Using A Protoplast Transformation System to Enable Functional Studies in Mangifera indica L.
Abstract
1. Introduction
2. Results
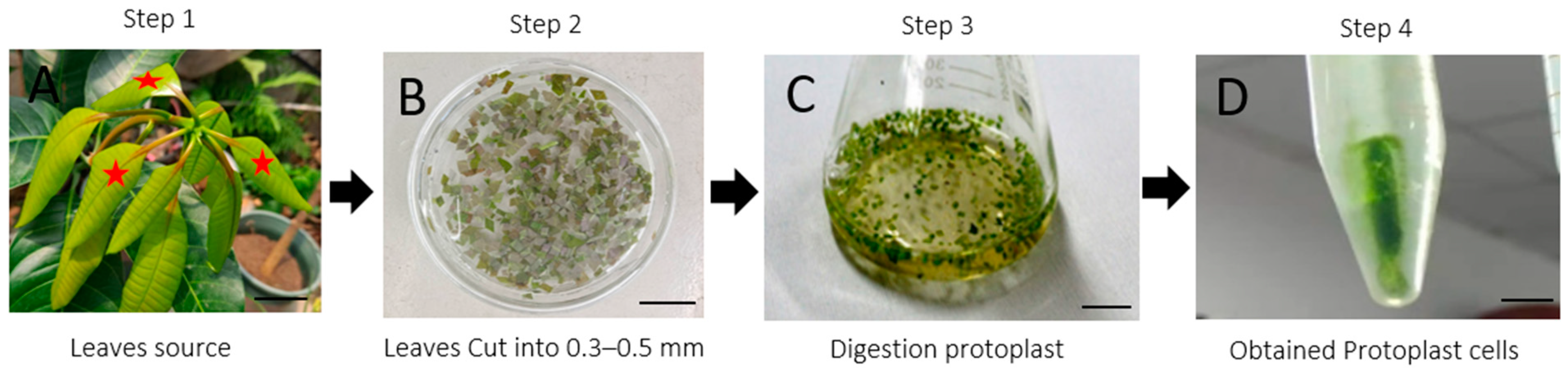
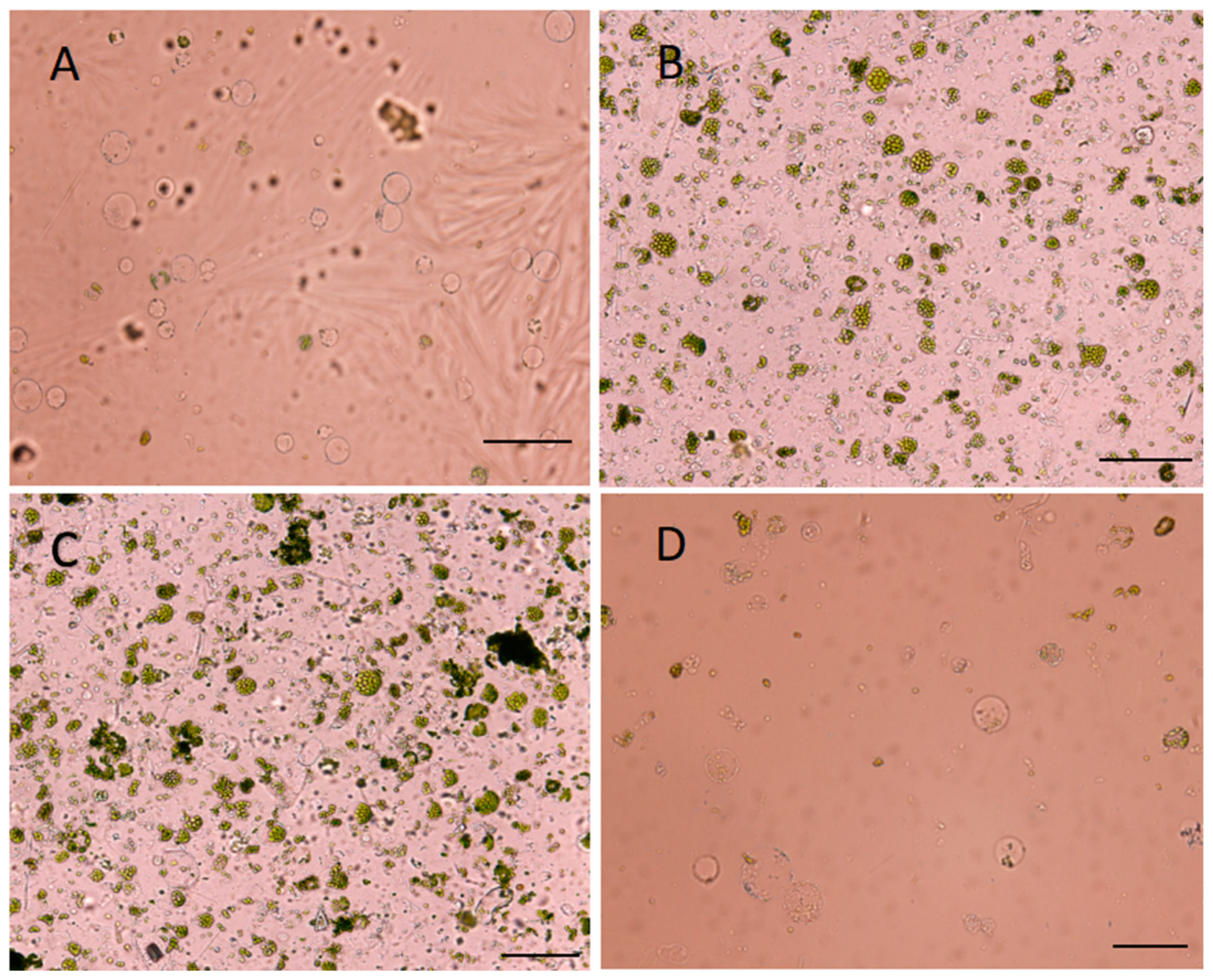
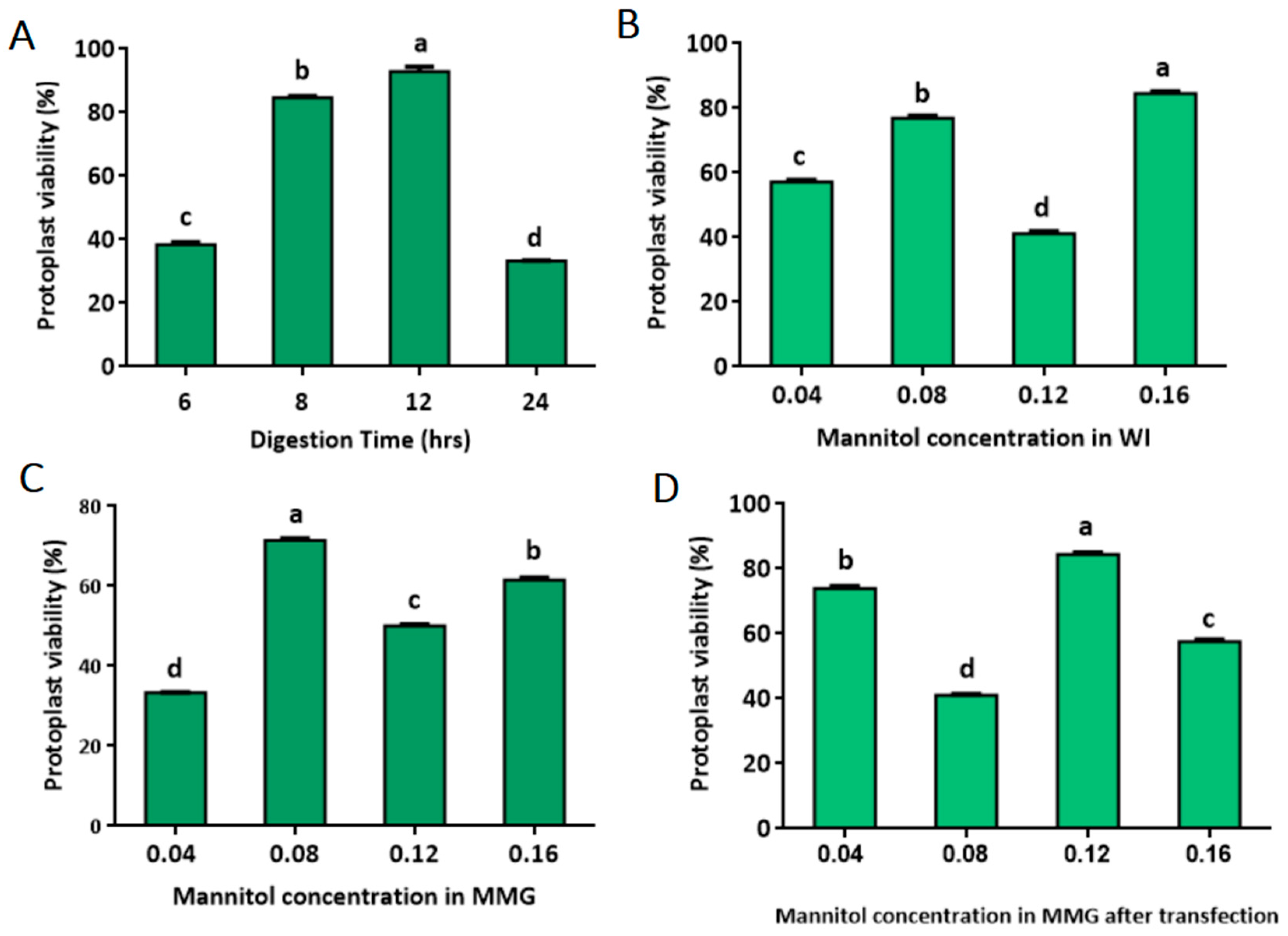
2.1. Development of Protoplast Isolation System
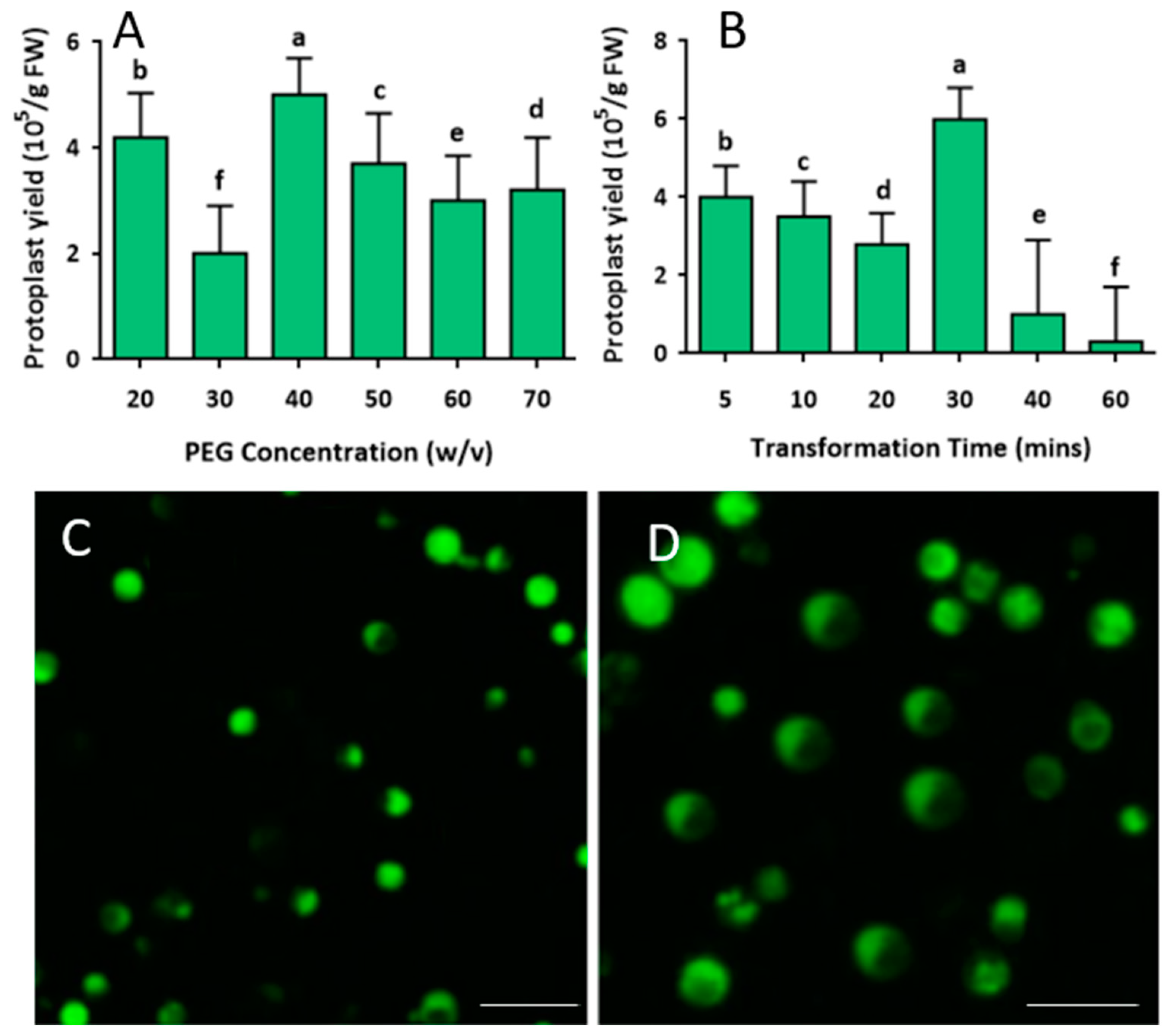
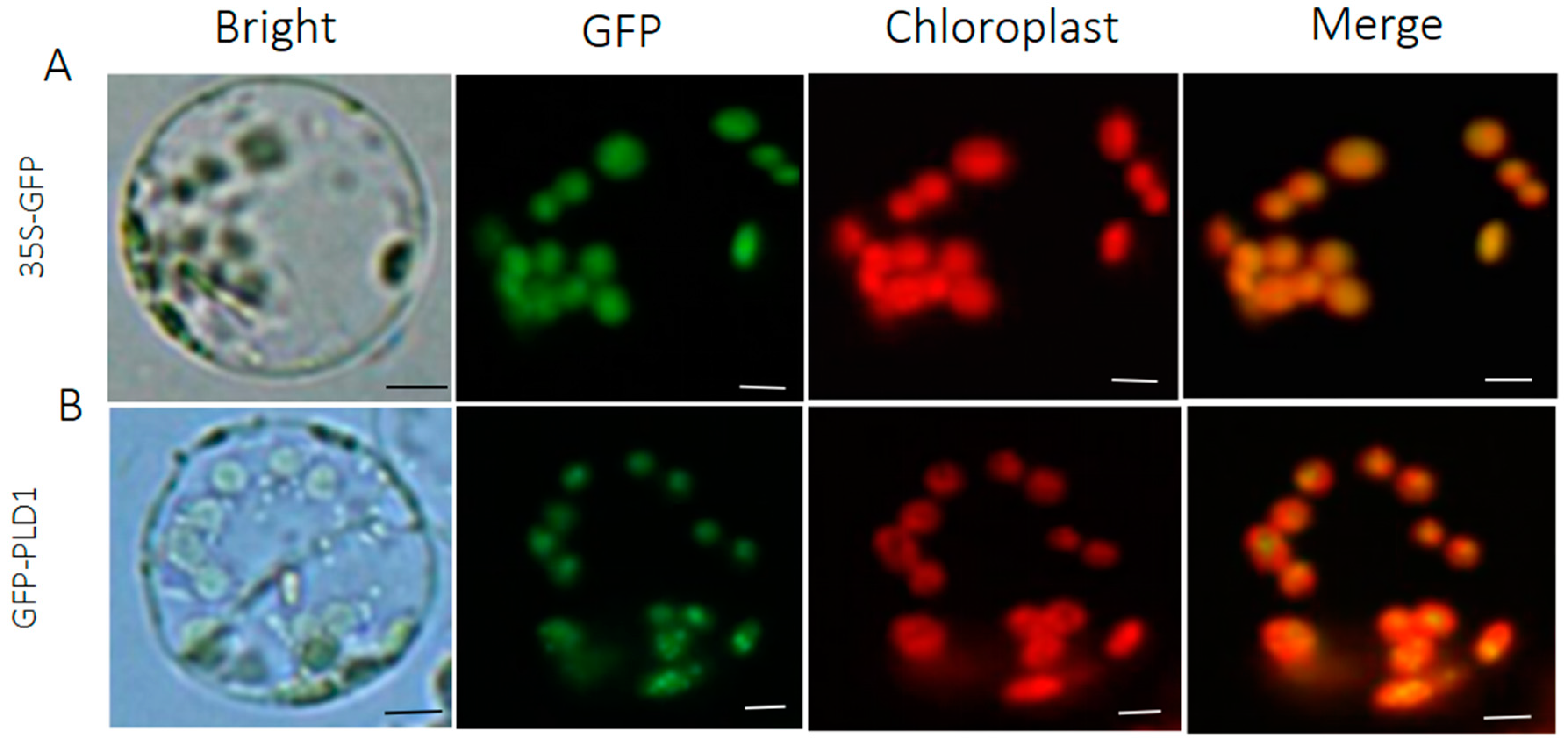
2.2. Development of Protoplast Transfection System
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Protoplast Isolation
4.3. PEG-Calcium Preparation and Transformation Process
4.4. Protoplast Viability Test
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Busatto, N.; Vittani, L.; Farneti, B.; Khomenko, I.; Caffini, M.; Faccini, S.; Boschetti, M.; Costa, F. Physiological and molecular characterization of the late ripening stages in Mangifera indica cv Keitt. Postharvest Biol. Technol. 2022, 183, 111746. [Google Scholar] [CrossRef]
- Oliver-Simancas, R.; Labrador-Fernández, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Alañón, M.E. Comprehensive research on mango by-products applications in food industry. Trends Food Sci. Technol. 2021, 118, 179–188. [Google Scholar] [CrossRef]
- Marçal, S.; Pintado, M. Mango peels as food ingredient/additive: Nutritional value, processing, safety and applications. Trends Food Sci. Technol. 2021, 114, 472–489. [Google Scholar] [CrossRef]
- Dautt-Castro, M.; López-Virgen, A.G.; Ochoa-Leyva, A.; Contreras-Vergara, C.A.; Sortillón-Sortillón, A.P.; Martínez-Téllez, M.A.; González-Aguilar, G.A.; Casas-Flores, J.S.; Sañudo-Barajas, A.; Kuhn, D.N. Genome-wide identification of mango (Mangifera indica L.) polygalacturonases: Expression analysis of family members and total enzyme activity during fruit ripening. Front. Plant Sci. 2019, 10, 969. [Google Scholar] [CrossRef]
