 32 | 3.99 | C16H18O13 | 353.0862 | 353.0873 | 1.1 | 707.1831 ([2M-H]−), 353.0846, 191.0556, 179.0338, 173.0437, 135.0437, 85.0282 | 4-cis-Caffeoylquinic acid | [31] |
| 33 * | 4.04 | C16H18O11 | 385.0761 | 385.0771 | −2.6 | 385.0761, 223.0440, 191.0173 | 3-cis-Caffeoyl methyl glucaric acid | [37] |
| 34 + | 4.07 | C18H16O8 | 359.0760 | 359.0767 | −1.9 | 359.0760, 197.0435, 179.0332, 161.0224 | Rosmarinic acid | [34] |
| 35 | 4.14 | C8H14O5 | 189.0752 | 189.0763 | −5.8 | 189.0752, 127.0742, 99.0803 | 2-Hydroxyoctanedioic acid | [13] |
| 36 + | 4.20 | C16H18O14 | 353.0864 | 353.0873 | −0.6 | 707.1838 ([2M-H]−), 353.0876, 191.0549, 179.0335, 173.0439, 135.0436, 85.0280 | 4-trans-Caffeoylquinic acid | [31] |
| 37 | 4.38 | C16H18O15 | 353.0862 | 353.0873 | −1.1 | 707.1815 ([2M-H]−), 353.0857, 191.0542, 179.0328, 173.0438, 135.0437, 85.0277 | 1-cis-Caffeoylquinic acid | [31] |
| 38 * | 4.39 | C16H18O11 | 385.0753 | 385.0771 | −0.5 | 771.1613 ([2M-H]−), 385.0769, 223.0441, 205.0325, 191.0175 | 2-trans-Caffeoyl methyl glucaric acid | [37] |
| 39 * | 4.45 | C16H18O11 | 385.0767 | 385.0771 | −1.0 | 771.1616 ([2M-H]−), 385.0767, 223.0443, 205.0339, 191.0181 | 4-cis-Caffeoyl methyl glucaric acid | [37] |
| 40 + | 4.56 | C9H8O4 | 179.0334 | 179.0344 | −5.6 | 179.0334, 135.0441 | Caffeic acid | [39] |
| 41 * | 4.68 | C16H18O11 | 385.0761 | 385.0771 | −2.6 | 385.0761, 223.0443, 205.0333, 191.0183 | 2-cis-Caffeoyl methyl glucaric acid | [37] |
| 42 | 4.74 | C18H24O12 | 431.1188 | 431.1190 | −0.5 | 863.2485 ([2M-H]−), 431.1188,305.0687, 269.1235, 225.1118, 125.0226 | Asperulosidic acid | [34] |
| 43 | 4.97 | C15H16O9 | 339.0710 | 339.0716 | −2.7 | 339.071, 307.0846, 209.0291, 161.0232 | Daphnin | [13] |
| 44 | 5.02 | C16H18O17 | 353.0863 | 353.0873 | −2.8 | 707.1839 ([2M-H]−), 353.0863, 191.0545, 179.0333, 173.0436, 135.0438, 85.0280 | 1-trans-Caffeoylquinic acid | [31] |
| 45 * | 5.05 | C16H18O11 | 385.0774 | 385.0771 | 0.8 | 385.0774, 223.0438, 191.0186 | 5-cis-Caffeoyl methyl glucaric acid | [37] |
| 46 | 5.08 | C18H28O9 | 387.1642 | 387.1655 | −3.4 | 387.1642, 207.1004 | 7-Epi-12-Hydroxyjasmonic acid glucoside | [13] |
| 47 | 5.17 | C18H28O9 | 387.1657 | 387.1655 | 0.5 | 387.1657, 255.062, 207.1013 | Tuberonic acid glucoside | [13] |
| 48 | 5.27 | C16H18O8 | 337.0913 | 337.0923 | −3.0 | 675.1931 ([2M-H]−), 337.0913, 191.0543, 179.0343, 173.0450, 163.0386 | 3-p-Coumaroylquinic acid | [32] |
| 49 * | 5.61 | C16H18O11 | 385.0765 | 385.0771 | −1.6 | 385.0765, 223.0438, 191.0175 | 5-trans-Caffeoyl methyl glucaric acid | [37] |
| 50 | 5.71 | C29H34O18 | 669.1664 | 669.1667 | −0.4 | 669.1664, 507.1128, 345.0580, 344.0476, 330.0353, 329.0276, 301.0327, 300.0263, 285.0025 | Spinacetin 3-O-glucosyl-(1->6)-glucoside | [20] |
| 51 | 5.82 | C32H38O20 | 741.1891 | 741.1878 | 1.8 | 741.1891, 580.1392, 579.1348, 285.0388, 284.0316 | Kaempferol 3-sambubioside-7-glucoside | [20] |
| 52 | 5.88 | C17H20O9 | 367.1023 | 367.1029 | −1.6 | 367.1023, 193.0494, 191.0545, 173.0443, 135.0429, 134.0357, 93.0329 | 3-Feruloylquinic acid | [29] |
| 53 | 5.96 | C33H40O20 | 755.2041 | 755.2035 | 0.8 | 755.2041, 593.1494, 285.0388, 284.0320 | Kaempferol 7-caffeoylrhamnosyl-glucoside | [29] |
| 54 | 6.05 | C16H18O8 | 337.0922 | 337.0923 | −0.3 | 675.1938 ([2M-H]−), 337.0922, 191.0548, 179.0343, 173.0442, 163.0397 | 5-p-Coumaroylquinic acid | [32] |
| 55 | 6.25 | C17H20O9 | 367.1034 | 367.1029 | 1.4 | 367.1034, 193.0496, 191.0545, 173.0456, 135.0435, 134.0360, 93.0334 | 5-Feruloylquinic acid | [29] |
| 56 | 6.54 | C17H20O9 | 367.1028 | 367.1029 | −0.3 | 367.1028, 193.0488, 191.0541, 173.0442, 135.0431, 134.0345, 93.0322 | 4-Feruloylquinic acid | [29] |
