Blockade of Melatonin Receptors Abolishes Its Antiarrhythmic Effect and Slows Ventricular Conduction in Rat Hearts
Abstract
1. Introduction
2. Results
2.1. In Vivo Heart Electrophysiology (Protocol A)
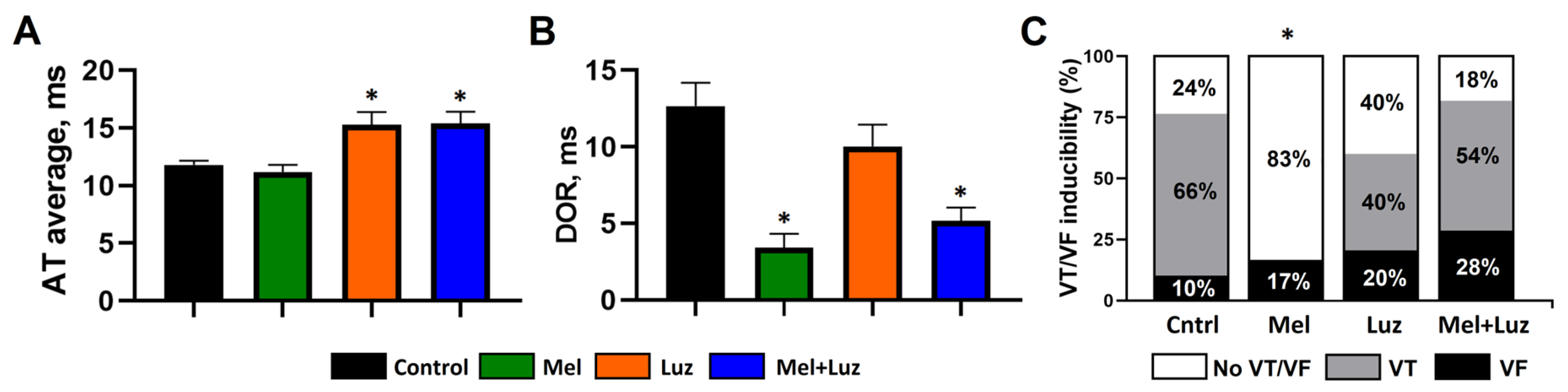
2.2. Incidence of Ventricular Arrhythmias (Protocol A)
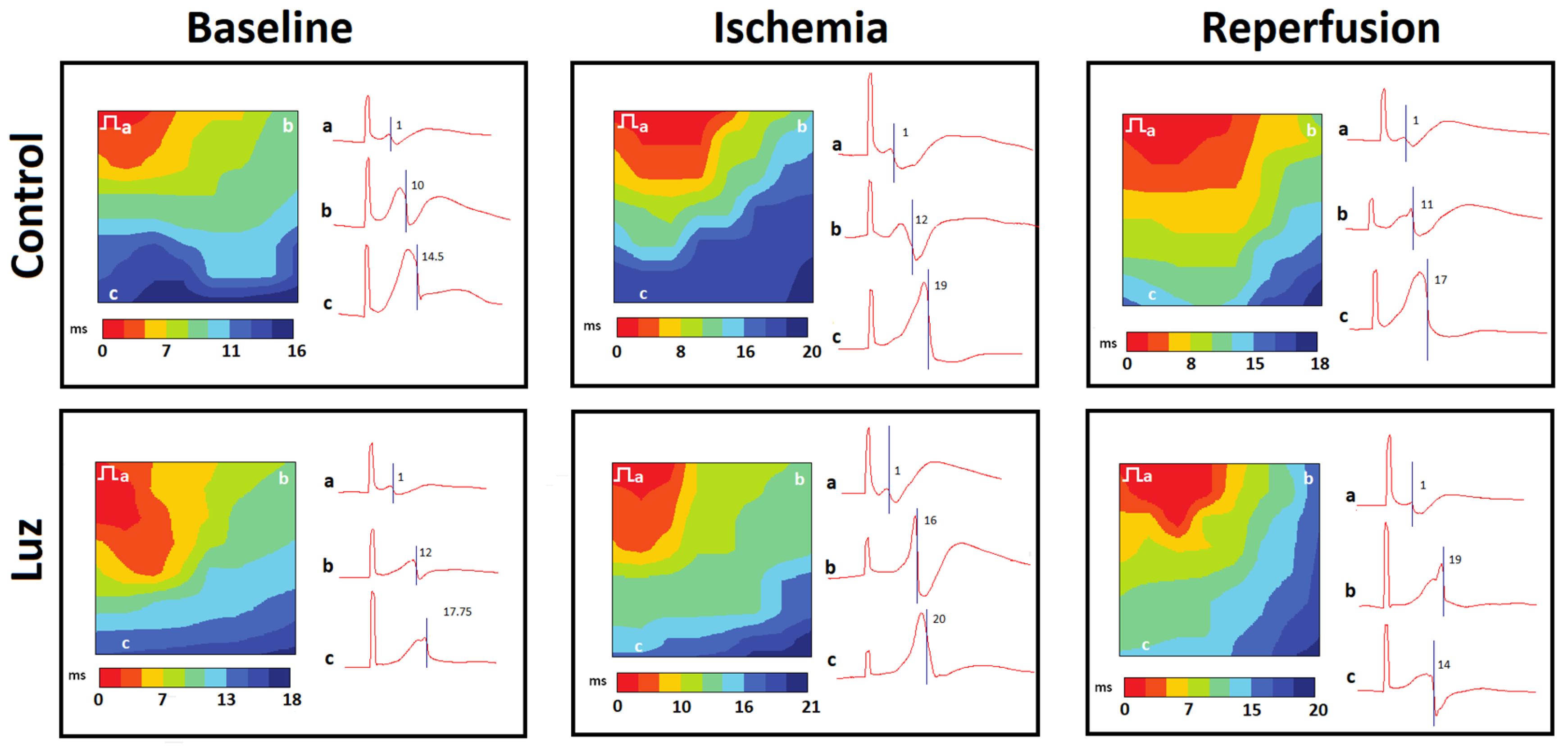
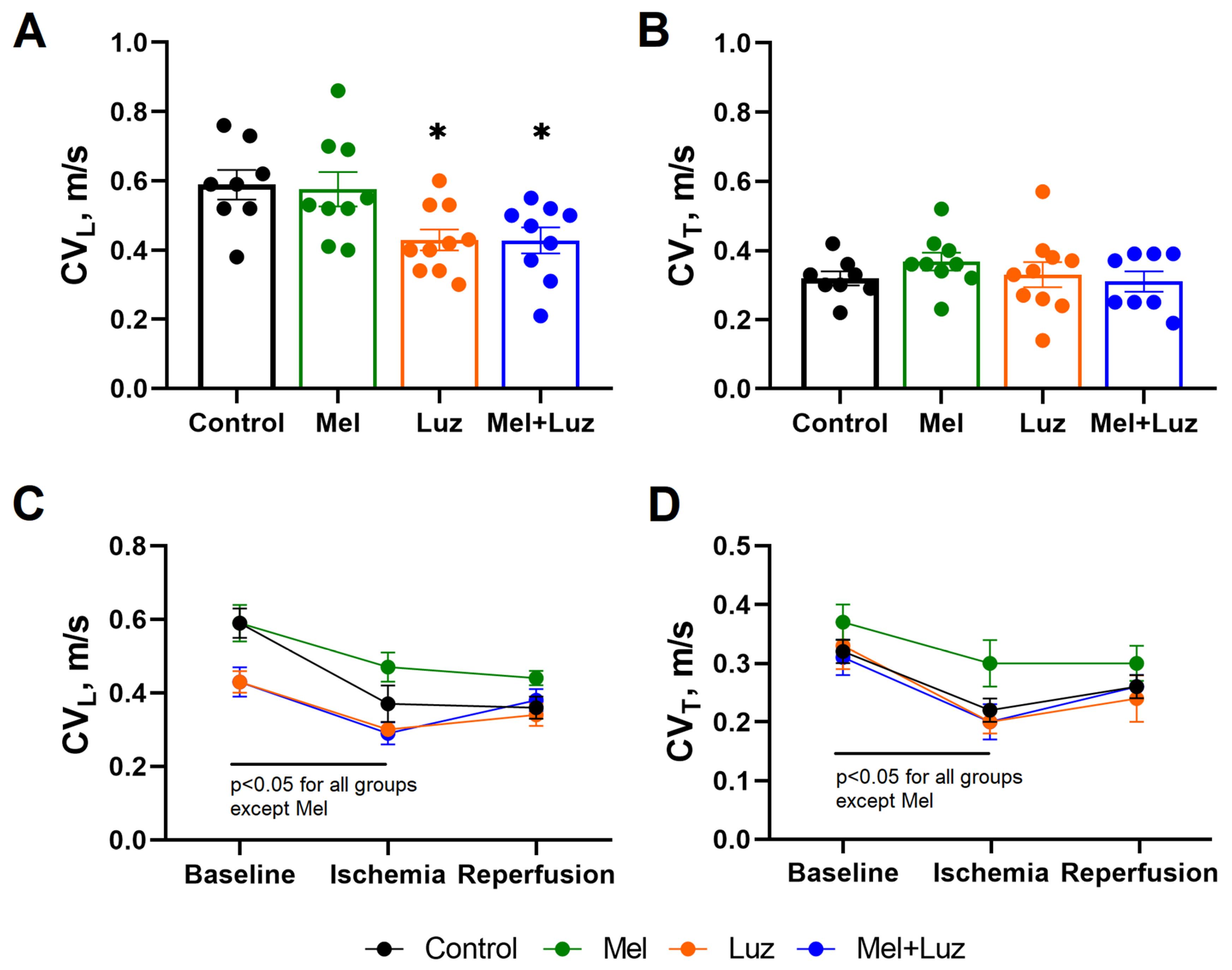
2.3. Myocardial Conduction Velocity (Protocol B)
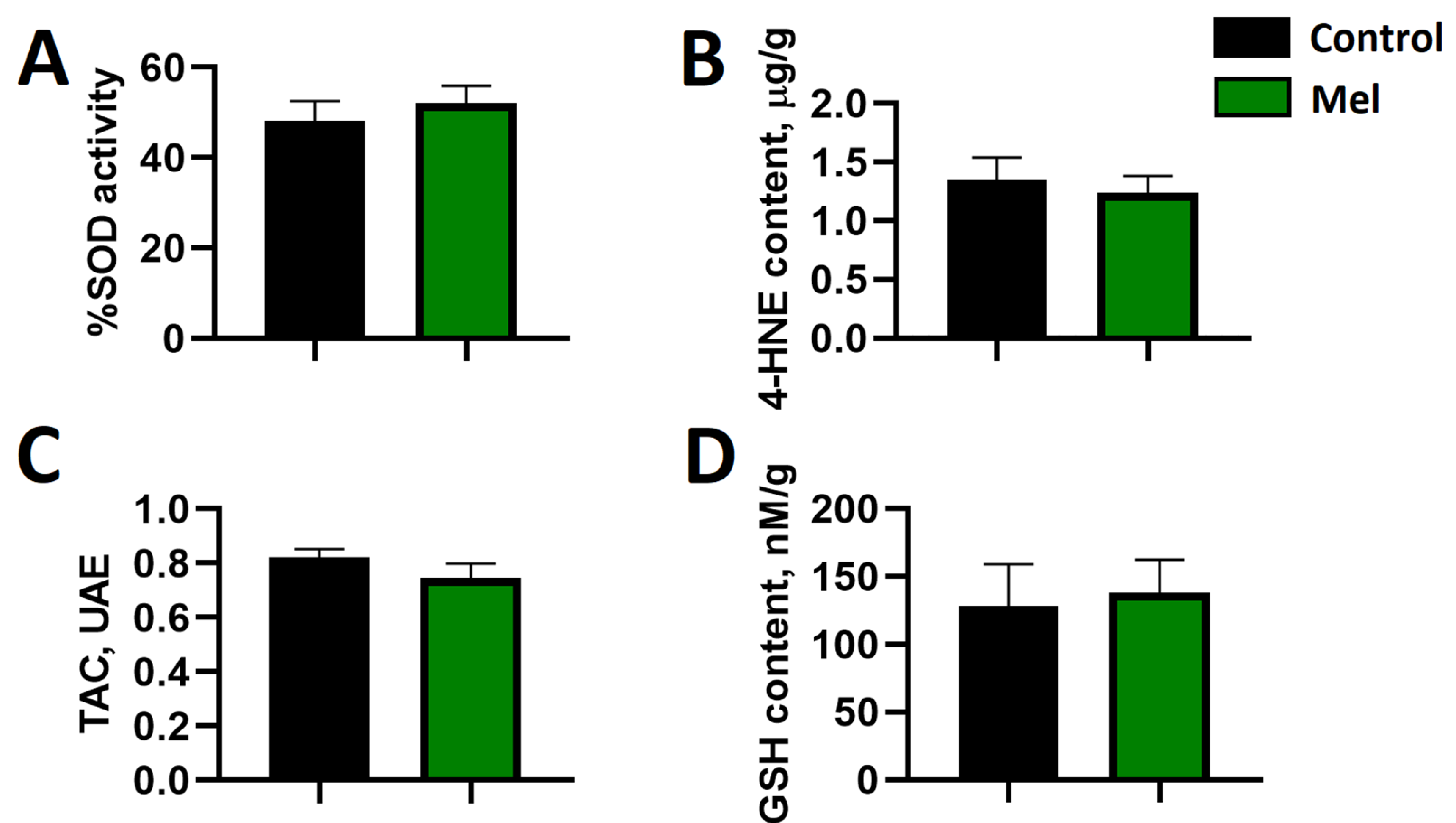
2.4. Markers of Myocardial Oxidative Stress
2.5. Cardiomyocyte Patch Clamp Measurements
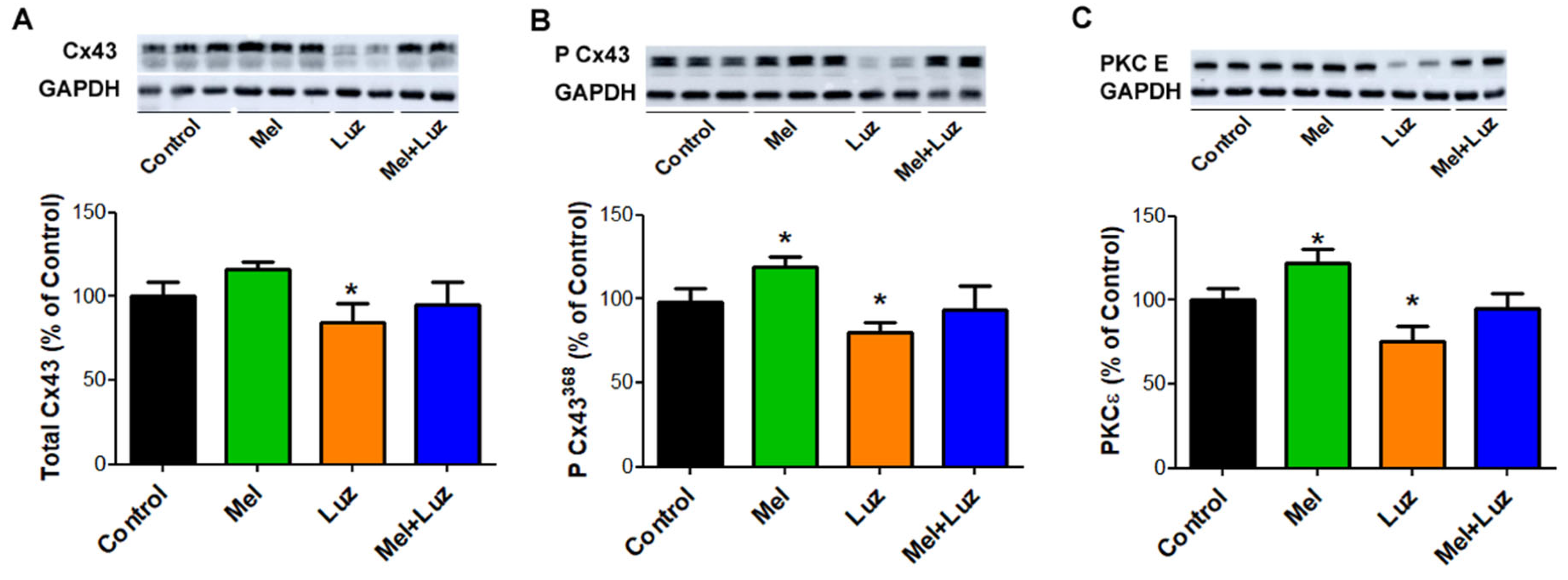
2.6. Myocardial Cx43, P-Cx43368, and PKCƐ Protein Levels
3. Discussion
4. Materials and Methods
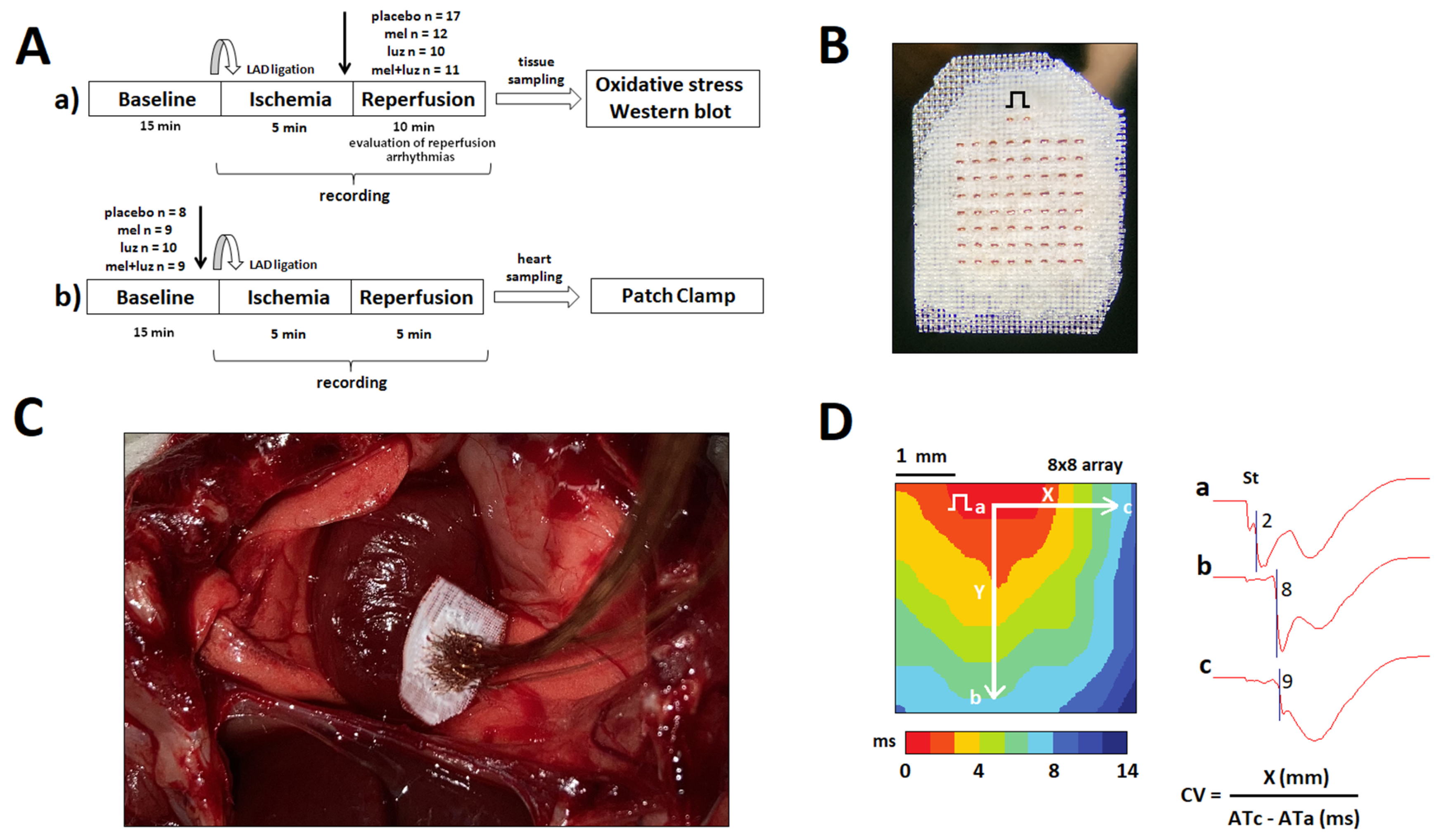
4.1. Animals and Experimental Design
4.2. Epicardial Mapping
4.3. Whole-Cell Patch-Clamp Recording
4.4. Determination of Oxidative Stress Markers
4.5. Cx43 and PKC ε Levels Assessed by Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Qi, W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: A review of the evidence. Cell Biochem. Biophys. 2001, 34, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Antolin, I.; Rodriguez, C.; Sainz, R.M.; Mayo, J.C.; Uria, H.; Kotler, M.L.; Rodriguez-Colunga, M.J.; Tolivia, D.; Menendez-Pelaez, A. Neurohormone melatonin prevents cell damage: Effect on gene expression for antioxidant enzymes. Faseb J. 1996, 10, 882–890. [Google Scholar] [CrossRef]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pablos, M.I.; Reiter, R.J.; Ortiz, G.G.; Guerrero, J.M.; Agapito, M.T.; Chuang, J.I.; Sewerynek, E. Rhythms of glutathione peroxidase and glutathione reductase in brain of chick and their inhibition by light. Neurochem. Int. 1998, 32, 69–75. [Google Scholar] [CrossRef]
- Liu, L.; Labani, N.; Cecon, E.; Jockers, R. Melatonin target proteins: Too many or not enough? Front. Endocrinol. 2019, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Stauch, B.; Johansson, L.C.; Cherezov, V. Structural insights into melatonin receptors. FEBS J. 2020, 287, 1496–1510. [Google Scholar] [CrossRef]
