Protective Properties of Spheroidal Taxifolin Form in Streptozotocin-Induced Diabetic Rats
Abstract
1. Introduction
2. Results
2.1. General Outcomes
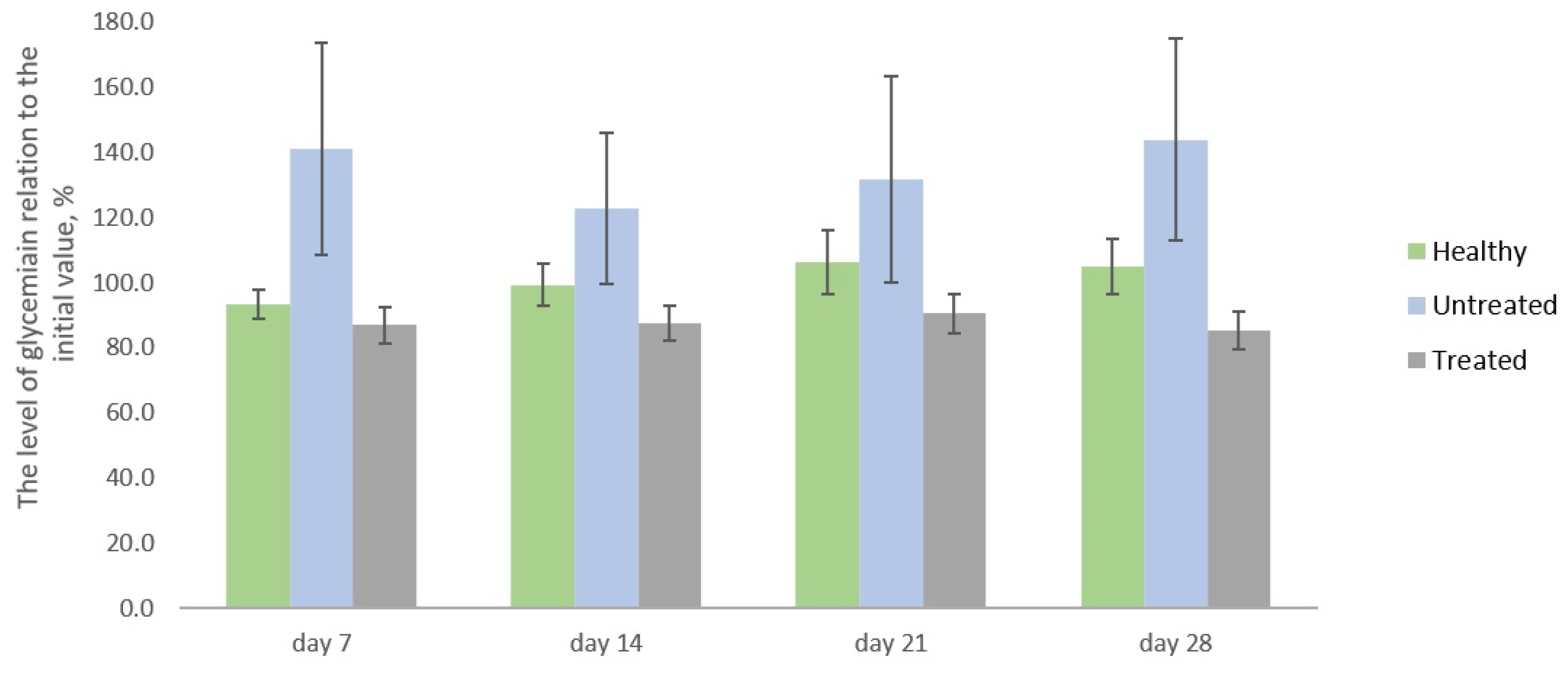
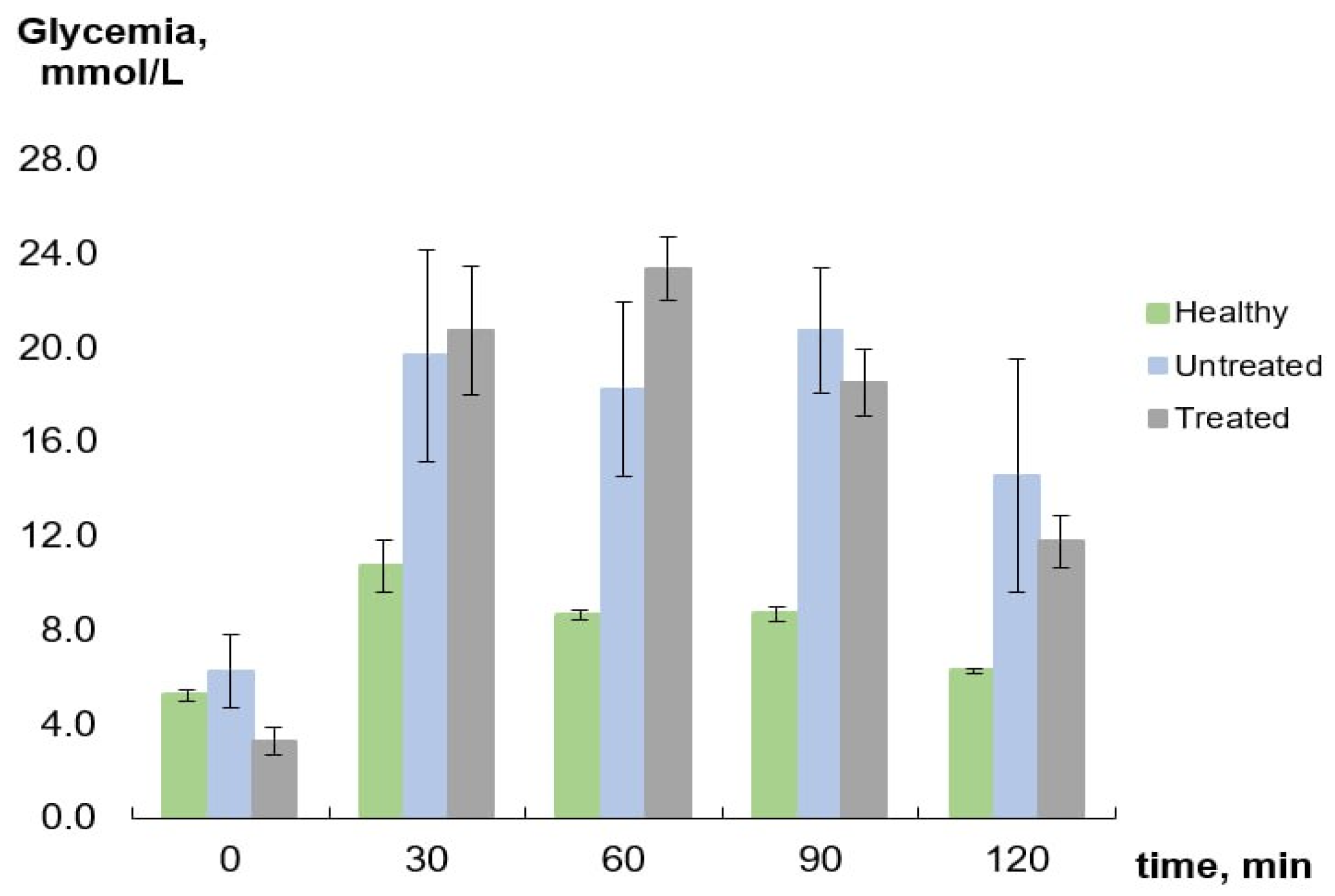
2.2. Blood Glucose Monitoring
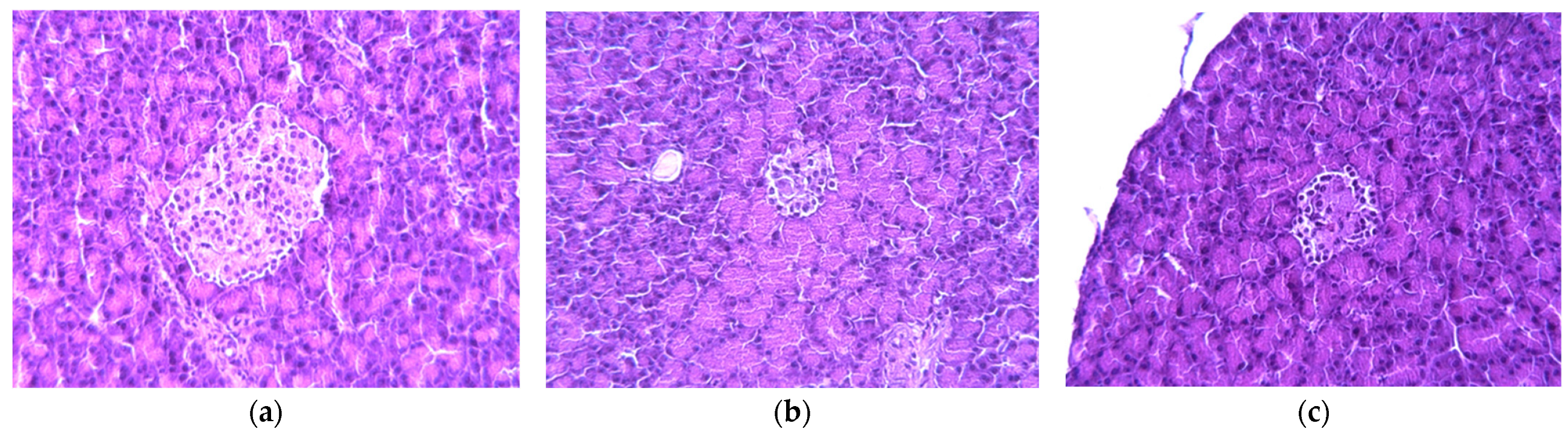
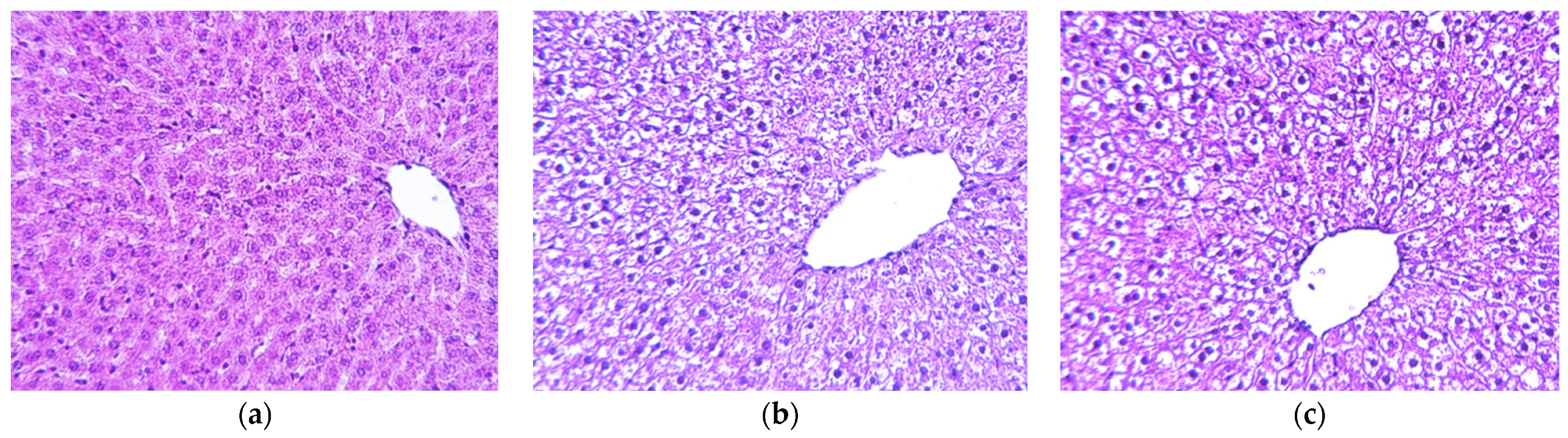
2.3. Histology Analysis
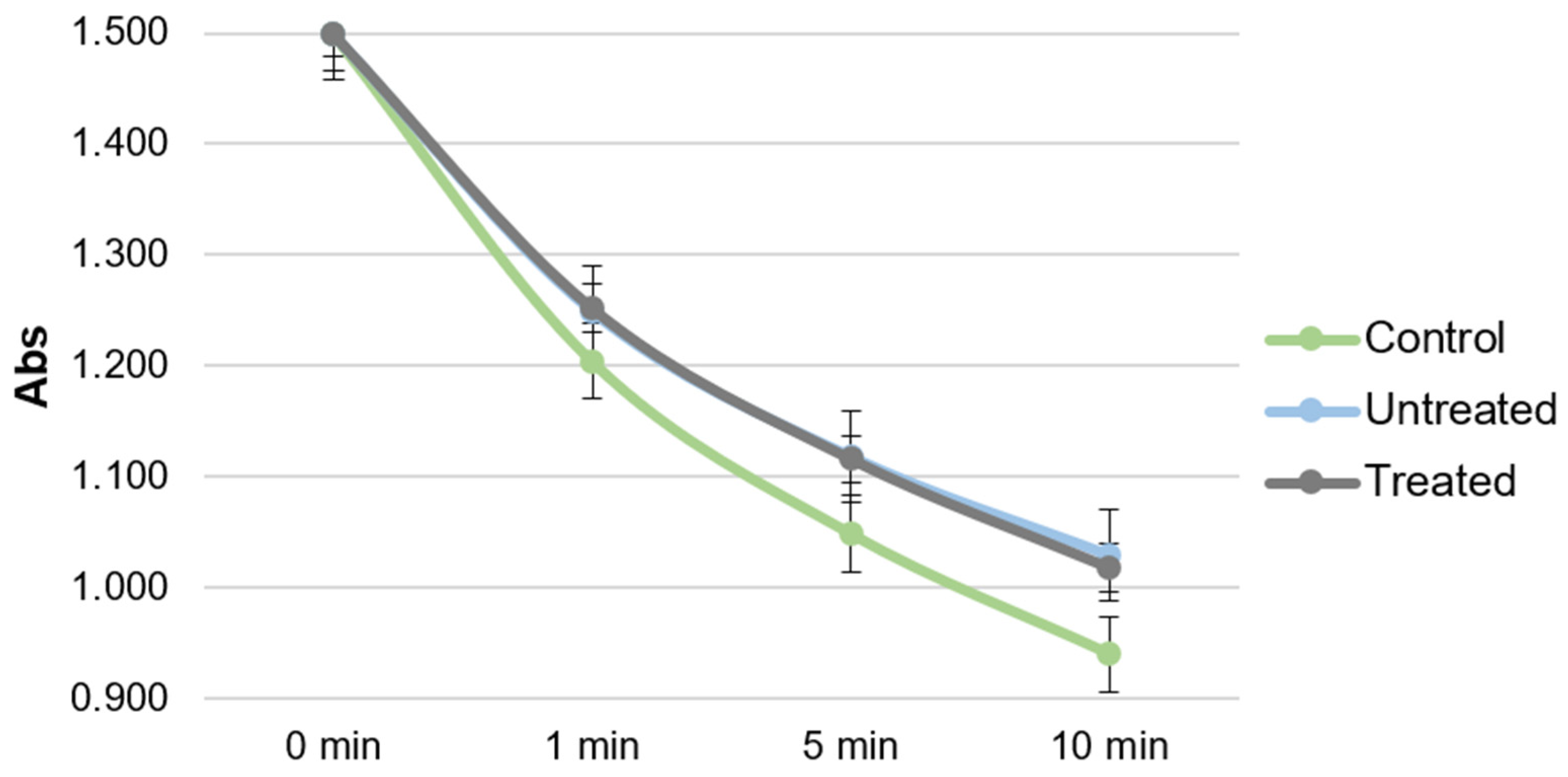
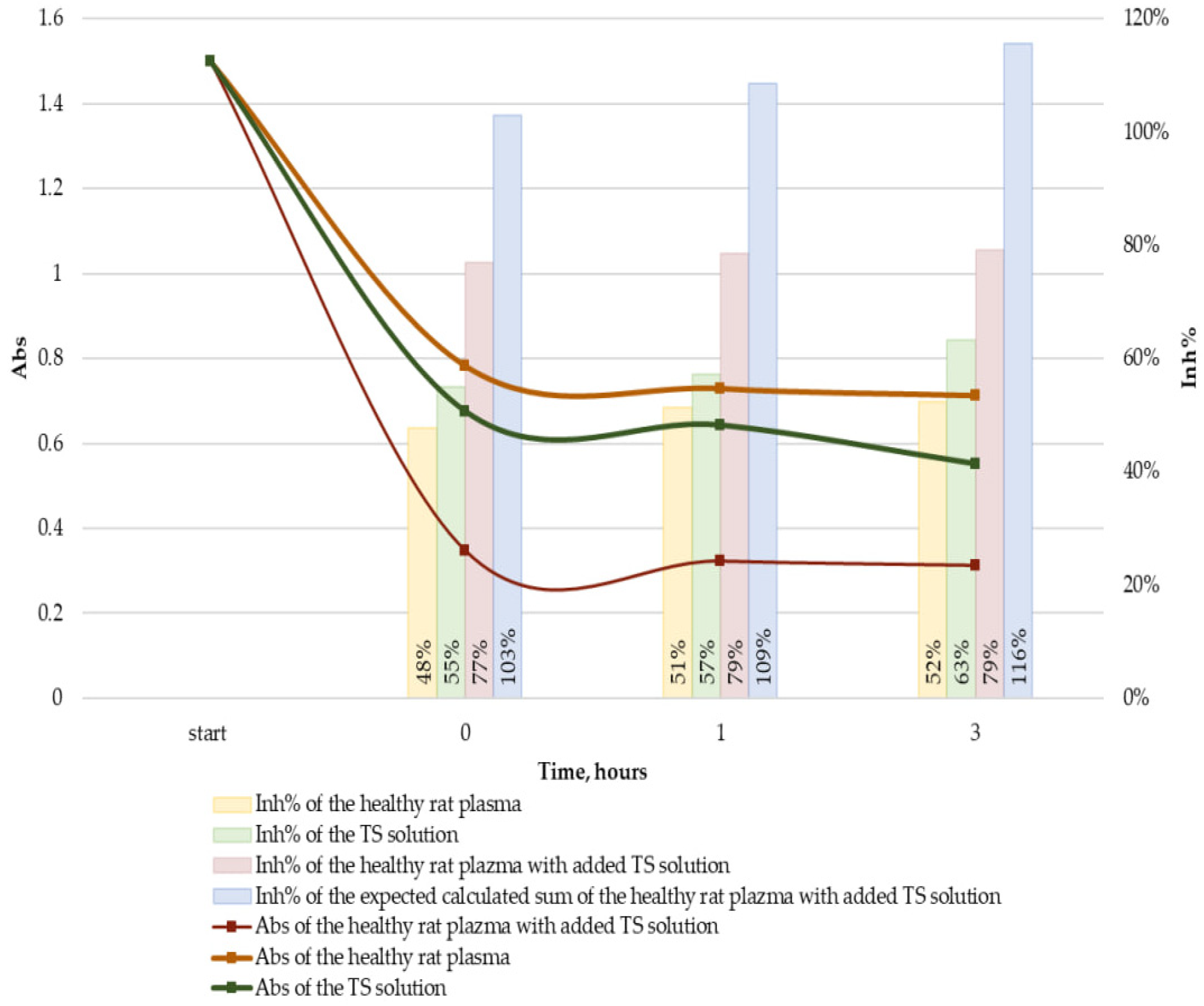
2.4. Antioxidant Activity of Plasma
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Materials
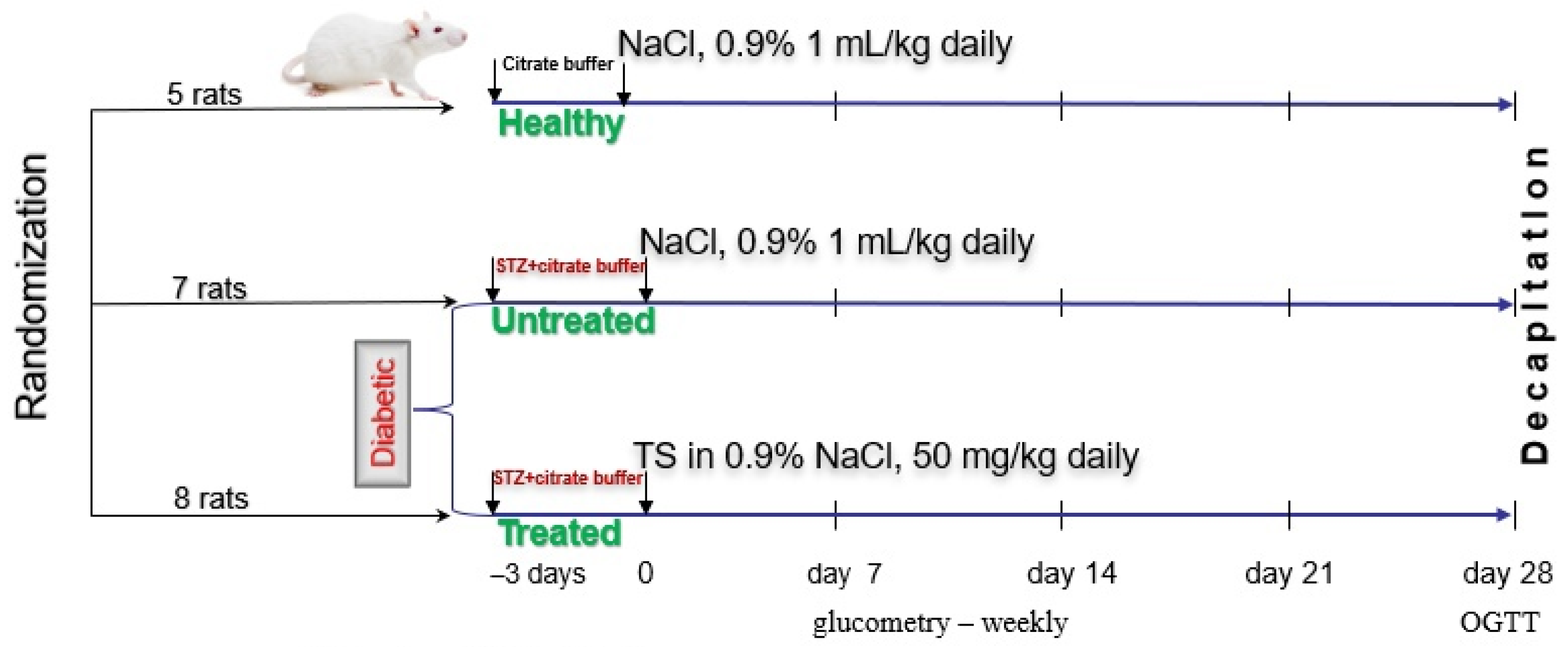
4.3. Study Design
4.4. OGTT
4.5. Pancreas and Liver Morphological Analysis
4.6. Measurement of Antioxidant Activity of Plasma
4.7. Modeling TS Binding to Plasma Proteins
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2019, 10, 107. [Google Scholar] [CrossRef]
- Ogurtsova, K.; Guariguata, L.; Barengo, N.C.; Ruiz, P.L.-D.; Sacre, J.W.; Karuranga, S.; Sun, H.; Boyko, E.J.; Magliano, D.J. IDF Diabetes Atlas: Global Estimates of Undiagnosed Diabetes in Adults for 2021. Diabetes Res. Clin. Pr. 2022, 183, 109118. [Google Scholar] [CrossRef]
- Argaev-Frenkel, L.; Rosenzweig, T. Redox Balance in Type 2 Diabetes: Therapeutic Potential and the Challenge of Antioxidant-Based Therapy. Antioxidants 2023, 12, 994. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Rolo, A.P.; Palmeira, C.M. Diabetes and Mitochondrial Function: Role of Hyperglycemia and Oxidative Stress. Toxicol. Appl. Pharmacol. 2006, 212, 167–178. