Structural Insight into the Amino Acid Environment of the Two-Domain Laccase’s Trinuclear Copper Cluster
Abstract
1. Introduction
2. Results
2.1. SgfSL Mutant Forms’ Construction and Purification
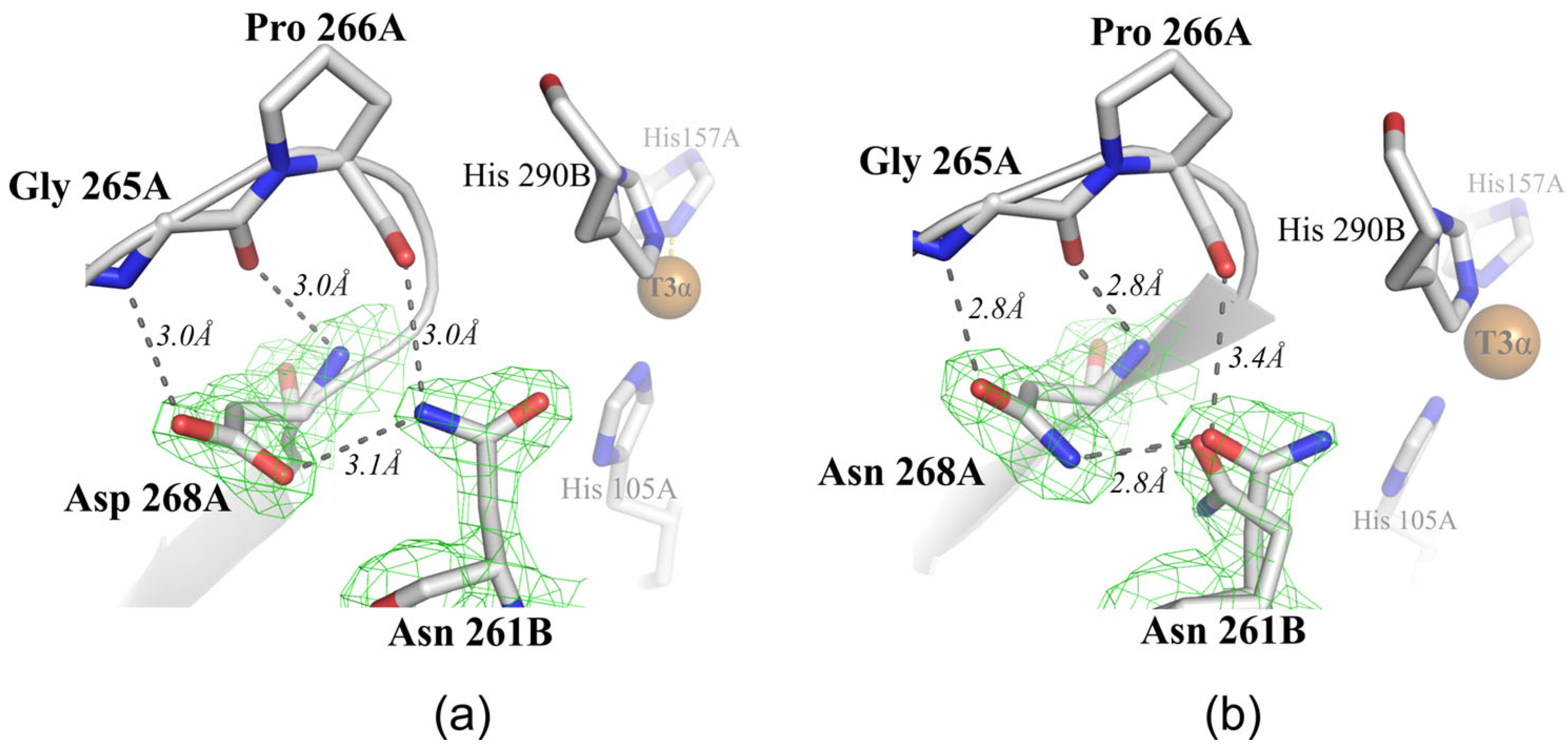
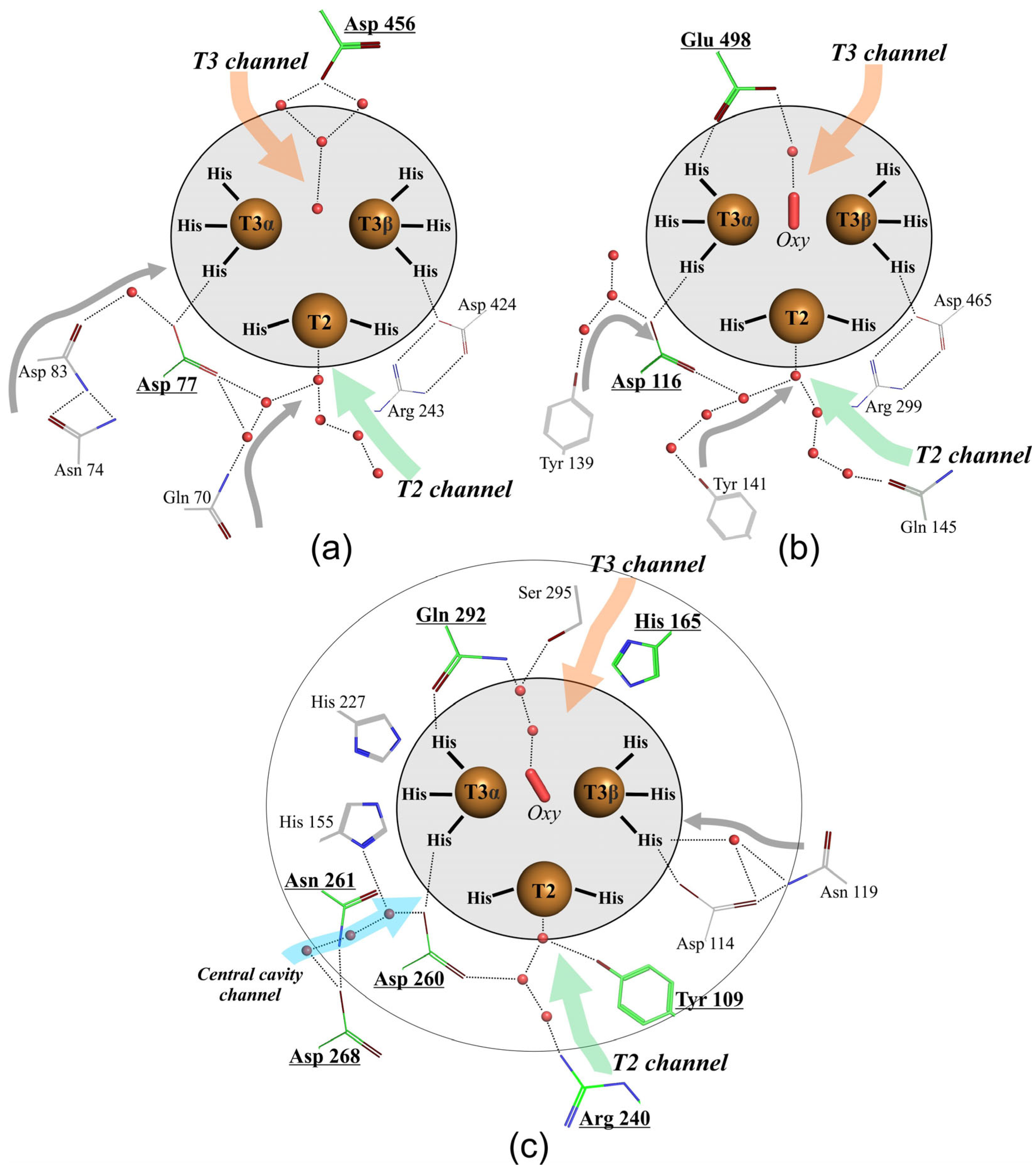
2.2. Structural Analysis of the Mutant Enzymes
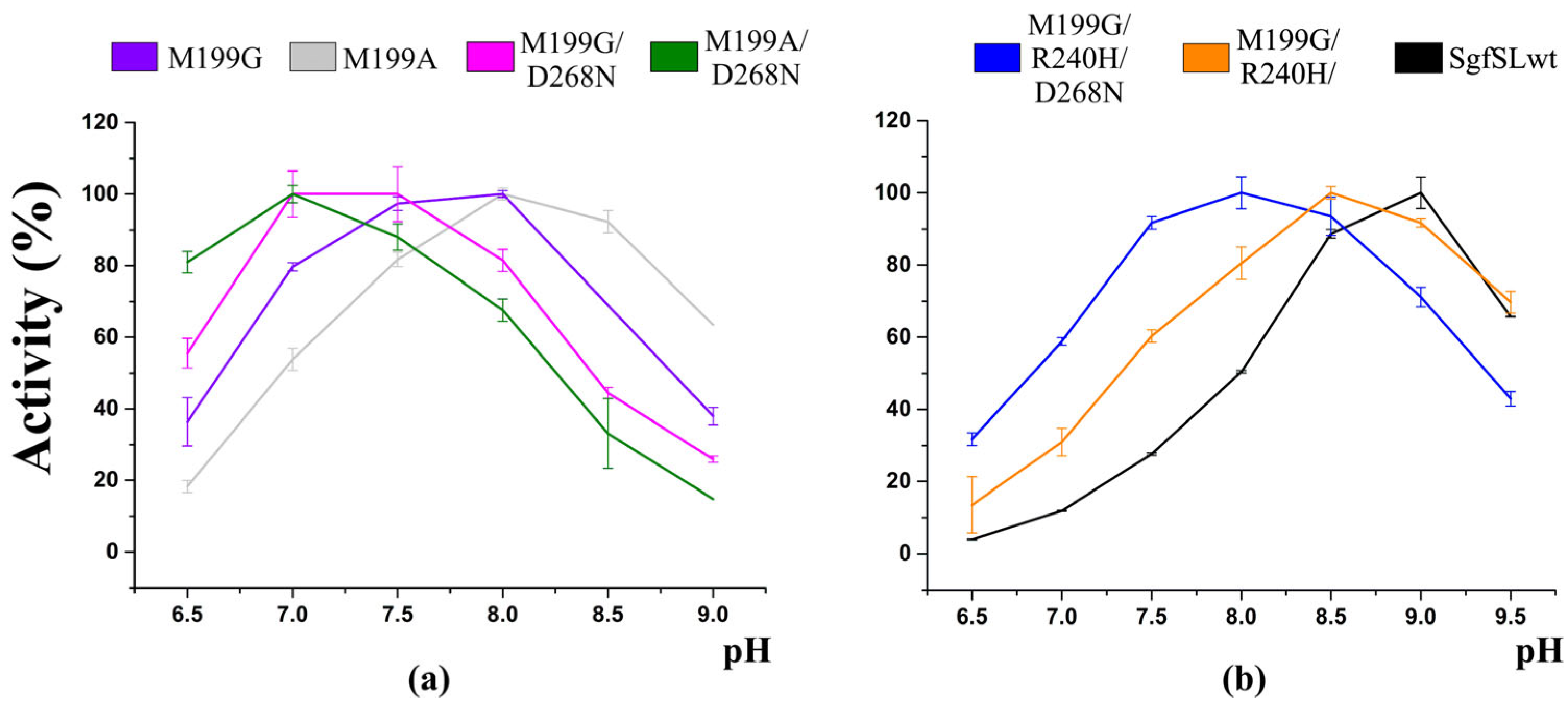
2.3. Kinetic Analysis of the Mutant Enzymes
3. Discussion
4. Materials and Methods
4.1. Plasmid Construction
- Asp268Asn_ For: 5′-ATCTGCGGCCCAGCAAACTCCTTCGGCTTCC-3′
- Asp268Asn_ Rev:5′-GGAAGCCGAAGGAGTTTGCTGGGCCGCAGAT-3′
4.2. Purification of SgfSL Mutants
4.3. Kinetic Parameters of SgfSL Mutants
4.4. Crystallization and Crystallography
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TNC | TriNuclear Center |
| 3D | Three-domain |
| 2D | Two-domain |
| SgfSL | Streptomyces griseoflavus Small Laccase |
| 2,6-DMP | 2,6-Dimethoxyphenol |
| ABTS | 2,2’-Azino-di-(3-ethylbenzothiazoline)-6-sulfonic acid |
References
- Yoshida, H. LXIII.—Chemistry of lacquer (Urushi). Part I. Communication from the Chemical Society of Tokio. J. Chem. Soc. Trans. 1883, 43, 472–486. [Google Scholar] [CrossRef]
- Wisniak, J. Gabriel Bertrand. Rev. CENIC Cienc. Biol. 2014, 45, 230–245. [Google Scholar]
- Jones, S.M.; Solomon, E.I. Electron Transfer and Reaction Mechanism of Laccases. Cell. Mol. Life Sci. 2015, 72, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Morozova, O.V.; Shumakovich, G.P.; Gorbacheva, M.A.; Shleev, S.V.; Yaropolov, A.I. “Blue” Laccases. Biochemistry 2007, 72, 1136–1150. [Google Scholar] [CrossRef] [PubMed]
- Morozova, O.V.; Shumakovich, G.P.; Shleev, S.V.; Yaropolov, Y.I. Laccase-Mediator Systems and Their Applications: A Review. Appl. Biochem. Microbiol. 2007, 43, 523–535. [Google Scholar] [CrossRef]
- Zerva, A.; Simić, S.; Topakas, E.; Nikodinovic-Runic, J. Applications of Microbial Laccases: Patent Review of the Past Decade (2009–2019). Catalysts 2019, 9, 1023. [Google Scholar] [CrossRef]
- Rodríguez-Delgado, M.M.; Alemán-Nava, G.S.; Rodríguez-Delgado, J.M.; Dieck-Assad, G.; Martínez-Chapa, S.O.; Barceló, D.; Parra, R. Laccase-Based Biosensors for Detection of Phenolic Compounds. TrAC Trends Anal. Chem. 2015, 74, 21–45. [Google Scholar] [CrossRef]
- Leynaud Kieffer Curran, L.M.C.; Pham, L.T.M.; Sale, K.L.; Simmons, B.A. Review of Advances in the Development of Laccases for the Valorization of Lignin to Enable the Production of Lignocellulosic Biofuels and Bioproducts. Biotechnol. Adv. 2022, 54, 107809. [Google Scholar] [CrossRef]
- Kudanga, T.; Nemadziva, B.; Le Roes-Hill, M. Laccase Catalysis for the Synthesis of Bioactive Compounds. Appl. Microbiol. Biotechnol. 2017, 101, 13–33. [Google Scholar] [CrossRef]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; Herrera De Los Santos, M.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.N.; et al. Laccases: Structure, Function, and Potential Application in Water Bioremediation. Microb. Cell Fact. 2019, 18, 200. [Google Scholar] [CrossRef]