| 57 | 6.83 | C21H20O12 | 463.0879 | 463.0877 | 0.4 | 463.0879, 301.0331, 300.0267, 271.0231, 255.0271, 243.0290 | Quercetin 7-glucoside | [20] |
| 58 | 6.86 | C16H18O8 | 337.0913 | 337.0923 | −3.0 | 675.1944 ([2M-H]−), 337.0913, 191.0542, 179.0323, 173.0433, 163.0367 | 4-p-Coumaroylquinic acid | [32] |
| 59 | 7.04 | C26H28O15 | 579.1357 | 579.1350 | 1.2 | 579.1357, 413.1424, 285.0388, 284.0305 | Kaempferol 7-xylosylglucoside | [28] |
| 60 | 7.11 | C26H28O15 | 579.1346 | 579.1350 | −0.7 | 579.1346, 461.0720, 285.0387, 284.0313 | Kaempferol 7-sambubioside | [28] |
| 61 + | 7.13 | C27H30O16 | 609.1456 | 609.1456 | 0.0 | 609.1456, 301.0328, 300.0262, 271.0235, 255.0284, 243.0285 | Rutin | [26] |
| 62 | 7.31 | C27H30O15 | 593.1504 | 593.1506 | −0.3 | 593.1504, 447.0935, 285.0388, 284.0311 | Kaempferol 3-rhamnoside-7-galactoside | [20] |
| 63 | 7.36 | C28H32O17 | 639.1560 | 639.1561 | −0.2 | 639.1560, 331.0426, 330.0359, 315.0125, 287.0179, 271.0226, 243.0279 | Mearnsetin 3-rutinoside | [28] |
| 64 + | 7.38 | C21H20O12 | 463.0881 | 463.0877 | 0.9 | 463.0881, 301.0330, 300.0269, 271.0236, 255.0285, 243.0287 | Isoquercitrin | [26] |
| 65 | 7.50 | C21H20O11 | 447.0927 | 447.0927 | 0.0 | 447.0927, 402.1311, 285.0385, 284.0312, 227.0316 | Kaempferol 7-glucoside | [20] |
| 66 | 7.62 | C22H22O13 | 439.0984 | 439.0982 | 0.2 | 493.0984, 331.0429, 330.0367, 315.0314, 287.0186 | Mearnsetin 3-glucoside | [28] |
| 67 + | 7.90 | C25H24O12 | 515.1187 | 515.1190 | 0.2 | 1031.2423 ([2M-H]−), 515.1182, 353.0863, 191.0542, 179.0332, 135.0432 | 3,4-Dicaffeoylquinic acid | [33] |
| 68 | 7.99 | C27H30O15 | 593.1510 | 593.1506 | 0.7 | 593.1510, 447.0916, 285.0388, 284.0310, 255.0287, 227.0333 | Kaempferol 7-rutinoside | [20] |
| 69 | 8.06 | C26H28O14 | 563.1387 | 563.1401 | −2.5 | 563.1401, 269.0439, 268.0363 | Apigenin-7-apioglucoside | [20] |
| 70 | 8.2 | C25H24O12 | 515.1195 | 515.1190 | 0.4 | 1031.2491 ([2M-H]−), 515.1182, 353.0863, 191.0542, 179.0332, 135.0432 | 3,5-Dicaffeoylquinic acid | [33] |
| 71 | 8.25 | C27H30O14 | 577.1557 | 577.1557 | 0 | 577.1557, 271.0231, 269.0437, 268.0366 | Apigenin 7-neohesperidoside | [20] |
| 72 | 8.27 | C28H32O16 | 623.1632 | 623.1647 | −2.4 | 623.1632, 315.0502, 314.0428, 300.0285, 299.0190, 272.0292, 271.0237, 255.0287, 243.0287 | Isorhamnetin 7-rutinoside | [20] |
| 73 | 8.37 | C25H24O12 | 515.1193 | 515.1190 | 0.6 | 1031.2510 ([2M-H]−), 515.1189, 353.0860, 191.0545, 179.0335, 135.0437 | 1,3-Dicaffeoylquinic acid | [33] |
| 74 | 8.40 | C27H28O14 | 575.1410 | 575.1401 | 1.6 | 575.1410, 431.1005, 413.0874, 269.0442, 268.0300 | Apigenin 7-[6″-(3-hydroxy-3-methylglutaryl)glucoside] | [29] |
| 75 | 8.62 | C29H34O17 | 653.1725 | 653.1718 | 1.1 | 653.1731, 345.0607, 344.0529, 330.0362, 329.0295, 315.0114, 314.0060, 302.0411, 301.0345, 287.0181, 286.0107, 259.0255, 258.0158 | Spinacetin 7-rutinoside | [20] |
| 76 | 8.67 | C22H22O12 | 477.1039 | 477.1033 | 1.3 | 477.1039, 315.0486, 314.0418, 300.0252, 299.0188, 272.0273, 271.0237, 258.0156, 243.0282, 242.0204, 215.0331 | Isorhamnetin 3-glucoside | [20] |
| 77 | 8.82 | C29H34O17 | 653.1730 | 653.1718 | 1.8 | 653.1730, 345.0605, 344.0525, 330.0350, 329.0289, 287.0191, 271.0245, 258.0155 | Spinacetin 7-robinobioside | [20] |
| 78 | 9.00 | C18H30O8 | 373.1862 | 373.1862 | 0 | 373.1862, 211.1326, 193.1219 | 6-Epi-7-isocucurbic acid glucoside | [20] |
| 79 + | 9.01 | C25H24O12 | 515.1195 | 515.1190 | 1.0 | 1031.2501 ([2M-H]−), 515.1182/353.0863/191.0542/179.0333/135.0432 | 4,5-Dicaffeoylquinic acid | [33] |