- Nikolaev, G.; Robeva, R.; Konakchieva, R. Membrane melatonin receptors activated cell signaling in physiology and disease. Int. J. Mol. Sci. 2021, 23, 471. [Google Scholar] [CrossRef]
- Cecon, E.; Oishi, A.; Jockers, R. Melatonin receptors: Molecular pharmacology and signalling in the context of system bias. Br. J. Pharmacol. 2018, 175, 3263–3280. [Google Scholar] [CrossRef]
- Tobeiha, M.; Jafari, A.; Fadaei, S.; Mirazimi, S.M.A.; Dashti, F.; Amiri, A.; Khan, H.; Asemi, Z.; Reiter, R.J.; Hamblin, M.R.; et al. Evidence for the benefits of melatonin in cardiovascular disease. Front. Cardiovasc. Med. 2022, 9, 888319. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and retinoid orphan receptors: Demand for new interpretations after their exclusion as nuclear melatonin receptors. Melatonin Res. 2018, 1, 78–93. [Google Scholar] [CrossRef]
- Ma, H.; Kang, J.; Fan, W.; He, H.; Huang, F. Ror: Nuclear receptor for melatonin or not? Molecules 2021, 26, 2693. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Hu, C.; Sun, W.; Xu, Y.; Gu, Y.; Wu, L.; Peng, Q.; Reiter, R.J.; Liu, L. Identification of a novel melatonin-binding nuclear receptor: Vitamin d receptor. J. Pineal Res. 2020, 68, e12618. [Google Scholar] [CrossRef] [PubMed]
- Lochner, A.; Marais, E.; Huisamen, B. Melatonin and cardioprotection against ischaemia/reperfusion injury: What’s new? A review. J. Pineal Res. 2018, 65, e12490. [Google Scholar] [CrossRef]
- Argueta, J.; Solís-Chagoyán, H.; Estrada-Reyes, R.; Constantino-Jonapa, L.A.; Oikawa-Sala, J.; Velázquez-Moctezuma, J.; Benítez-King, G. Further evidence of the melatonin calmodulin interaction: Effect on camkii activity. Int. J. Mol. Sci. 2022, 23, 2479. [Google Scholar] [CrossRef]
- Prado, N.J.; Egan Beňová, T.; Diez, E.R.; Knezl, V.; Lipták, B.; Ponce Zumino, A.Z.; Llamedo-Soria, M.; Szeiffová Bačová, B.; Miatello, R.M.; Tribulová, N. Melatonin receptor activation protects against low potassium-induced ventricular fibrillation by preserving action potentials and connexin-43 topology in isolated rat hearts. J. Pineal Res. 2019, 67, e12605. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Paredes, S.D.; Fuentes-Broto, L. Beneficial effects of melatonin in cardiovascular disease. Ann. Med. 2010, 42, 276–285. [Google Scholar] [CrossRef]