- Brijwani, K.; Rigdon, A.; Vadlani, P.V. Fungal Laccases: Production, Function, and Applications in Food Processing. Enzyme Res. 2010, 2010, 149748. [Google Scholar] [CrossRef]
- Pardo, I.; Santiago, G.; Gentili, P.; Lucas, F.; Monza, E.; Medrano, F.J.; Galli, C.; Martínez, A.T.; Guallar, V.; Camarero, S. Re-Designing the Substrate Binding Pocket of Laccase for Enhanced Oxidation of Sinapic Acid. Catal. Sci. Technol. 2016, 6, 3900–3910. [Google Scholar] [CrossRef]
- Camarero, S.; Pardo, I.; Cañas, A.I.; Molina, P.; Record, E.; Martínez, A.T.; Martínez, M.J.; Alcalde, M. Engineering Platforms for Directed Evolution of Laccase from Pycnoporus Cinnabarinus. Appl. Environ. Microbiol. 2012, 78, 1370–1384. [Google Scholar] [CrossRef] [PubMed]
- Pardo, I.; Camarero, S. Laccase Engineering by Rational and Evolutionary Design. Cell. Mol. Life Sci. 2015, 72, 897–910. [Google Scholar] [CrossRef]
- Mate, D.M.; Alcalde, M. Laccase Engineering: From Rational Design to Directed Evolution. Biotechnol. Adv. 2015, 33, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Pardo, I.; Rodríguez-Escribano, D.; Aza, P.; de Salas, F.; Martínez, A.T.; Camarero, S. A Highly Stable Laccase Obtained by Swapping the Second Cupredoxin Domain. Sci. Rep. 2018, 8, 15669. [Google Scholar] [CrossRef]
- Barber-Zucker, S.; Mateljak, I.; Goldsmith, M.; Kupervaser, M.; Alcalde, M.; Fleishman, S.J. Designed High-Redox Potential Laccases Exhibit High Functional Diversity. ACS Catal. 2022, 12, 13164–13173. [Google Scholar] [CrossRef]
- Mateljak, I.; Alcalde, M. Engineering a Highly Thermostable High-Redox Potential Laccase. ACS Sustain. Chem. Eng. 2021, 9, 9632–9637. [Google Scholar] [CrossRef]
- Othman, A.M.; Sanroman, A.; Moldes, D. Laccase-Oriented Immobilization Using Concanavalin A as an Approach for Efficient Glycoproteins Immobilization and Its Application to the Removal of Aqueous Phenolics. Sustainability 2022, 14, 13306. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, D.; Jiang, S.; Yu, H.; Cheng, Y.; Zhang, S.; Zhang, X.; Chen, W. The Study of Laccase Immobilization Optimization and Stability Improvement on CTAB-KOH Modified Biochar. BMC Biotechnol. 2021, 21, 47. [Google Scholar] [CrossRef]
- Mani, P.; Fidal, V.T.; Keshavarz, T.; Chandra, T.S.; Kyazze, G. Laccase Immobilization Strategies for Application as a Cathode Catalyst in Microbial Fuel Cells for Azo Dye Decolourization. Front. Microbiol. 2021, 11, 620075. [Google Scholar] [CrossRef]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A Never-Ending Story. Cell. Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef]