| 80 | 9.09 | C23H24O13 | 507.1150 | 507.1139 | 2.2 | 507.1150, 345.0602, 344.0530, 330.0358, 329.0293, 302.0395, 301.0346, 287.0168, 286.0107 | Spinacetin-3-glucosid | [28] |
| 81 | 9.46 | C22H22O11 | 461.1082 | 461.1084 | −0.7 | 461.1082, 299.0536, 298.0471, 255.0282 | Kaempferide 3-glucoside | [20] |
| 82 | 10.22 | C24H24O13 | 519.1152 | 519.1139 | 2.5 | 519.1152, 315.0502, 314.0076, 300.0272, 299.0198, 271.0237, 258.0151 | Isorhamnetin 7-(2″-acetylglucoside) | [20] |
| 83 | 12.27 | C34H30O15 | 677.1506 | 677.1497 | 2.5 | 677.1497, 515.1186, 353.0826, 191.0544, 179.0338, 161.0225, 135.0428 | 3,4,5-Tricaffeoylquinic acid | [31] |
| 84 + | 13.47 | C15H10O5 | 269.0442 | 269.0450 | −3.0 | 269.0442, 117.0365 | Apigenin | [34] |
| 85 + | 13.73 | C15H10O6 | 285.0395 | 285.0399 | −1.4 | 285.0395, 255.0284, 227.0349 | Kaempferol | [13] |
| 86 | 14.16 | C16H12O6 | 299.0544 | 299.0556 | −2.7 | 299.0544, 241.0953, 215.0549 | Chrysoeriol | [26] |
| 87 | 17.93 | C18H16O7 | 343.0823 | 343.0818 | 1.5 | 343.0818, 328.0570, 313.0321, 298.0078 | Eupatilin | [26] |
| 88 + | 18.50 | C19H18O8 | 373.0919 | 373.0923 | −0.5 | 373.0921, 358.0681, 343.0445, 257.0088, 229.0125 | Casticin | [34] |
| 89 + | 18.64 | C16H12O6 | 299.0547 | 299.0556 | −2.7 | 299.0547, 285.0353, 284.0323, 257.1288 | Kaempferide | [34] |
| 90 | 19.01 | C20H20O8 | 387.1074 | 387.1080 | −1.5 | 387.1074, 356.9952 | Artemetin | [34] |
2.2.4. A New Class of Caffeoyl Methyl Glucaric Acid Isomers
2.2.5. Other Compounds
2.3. Content of 14 Active Compounds in A. absinthium
2.4. Antimicrobial Activity of 14 Active Compounds in A. absinthium
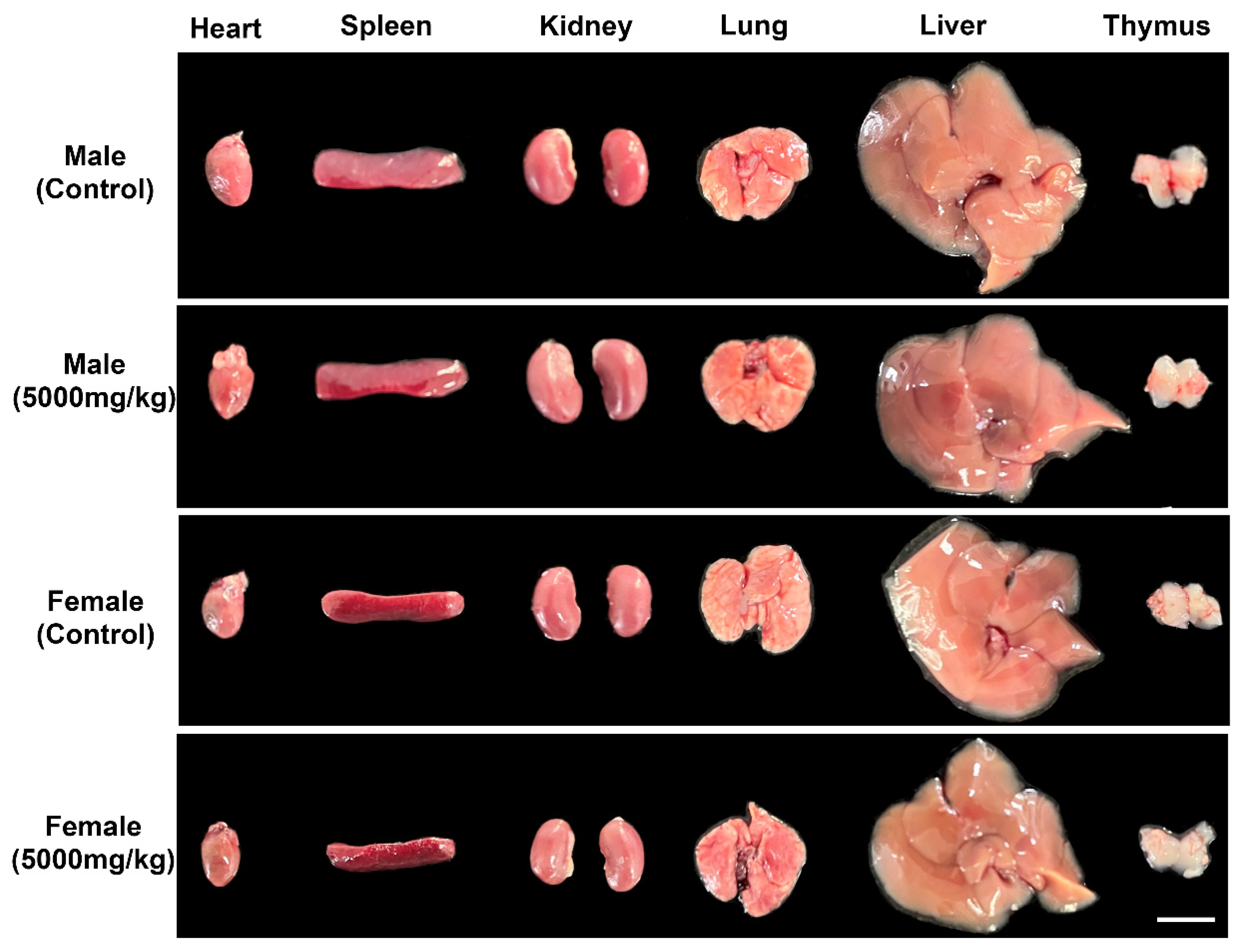
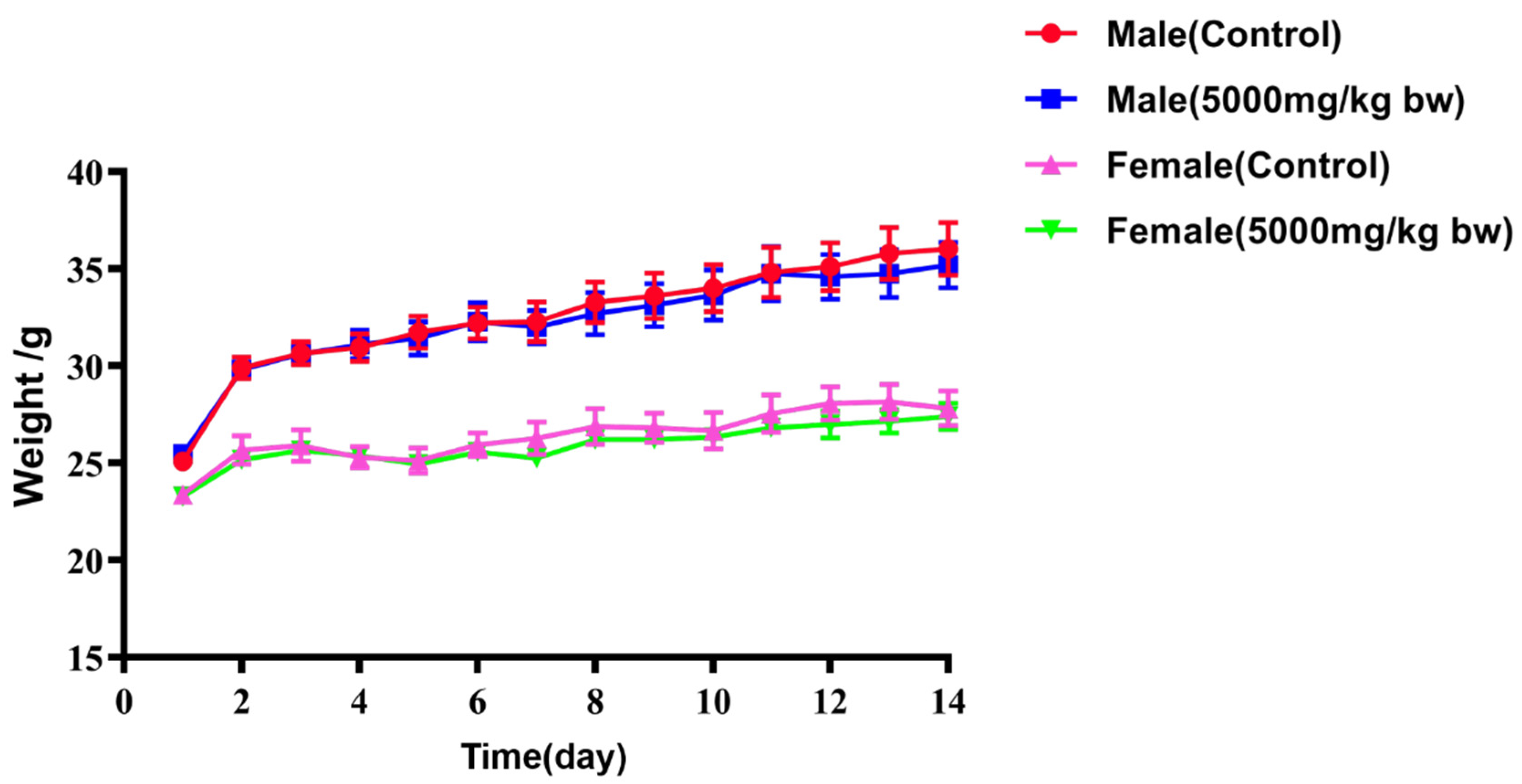
2.5. Acute Toxicity
3. Materials and Methods
3.1. Plant Materials and Chemicals
3.2. Preparation of Plant Extracts
3.3. Experimental Animals
3.4. Chemical Profile Analysis by UPLC-Q-TOF-MS
3.5. Quantitative Analysis of the Main Active Compounds by UPLC-MS/MS
3.6. Microbial Strains and Culture Media
3.7. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3.8. Acute Toxicity Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Compound | Time (min) | Q1 Mass (Da) | Q3 Mass (Da) | DP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|
| Neochlorogenic acid | 1.56 | 352.900 | 191.000 | −58.000 | −26.000 | −18.000 |
| Chlorogenic acid | 2.10 | 352.910 | 191.000 | −45.000 | −22.000 | −18.000 |
| Cryptochlorogenic acid | 2.25 | 353.100 | 172.900 | −43.000 | −22.000 | −11.000 |
| Caffeic acid | 2.41 | 179.200 | 135.000 | −68.800 | −21.400 | −11.300 |
| Rutin | 3.69 | 609.100 | 300.000 | −245.000 | −48.000 | −38.000 |
| Isoquercitrin | 3.83 | 463.100 | 300.000 | −191.000 | −36.000 | −22.000 |
| 3,4-Dicaffeoylquinic acid | 4.17 | 515.000 | 353.100 | −79.000 | −27.000 | −33.000 |
| Salicylic acid | 4.54 | 137.000 | 92.900 | −48.000 | −21.000 | −11.000 |
| 4,5-Dicaffeoylquinic acid | 4.54 | 515.200 | 173.000 | −60.000 | −35.000 | −15.000 |
| Rosmarinic acid | 4.62 | 359.100 | 161.000 | −140.000 | −22.000 | −13.000 |
| Apigenin | 6.12 | 269.100 | 117.000 | −171.000 | −43.000 | −19.000 |
| Kaempferol | 6.24 | 285.000 | 210.700 | −30.000 | −46.000 | −23.000 |
| Casticin | 7.50 | 373.200 | 358.300 | −121.000 | −32.200 | −12.500 |
| Kaempferide | 7.64 | 299.100 | 284.100 | −149.800 | −28.900 | −32.700 |
References
- Li, T.; Wang, Z.; Guo, J.; Fuente-Nunez, C.; Wang, J.; Han, B.; Tao, H.; Liu, J.; Wang, X. Bacterial resistance to antibacterial agents: Mechanisms, control strategies, and implications for global health. Sci. Total Environ. 2023, 860, 160461. [Google Scholar] [CrossRef]