- Paulis, L.; Simko, F. Blood pressure modulation and cardiovascular protection by melatonin: Potential mechanisms behind. Physiol. Res. 2007, 56, 671–684. [Google Scholar] [CrossRef]
- Yang, J.-B.; Kang, Y.-M.; Zhang, C.; Yu, X.-J.; Chen, W.-S. Infusion of melatonin into the paraventricular nucleus ameliorates myocardial ischemia–reperfusion injury by regulating oxidative stress and inflammatory cytokines. J. Cardiovasc. Pharmacol. 2019, 74, 336–347. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, J.-B.; Quan, W.; Feng, Y.-D.; Feng, J.-Y.; Cheng, L.-S.; Li, X.-Q.; Zhang, H.-N.; Chen, W.-S. Activation of paraventricular melatonin receptor 2 mediates melatonin- conferred cardio-protection against myocardial ischemia/reperfusion injury. J. Cardiovasc. Pharmacol. 2020, 76, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Blatt, C.M.; Rabinowitz, S.H.; Lown, B. Central serotonergic agents raise the repetitive extrasystole threshold of the vulnerable period of the canine ventricular myocardium. Circ. Res. 1979, 44, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.; Kim, S.J.; El-Sokkary, G.H. Ischemia/reperfusion-induced arrhythmias in the isolated rat heart: Prevention by melatonin. J. Pineal Res. 1998, 25, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Vazan, R.; Pancza, D.; Beder, I.; Styk, J. Ischemia-reperfusion injury—antiarrhythmic effect of melatonin associated with reduced recovering of contractility. Gen. Physiol. Biophys. 2005, 24, 355–359. [Google Scholar]
- Diez, E.R.; Prados, L.V.; Carrion, A.; Ponce, Z.A.; Miatello, R.M. A novel electrophysiologic effect of melatonin on ischemia/reperfusion-induced arrhythmias in isolated rat hearts. J. Pineal Res. 2009, 46, 155–160. [Google Scholar] [CrossRef]
- Jeong, E.-M.; Liu, M.; Sturdy, M.; Gao, G.; Varghese, S.T.; Sovari, A.A.; Dudley, S.C. Metabolic stress, reactive oxygen species, and arrhythmia. J. Mol. Cell. Cardiol. 2012, 52, 454–463. [Google Scholar] [CrossRef]