- Luna-Acosta, A.; Breitwieser, M.; Renault, T.; Thomas-Guyon, H. Recent Findings on Phenoloxidases in Bivalves. Mar. Pollut. Bull. 2017, 122, 5–16. [Google Scholar] [CrossRef]
- Lisov, A.V.; Zavarzina, A.G.; Zavarzin, A.A.; Leontievsky, A.A. Laccases Produced by Lichens of the Order Peltigerales. FEMS Microbiol. Lett. 2007, 275, 46–52. [Google Scholar] [CrossRef]
- Martínez-Alvarez, O.; Montero, P.; Gómez-Guillén, C. Evidence of an Active Laccase-like Enzyme in Deepwater Pink Shrimp (Parapenaeus longirostris). Food Chem. 2008, 108, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.K.; Kuhad, R.C. An Evidence of Laccases in Archaea. Indian J. Microbiol. 2009, 49, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Ausec, L.; Zakrzewski, M.; Goesmann, A.; Schlüter, A.; Mandic-Mulec, I. Bioinformatic Analysis Reveals High Diversity of Bacterial Genes for Laccase-like Enzymes. PLoS ONE 2011, 6, e25724. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zeng, G.; Fan, C.; Zhang, J.; Chen, A.; Chen, M.; Jiang, M.; Yuan, Y.; Wu, H.; Lai, M.; et al. Diversity of Two-Domain Laccase-like Multicopper Oxidase Genes in Streptomyces Spp.: Identification of Genes Potentially Involved in Extracellular Activities and Lignocellulose Degradation during Composting of Agricultural Waste. Appl. Environ. Microbiol. 2014, 80, 3305–3314. [Google Scholar] [CrossRef]
- Trubitsina, L.I.; Tishchenko, S.V.; Gabdulkhakov, A.G.; Lisov, A.V.; Zakharova, M.V.; Leontievsky, A.A. Structural and Functional Characterization of Two-Domain Laccase from Streptomyces Viridochromogenes. Biochimie 2015, 112, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.I.; Heppner, D.E.; Johnston, E.M.; Ginsbach, J.W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M.T.; Kjaergaard, C.H.; Hadt, R.G.; Tian, L. Copper Active Sites in Biology. Chem. Rev. 2014, 114, 3659–3853. [Google Scholar] [CrossRef]
- Polyakov, K.M.; Gavryushov, S.; Ivanova, S.; Fedorova, T.V.; Glazunova, O.A.; Popov, A.N.; Koroleva, O.V. Structural Study of the X-Ray-Induced Enzymatic Reduction of Molecular Oxygen to Water by Steccherinum murashkinskyi Laccase: Insights into the Reaction Mechanism. Acta Crystallogr. Sect. D Struct. Biol. 2017, 73, 388–401. [Google Scholar] [CrossRef]
- Augustine, A.J.; Quintanar, L.; Stoj, C.S.; Kosman, D.J.; Solomon, E.I. Spectroscopic and Kinetic Studies of Perturbed Trinuclear Copper Clusters: The Role of Protons in Reductive Cleavage of the O-O Bond in the Multicopper Oxidase Fet3p. J. Am. Chem. Soc. 2007, 129, 13118–13126. [Google Scholar] [CrossRef] [PubMed]
- Quintanar, L.; Stoj, C.; Wang, T.P.; Kosman, D.J.; Solomon, E.I. Role of Aspartate 94 in the Decay of the Peroxide Intermediate in the Multicopper Oxidase Fet3p. Biochemistry 2005, 44, 6081–6091. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Liboiron, B.D.; Sarangi, R.; Hodgson, K.O.; Hedman, B.; Solomon, E.I. The Two Oxidized Forms of the Trinuclear Cu Cluster in the Multicopper Oxidases and Mechanism for the Decay of the Native Intermediate. Proc. Natl. Acad. Sci. USA 2007, 104, 13609–13614. [Google Scholar] [CrossRef]