- Charlwood, B.V.; Pletsch, M. Manipulation of natural product accumulation in plants through genetic engineering. J. Herbs Spices Med. Plants 2002, 9, 139–151. [Google Scholar] [CrossRef]
- Yoo, S.-D.; Cho, Y.-H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, Y.; Li, D.; Shi, S.; Li, S.; Li, L.; He, Y.; Li, J.; Chen, H.; Ge, H. A highly efficient mesophyll protoplast isolation and PEG-mediated transient expression system in eggplant. Sci. Hortic. 2022, 304, 111303. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Chen, J.-C.; Fang, S.-C. A protoplast transient expression system to enable molecular, cellular, and functional studies in Phalaenopsis orchids. Front. Plant Sci. 2018, 9, 843. [Google Scholar] [CrossRef]
- Adedeji, O.S.; Naing, A.H.; Kang, H.; Chung, M.Y.; Lim, K.B.; Kim, C.K. Optimization of protocol for efficient protoplast isolation and transient gene expression in carnation. Sci. Hortic. 2022, 299, 111057. [Google Scholar] [CrossRef]
- Wang, P.; Pu, Y.; Abid, M.A.; Kang, L.; Ye, Y.; Zhang, M.; Liang, C.; Wei, Y.; Zhang, R.; Meng, Z. A rapid and efficient method for isolation and transformation of cotton callus protoplast. Int. J. Mol. Sci. 2022, 23, 8368. [Google Scholar] [CrossRef]
- Yang, W.; Ren, J.; Liu, W.; Liu, D.; Xie, K.; Zhang, F.; Wang, P.; Guo, W.; Wu, X. An efficient transient gene expression system for protein subcellular localization assay and genome editing in citrus protoplasts. Hortic. Plant J. 2022, 9, 425–436. [Google Scholar] [CrossRef]
- Masani, M.Y.A.; Parveez, G.K.A.; Noll, G.; Fizree, M.P.M.A.A.; Sambanthamurthi, R.; Pruefer, D. Protoplast Isolation and Transformation in Oil Palm Oil palms. In Protoplast Technology: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2022; pp. 187–202. [Google Scholar]