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Howarth, F.C.; Raza, H. Exercise Alleviates Diabetic Complications by Inhibiting Oxidative Stress-Mediated Signaling Cascade and Mitochondrial Metabolic Stress in GK Diabetic Rat Tissues. Front. Physiol. 2022, 13, 1052608. [Google Scholar] [CrossRef]
- Dinić, S.; Arambašić Jovanović, J.; Uskoković, A.; Mihailović, M.; Grdović, N.; Tolić, A.; Rajić, J.; Đorđević, M.; Vidaković, M. Oxidative Stress-Mediated Beta Cell Death and Dysfunction as a Target for Diabetes Management. Front. Endocrinol. 2022, 13, 1006376. [Google Scholar] [CrossRef] [PubMed]
- Terekhov, R.P.; Selivanova, I.A. Molecular Modeling of the Interaction of the Dihydroquercetin and Its Metabolites with Cyclooxygenase-2. Bull. Sib. Med. 2019, 18, 101–106. [Google Scholar] [CrossRef]
- Oi, N.; Chen, H.; Ok Kim, M.; Lubet, R.A.; Bode, A.M.; Dong, Z. Taxifolin Suppresses UV-Induced Skin Carcinogenesis by Targeting EGFR and PI3K. Cancer Prev. Res. 2012, 5, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Raj, U.; Varadwaj, P.K. Flavonoids as Multi-Target Inhibitors for Proteins Associated with Ebola Virus: In Silico Discovery Using Virtual Screening and Molecular Docking Studies. Interdiscip. Sci. Comput. Life Sci. 2016, 8, 132–141. [Google Scholar] [CrossRef]
- Fischer, A.; Sellner, M.; Neranjan, S.; Smieško, M.; Lill, M.A. Potential Inhibitors for Novel Coronavirus Protease Identified by Virtual Screening of 606 Million Compounds. Int. J. Mol. Sci. 2020, 21, 3626. [Google Scholar] [CrossRef]