- Sekretareva, A.; Tian, S.; Gounel, S.; Mano, N.; Solomon, E.I. Electron Transfer to the Trinuclear Copper Cluster in Electrocatalysis by the Multicopper Oxidases. J. Am. Chem. Soc. 2021, 143, 17236–17249. [Google Scholar] [CrossRef]
- Osipov, E.; Polyakov, K.; Kittl, R.; Shleev, S.; Dorovatovsky, P.; Tikhonova, T.; Hann, S.; Ludwig, R.; Popov, V. Effect of the L499M Mutation of the Ascomycetous Botrytis Aclada Laccase on Redox Potential and Catalytic Properties. Acta Crystallogr. Sect. D Biol. Crystallogr. 2014, 70, 2913–2923. [Google Scholar] [CrossRef]
- Mateljak, I.; Monza, E.; Lucas, M.F.; Guallar, V.; Aleksejeva, O.; Ludwig, R.; Leech, D.; Shleev, S.; Alcalde, M. Increasing Redox Potential, Redox Mediator Activity, and Stability in a Fungal Laccase by Computer-Guided Mutagenesis and Directed Evolution. ACS Catal. 2019, 9, 4561–4572. [Google Scholar] [CrossRef]
- Prins, A.; Kleinsmidt, L.; Khan, N.; Kirby, B.; Kudanga, T.; Vollmer, J.; Pleiss, J.; Burton, S.; Le Roes-Hill, M. The Effect of Mutations near the T1 Copper Site on the Biochemical Characteristics of the Small Laccase from Streptomyces Coelicolor A3(2). Enzyme Microb. Technol. 2015, 68, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.D.; De Maria, L.; Lobedanz, S.; Østergaard, L.H. Optimization of a Small Laccase by Active-Site Redesign. ChemBioChem 2013, 14, 1209–1211. [Google Scholar] [CrossRef]
- Kolyadenko, I.; Scherbakova, A.; Kovalev, K.; Gabdulkhakov, A.; Tishchenko, S. Engineering the Catalytic Properties of Two-Domain Laccase from Streptomyces griseoflavus Ac-993. Int. J. Mol. Sci. 2022, 23, 65. [Google Scholar] [CrossRef] [PubMed]
- Gabdulkhakov, A.; Kolyadenko, I.; Kostareva, O.; Mikhaylina, A.; Oliveira, P.; Tamagnini, P.; Lisov, A.; Tishchenko, S. Investigations of Accessibility of T2/T3 Copper Center of Two-Domain Laccase from Streptomyces griseoflavus Ac-993. Int. J. Mol. Sci. 2019, 20, 3184. [Google Scholar] [CrossRef]
- Gabdulkhakov, A.; Kolyadenko, I.; Oliveira, P.; Tamagnini, P.; Mikhaylina, A.; Tishchenko, S. The Role of Positive Charged Residue in the Proton-Transfer Mechanism of Two-Domain Laccase from Streptomyces griseoflavus Ac-993. J. Biomol. Struct. Dyn. 2022, 40, 8324–8331. [Google Scholar] [CrossRef]
- Chen, Z.; Durão, P.; Silva, C.S.; Pereira, M.M.; Todorovic, S.; Hildebrandt, P.; Bento, I.; Lindley, P.F.; Martins, L.O. The Role of Glu498 in the Dioxygen Reactivity of CotA-Laccase from Bacillus subtilis. Dalton Trans. 2010, 39, 2875–2882. [Google Scholar] [CrossRef]