- PPriyadarshani, S.V.G.N.; Hu, B.; Li, W.; Ali, H.; Jia, H.; Zhao, L.; Ojolo, S.P.; Azam, S.M.; Xiong, J.; Yan, M.; et al. Simple protoplast isolation system for gene expression and protein interaction studies in pineapple (Ananas comosus L.). Plant Methods 2018, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Ara, H.; Jaiswal, U.; Jaiswal, V. Plant regeneration from protoplasts of mango (Mangifera indica L.) through somatic embryogenesis. Plant Cell Rep. 2000, 19, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, S.; Du, C.; Gao, H.; Yang, D.; Fu, G.; Cui, H. Establishment of a Protoplasts-Based Transient Expression System in Banana (Musa spp.). Agronomy 2022, 12, 2648. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Igwe, D.O.; Popoola, J.O. Transgenic plant-mediated phytoremediation: Applications, challenges, and prospects. Assist. Phytoremediat. 2022, 179–202. [Google Scholar]
- Naing, A.H.; Adedeji, O.S.; Kim, C.K. Protoplast technology in ornamental plants: Current progress and potential applications on genetic improvement. Sci. Hortic. 2021, 283, 110043. [Google Scholar] [CrossRef]
- Rehman, L.; Su, X.; Guo, H.; Qi, X.; Cheng, H. Protoplast transformation as a potential platform for exploring gene function in Verticillium dahliae. BMC Biotechnol. 2016, 16, 57. [Google Scholar] [CrossRef]
- Rezazadeh, R.; Harrison, D.K.; Williams, R.R. Intraspecific somatic hybridization of mango (Mangifera indica L.) through protoplast fusion. J. Appl. Hortic. 2011, 13, 101–107. [Google Scholar] [CrossRef]
