Structural Speciation of Ti(IV)-(α-Hydroxycarboxylic Acid) Complexes in Metabolism-Related (Patho)Physiology—In Vitro Approaches to (Pre)Adipocyte Differentiation and Mineralization
Abstract
1. Introduction
2. Results
2.1. Synthesis
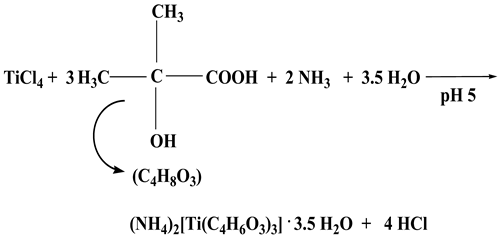

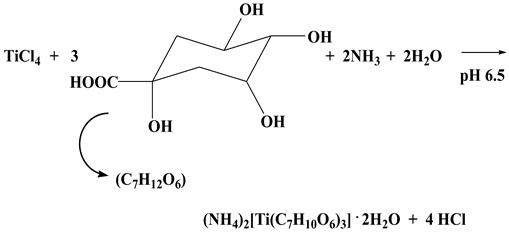
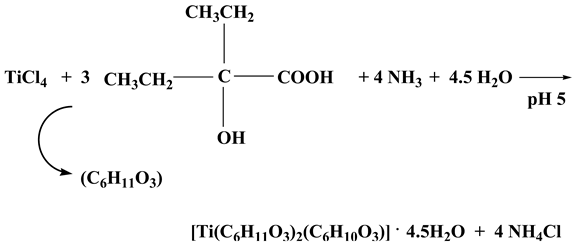
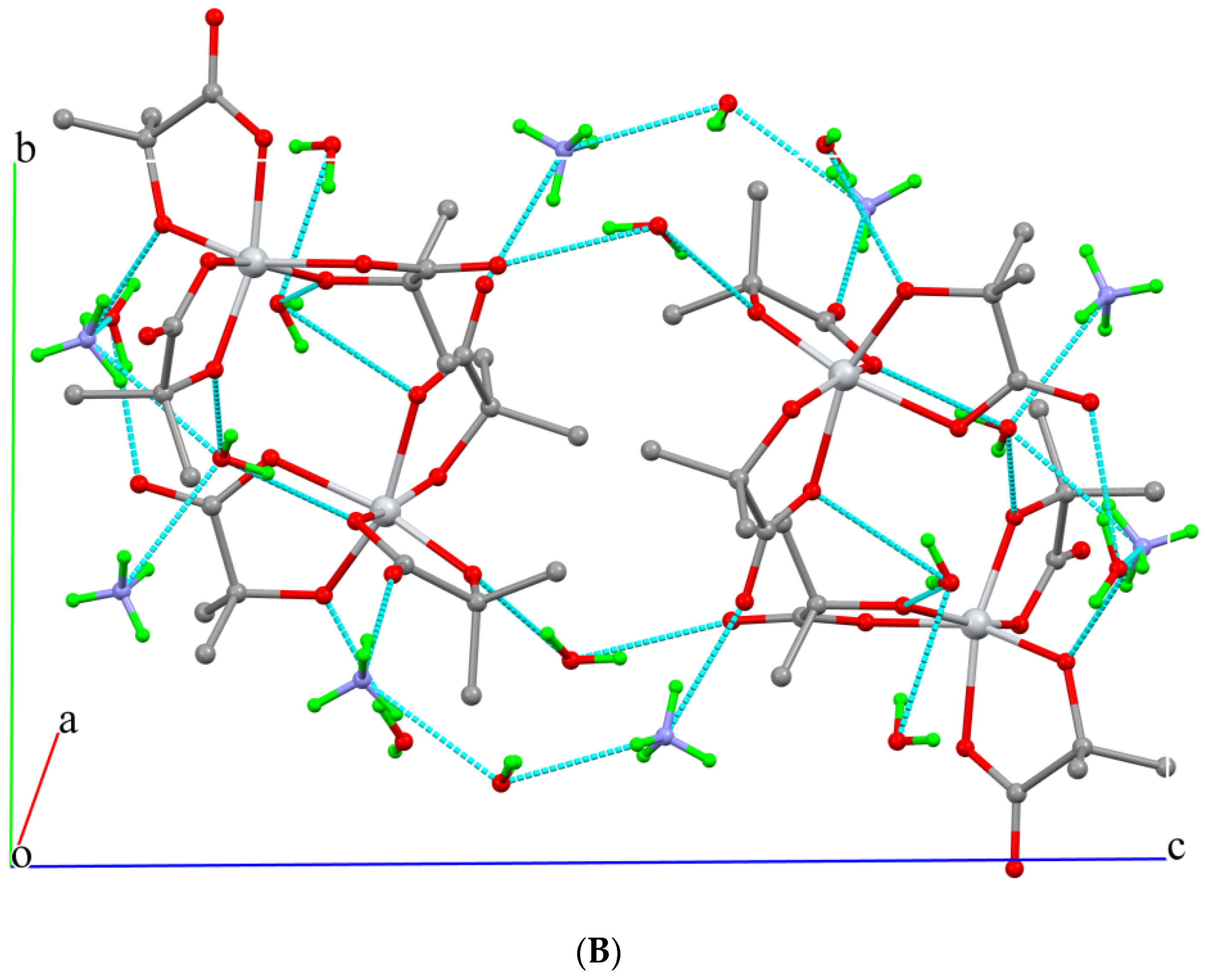
2.2. Description of X-ray Crystallographic Structures
2.3. FT-IR Spectroscopy
2.4. Thermal Studies
2.5. Mass Spectrometry Measurements
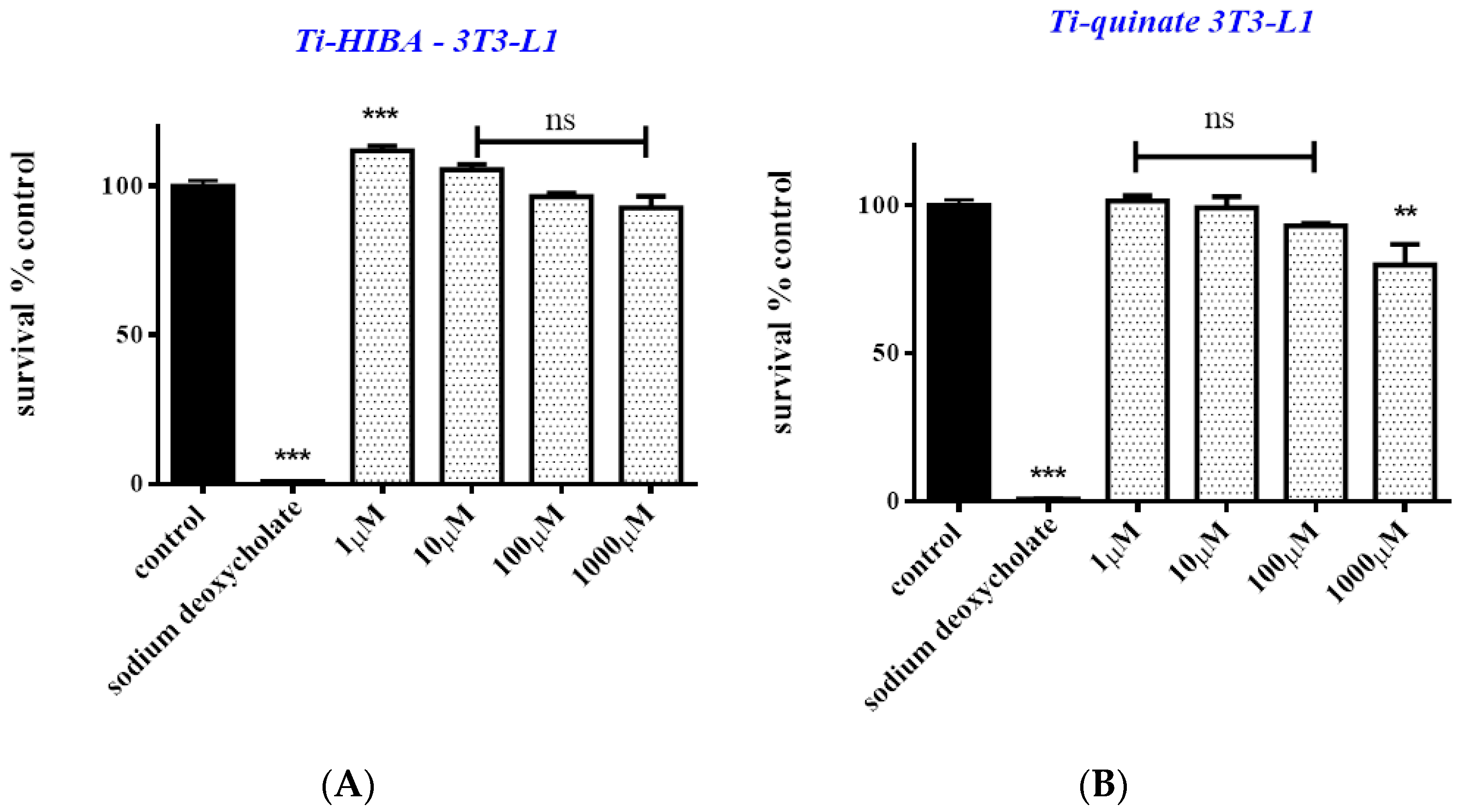
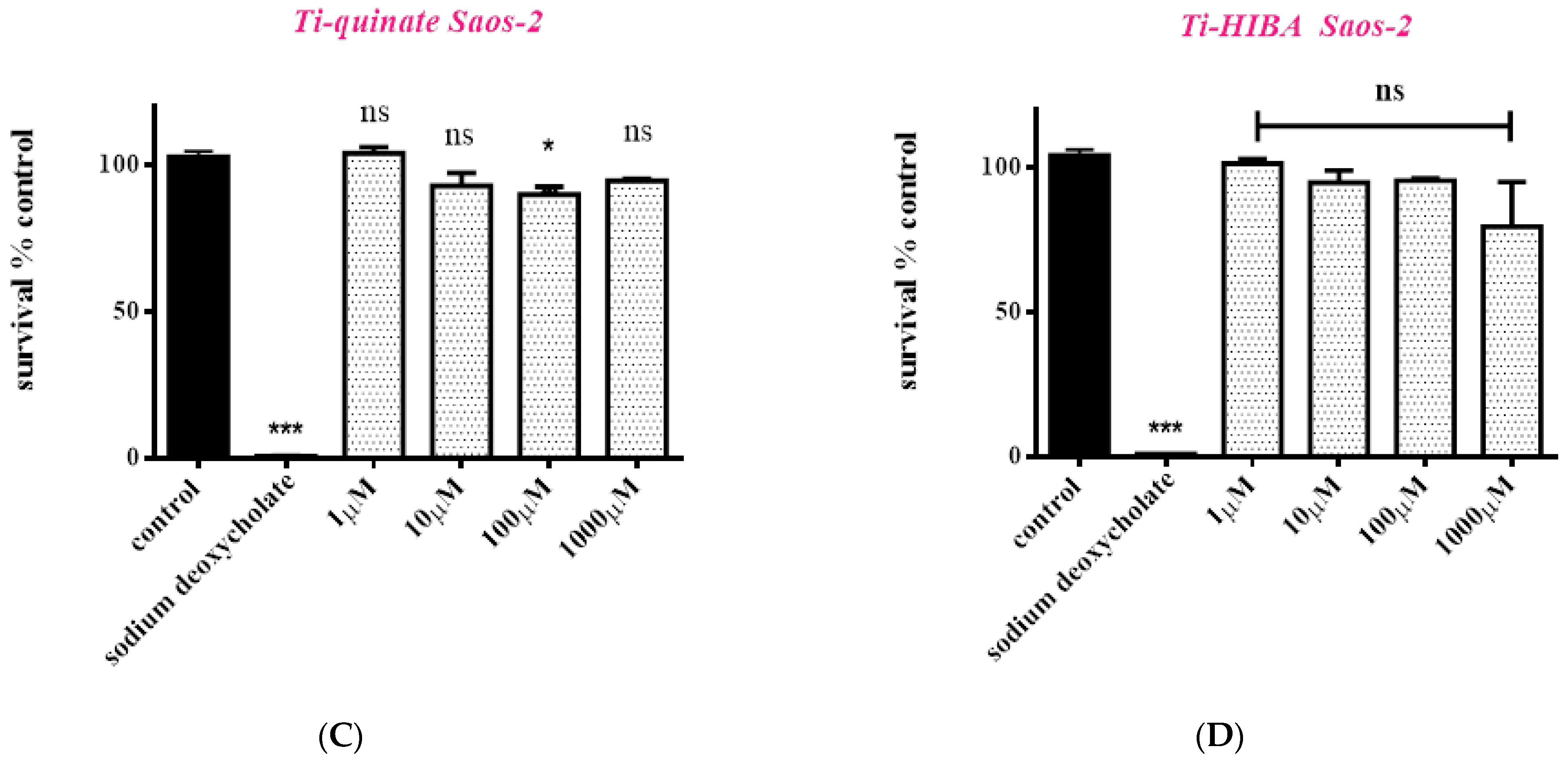
2.6. Cytotoxicity Studies
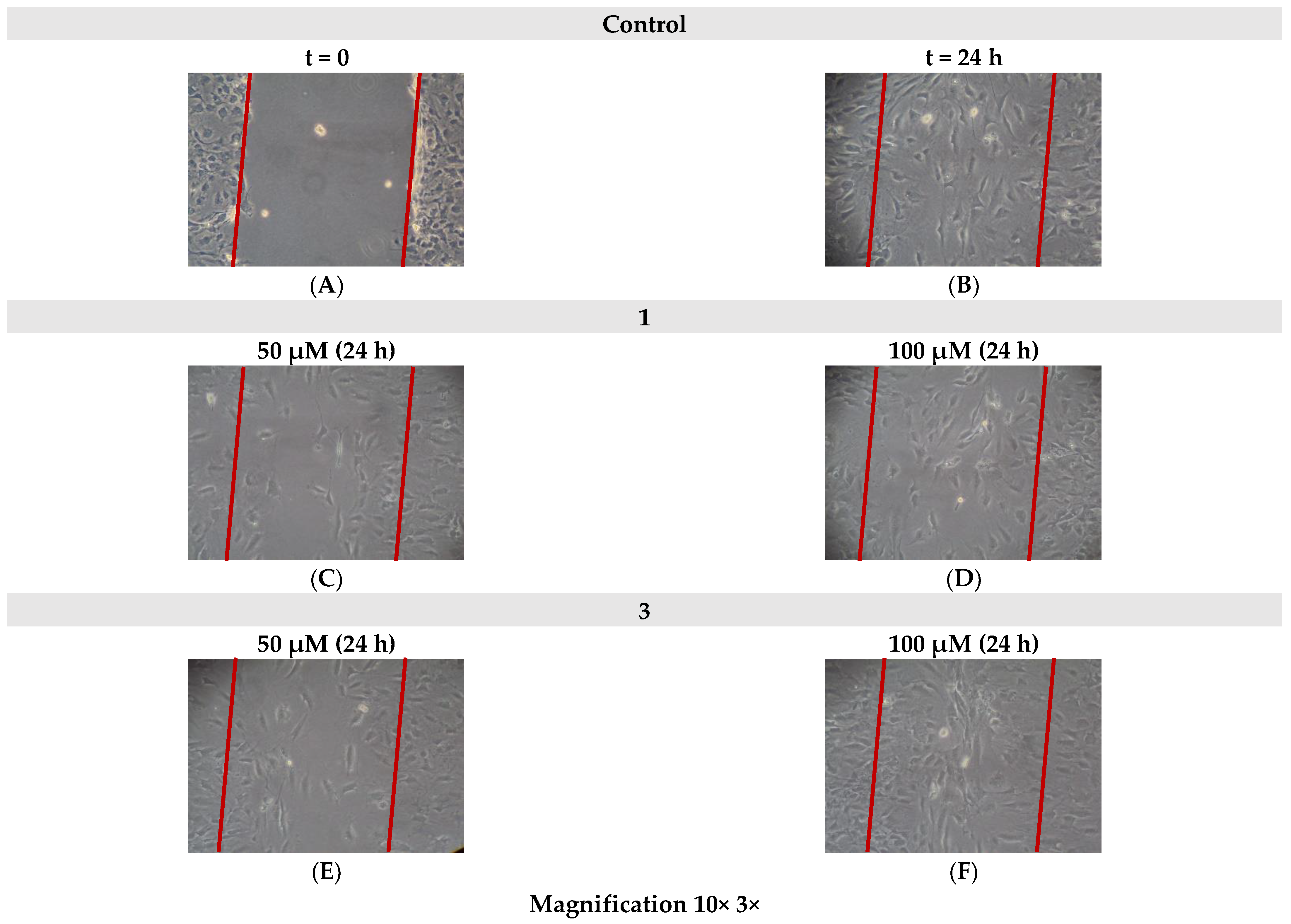
2.7. Cell Migration
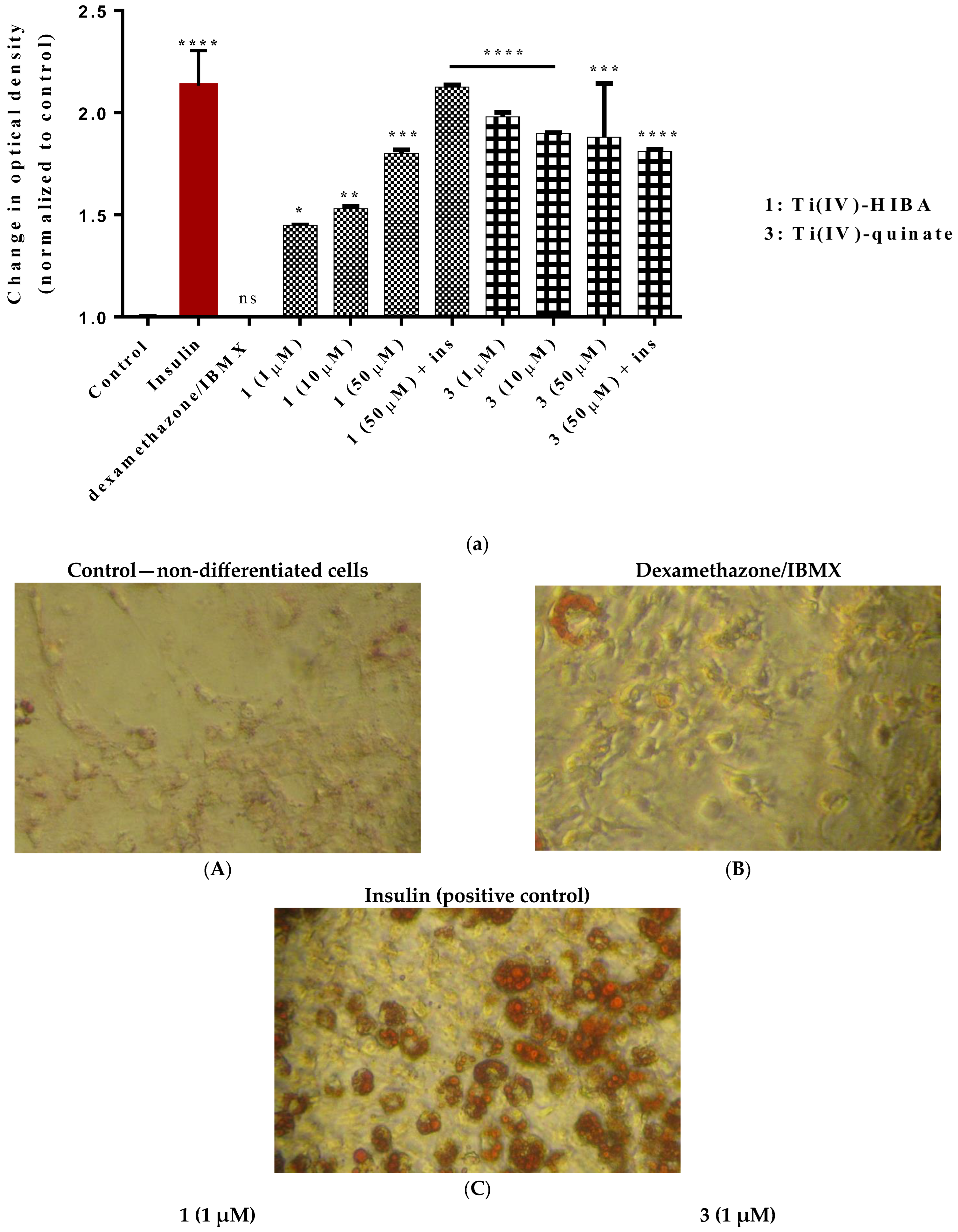
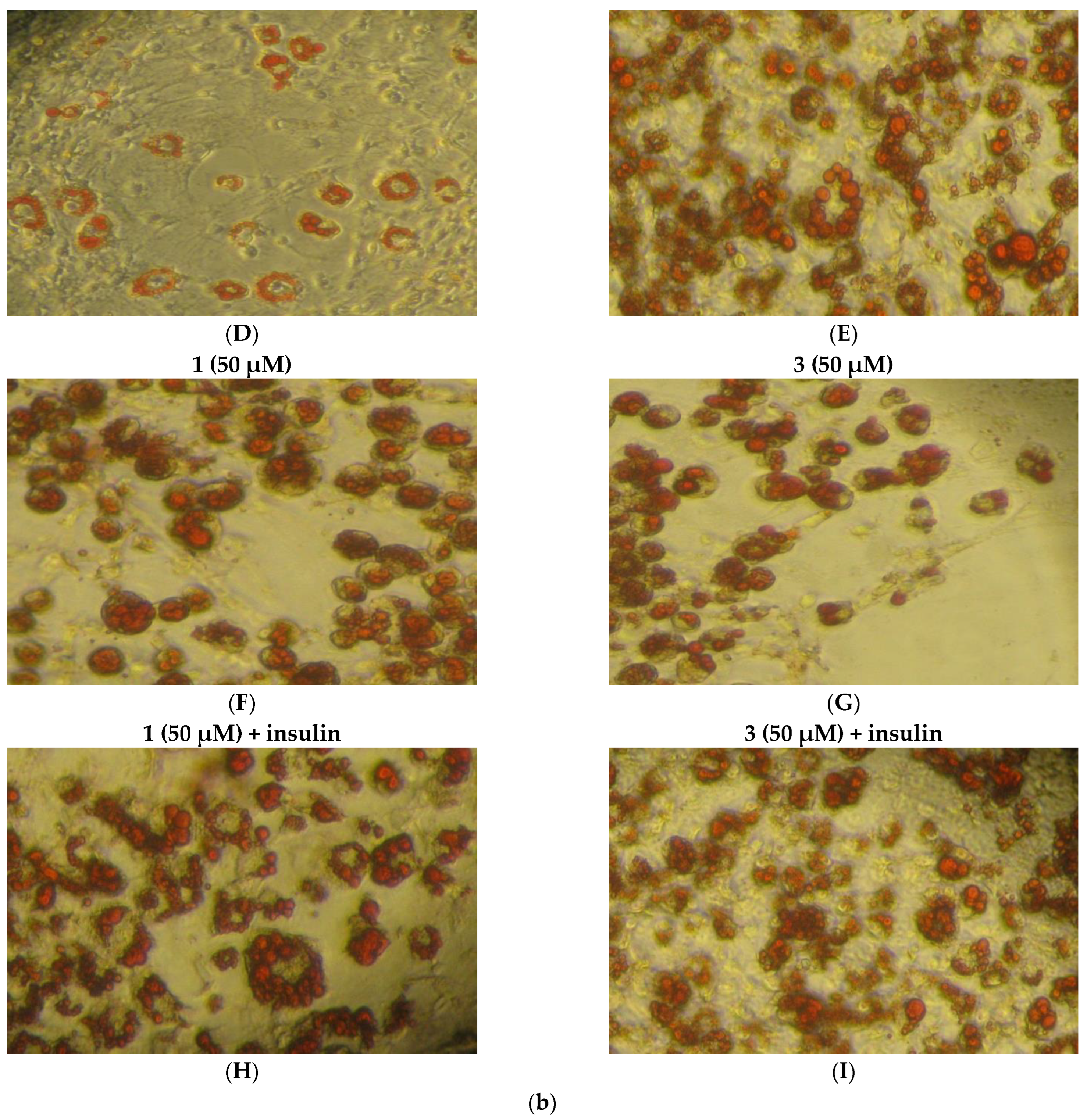
2.8. Study of In Vitro Adipogenesis
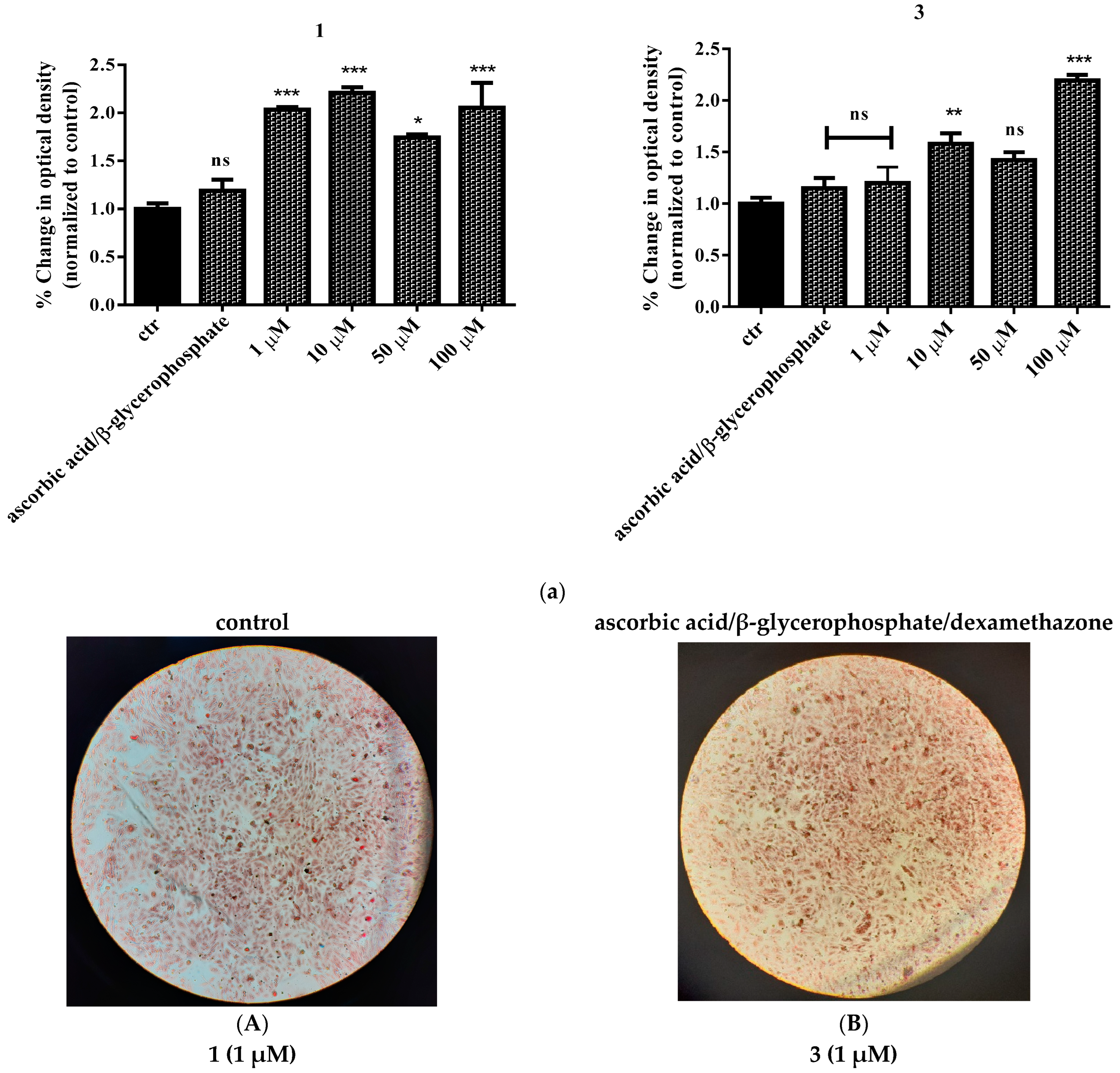
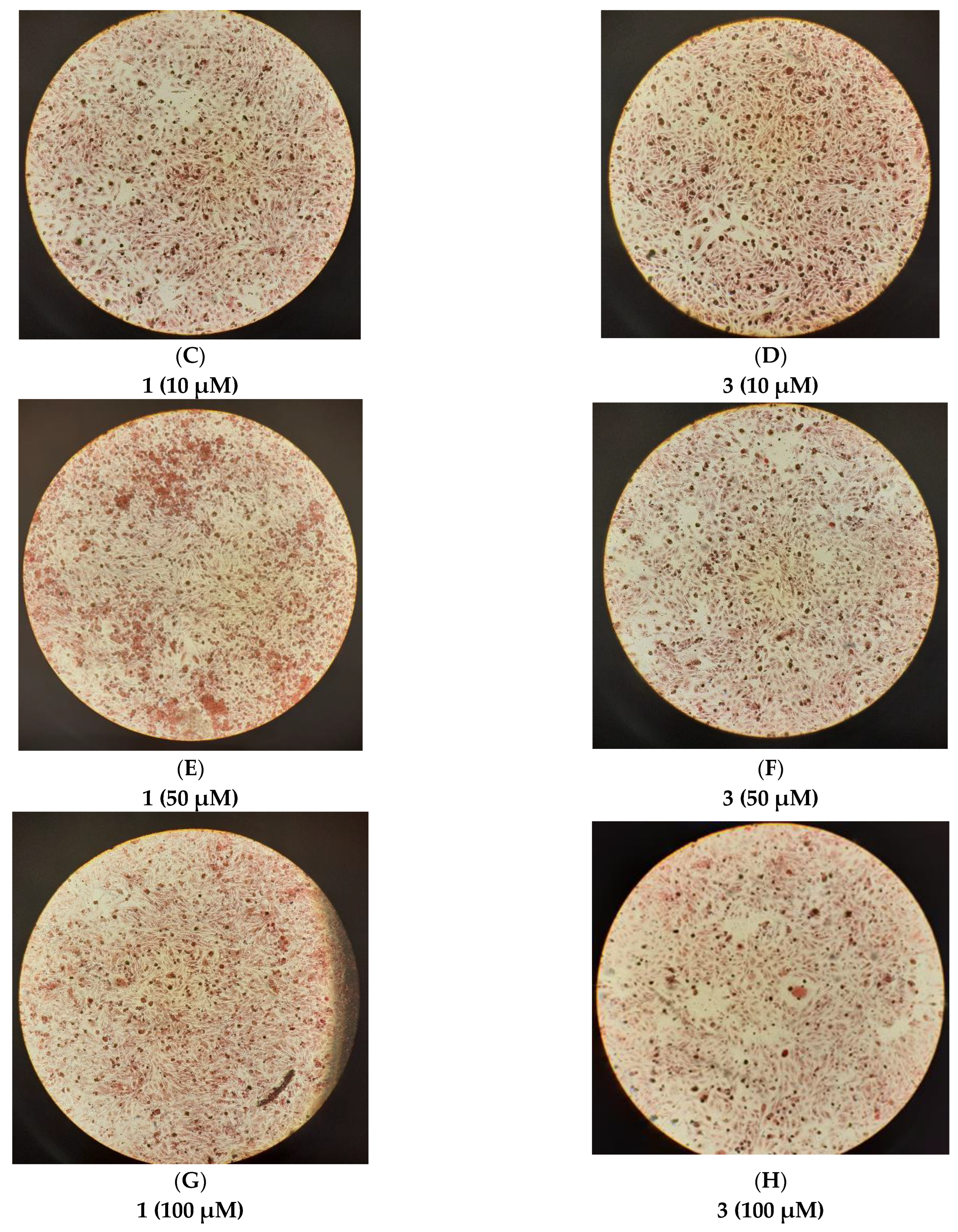
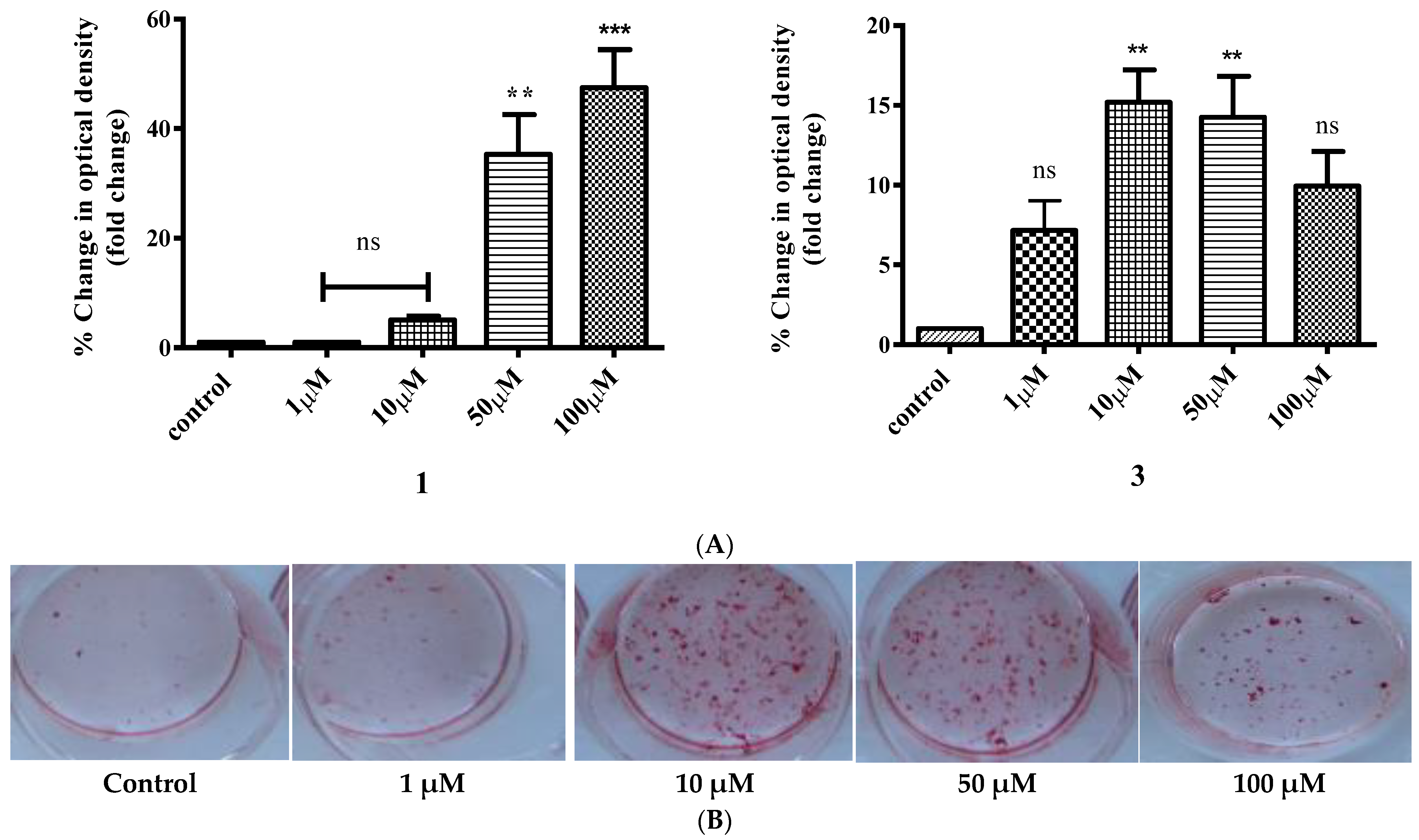
2.9. Study of In Vitro Mineralization
3. Discussion
3.1. Synthetic Challenges in the Pursuit of Biological Titanium Candidates
3.2. Biological Profile Studies
4. Methods and Materials
4.1. Material and Methods
4.1.1. Physical Measurements
4.1.2. Thermal Studies
4.1.3. Mass Spectrometry Measurements (ESI-MS)
4.2. Synthesis of Binary Ti(IV) Compounds
4.2.1. (NH4)2[Ti(C4H6O3)3]·3.5H2O (1)
4.2.2. (CH6N3)2[Ti(C4H6O3)3]∙1.5H2O (2)
4.2.3. (NH4)2[Ti(C7H10O6)3]∙2H2O (3)
4.2.4. Ti(C6H11O3)2(C6H10O3)·4.5H2O (4)
4.3. X-ray Crystal Structure Determination
4.4. Cell Cultures and Biological Tests
4.4.1. Cultivation of 3T3-L1, Saos-2, and KS483 Cells
4.4.2. Preparation of Ti(IV)-Compound Stock Solution
4.4.3. Cell Viability–Growth Assay
4.4.4. Cell Migration Assay
4.4.5. Cell Biocompatibility—Morphology