- Kolhir, V.K.; Bykov, V.A.; Baginskaja, A.I.; Sokolov, S.Y.; Glazova, N.G.; Leskova, T.E.; Sakovich, G.S.; Tjukavkina, N.A.; Kolesnik, Y.A.; Rulenko, I.A. Antioxidant Activity of a Dihydroquercetin Isolated FromLarix Gmelinii (Rupr.) Rupr. Wood. Phytother. Res. 1996, 10, 478–482. [Google Scholar] [CrossRef]
- Kolkhir, V.K.; Tyukavkina, N.A.; Bykov, V.A.; Glyzin, V.I.; Arzamastsev, A.P.; Baginskaya, A.I.; Sokolov, S.Y.; Kolesnik, Y.A.; Glazova, N.G.; Rulenko, I.A.; et al. Dicvertin: A New Antioxidant and Capillary-Protecting Drug. Pharm. Chem. J. 1995, 29, 657–660. [Google Scholar] [CrossRef]
- Yang, C.-L.; Lin, Y.-S.; Liu, K.-F.; Peng, W.-H.; Hsu, C.-M. Hepatoprotective Mechanisms of Taxifolin on Carbon Tetrachloride-Induced Acute Liver Injury in Mice. Nutrients 2019, 11, 2655. [Google Scholar] [CrossRef]
- Teselkin, Y.O.; Babenkova, I.V.; Kolhir, V.K.; Baginskaya, A.I.; Tjukavkina, N.A.; Kolesnik, Y.A.; Selivanova, I.A.; Eichholz, A.A. Dihydroquercetin as a Means of Antioxidative Defence in Rats with Tetrachloromethane Hepatitis. Phytother. Res. 2000, 14, 160–162. [Google Scholar] [CrossRef]
- Inoue, T.; Saito, S.; Tanaka, M.; Yamakage, H.; Kusakabe, T.; Shimatsu, A.; Ihara, M.; Satoh-Asahara, N. Pleiotropic Neuroprotective Effects of Taxifolin in Cerebral Amyloid Angiopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 10031–10038. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Saito, S.; Inoue, T.; Satoh-Asahara, N.; Ihara, M. Novel Therapeutic Potentials of Taxifolin for Amyloid-β-Associated Neurodegenerative Diseases and Other Diseases: Recent Advances and Future Perspectives. Int. J. Mol. Sci. 2019, 20, 2139. [Google Scholar] [CrossRef]