- Biswas, S.; Wahl, N.J.; Thomson, M.J.; Cason, J.M.; McCutchen, B.F.; Septiningsih, E.M. Optimization of protoplast isolation and transformation for a pilot study of genome editing in peanut by targeting the allergen gene Ara h 2. Int. J. Mol. Sci. 2022, 23, 837. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Bridgeland, A.; Irum, S.; Thomson, M.J.; Septiningsih, E.M. Optimization of prime editing in rice, peanut, chickpea, and cowpea protoplasts by restoration of GFP activity. Int. J. Mol. Sci. 2022, 23, 9809. [Google Scholar] [CrossRef]
- Zhu, P.; Zhao, Y.; You, X.; Zhang, Y.J.; Vasseur, L.; Haughn, G.; Liu, Y. A versatile protoplast system and its application in Cannabis sativa L. Botany 2022, 101, 291–300. [Google Scholar] [CrossRef]
- Fry, B.A.; Loria, R. Thaxtomin A: Evidence for a plant cell wall target. Physiol. Mol. Plant Pathol. 2002, 60, 1–8. [Google Scholar] [CrossRef]
- Bethke, G.; Thao, A.; Xiong, G.; Li, B.; Soltis, N.E.; Hatsugai, N.; Hillmer, R.A.; Katagiri, F.; Kliebenstein, D.J.; Pauly, M. Pectin biosynthesis is critical for cell wall integrity and immunity in Arabidopsis thaliana. Plant Cell 2016, 28, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-f.; Zhu, H.-y.; Ren, Y.-f.; Feng, C.; Ye, Z.-h.; Cai, H.-m.; Wan, X.-c.; Peng, C.-y. Efficient isolation and purification of tissue-specific protoplasts from tea plants (Camellia sinensis (L.) O. Kuntze). Plant Methods 2021, 17, 84. [Google Scholar] [CrossRef]
- Antoniadi, I.; Skalický, V.; Sun, G.; Ma, W.; Galbraith, D.W.; Novák, O.; Ljung, K. Fluorescence activated cell sorting—A selective tool for plant cell isolation and analysis. Cytom. Part A 2022, 101, 725–736. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Zhan, J.; Huang, W.; Xu, H. An efficient PEG-mediated transient gene expression system in grape protoplasts and its application in subcellular localization studies of flavonoids biosynthesis enzymes. Sci. Hortic. 2015, 191, 82–89. [Google Scholar] [CrossRef]