- Arque, X.; Torres, M.; Patino, T.; Boaro, A.; Sanchez, S.; Cesar, F.N. Autonomous Treatment of Bacterial Infections in Vivo Using Antimicrobial Micro- and Nanomotors. ACS Nano 2022, 16, 7547–7558. [Google Scholar] [CrossRef]
- Torres, M.; Cao, J.; Franco, O.L.; Lu, T.K.; Fuente-Nunez, C. Synthetic Biology and Computer-Based Frameworks for Antimicrobial Peptide Discovery. ACS Nano 2021, 15, 2143–2164. [Google Scholar] [CrossRef]
- Silva, O.N.; Torres, M.D.T.; Cao, J.; Alves, E.S.F.; Fuente-Nunez, C.D.L. Repurposing a peptide toxin from wasp venom into antiinfectives with dual antimicrobial and immunomodulatory properties. Proc. Natl. Acad. Sci. USA 2020, 117, 26936–26945. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, X.; Teng, D.; Mao, R.; Hao, Y.; Yang, N.; Wang, X.; Li, Z.; Wang, X.; Wang, J. Development of chimeric peptides to facilitate the neutralisation of lipopolysaccharides during bactericidal targeting of multidrug-resistant. Commun. Biol. 2020, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, S. The Role of Vaccines in Combating Antimicrobial Resistance (AMR) Bacteria. Saudi J. Biol. Sci. 2021, 28, 7505–7510. [Google Scholar] [CrossRef]
- Abdallah, A.; Zhang, P.; Zhong, Q.; Sun, Z. Application of Traditional Chinese Herbal Medicine By-products as Dietary Feed Supplements and Antibiotic Replacements in Animal Production. Curr. Drug Metab. 2019, 20, 54–64. [Google Scholar] [CrossRef]
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Chellappan, D. Phytochemistry and pharmacological activity of the genus Artemisia. Arch. Pharm. Res. 2021, 44, 439–474. [Google Scholar] [CrossRef] [PubMed]
- Axelle, S.M.; Mahary, L.R.; Marodon, C.; Bedoui, Y.; Gasque, P. Artemisia annua, a Traditional Plant Brought to Light. Int. J. Mol. Sci. 2020, 21, 4986. [Google Scholar]
- Yang, M.T.; Kuo, T.F.; Chung, K.F.; Liang, Y.C.; Yang, W.C. Authentication, phytochemical characterization and anti-bacterial activity of two Artemisia species. Food Chem. 2020, 333, 127458. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Rather, M.A.; Shah, W.A.; Bilal, A.B. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem. 2013, 138, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.S.; Ahmed, O.; Amany, E.M.; Hetta, H.F.; Al-Rejaie, S.; Alghamdi, S.; Zahoor, M.; Magdy, B.A.; Murata, T.; Zaragoza-Bastida, A.; et al. Artemisia absinthium Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Wormwood. Antibiotics 2020, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Pajor, J.; Klin, P.; Rzepiela, A.; Hosam, O.E.; Al-Mana, F.A.; Mattar, M.A.; Ekiert, H. Artemisia absinthium L.—Importance in the history of medicine, the latest advances in phytochemistry and therapeutical, cosmetological and culinary uses. Plants 2020, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, J.; Mir, S.R.; Amin, S. A pharmacognostic review on artemisia absinthium. Int. Res. J. Pharm. 2019, 10, 25–31. [Google Scholar] [CrossRef]
- Cao, J.L.; Wei, J.C.; Hu, Y.J.; He, C.W.; Chen, M.W.; Wan, J.B.; Li, P. Qualitative and quantitative characterization of phenolic and diterpenoid constituents in Danshen (Salvia miltiorrhiza) by comprehensive two-dimensional liquid chromatography coupled with hybrid linear ion trap Orbitrap mass. J. Chromatogr. A 2016, 1427, 79–89. [Google Scholar] [CrossRef]
- Yang, W.Z.; Ye, M.; Qiao, X.; Liu, C.F.; Miao, W.J.; Bo, T.; Tao, H.Y.; Guo, D.A. A strategy for efficient discovery of new natural compounds by integrating orthogonal column chromatography and liquid chromatography/mass spectrometry analysis: Its application in Panax ginseng, Panax quinquefolium and Panax notoginseng to characterize. Anal. Chim. Acta 2012, 739, 56–66. [Google Scholar]