4.4.6. Induction of Adipogenesis with Ti(IV)-Complexes In Vitro
4.4.7. Oil Red O Staining
4.4.8. In Vitro Mineralization
4.4.9. Alizarin Red Staining
4.4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | One-way analysis of variance |
| DMEM | Dulbecco’s modified Eagle’s medium |
| EHBAH2 | 2-Ethyl-2-hydroxy-butyric acid |
| ESI-MS | Electron spray ionization mass spectrometry |
| FBS | Fetal bovine serum |
| FT-IR | Fourier transform infrared |
| HIBAH2 | α-Hydroxy isobutyric acid |
| αMEM | α minimum essential medium |
| QA | Quinic acid |
| SEM | Standard error mean |
| TGA | Thermogravimetric analysis |
References
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 726572–726579. [Google Scholar] [CrossRef]
- Jia, P.; Ouyang, R.; Cao, P.; Tong, X.; Zhou, X.; Lei, T.; Zhao, Y.; Guo, N.; Chang, H.; Miao, Y.; et al. Review: Recent advances and future development of metal complexes as anticancer agents. J. Coord. Chem. 2017, 70, 2175–2201. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.G.; Dowson, C.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef]
- Kiss, T.; Enyedy, É.A.; Jakusch, T.; Dömötör, O. Speciation of Metal Complexes of Medicinal Interest: Relationship between Solution Equilibria and Pharmaceutical Properties. Curr. Med. Chem 2019, 26, 580–606. [Google Scholar] [CrossRef]
- Muhammad, N.; Guo, Z. Metal-based anticancer chemotherapeutic agents. Curr. Opin. Chem. Biol. 2014, 19, 144–153. [Google Scholar] [CrossRef]
- Hanif, M.; Babak, M.V.; Hartinger, C.G. Development of anticancer agents: Wizardry with osmium. Drug Discov. Today 2014, 19, 1640–1648. [Google Scholar] [CrossRef]
- Jopp, M.; Becker, J.; Becker, S.; Miska, A.; Gandin, V.; Marzano, C.; Schindler, S. Anticancer activity of a series of copper(II) complexes with tripodal ligands. Eur. J. Med. Chem. 2017, 132, 274–281. [Google Scholar] [CrossRef]