- Brissos, V.; Chen, Z.; Martins, L.O. The Kinetic Role of Carboxylate Residues in the Proximity of the Trinuclear Centre in the O2 Reactivity of CotA-Laccase. Dalt. Trans. 2012, 41, 6247. [Google Scholar] [CrossRef]
- Sherif, M.; Waung, D.; Korbeci, B.; Mavisakalyan, V.; Flick, R.; Brown, G.; Abou-Zaid, M.; Yakunin, A.F.; Master, E.R. Biochemical Studies of the Multicopper Oxidase (Small Laccase) from Streptomyces Coelicolor Using Bioactive Phytochemicals and Site-Directed Mutagenesis. Microb. Biotechnol. 2013, 6, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Machczynski, M.C.; Vijgenboom, E.; Samyn, B.; Canters, G.W. Characterization of SLAC: A Small Laccase from Streptomyces Coelicolor with Unprecedented Activity. Protein Sci. 2004, 13, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Hayashi, Y.; Hibi, T.; Hosono, K.; Beppu, T.; Ueda, K. Enzymological Characterization of EpoA, a Laccase-like Phenol Oxidase Produced by Streptomyces griseus. J. Biochem. 2003, 133, 671–677. [Google Scholar] [CrossRef]
- Gunne, M.; Urlacher, V.B. Characterization of the Alkaline Laccase Ssl1 from Streptomyces sviceus with Unusual Properties Discovered by Genome Mining. PLoS ONE 2012, 7, e52360. [Google Scholar] [CrossRef]
- Olbrich, A.C.; Schild, J.N.; Urlacher, V.B. Correlation between the T1 Copper Reduction Potential and Catalytic Activity of a Small Laccase. J. Inorg. Biochem. 2019, 201, 110843. [Google Scholar] [CrossRef] [PubMed]
- Skálová, T.; Dohnálek, J.; Østergaard, L.H.; Østergaard, P.R.; Kolenko, P.; Dušková, J.; Štěpánková, A.; Hašek, J. The Structure of the Small Laccase from Streptomyces Coelicolor Reveals a Link between Laccases and Nitrite Reductases. J. Mol. Biol. 2009, 385, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.K. Enzymes: Principles and Biotechnological Applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef]
- Wraight, C.A. Chance and Design-Proton Transfer in Water, Channels and Bioenergetic Proteins. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 886–912. [Google Scholar] [CrossRef]
- Hayes, D.M.; Kollman, P.A. Electrostatic Potentials of Proteins. 2. Role of Electrostatics in a Possible Catalytic Mechanism for Carboxypeptidase A. J. Am. Chem. Soc. 1975, 98, 7811–7816. [Google Scholar] [CrossRef] [PubMed]
- Goings, J.J.; Reinhardt, C.R.; Hammes-Schiffer, S. Propensity for Proton Relay and Electrostatic Impact of Protein Reorganization in Slr1694 BLUF Photoreceptor. J. Am. Chem. Soc. 2018, 140, 15241–15251. [Google Scholar] [CrossRef] [PubMed]
- Bondar, A.N.; Fischer, S.; Smith, J.C.; Elstner, M.; Suhai, S. Key Role of Electrostatic Interactions in Bacteriorhodopsin Proton Transfer. J. Am. Chem. Soc. 2004, 126, 14668–14677. [Google Scholar] [CrossRef]