- Wang, L.; Liu, S.; Zhang, X.; Xing, J.; Liu, Z.; Song, F. A strategy for identification and structural characterization of compounds from Gardenia jasminoides by integrating macroporous resin column chromatography and liquid chromatography-tandem mass spectrometry combined with ion-mobility spectrometry. J. Chromatogr. A 2016, 1452, 47–57. [Google Scholar] [CrossRef]
- Qiao, X.; Li, R.; Song, W.; Miao, W.J.; Liu, J.; Chen, H.B.; Guo, D.A.; Ye, M. A targeted strategy to analyze untargeted mass spectral data: Rapid chemical profiling of Scutellaria baicalensis using ultra-high performance liquid chromatography coupled with hybrid quadrupole orbitrap mass spectrometry and key ion filtering. J. Chromatogr. A 2016, 1441, 83–95. [Google Scholar] [CrossRef]
- Qiao, X.; Lin, X.H.; Ji, S.; Zhang, Z.X.; Bo, T.; Guo, D.A.; Ye, M. Global Profiling and Novel Structure Discovery Using Multiple Neutral Loss/Precursor Ion Scanning Combined with Substructure Recognition and Statistical Analysis (MNPSS): Characterization of Terpene-Conjugated Curcuminoids in Curcuma longa as a Case Study. Anal. Chem. 2016, 88, 703–710. [Google Scholar]
- Gonzales, G.B.; Raes, K.; Coelus, S.; Struijs, K.; Smagghe, G.; Van Camp, J. Ultra(high)-pressure liquid chromatography-electrospray ionization-time-of-flight-ion mobility-high definition mass spectrometry for the rapid identification and structural characterization of flavonoid glycosides from cauliflower waste. J. Chromatogr. A 2014, 1323, 39–48. [Google Scholar] [CrossRef]
- Allard, P.M.; Péresse, T.; Bisson, J.; Gindro, K.; Marcourt, L.; Pham, V.C.; Roussi, F.; Litaudon, M.; Wolfender, J.L. Integration of Molecular Networking and In-Silico MS/MS Fragmentation for Natural Products Dereplication. Anal. Chem. 2016, 88, 3317–3323. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Gao, B.; Shi, H.; Cao, J.; Yin, C.; Lu, W.; Yu, L.; Cheng, Z. Characterization of flavonol mono-, di-, tri- and tetra-O-glycosides by ultra-performance liquid chromatography-electrospray ionization-quadrupole time-of-flight mass spectrometry and its application for identification of flavonol glycosides in Viola tia. J. Pharm. Biomed. Anal. 2017, 142, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huo, M.; Zhang, Y.; Qiao, Y.; Gao, X. A strategy to improve the identification reliability of the chemical constituents by high-resolution mass spectrometry-based isomer structure prediction combined with a quantitative structure retention relationship analysis: Phthalide compounds in Chuanxiong as a test case. J. Chromatogr. A 2018, 1552, 17–28. [Google Scholar] [PubMed]
- Lai, C.J.S.; Zha, L.P.; Liu, D.H.; Kang, L.; Ma, X.; Zhan, Z.L.; Nan, T.G.; Yang, J.; Li, F.; Yuan, Y.; et al. Global profiling and rapid matching of natural products using diagnostic product ion network and in silico analogue database: Gastrodia elata as a case study. J. Chromatogr. A 2016, 1456, 187–195. [Google Scholar]
- Torres, S.; Pandey, A.; Castro, G.R. Organic solvent adaptation of Gram positive bacteria: Applications and biotechnological potentials. Biotechnol. Adv. 2011, 29, 442–452. [Google Scholar] [CrossRef]
- Nikaido, H. Preventing drug access to targets: Cell surface permeability barriers and active efflux in bacteria. Semin. Cell Dev. Biol. 2001, 12, 215–223. [Google Scholar] [CrossRef]
- Ku, Y.S.; Ng, M.S.; Cheng, S.S.; Lo, A.W.; Xiao, Z.; Shin, T.S.; Chung, G.; Lam, H.M. Understanding the Composition, Biosynthesis, Accumulation and Transport of Flavonoids in Crops for the Promotion of Crops as Healthy Sources of Flavonoids for Human Consumption. Nutrients 2020, 12, 1717. [Google Scholar] [CrossRef]