- Jorge, J.R.; Barão, V.A.; Delben, J.A.; Faverani, L.P.; Queiroz, T.P.; Assunção, W.G. Titanium in dentistry: Historical development, state of the art and future perspectives. J. Indian Prosthodont. Soc. 2013, 13, 71–77. [Google Scholar] [CrossRef]
- Maia, A. Titanio. Balanc. Miner. Bras. 2003, 1, 1–23. [Google Scholar]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A 2002, 33, 477–486. [Google Scholar] [CrossRef]
- Okuno, O. Titanium alloys for dental applications. J. Jpn. Soc. Biomater. 1996, 14, 267–273. [Google Scholar]
- Sun, Y.; Zhao, Y.Q.; Zeng, Q.; Wu, Y.W.; Hu, Y.; Duan, S.; Tang, Z.; Xu, F.J. Dual-Functional Implants with Antibacterial and Osteointegration-Promoting Performances. ACS Appl. Mater. Interfaces 2019, 11, 36449–36457. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef]
- Köpf-Maier, P.; Köpf, H. Non-platinum group metal antitumor agents. History, current status, and perspectives. Chem. Rev. 1987, 87, 1137. [Google Scholar] [CrossRef]
- Tinoco, A.D.; Thomas, H.R.; Incarvito, C.D.; Saghatelian, A.; Valentine, A.M. Cytotoxicity of a Ti(IV) compound is independent of serum proteins. Proc. Nat. Acad. Sci. USA 2012, 109, 5016–5021. [Google Scholar] [CrossRef]
- Caruso, F.; Rossi, M. Antitumor titanium compounds. Mini Rev. Med. Chem. 2004, 4, 49–60. [Google Scholar] [CrossRef]
- Meléndez, E. Titanium complexes in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 309–315. [Google Scholar] [CrossRef]
- Berdel, E.; Schmoll, H.J.; Scheulen, M.E.; Korfel, A.; Knoche, M.F.; Harstrci, A.; Bach, F.; Baumgart, J.; Sass, G.J. Phase I clinical trial of titanocene dichloride in adults with advanced solid tumors. Cancer Res. Clin. Oncol. 1994, 120 (Suppl.), R172. [Google Scholar]