- Lyashenko, A.V.; Bento, I.; Zaitsev, V.N.; Zhukhlistova, N.E.; Zhukova, Y.N.; Gabdoulkhakov, A.G.; Morgunova, E.Y.; Voelter, W.; Kachalova, G.S.; Stepanova, E.V.; et al. X-ray Structural Studies of the Fungal Laccase from Cerrena Maxima. J. Biol. Inorg. Chem. 2006, 11, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, K.M.; Gavryushov, S.; Fedorov, T.V.; Glazunova, O.A.; Popov, A.N. The Subatomic Resolution Study of Laccase Inhibition by Chloride and Fluoride Anions Using Single-Crystal Serial Crystallography: Insights into the Enzymatic Reaction Mechanism. Acta Crystallogr. Sect. D Struct. Biol. 2019, 75, 804–816. [Google Scholar] [CrossRef]
- Gavryushov, S.; Kuzmich, N.N.; Polyakov, K.M. Quantum Mechanical Study of Oxygen Ligands Protonation for the Stable States of the Laccase Active Site. Int. J. Mol. Sci. 2023, 24, 2990. [Google Scholar] [CrossRef]
- Boer, D.D.; de Heer, H.C.; Buda, F.; Hetterscheid, D.G.H. Challenges in Elucidating the Free Energy Scheme of the Laccase Catalyzed Reduction of Oxygen. ChemCatChem 2023, 15, e202200878. [Google Scholar] [CrossRef]
- Torres-Salas, P.; Mate, D.M.; Ghazi, I.; Plou, F.J.; Ballesteros, A.O.; Alcalde, M. Widening the PH Activity Profile of a Fungal Laccase by Directed Evolution. ChemBioChem 2013, 14, 934–937. [Google Scholar] [CrossRef]
- Olmeda, I.; Casino, P.; Collins, R.E.; Sendra, R.; Callejón, S.; Huesa, J.; Soares, A.S.; Ferrer, S.; Pardo, I. Structural Analysis and Biochemical Properties of Laccase Enzymes from Two Pediococcus Species. Microb. Biotechnol. 2021, 14, 1026–1043. [Google Scholar] [CrossRef] [PubMed]
- Novoa, C.; Dhoke, G.V.; Mate, D.M.; Martínez, R.; Haarmann, T.; Schreiter, M.; Eidner, J.; Schwerdtfeger, R.; Lorenz, P.; Davari, M.D.; et al. KnowVolution of a Fungal Laccase toward Alkaline PH. ChemBioChem 2019, 20, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Zhou, G.; Peng, C.; Zhang, Y.; Kües, U.; Liu, J.; Xiao, Y.; Fang, Z. The First Fungal Laccase with an Alkaline PH Optimum Obtained by Directed Evolution and Its Application in Indigo Dye Decolorization. AMB Express 2019, 9, 151. [Google Scholar] [CrossRef]
- Gupta, A.; Nederlof, I.; Sottini, S.; Tepper, A.W.J.W.; Groenen, E.J.J.; Thomassen, E.A.J.; Canters, G.W. Involvement of Tyr108 in the Enzyme Mechanism of the Small Laccase from Streptomyces Coelicolor. J. Am. Chem. Soc. 2012, 134, 18213–18216. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, R.; Gupta, K.B.S.S.; de Groot, H.J.M.; Ubbink, M. The Resting Oxidized State of Small Laccase Analyzed with Paramagnetic NMR Spectroscopy. ChemPhysChem 2021, 22, 733–740. [Google Scholar] [CrossRef]
- Dasgupta, R.; Gupta, K.B.S.S.; de Groot, H.J.M.; Ubbink, M. Towards Resolving the Complex Paramagnetic Nuclear Magnetic Resonance (NMR) Spectrum of Small Laccase: Assignments of Resonances to Residue-Specific Nuclei. Magn. Reson. 2021, 2, 15–23. [Google Scholar] [CrossRef]
- Gabdulkhakov, A.G.; Kostareva, O.S.; Kolyadenko, I.A.; Mikhaylina, A.O.; Trubitsina, L.I.; Tishchenko, S.V. Incorporation of Copper Ions into T2/T3 Centers of Two-Domain Laccases. Mol. Biol. 2018, 52, 23–29. [Google Scholar] [CrossRef]