- Sedova, K.A.; Bernikova, O.G.; Cuprova, J.I.; Ivanova, A.D.; Kutaeva, G.A.; Pliss, M.G.; Lopatina, E.V.; Vaykshnorayte, M.A.; Diez, E.R.; Azarov, J.E. Association between antiarrhythmic, electrophysiological, and antioxidative effects of melatonin in ischemia/reperfusion. Int. J. Mol. Sci. 2019, 20, 6331. [Google Scholar] [CrossRef]
- Durkina, A.V.; Bernikova, O.G.; Gonotkov, M.A.; Mikhaleva, N.J.; Sedova, K.A.; Malykhina, I.A.; Kuzmin, V.S.; Velegzhaninov, I.O.; Azarov, J.E. Melatonin treatment improves ventricular conduction via upregulation of nav1.5 channel proteins and sodium current in the normal rat heart. J. Pineal Res. 2022, 73, e12798. [Google Scholar] [CrossRef]
- Szeiffova Bacova, B.; Viczenczova, C.; Andelova, K.; Sykora, M.; Chaudagar, K.; Barancik, M.; Adamcova, M.; Knezl, V.; Egan Benova, T.; Weismann, P.; et al. Antiarrhythmic effects of melatonin and omega-3 are linked with protection of myocardial cx43 topology and suppression of fibrosis in catecholamine stressed normotensive and hypertensive rats. Antioxidants 2020, 9, 546. [Google Scholar] [CrossRef]
- Durkina, A.V.; Bernikova, O.G.; Mikhaleva, N.J.; Paderin, N.M.; Sedova, K.A.; Gonotkov, M.A.; Kuzmin, V.S.; Azarov, J.E. Melatonin pretreatment does not modify extrasystolic burden in the rat ischemia-reperfusion model. J. Physiol. Pharmacol. 2021, 72, 141–148. [Google Scholar] [CrossRef]
- Tsvetkova, A.S.; Bernikova, O.G.; Mikhaleva, N.J.; Khramova, D.S.; Ovechkin, A.O.; Demidova, M.M.; Platonov, P.G.; Azarov, J.E. Melatonin prevents early but not delayed ventricular fibrillation in the experimental porcine model of acute ischemia. Int. J. Mol. Sci. 2021, 22, 328. [Google Scholar] [CrossRef]
- Bernikova, O.G.; Tsvetkova, A.S.; Ovechkin, A.O.; Demidova, M.M.; Azarov, J.E.; Platonov, P.G. Ecg markers of acute melatonin treatment in a porcine model of acute myocardial ischemia. Int. J. Mol. Sci. 2022, 23, 11800. [Google Scholar] [CrossRef] [PubMed]