- Shubina, V.S.; Shatalin, Y.V. Skin Regeneration after Chemical Burn under the Effect of Taxifolin-Based Preparations. Bull. Exp. Biol. Med. 2012, 154, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Terekhov, R.P.; Selivanova, I.A.; Tyukavkina, N.A.; Shylov, G.V.; Utenishev, A.N.; Porozov, Y.B. Taxifolin Tubes: Crystal Engineering and Characteristics. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2019, 75, 175–182. [Google Scholar] [CrossRef]
- Stenger Moura, F.C.; Pinna, N.; Vivani, R.; Nunes, G.E.; Schoubben, A.; Bellé Bresolin, T.M.; Bechold, I.H.; Ricci, M. Exploring Taxifolin Polymorphs: Insights on Hydrate and Anhydrous Forms. Pharmaceutics 2021, 13, 1328. [Google Scholar] [CrossRef]
- Terekhov, R.P.; Ilyasov, I.R.; Beloborodov, V.L.; Zhevlakova, A.K.; Pankov, D.I.; Dzuban, A.V.; Bogdanov, A.G.; Davidovich, G.N.; Shilov, G.V.; Utenyshev, A.N.; et al. Solubility Enhancement of Dihydroquercetin via “Green” Phase Modification. Int. J. Mol. Sci. 2022, 23, 15965. [Google Scholar] [CrossRef]
- Hickey, M.B.; Peterson, M.L.; Scoppettuolo, L.A.; Morrisette, S.L.; Vetter, A.; Guzmán, H.; Remenar, J.F.; Zhang, Z.; Tawa, M.D.; Haley, S. Performance Comparison of a Co-Crystal of Carbamazepine with Marketed Product. Eur. J. Pharm. Biopharm. 2007, 67, 112–119. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Miroshnyk, I.; Mirza, S.; Urakova, I.N.; Hirsjärvi, S.; Makarov, V.G.; Heinämäki, J.; Yliruusi, J.; Hiltunen, R. Nanodispersions of Taxifolin: Impact of Solid-State Properties on Dissolution Behavior. Int. J. Pharm. 2009, 377, 148–152. [Google Scholar] [CrossRef]
- Tzeng, C.-W.; Yen, F.-L.; Wu, T.-H.; Ko, H.-H.; Lee, C.-W.; Tzeng, W.-S.; Lin, C.-C. Enhancement of Dissolution and Antioxidant Activity of Kaempferol Using a Nanoparticle Engineering Process. J. Agric. Food Chem. 2011, 59, 5073–5080. [Google Scholar] [CrossRef] [PubMed]
- Terekhov, R.P.; Selivanova, I.A.; Tyukavkina, N.A.; Ilyasov, I.R.; Zhevlakova, A.K.; Dzuban, A.V.; Bogdanov, A.G.; Davidovich, G.N.; Shylov, G.V.; Utenishev, A.N.; et al. Assembling the Puzzle of Taxifolin Polymorphism. Molecules 2020, 25, 5437. [Google Scholar] [CrossRef]
- Taldaev, A.; Terekhov, R.P.; Selivanova, I.A.; Pankov, D.I.; Anurova, M.N.; Markovina, I.Y.; Cong, Z.; Ma, S.; Dong, Z.; Yang, F.; et al. Modification of Taxifolin Properties by Spray Drying. Sci. Pharm. 2022, 90, 67. [Google Scholar] [CrossRef]
- Ostrovskaya, R.U.; Zolotov, N.N.; Ozerova, I.V.; Ivanova, E.A.; Kapitsa, I.G.; Taraban, K.V.; Michunskaya, A.M.; Voronina, T.A.; Gudasheva, T.A.; Seredenin, S.B. Noopept Normalizes Parameters of the Incretin System in Rats with Experimental Diabetes. Bull. Exp. Biol. Med. 2014, 157, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Gheibi, S.; Kashfi, K.; Ghasemi, A. A Practical Guide for Induction of Type-2 Diabetes in Rat: Incorporating a High-Fat Diet and Streptozotocin. Biomed. Pharmacother. 