- Aleksanteri, P.; Jorma, J.; Ari, T. Identification of flavonoids of Rhodiola rosea by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2006, 1112, 224–231. [Google Scholar]
- Garran, T.A.; Ji, R.; Chen, J.L.; Xie, D.M.; Guo, L.P.; Huang, L.Q.; Lai, C.J.S. Elucidation of metabolite isomers of Leonurus japonicus and Leonurus cardiaca using discriminating metabolite isomerism strategy based on ultra-high performance liquid chromatography tandem quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2019, 1598, 141–153. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K. Caffeoylglucaric Acids and Other Phenylpropanoids From Siberian Leonurus Species. Chem. Nat. Compd. 2016, 52, 915–917. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhang, Q.; Li, N.; Wang, Z.J.; Lu, J.Q.; Qiao, Y.J. Diagnostic fragment-ion-based and extension strategy coupled to DFIs intensity analysis for identification of chlorogenic acids isomers in Flos Lonicerae Japonicae by HPLC-ESI-MSn. Talanta 2013, 104, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Wang, Z.J.; Li, Y.; Liu, Y.; Cai, W.; Li, C.; Lu, J.Q.; Qiao, Y.J. A strategy for comprehensive identification of sequential constituents using ultra-high-performance liquid chromatography coupled with linear ion trap–Orbitrap mass spectrometer, application study on chlorogenic acids in Flos Lonicerae Japonicae. Talanta 2016, 147, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and Efflux Pump Inhibitory Activity of Caffeoylquinic Acids from Artemisia absinthium against Gram-Positive Pathogenic Bacteria. PLoS ONE 2011, 6, e18127. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Gašić, U.; Stojković, D.; Kostić, M.; Mišić, D.; Soković, M. New Evidence for Artemisia absinthium L. Application in Gastrointestinal Ailments: Ethnopharmacology, Antimicrobial Capacity, Cytotoxicity, and Phenolic Profile. Evid. Based Complement. Alternat Med. 2021, 2021, 9961089. [Google Scholar] [CrossRef]
- Han, B.; Xin, Z.; Ma, S.; Liu, W.; Zhang, B.; Ran, L.; Yi, L.; Ren, D. Comprehensive characterization and identification of antioxidants in Folium Artemisiae Argyi using high-resolution tandem mass spectrometry. J. Chromatogr. B Analyt Technol. Biomed. Life Sci/ 2017, 1063, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, Y.; Feng, Z.; Yang, Y.; Zhang, P. Glucaric acids from Leonurus japonicus. Fitoterapia 2015, 107, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Mardones, C.; Vergara, C.; von Baer, D.; Gómez-Alonso, S.; Gómez, M.V.; Hermosín-Gutiérrez, I. Isolation and structural elucidation of anthocyanidin 3,7-β-O-diglucosides and caffeoyl-glucaric acids from calafate berries. Food Chem. 2014, 62, 6918–6925. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; Jamali, A.; Grand, E.; Morreel, K.; Marcelo, P.; Gontier, E.; Dauwe, R. Phenylpropanoid profiling reveals a class of hydroxycinnamoyl glucaric acid conjugates in Isatis tinctoria leaves. Phytochemistry 2017, 144, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.P.; Lim, Y.H.; Su, J.; Shen, H.M.; Ong, C.N. Identification and characterization of major flavonoids and caffeoylquinic acids in three Compositae plants by LC/DAD-APCI/MS. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2007, 848, 215–225. [Google Scholar] [CrossRef]
- Sahin, S.; Aybastıer, O.; Işık, E. Optimisation of ultrasonic-assisted extraction of antioxidant compounds from Artemisia absinthium using response surface methodology. Food Chem. 2013, 141, 1361–1368. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Vasil’eva, A.G.; Gadimli, A.I.; Isaev, J.I.; Vennos, C. Caffeoylquinic Acids and Flavonoids of Fringed Sagewort (Artemisia frigida Willd.): HPLC-DAD-ESI-QQQ-MS Profile, HPLC-DAD Quantification, in Vitro Digestion Stability, and Antioxidant Capacity. Antioxidants 2019, 8, 307. [Google Scholar] [CrossRef]