- Moose, S.P.; Mumm, R.H. Molecular plant breeding as the foundation for 21st century crop improvement. Plant Physiol. 2008, 147, 969–977. [Google Scholar] [CrossRef]
- Wu, F.-H.; Shen, S.-C.; Lee, L.-Y.; Lee, S.-H.; Chan, M.-T.; Lin, C.-S. Tape-Arabidopsis Sandwich-a simpler Arabidopsis protoplast isolation method. Plant Methods 2009, 5, 16. [Google Scholar] [CrossRef]
- He, F.; Chen, S.; Ning, Y.; Wang, G.L. Rice (Oryza sativa) protoplast isolation and its application for transient expression analysis. Curr. Protoc. Plant Biol. 2016, 1, 373–383. [Google Scholar] [CrossRef]
- Gao, J.; Wang, G.; Ma, S.; Xie, X.; Wu, X.; Zhang, X.; Wu, Y.; Zhao, P.; Xia, Q. CRISPR/Cas9-mediated targeted mutagenesis in Nicotiana tabacum. Plant Mol. Biol. 2015, 87, 99–110. [Google Scholar] [CrossRef]
- Yao, L.; Liao, X.; Gan, Z.; Peng, X.; Wang, P.; Li, S.; Li, T. Protoplast isolation and development of a transient expression system for sweet cherry (Prunus avium L.). Sci. Hortic. 2016, 209, 14–21. [Google Scholar] [CrossRef]
- Lakhani, H.; Thakur, N.; Tiwari, S. Genome editing for vegetatively propagated crops improvement: A new horizon of possibilities. J. Plant Biochem. Biotechnol. 2022, 1–12. [Google Scholar] [CrossRef]
- Hu, Y.; Song, D.; Gao, L.; Ajayo, B.S.; Wang, Y.; Huang, H.; Zhang, J.; Liu, H.; Liu, Y.; Yu, G. Optimization of isolation and transfection conditions of maize endosperm protoplasts. Plant Methods 2020, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, X.; Qu, J.; Han, R. Optimization conditions of wheat mesophyll protoplast isolation. Agric. Sci. 2016, 7, 850–858. [Google Scholar] [CrossRef]