2017, 95, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Adachi, S.; Yoshizawa, F.; Yagasaki, K. Antidiabetic Effect of Taxifolin in Cultured L6 Myotubes and Type 2 Diabetic Model KK-Ay/Ta Mice with Hyperglycemia and Hyperuricemia. Curr. Issues Mol. Biol. 2021, 43, 1293–1306. [Google Scholar] [CrossRef]
- Terekhov, R.P.; Selivanova, I.A.; Anurova, M.N.; Zhevlakova, A.K.; Nikitin, I.D.; Cong, Z.; Ma, S.; Yang, F.; Dong, Z.; Liao, Y. Comparative Study of Wound-Healing Activity of Dihydroquercetin Pseudopolymorphic Modifications. Bull. Exp. Biol. Med. 2021, 170, 444–447. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef]
- Arts, M.J.T.J.; Haenen, G.R.M.M.; Voss, H.-P.; Bast, A. Masking of Antioxidant Capacity by the Interaction of Flavonoids with Protein. Food Chem. Toxicol. 2001, 39, 787–791. [Google Scholar] [CrossRef]
- Shen, Y.; Li, M.; Wang, K.; Qi, G.; Liu, H.; Wang, W.; Ji, Y.; Chang, M.; Deng, C.; Xu, F.; et al. Diabetic Muscular Atrophy: Molecular Mechanisms and Promising Therapies. Front. Endocrinol. 2022, 13, 917113. [Google Scholar] [CrossRef] [PubMed]
- Benioudakis, E.S.; Karlafti, E.; Georgiou, E.D.; Kalaitzaki, A.; Kaiafa, G.; Savopoulos, C.; Didangelos, T. Diabetes Mellitus Type 1 During COVID-19: Psychological Symptoms AndEating Attitudes. Curr. Diabetes Rev. 2023, 19, e160522204817. [Google Scholar] [CrossRef] [PubMed]