- Christodoulou, C.V.; Ferry, D.R.; Fyfe, D.W.; Young, A.; Doran, J.; Sheehan, T.M.; Hale, K.; Baumgart, J.; Sass, G.; Ker, D.J. Phase I trial of weekly scheduling and pharmacokinetics of titanocene dichloride in patients with advanced cancer. J. Clin. Oncol. 1998, 16, 2761–2769. [Google Scholar] [CrossRef]
- Schilling, T.; Keppler, B.K.; Heim, M.E.; Niebch, G.; Dietzfelbinger, H.; Rastetter, J.; Hanauske, A.R. Clinical phase I and pharmacokinetic trial of the new titanium complex budotitane. Investig. New Drugs 1996, 13, 327–332. [Google Scholar] [CrossRef]
- Immel, T.A.; Groth, U.; Huhn, T.; Öhlschläger, P. Titanium salan complexes displays strong antitumor properties in vitro and in vivo in mice. PLoS ONE 2011, 6, e17869. [Google Scholar] [CrossRef][Green Version]
- Lümmen, G.; Sperling, H.; Luboldt, H.; Otto, T.; Rübben, H. Phase II trial of titanocene dichloride in advanced renal-cell carcinoma. Cancer Chemother. Pharmacol. 1998, 42, 415–417. [Google Scholar] [CrossRef]
- Tsave, O.; Yavropoulou, M.P.; Kafantari, M.; Gabriel, C.; Yovos, J.G.; Salifoglou, A. The adipogenic potential of Cr(III). A molecular approach exemplifying metal-induced enhancement of insulin mimesis in diabetes mellitus II. J. Inorg. Biochem. 2016, 163, 323–331. [Google Scholar] [CrossRef]
- Domingo, J.L.; Gómez, M. Vanadium compounds for the treatment of human diabetes mellitus: A scientific curiosity? A review of thirty years of research. Food Chem. Toxicol. 2016, 95, 137–141. [Google Scholar] [CrossRef]