- Wang, A.; Jin, Q.; Xu, X.; Miao, A.; White, J.C.; Gardea-Torresdey, J.L.; Ji, R.; Zhao, L. High-throughput screening for engineered nanoparticles that enhance photosynthesis using mesophyll protoplasts. J. Agric. Food Chem. 2020, 68, 3382–3389. [Google Scholar] [CrossRef]
- Ma, W.; Yi, F.; Xiao, Y.; Yang, G.; Chen, F.; Wang, J. Isolation of leaf mesophyll protoplasts optimized by orthogonal design for transient gene expression in Catalpa bungei. Sci. Hortic. 2020, 274, 109684. [Google Scholar] [CrossRef]
- Adedeji, O.S.; Naing, A.H.; Kim, C.K. Protoplast isolation and shoot regeneration from protoplast-derived calli of Chrysanthemum cv. White ND. Plant Cell Tissue Organ Cult. (PCTOC) 2020, 141, 571–581. [Google Scholar] [CrossRef]
- Li, S.; Zhao, R.; Ye, T.; Guan, R.; Xu, L.; Ma, X.; Zhang, J.; Xiao, S.; Yuan, D. Isolation, purification and PEG-mediated transient expression of mesophyll protoplasts in Camellia oleifera. Plant Methods 2022, 18, 141. [Google Scholar] [CrossRef]
- Shawkat, H.; Westwood, M.-M.; Mortimer, A. Mannitol: A review of its clinical uses. Contin. Educ. Anaesth. Crit. Care Pain 2012, 12, 82–85. [Google Scholar] [CrossRef]
- Kumar, M.K.; Sandeep, B.; Rao, P.S. Development of salt tolerant callus cultures by somatic hybridization between Oryza sativa and mangrove grass Myriostachya wightiana. Ann. Agrar. Sci. 2018, 16, 396–404. [Google Scholar] [CrossRef]
- Zhang, L.; Yung, W.-S.; Wang, Z.; Li, M.-W.; Huang, M. Optimization of an Efficient Protoplast Transformation System for Transient Expression Analysis Using Leaves of Torenia fournieri. Plants 2022, 11, 2106. [Google Scholar] [CrossRef]