- Vandeyar, M.A.; Weiner, M.P.; Hutton, C.J.; Batt, C.A. A Simple and Rapid Method for the Selection of Oligodeoxynucleotide-Directed Mutants. Gene 1988, 65, 129–133. [Google Scholar] [CrossRef]
- Heinfling, A.; Martínez, M.J.; Martínez, A.T.; Bergbauer, M.; Szewzyk, U. Purification and Characterization of Peroxidases from the Dye-Decolorizing Fungus Bjerkandera Adusta. FEMS Microbiol. Lett. 1998, 165, 43–50. [Google Scholar] [CrossRef]
- Wariishi, H.; Valli, K.; Gold, M.H. Manganese(II) Oxidation by Manganese Peroxidase from the Basidiomycete Phanerochaete Chrysosporium. Kinetic Mechanism and Role of Chelators. J. Biol. Chem. 1992, 267, 23688–23695. [Google Scholar] [CrossRef]
- D’Arcy, A.; Bergfors, T.; Cowan-Jacob, S.W.; Marsh, M. Microseed Matrix Screening for Optimization in Protein Crystallization: What Have We Learned? Acta Crystallogr. Sect. Struct. Biol. Commun. 2014, 70, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Kraft, P.; Bergamaschi, A.; Broennimann, C.; Dinapoli, R.; Eikenberry, E.F.; Henrich, B.; Johnson, I.; Mozzanica, A.; Schlepütz, C.M.; Willmott, P.R.; et al. Performance of Single-Photon-Counting PILATUS Detector Modules. J. Synchrotron Radiat. 2009, 16, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Mueller, U.; Förster, R.; Hellmig, M.; Huschmann, F.U.; Kastner, A.; Malecki, P.; Pühringer, S.; Röwer, M.; Sparta, K.; Steffien, M.; et al. The Macromolecular Crystallography Beamlines at BESSY II of the Helmholtz-Zentrum Berlin: Current Status and Perspectives. Eur. Phys. J. Plus 2015, 130, 141. [Google Scholar] [CrossRef]
- Rigaku, O.D. CrysAlis PRO; Rigaku Oxford Diffraction: Yarnton, UK, 2019. [Google Scholar]
- Vagin, A.; Teplyakov, A. MOLREP: An Automated Program for Molecular Replacement. J. Appl. Crystallogr. 1997, 30, 1022–1025. [Google Scholar] [CrossRef]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser Crystallographic Software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Murshudov, G.N.; Skubák, P.; Lebedev, A.A.; Pannu, N.S.; Steiner, R.A.; Nicholls, R.A.; Winn, M.D.; Long, F.; Vagin, A.A. REFMAC5 for the Refinement of Macromolecular Crystal Structures. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and Development of Coot. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Afonine, P.V.; Grosse-Kunstleve, R.W.; Echols, N.; Headd, J.J.; Moriarty, N.W.; Mustyakimov, M.; Terwilliger, T.C.; Urzhumtsev, A.; Zwart, P.H.; Adams, P.D. Towards Automated Crystallographic Structure Refinement with Phenix.Refine. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 352–367. [Google Scholar] [CrossRef]
- Schrödinger, L.; DeLano, W. PyMOL. 2020. Available online: http://www.pymol.org/pymol (accessed on 8 June 2023).