- Dakanali, M.; Kefalas, E.T.; Raptopoulou, C.P.; Terzis, A.; Voyiatzis, G.; Kyrikou, I.; Kyrikou, I.; Mavromoustakos, T.; Salifoglou, A. A new dinuclear Ti(IV)-peroxo-citrate complex from aqueous solutions. Synthetic, structural, and spectroscopic studies in relevance to aqueous titanium(IV)-peroxo-citrate speciation. Inorg. Chem. 2003, 42, 4632–4639. [Google Scholar] [CrossRef]
- Tsave, O.; Salifoglou, A. Biomimetic activity of soluble, well-defined, aqueous Ti(IV)-citrate species toward adipogenesis. An in vitro study. J. Inorg. Biochem. 2021, 214, 111290. [Google Scholar] [CrossRef]
- Gabriel, C.; Menelaou, M.; Daskalakis, M.; Lakatos, A.; Kiss, T.; Mateescu, C.; Raptis, R.G.; Zoumpoulakis, P.; Salifoglou, A. Synthetic, structural, spectroscopic and solution speciation studies of the binary Al(III)-quinic acid system. Relevance of soluble Al(III)-hydroxycarboxylate species to molecular toxicity. Polyhedron 2008, 27, 2911–2920. [Google Scholar] [CrossRef]
- Halevas, E.; Hatzidimitriou, A.; Bertmer, M.; Vangelis, A.A.; Antzara, A.; Mateescu, C.; Salifoglou, A. Structure Lattice-Dimensionality and Spectroscopic Property Correlations in Novel Binary and Ternary Materials of Group 13 Elements with α-Hydroxycarboxylic Benzilic Acid and Phenanthroline. Cryst. Growth Des. 2014, 14, 4041–4059. [Google Scholar] [CrossRef]
- Iordanidou, C.; Tsave, O.; Gabriel, C.; Hatzidimitriou, A.; Yavropoulou, M.P.; Mateescu, C.; Salifoglou, A. Synthetic endeavors on cadmium species bearing glycolate and aromatic chelators with structure-specific biotoxic correlations in vitro. J. Inorg. Biochem. 2017, 176, 38–52. [Google Scholar] [CrossRef]
- Iordanidou, C.; Tsave, O.; Gabriel, C.; Hatzidimitriou, A.; Salifoglou, A. Synthetic exploration of the binary cadmium-quinic acid system linked to in vitro cytotoxicity and chelation cytoprotection investigation. Inorg. Chim. Acta 2018, 482, 364–374. [Google Scholar] [CrossRef]