- Sehrawat, R.; Rathee, P.; Akkol, E.K.; Khatkar, S.; Lather, A.; Redhu, N.; Khatkar, A. Phenolic Acids—Versatile Natural Moiety with Numerous Biological Applications. Curr. Top. Med. Chem. 2022, 22, 1472–1484. [Google Scholar]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.B.; Teng, H. Absorption, metabolism and bioavailability of flavonoids: A review. Crit. Rev. Food Sci. Nutr. 2021, 62, 7730–7742. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China 1; China Medical Science and Technology Press: Beijing, China, 2020; pp. 230, 290.
- Li, S.L.; Pi, J.; Zhu, H.; Yang, L.; Zhang, X.; Ding, W. Ralstonia solanacearum Caffeic Acid in Tobacco Root Exudate Defends Tobacco Plants From Infection by. Front. Plant Sci. 2021, 12, 690586. [Google Scholar] [CrossRef] [PubMed]
- Ramchandani, S.; Naz, I.; Lee, J.H.; Khan, M.R.; Ahn, K.S. An Overview of the Potential Antineoplastic Effects of Casticin. Molecules 2020, 25, 1287. [Google Scholar] [CrossRef]
- Mekonen, K.; Afework, M.; Makonnen, E.; Debela, A.; Ergete, W.; Tolessa, T. Evaluation of Acute and Sub-Acute Toxicity of Aqueous Extracts of Artemisia afra Leaves on Brain, Heart and Suprarenal Glands in Swiss Albino Mice. Ethiop. J. Health Sci. 2020, 30, 981–990. [Google Scholar] [PubMed]
- Dib, I.; Tits, M.; Angenot, L.; Wauters, J.N.; Assaidi, A.; Mekhfi, H.; Aziz, M.; Bnouham, M.; Legssyer, A.; Frederich, M.; et al. Antihypertensive and vasorelaxant effects of aqueous extract of Artemisia campestris L. from Eastern Morocco. J. Ethnopharmacol. 2017, 206, 224–235. [Google Scholar] [CrossRef]
- Liu, Z.H.; Chen, Z.Y.; Li, X.L.; Jiang, C.; Jin, Y.; Zhao, Y.Y.; Yuan, Y. Specific PCR Identification of Artemisia absinthium, a New Foreign Medicinal Resource of Artemisia. Chin. J. Exp. Tradit. Med. Formulae 2022, 28, 127–132. [Google Scholar]
- OECD. Guidelines for the Testing of Chemicals. Acute Oral Toxicity–Fixed Dose Procedure; OECD: Paris, France, 2008; pp. 425–427. [Google Scholar]
- Wang, X.J.; Deng, Y.H.; Zhang, L.P.; Zheng, G.H.; Chen, L.L.; Fang, Y. Identification and determination of phenolic acids and flavonoids in Artemisiae Argyi Folium by UPLC-DAD-MS. Zhongguo Zhong Yao Za Zhi 2019, 44, 983–989. [Google Scholar]
- Mihajilov-Krstev, T.; Jovanović, B.; Jović, J.; Ilić, B.; Miladinović, D.; Matejić, J.; Rajković, J.; Dorđević, L.; Cvetković, V.; Zlatković, B. Antimicrobial, antioxidative, and insect repellent effects of Artemisia absinthium essential oil. Planta Med. 2014, 80, 1698–1705. [Google Scholar] [CrossRef]

| Microorganism | Methanol Extracts | Tetracycline | Fluconazole | ||||
|---|---|---|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | ||
| G+ bacteria | S. aureus | 1.25 | 1.25 | <1.56 | <1.56 | NT | NT |
| S. epidermidis | 0.625 | 1.25 | <1.56 | <1.56 | NT | NT | |
| B. subtilis | 2.5 | 2.5 | <1.56 | <1.56 | NT | NT | |
| B. cereus | 1.25 | 1.25 | 12.5 | 12.5 | NT | NT | |
| G− bacteria | P. aeruginosa | 1.25 | 1.25 | 12.5 | 25 | NT | NT |
| E. coli | 2.5 | 2.5 | 3.125 | 3.125 | NT | NT | |
| K. aerogenes | 2.5 | 2.5 | 3.125 | 3.125 | NT | NT | |
| K. pneumoniae | 2.5 | 2.5 | 3.125 | 3.125 | NT | NT | |
| Fungal strains | C. albicans | 2.5 | 5.0 | NT | NT | 5.0 | 10 |
| Compound | Linear Formula | R2 | Content/(μg/g) |
|---|---|---|---|
| Salicylic acid | y = 3694.3x + 283,353 | 0.9995 | 0.3601 ± 0.019 |
| Caffeic acid | y = 5170.6x + 305,047 | 0.9996 | 1.8934 ± 0.032 |