- Yahya, A.S.; Khawaja, S.; Chukwuma, J.; Chukwuma, C. Early Diagnosis and Management of Bulimia Nervosa in Type 1 Diabetes. Prim. Care Companion CNS Disord. 2020, 22, 26721. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A. Three ABTS•+ Radical Cation-Based Approaches for the Evaluation of Antioxidant Activity: Fast- and Slow-Reacting Antioxidant Behavior. Chem. Pap. 2018, 72, 1917–1925. [Google Scholar] [CrossRef]
- Ilyasov, I.; Beloborodov, V.; Antonov, D.; Dubrovskaya, A.; Terekhov, R.; Zhevlakova, A.; Saydasheva, A.; Evteev, V.; Selivanova, I. Flavonoids with Glutathione Antioxidant Synergy: Influence of Free Radicals Inflow. Antioxidants 2020, 9, 695. [Google Scholar] [CrossRef]
| Group | Body Weight, g | ||||
|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | |
| Healthy | 291.6 ± 4.9 | 327.4 ± 8.3 | 341.6 ± 6.1 | 354.4 ± 6.8 | 355.0 ± 6.5 |
| Untreated | 276.7 ± 10.2 | 248.6 ± 19.5 | 248.1 ± 24.8 | 258.3 ± 27.5 | 252.8 ± 26.2 |
| Treated | 283.3 ± 6.9 | 220.9 ± 5.6 | 212.8 ± 7.6 | 213.1 ± 10.0 | 211.3 ± 9.4 |
| Group | Food Intake, g/Day/Rat | ||||
|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | |
| Healthy | 23.2 ± 0.9 | 26.9 ± 1.8 | 25.6 ± 1.4 | 23.4 ± 3.0 | 27.9 ± 3.1 |
| Untreated | 23.4 ± 1.5 | 25.9 ± 1.4 | 26.3 ± 1.4 | 27.5 ± 1.2 | 29.6 ± 2.3 |
| Treated | 23.3 ± 1.2 | 20.6 ± 2.1 | 21.9 ± 0.9 | 23.5 ± 1.0 | 21.9 ± 3.0 |
| Time, h | ∆Abs Values | The Observed Value Difference from the Expected Value, % | |||
|---|---|---|---|---|---|
| Healthy Rat Plasma | TS Solution | Healthy Rat Plasma with Added TS Solution | |||
| Observed | Expected | ||||
| 0 | 0.72 ± 0.08 | 0.83 ± 0.01 | 1.15 ± 0.05 | 1.54 ± 0.09 | −25 ± 0 * |