- Panagiotidis, P.; Kefalas, E.T.; Raptopoulou, C.P.; Terzis, A.; Mavromoustakos, T.; Salifoglou, A. Delving into The Complex Picture of Ti(IV)-Citrate Speciation in Aqueous Media: Synthetic, Structural, and Electrochemical Considerations in Mononuclear Ti(IV) Complexes Containing Variably Deprotonated Citrate Ligands. Inorg. Chim. Acta 2008, 361, 2210–2224. [Google Scholar] [CrossRef]
- Deng, Y.-F.; Jiang, Y.-Q.; Hong, Q.-M.; Zhou, Z.-H. Speciation of water-soluble titanium citrate: Synthesis, structural, spectroscopic properties and biological relevance. Polyhedron 2007, 26, 1561–1569. [Google Scholar] [CrossRef]
- Kefalas, E.T.; Panagiotidis, P.; Raptopoulou, C.P.; Terzis, A.; Mavromoustakos, T.; Salifoglou, A. Mononuclear Titanium(IV)-Citrate Complexes from Aqueous Solutions: pH-Specific Synthesis and Structural and Spectroscopic Studies in Relevance to Aqueous Titanium(IV)-Citrate Speciation. Inorg. Chem. 2005, 44, 2596–2605. [Google Scholar] [CrossRef] [PubMed]
- Claffey, J.; Hogan, M.; Muller-Bunz, H.; Pampillon, C.; Tacke, M. Oxali-titanocene Y: A potent anticancer drug. ChemMedChem 2008, 3, 729–731. [Google Scholar] [CrossRef]
- Fichtner, I.; Behrens, D.; Claffey, J.; Gleeson, B.; Hogan, M.; Wallis, D.; Weber, H.; Tacke, M. Antitumor activity of oxali-titanocene Y in xenografted CAKI-1 tumors in mice. Lett. Drug Des. Discov. 2008, 5, 489–493. [Google Scholar] [CrossRef]
- Shavit, M.; Tshuva, E.Y. Preparation and X-ray Structures of TiIV Complexes of Bis(carboxylato) Ligands–Formation of Mono-, Di-, Tetra-, and Hexanuclear Complexes with or without OR and μ-O Ligands. Eur. J. Inorg. Chem. 2008, 2008, 1467–1474. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; Gallego, B.; Žižak, Ž.; Hawkins, E.H.; Juranić, Z.D.; Kaluderović, G.N. Titanium(IV) carboxylate complexes: Synthesis, structural characterization and cytotoxic activity. Polyhedron 2010, 29, 354–360. [Google Scholar] [CrossRef]