- Diez, E.R.; Renna, N.F.; Prado, N.J.; Lembo, C.; Ponce Zumino, A.Z.; Vazquez-Prieto, M.; Miatello, R.M. Melatonin, given at the time of reperfusion, prevents ventricular arrhythmias in isolated hearts from fructose-fed rats and spontaneously hypertensive rats. J. Pineal Res. 2013, 55, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Sahna, E.; Parlakpinar, H.; Turkoz, Y.; Acet, A. Protective effects of melatonin on myocardial ischemia/reperfusion induced infarct size and oxidative changes. Physiol. Res. 2005, 54, 491–495. [Google Scholar] [CrossRef]
- Gonzaléz-Candia, A.; Arias, P.V.; Aguilar, S.A.; Figueroa, E.G.; Reyes, R.V.; Ebensperger, G.; Llanos, A.J.; Herrera, E.A. Melatonin reduces oxidative stress in the right ventricle of newborn sheep gestated under chronic hypoxia. Antioxidants 2021, 10, 1658. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J. International union of basic and clinical pharmacology. Lxxv. Nomenclature, classification, and pharmacology of g protein-coupled melatonin receptors. Pharmacol. Rev. 2010, 62, 343–380. [Google Scholar] [CrossRef]
- MacKenzie, R.S.; Melan, M.A.; Passey, D.K.; Witt-Enderby, P.A. Dual coupling of mt(1) and mt(2) melatonin receptors to cyclic amp and phosphoinositide signal transduction cascades and their regulation following melatonin exposure. Biochem. Pharmacol. 2002, 63, 587–595. [Google Scholar] [CrossRef] [PubMed]
- McArthur, A.J.; Hunt, A.E.; Gillette, M.U. Melatonin action and signal transduction in the rat suprachiasmatic circadian clock: Activation of protein kinase c at dusk and dawn. Endocrinology 1997, 138, 627–634. [Google Scholar] [CrossRef]
- Radio, N.M.; Doctor, J.S.; Witt-Enderby, P.A. Melatonin enhances alkaline phosphatase activity in differentiating human adult mesenchymal stem cells grown in osteogenic medium via mt2melatonin receptors and the mek/erk (1/2) signaling cascade. J. Pineal Res. 2006, 40, 332–342. [Google Scholar] [CrossRef]
- Bao, X.; Reuss, L.; Altenberg, G.A. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase c-mediated phosphorylation of serine 368. J. Biol. Chem. 2004, 279, 20058–20066. [Google Scholar] [CrossRef]