- Cui, J.; Kuligowska Mackenzie, K.; Eeckhaut, T.; Müller, R.; Lütken, H. Protoplast isolation and culture from Kalanchoë species: Optimization of plant growth regulator concentration for efficient callus production. Plant Cell Tissue Organ Cult. (PCTOC) 2019, 138, 287–297. [Google Scholar] [CrossRef]
- Shan, X.; Li, Y.; Zhou, L.; Tong, L.; Wei, C.; Qiu, L.; Gao, X.; Wang, L. Efficient isolation of protoplasts from freesia callus and its application in transient expression assays. Plant Cell Tissue Organ Cult. (PCTOC) 2019, 138, 529–541. [Google Scholar] [CrossRef]
- Chen, K.; Hu, K.; Xi, F.; Wang, H.; Kohnen, M.V.; Gao, P.; Liao, J.; Wei, W.; Liu, X.; Zhang, H. High-Efficient and Transient Transformation of Moso Bamboo (Phyllostachys edulis) and Ma Bamboo (Dendrocalamus latiflorus Munro). J. Plant Biol. 2021, 66, 75–86. [Google Scholar] [CrossRef]
- Chen, S.; Tao, L.; Zeng, L.; VEGA-SANCHEZ, M.E.; Umemura, K.; WANG, G.L. A highly efficient transient protoplast system for analyzing defence gene expression and protein–protein interactions in rice. Mol. Plant Pathol. 2006, 7, 417–427. [Google Scholar] [CrossRef]
- Sangra, A.; Shahin, L.; Dhir, S.K. Optimization of isolation and culture of protoplasts in alfalfa (Medicago sativa) cultivar Regen-SY. Am. J. Plant Sci. 2019, 10, 1206. [Google Scholar] [CrossRef]
- Jung, H.-i.; Yan, J.; Zhai, Z.; Vatamaniuk, O.K. Gene functional analysis using protoplast transient assays. Plant Funct. Genom. Methods Protoc. 2015, 1284, 433–452. [Google Scholar]
- Green, M.R.; Sambrook, J. Estimation of cell number by hemocytometry counting. Cold Spring Harbor Protocols 2019, 2019, pdb.prot097980. [Google Scholar] [CrossRef]
- Mahon, M.J. pHluorin2: An enhanced, ratiometric, pH-sensitive green florescent protein. Adv. Biosci. Biotechnol. 2011, 2, 132. [Google Scholar] [CrossRef]