- Rajini, A.; Nookaraju, M.; Reddy, I.A.K.; Venkatathri, N.J. Synthesis, characterization, antimicrobial and cytotoxicity studies of a novel titanium dodecylamino phosphate. Saudi Chem. Soc. 2017, 21, S77–S85. [Google Scholar] [CrossRef]
- Taylor, R. Insulin resistance and type 2 diabetes. Diabetes 2012, 61, 778–779. [Google Scholar] [CrossRef]
- Liu, H.Y.; Wu, A.T.H.; Tsai, C.Y.; Chou, K.R.; Zeng, R.; Wang, M.F.; Chang, W.C.; Hwang, S.M.; Su, C.-H.; Deng, W.P. The balance between adipogenesis and osteogenesis in bone regeneration by platelet-rich plasma for age-related osteoporosis. Biomaterials 2011, 32, 6773–6780. [Google Scholar] [CrossRef]
- Duque, G. Bone and fat connection in aging bone. Curr. Opin. Rheumatol. 2008, 20, 429–434. [Google Scholar] [CrossRef]
- Bruker Analytical X-ray Systems, Inc. Apex2, Version 2 User Manual, M86-E01078; Bruker Analytical X-ray Systems, Inc.: Madison, WI, USA, 2006. [Google Scholar]
- Siemens Industrial Automation, Inc. SADABS: Area-Detector Absorption Correction; Siemens Industrial Automation, Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Palatinus, L.; Chapuis, G.J. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J.J. CRYSTALS version 12: Software for guided crystal structure analysis. Appl. Cryst. 2003, 36, 1487. [Google Scholar] [CrossRef]
- Crystal Impact. DIAMOND, Crystal and Molecular Structure Visualization, Ver. 3.1c; Crystal Impact: Bonn, Germany, 2006. [Google Scholar]