| 1 | 0.77 ± 0.03 | 0.86 ± 0.02 | 1.18 ± 0.02 | 1.63 ± 0.05 | −28 ± 1 * |
| 3 | 0.79 ± 0.04 | 0.95 ± 0.05 | 1.19 ± 0.09 | 1.74 ± 0.09 | −32 ± 2 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taldaev, A.; Savina, A.D.; Olicheva, V.V.; Ivanov, S.V.; Terekhov, R.P.; Ilyasov, I.R.; Zhevlakova, A.K.; Selivanova, I.A. Protective Properties of Spheroidal Taxifolin Form in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2023, 24, 11962. https://doi.org/10.3390/ijms241511962
Taldaev A, Savina AD, Olicheva VV, Ivanov SV, Terekhov RP, Ilyasov IR, Zhevlakova AK, Selivanova IA. Protective Properties of Spheroidal Taxifolin Form in Streptozotocin-Induced Diabetic Rats. International Journal of Molecular Sciences. 2023; 24(15):11962. https://doi.org/10.3390/ijms241511962
Chicago/Turabian StyleTaldaev, Amir, Anastasiya D. Savina, Vera V. Olicheva, Sergey V. Ivanov, Roman P. Terekhov, Igor R. Ilyasov, Anastasiya K. Zhevlakova, and Irina A. Selivanova. 2023. "Protective Properties of Spheroidal Taxifolin Form in Streptozotocin-Induced Diabetic Rats" International Journal of Molecular Sciences 24, no. 15: 11962. https://doi.org/10.3390/ijms241511962
APA StyleTaldaev, A., Savina, A. D., Olicheva, V. V., Ivanov, S. V., Terekhov, R. P., Ilyasov, I. R., Zhevlakova, A. K., & Selivanova, I. A. (2023). Protective Properties of Spheroidal Taxifolin Form in Streptozotocin-Induced Diabetic Rats. International Journal of Molecular Sciences, 24(15), 11962. https://doi.org/10.3390/ijms241511962