- Brandt, K.M.; Gunn, H.; Moretti, N.; Zemetra, R.S. A streamlined protocol for wheat (Triticum aestivum) protoplast isolation and transformation with CRISPR-Cas ribonucleoprotein complexes. Front. Plant Sci. 2020, 11, 769. [Google Scholar] [CrossRef]
- Ling, A.P.K.; Ong, C.P.L.; Tee, C.S.; Hussein, S. Establishment of protoplast isolation protocols of Orthosiphon staminues. Am.-Eurasian J. Sustain. Agric. 2009, 3, 587–596. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adjei, M.O.; Zhao, H.; Tao, X.; Yang, L.; Deng, S.; Li, X.; Mao, X.; Li, S.; Huang, J.; Luo, R.; et al. Using A Protoplast Transformation System to Enable Functional Studies in Mangifera indica L. Int. J. Mol. Sci. 2023, 24, 11984. https://doi.org/10.3390/ijms241511984
Adjei MO, Zhao H, Tao X, Yang L, Deng S, Li X, Mao X, Li S, Huang J, Luo R, et al. Using A Protoplast Transformation System to Enable Functional Studies in Mangifera indica L. International Journal of Molecular Sciences. 2023; 24(15):11984. https://doi.org/10.3390/ijms241511984
Chicago/Turabian StyleAdjei, Mark Owusu, Huan Zhao, Xiaoguang Tao, Li Yang, Shuyue Deng, Xiyan Li, Xinjing Mao, Shujiang Li, Jianfeng Huang, Ruixiong Luo, and et al. 2023. "Using A Protoplast Transformation System to Enable Functional Studies in Mangifera indica L." International Journal of Molecular Sciences 24, no. 15: 11984. https://doi.org/10.3390/ijms241511984
APA StyleAdjei, M. O., Zhao, H., Tao, X., Yang, L., Deng, S., Li, X., Mao, X., Li, S., Huang, J., Luo, R., Gao, A., & Ma, J. (2023). Using A Protoplast Transformation System to Enable Functional Studies in Mangifera indica L. International Journal of Molecular Sciences, 24(15), 11984. https://doi.org/10.3390/ijms241511984