- Kohutova, J.; Elsnicova, B.; Holzerova, K.; Neckar, J.; Sebesta, O.; Jezkova, J.; Vecka, M.; Vebr, P.; Hornikova, D.; Szeiffova Bacova, B.; et al. Anti-arrhythmic cardiac phenotype elicited by chronic intermittent hypoxia is associated with alterations in connexin-43 expression, phosphorylation, and distribution. Front. Endocrinol. 2019, 9, 789. [Google Scholar] [CrossRef] [PubMed]
- Belardinelli, L.; Antzelevitch, C.; Vos, M.A. Assessing predictors of drug-induced torsade de pointes. Trends Pharmacol. Sci. 2003, 24, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Benova, T.; Viczenczova, C.; Radosinska, J.; Bacova, B.; Knezl, V.; Dosenko, V.; Weismann, P.; Zeman, M.; Navarova, J.; Tribulova, N. Melatonin attenuates hypertension-related proarrhythmic myocardial maladaptation of connexin-43 and propensity of the heart to lethal arrhythmias. Can. J. Physiol. Pharmacol. 2013, 91, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.S.; Marino, J.L.; Allen, C.N. Melatonin receptors activate heteromeric g-protein coupled kir3 channels. Neuroreport 1996, 7, 717–720. [Google Scholar] [CrossRef]
- Whorton, M.R.; Mackinnon, R. X-ray structure of the mammalian girk2–βγ g-protein complex. Nature 2013, 498, 190–197. [Google Scholar] [CrossRef]
- Zhou, M.O.; Jiao, S.; Liu, Z.; Zhang, Z.H.; Mei, Y.A. Luzindole, a melatonin receptor antagonist, inhibits the transient outward k+ current in rat cerebellar granule cells. Brain Res. 2003, 970, 169–177. [Google Scholar] [CrossRef]
- Legros, C.; Dupré, C.; Brasseur, C.; Bonnaud, A.; Bruno, O.; Valour, D.; Shabajee, P.; Giganti, A.; Nosjean, O.; Kenakin, T.P.; et al. Characterization of the various functional pathways elicited by synthetic agonists or antagonists at the melatonin mt 1 and mt 2 receptors. Pharmacol. Res. Perspect 2020, 8, e00539. [Google Scholar] [CrossRef]
- Koumi, S.; Backer, C.L.; Arentzen, C.E.; Sato, R. Beta-adrenergic modulation of the inwardly rectifying potassium channel in isolated human ventricular myocytes. Alteration in channel response to beta-adrenergic stimulation in failing human hearts. J. Clin. Investig. 1995, 96, 2870–2881. [Google Scholar] [CrossRef][Green Version]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: Iuphar review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef]