| Rosmarinic acid | y = 861.98x + 6146.5 | 1.0000 | 0.1515 ± 0.039 |
| Chlorogenic acid | y = 1597.7x + 19,247 | 1.0000 | 25.5950 ± 0.544 |
| Cryptochlorogenic acid | y = 2623.3x − 6771.2 | 1.0000 | 0.6693 ± 0.011 |
| Neochlorogenic acid | y = 2968.9x − 67,665 | 0.9999 | 1.3589 ± 0.029 |
| 3,4-Dicaffeoylquinic acid | y = 2513.4x − 18,469 | 1.0000 | 0.9548 ± 0.055 |
| 4,5-Dicaffeoylquinic acid | y = 1952.4x − 41,101 | 0.9999 | 1.9678 ± 0.016 |
| Kaempferol | y = 144.18x + 7766 | 0.9995 | 0.2022 ± 0.057 |
| Apigenin | y = 852.56x + 54,793 | 0.9995 | 0.5389 ± 0.099 |
| Casticin | y = 201.8x + 7446.7 | 0.9998 | 3.5470 ± 0.148 |
| Rutin | y = 1265.4x − 7408.8 | 1.0000 | 11.3730 ± 0.368 |
| Isoquercitrin | y = 2258.3x + 12,910 | 1.0000 | 0.7482 ± 0.024 |
| Kaempferide | y = 728.34x + 11,583 | 1.0000 | 8.8500 ± 0.258 |
| Microorganism | Salicylic Acid | Caffeic Acid | Casticin | 3,4-Dicaffeoylquinic Acid | ||||
|---|---|---|---|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| S.aureus | 0.25 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| S. epidermidis | 0.25 | 0.5 | 0.5 | 1 | 1 | 1 | 1 | >1 |
| B. subtilis | 0.5 | 0.5 | 1 | 1 | 0.5 | 1 | 0.5 | 1 |
| B. cereus | 0.5 | 0.5 | 0.5 | 1 | 1 | 1 | 1 | >1 |
| P. aeruginosa | 0.25 | 0.5 | 0.5 | 1 | 1 | 1 | 0.5 | 1 |
| E. coli | 0.5 | 0.5 | 1 | >1 | 1 | >1 | 1 | 1 |
| K. aerogenes | 0.5 | 1 | 0.5 | 1 | 1 | >1 | >1 | NT |
| K. pneumoniae | 0.25 | 0.5 | 1 | 1 | 1 | 1 | 1 | >1 |
| C. albicans | 0.5 | 1 | 1 | >1 | >1 | NT | >1 | NT |
| Group | Weight (g) | |
|---|---|---|
| Male | Female | |
| Control | 36.04 ± 1.35 ns | 27.82 ± 0.90 ns |
| 5000 mg/kg bw | 35.20 ± 117 ns | 27.40 ± 0.66 ns |
| Group | Heart | Liver | Spleen | Lung | Kidney | Thymus | |
|---|---|---|---|---|---|---|---|
| Male | Control | 5.62 ± 0.22 ns | 55.74 ± 1.06 ns | 3.96 ± 0.27 ns | 6.39 ± 0.31 ns | 16.34 ± 0.90 ns | 2.75 ± 0.32 ns |
| 5000 mg/kg bw | 5.66 ± 0.49 ns | 53.15 ± 0.40 ns | 4.21 ± 0.28 ns | 6.11 ± 0.40 ns | 17.03 ± 1.37 ns | 2.22 ± 0.26 ns | |
| Female | Control | 6.66 ± 0.41 ns | 49.45 ± 0.46 ns | 5.06 ± 0.29 ns | 6.64 ± 0.34 ns | 12.31 ± 0.49 ns | 3.32 ± 0.31 ns |
| 5000 mg/kg bw | 5.75 ± 0.25 ns | 47.76 ± 0.61 ns | 4.58 ± 0.39 ns | 6.57 ± 0.23 ns | 12.63 ± 0.25 ns | 3.37 ± 0.20 ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, X.; Jin, Y.; Nan, T.; Zhao, Y.; Huang, L.; Yuan, Y. New Evidence for Artemisia absinthium as an Alternative to Classical Antibiotics: Chemical Analysis of Phenolic Compounds, Screening for Antimicrobial Activity. Int. J. Mol. Sci. 2023, 24, 12044. https://doi.org/10.3390/ijms241512044
Liu Z, Li X, Jin Y, Nan T, Zhao Y, Huang L, Yuan Y. New Evidence for Artemisia absinthium as an Alternative to Classical Antibiotics: Chemical Analysis of Phenolic Compounds, Screening for Antimicrobial Activity. International Journal of Molecular Sciences. 2023; 24(15):12044. https://doi.org/10.3390/ijms241512044
Chicago/Turabian StyleLiu, Zhihao, Xiaolin Li, Yan Jin, Tiegui Nan, Yuyang Zhao, Luqi Huang, and Yuan Yuan. 2023. "New Evidence for Artemisia absinthium as an Alternative to Classical Antibiotics: Chemical Analysis of Phenolic Compounds, Screening for Antimicrobial Activity" International Journal of Molecular Sciences 24, no. 15: 12044. https://doi.org/10.3390/ijms241512044
APA StyleLiu, Z., Li, X., Jin, Y., Nan, T., Zhao, Y., Huang, L., & Yuan, Y. (2023). New Evidence for Artemisia absinthium as an Alternative to Classical Antibiotics: Chemical Analysis of Phenolic Compounds, Screening for Antimicrobial Activity. International Journal of Molecular Sciences, 24(15), 12044. https://doi.org/10.3390/ijms241512044