| M199A/D268N | M199G/R240H/D268N | |
|---|---|---|
| Data collection | ||
| Wavelength (Å) | 1.54 | 1.54 |
| Resolution range (Å) | 23.54–2.00 (2.07–2.00) a | 23.70–2.20 (2.28–2.20) a |
| Space group | P21 | P21 |
| Cell parameters a,b,c (Å) α = γ = 90, β (°) | 74.30, 94.02, 118.92 91.10 | 74.36, 93.91, 119.59 91.10 |
| Collection temperature (K) | 120 | 120 |
| Total reflections | 299,870 (30,309) | 376,173 (38,889) |
| Unique reflections | 104,468 (10,454) | 82,258 (8,233) |
| Multiplicity | 2.9 (2.9) | 4.6 (4.7) |
| Completeness (%) | 94.60 (95.30) | 98.40 (96.66) |
| Mean I/sigma (I) | 8.01 (1.43) | 4.89 (1.35) |
| Wilson B-factor (Å2) | 21.5 | 17.8 |
| CC1/2 | 0.98 (0.54) | 0.96 (0.49) |
| Refinement | ||
| Resolution range | 23.54–2.00 (2.02–2.00) | 23.70–2.20 (2.23–2.20) |
| Reflections used in refinement | 104,300 (3,273) | 82,065 (2,593) |
| Reflections used for R-free | 5231 (161) | 4124 (139) |
| R-work, % | 20.11 (35.49) | 21.49 (27.12) |
| R-free, % | 24.18 (37.39) | 25.81 (32.68) |
| RMSD bond lengths (Å) | 0.008 | 0.009 |
| RMSD bond angles (◦) | 0.89 | 0.99 |
| Ramachandran favored (%) | 96.61 | 96.66 |
| Ramachandran allowed (%) | 3.33 | 3.28 |
| Ramachandran outliers (%) | 0.06 | 0.06 |
| Average B-factor (Å2) | 27.42 | 20.69 |
| macromolecules | 27.49 | 20.73 |
| ligands | 29.76 | 23.25 |
| solvent | 26.03 | 15.48 |
| PDB ID | 8P9U | 8P9V |
| Substrate | Object | pH | KM (mM) | Kcat (sec−1) | kcat/KM (sec−1 mM−1) |
|---|---|---|---|---|---|
| ABTS | SgfSLwt * | 4.0 | 0.36 ± 0.07 | 15.11 ± 1.07 | 41.98 |
| Met199Ala/Asp268Asn | 4.0 | 0.11 ± 0.03 | 21.41 ± 1.12 | 194.62 | |
| Met199Gly/ Asp268Asn | 4.0 | 0.22 ± 0.03 | 37.70 ± 2.41 | 171.40 | |
| Met199Gly/Arg240His/ Asp268Asn | 4.0 | 0.25 ± 0.02 | 38.90 ± 0.99 | 155.65 | |
| 2,6-DMP | SgfSLwt * | 9.0 | 0.32 ± 0.08 | 0.35 ± 0.02 | 1.09 |
| Met199Ala/Asp268Asn | 7.0 | 0.87 ± 0.1 | 0.80 ± 0.03 | 0.92 | |
| Met199Gly/ Asp268Asn | 7.5 | 0.29 ± 0.02 | 1.88 ± 0.05 | 6.49 | |
| Met199Gly/Arg240His/ Asp268Asn | 7.5 | 1.16 ± 0.17 | 12.84 ± 0.65 | 11.07 | |
| 8.0 | 0.50 ± 0.08 | 11.10 ± 0.45 | 22.19 | ||
| 8.5 | 0.24 ± 0.03 | 8.19 ± 0.13 | 34.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolyadenko, I.; Tishchenko, S.; Gabdulkhakov, A. Structural Insight into the Amino Acid Environment of the Two-Domain Laccase’s Trinuclear Copper Cluster. Int. J. Mol. Sci. 2023, 24, 11909. https://doi.org/10.3390/ijms241511909
Kolyadenko I, Tishchenko S, Gabdulkhakov A. Structural Insight into the Amino Acid Environment of the Two-Domain Laccase’s Trinuclear Copper Cluster. International Journal of Molecular Sciences. 2023; 24(15):11909. https://doi.org/10.3390/ijms241511909
Chicago/Turabian StyleKolyadenko, Ilya, Svetlana Tishchenko, and Azat Gabdulkhakov. 2023. "Structural Insight into the Amino Acid Environment of the Two-Domain Laccase’s Trinuclear Copper Cluster" International Journal of Molecular Sciences 24, no. 15: 11909. https://doi.org/10.3390/ijms241511909
APA StyleKolyadenko, I., Tishchenko, S., & Gabdulkhakov, A. (2023). Structural Insight into the Amino Acid Environment of the Two-Domain Laccase’s Trinuclear Copper Cluster. International Journal of Molecular Sciences, 24(15), 11909. https://doi.org/10.3390/ijms241511909