- Johansson, L.C.; Stauch, B.; McCorvy, J.D.; Han, G.W.; Patel, N.; Huang, X.P.; Batyuk, A.; Gati, C.; Slocum, S.T.; Li, C.; et al. Xfel structures of the human mt(2) melatonin receptor reveal the basis of subtype selectivity. Nature 2019, 569, 289–292. [Google Scholar] [CrossRef]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Stauch, B.; Johansson, L.C.; McCorvy, J.D.; Patel, N.; Han, G.W.; Huang, X.P.; Gati, C.; Batyuk, A.; Slocum, S.T.; Ishchenko, A.; et al. Structural basis of ligand recognition at the human mt(1) melatonin receptor. Nature 2019, 569, 284–288. [Google Scholar] [CrossRef]
- Lin, X.; Liu, N.; Lu, J.; Zhang, J.; Anumonwo, J.M.B.; Isom, L.L.; Fishman, G.I.; Delmar, M. Subcellular heterogeneity of sodium current properties in adult cardiac ventricular myocytes. Heart Rhythm. 2011, 8, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Bernikova, O.G.; Sedova, K.A.; Durkina, A.V.; Azarov, J.E. Managing of ventricular reperfusion tachyarrhythmias-focus on a perfused myocardium. J. Physiol. Pharmacol. 2019, 70, 757–763. [Google Scholar] [CrossRef]
- Bernikova, O.G.; Durkina, A.V.; Sedova, K.A.; Azarov, J.E. Determinants of reperfusion arrhythmias: Action potential duration versus dispersion of repolarization. J. Physiol. Pharmacol. 2021, 72, 691–697. [Google Scholar] [CrossRef]
- Govender, J.; Loos, B.; Marais, E.; Engelbrecht, A.M. Melatonin improves cardiac and mitochondrial function during doxorubicin-induced cardiotoxicity: A possible role for peroxisome proliferator-activated receptor gamma coactivator 1-alpha and sirtuin activity? Toxicol. Appl. Pharmacol. 2018, 358, 86–101. [Google Scholar] [CrossRef]
- Andelova, K.; Szeiffova Bacova, B.; Sykora, M.; Pavelka, S.; Rauchova, H.; Tribulova, N. Cardiac cx43 signaling is enhanced and tgf-β1/smad2/3 suppressed in response to cold acclimation and modulated by thyroid status in hairless shrm. Biomedicines 2022, 10, 1707. [Google Scholar] [CrossRef] [PubMed]
- Szeiffová Bačová, B.; Egan Beňová, T.; Viczenczová, C.; Soukup, T.; Rauchová, H.; Pavelka, S.; Knezl, V.; Barančík, M.; Tribulová, N. Cardiac connexin-43 and pkc signaling in rats with altered thyroid status without and with omega-3 fatty acids intake. Physiol. Res. 2016, 65, S77–S90. [Google Scholar] [CrossRef] [PubMed]

| Outcome | AT | DOR | ||
|---|---|---|---|---|
| Odd Ratio | p | Odd Ratio | p | |
| VT | 1.591 95% CI 1.061–2.386 | 0.025 | 0.932 95% CI 0.793–1.096 | 0.397 |
| VF | 1.052 95% CI 0.731–1.516 | 0.784 | 1.100 95% CI 0.883–1.370 | 0.395 |
| VT/VF | 1.695 95% CI 1.046–2.748 | 0.032 | 1.088 95% CI 0.922–1.284 | 0.316 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durkina, A.V.; Szeiffova Bacova, B.; Bernikova, O.G.; Gonotkov, M.A.; Sedova, K.A.; Cuprova, J.; Vaykshnorayte, M.A.; Diez, E.R.; Prado, N.J.; Azarov, J.E. Blockade of Melatonin Receptors Abolishes Its Antiarrhythmic Effect and Slows Ventricular Conduction in Rat Hearts. Int. J. Mol. Sci. 2023, 24, 11931. https://doi.org/10.3390/ijms241511931
Durkina AV, Szeiffova Bacova B, Bernikova OG, Gonotkov MA, Sedova KA, Cuprova J, Vaykshnorayte MA, Diez ER, Prado NJ, Azarov JE. Blockade of Melatonin Receptors Abolishes Its Antiarrhythmic Effect and Slows Ventricular Conduction in Rat Hearts. International Journal of Molecular Sciences. 2023; 24(15):11931. https://doi.org/10.3390/ijms241511931
Chicago/Turabian StyleDurkina, Aleksandra V., Barbara Szeiffova Bacova, Olesya G. Bernikova, Mikhail A. Gonotkov, Ksenia A. Sedova, Julie Cuprova, Marina A. Vaykshnorayte, Emiliano R. Diez, Natalia J. Prado, and Jan E. Azarov. 2023. "Blockade of Melatonin Receptors Abolishes Its Antiarrhythmic Effect and Slows Ventricular Conduction in Rat Hearts" International Journal of Molecular Sciences 24, no. 15: 11931. https://doi.org/10.3390/ijms241511931
APA StyleDurkina, A. V., Szeiffova Bacova, B., Bernikova, O. G., Gonotkov, M. A., Sedova, K. A., Cuprova, J., Vaykshnorayte, M. A., Diez, E. R., Prado, N. J., & Azarov, J. E. (2023). Blockade of Melatonin Receptors Abolishes Its Antiarrhythmic Effect and Slows Ventricular Conduction in Rat Hearts. International Journal of Molecular Sciences, 24(15), 11931. https://doi.org/10.3390/ijms241511931