- Tsave, O.; Yavropoulou, M.P.; Kafantari, M.; Gabriel, C.; Yovos, J.G.; Salifoglou, A. Comparative assessment of metal-specific adipogenic activity in zinc and vanadium-citrates through associated gene expression. J. Inorg. Biochem. 2018, 186, 217–227. [Google Scholar] [CrossRef] [PubMed]
- De Meulenaer, J.; Tompa, H. The absorption correction in crystal structure analysis. Acta Cryst. 1965, 19, 1014–1018. [Google Scholar] [CrossRef]
- Prince, E. Mathematical Techniques in Crystallography and Materials Science; Springer: New York, NY, USA, 1982. [Google Scholar]
- Watkin, D.J. The control of difficult refinements. Acta Cryst. 1994, A50, 411–437. [Google Scholar] [CrossRef]
- Watkin, D.J.; Prout, C.K.; Pearce, L.J. CAMERON; Chemical Crystallography Laboratory: Oxford, UK, 1996. [Google Scholar]
- Flack, H.D. On enantiomorph-polarity estimation. Acta Cryst. 1983, A39, 876–881. [Google Scholar] [CrossRef]


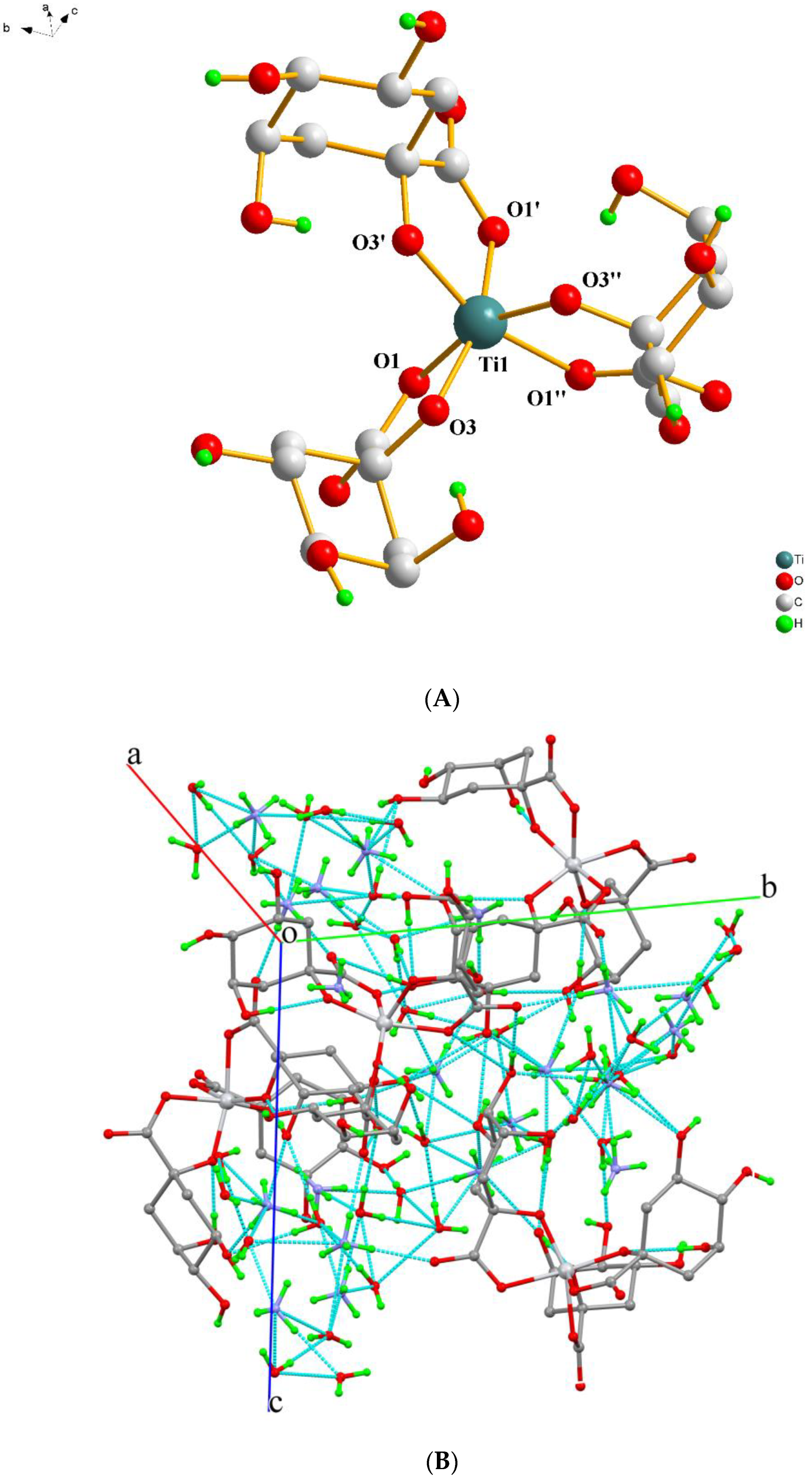






| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Chemical formula | C24H66N4O25Ti2 | C28H66N12O21Ti2 | C21H42N2O20Ti | C18H41O13.50Ti |
| Mr | 906.60 | 1002.70 | 690.35 | 521.42 |
| Crystal system | Triclinic | Monoclinic | Cubic | Monoclinic |
| Space group | Pī | P21/n | P213 | C2/c |
| Temperature (K) | 295 | 295 | 295 | 130 |
| a (Å) | 10.530 (6) | 21.458 (7) | 15.1689 (6) | 17.017 (2) |
| b (Å) | 11.531 (6) | 10.212 (3) | 15.1689 (6) | 11.3810 (15) |
| c (Å) | 18.946 (11) | 23.248 (7) | 15.1689 (6) | 27.743 (3) |
| α (°) | 89.09 (3) | 90 | 90 | 90 |
| β (°) | 89.82 (3) | 94.828 (8) | 90 | 101.800 (3) |
| γ (°) | 80.80 (3) | 90 | 90 | 90 |
| V (Å3) | 2270 (2) | 5076 (3) | 3490.3 (4) | 5259.3 (11) |
| Z | 2 | 4 | 4 | 8 |
| Radiation type | Mo Kα | Mo Kα | Mo Kα | Mo Kα |
| µ (mm−1) | 0.43 | 0.39 | 0.32 | 0.39 |
| Crystal size (mm) | 0.26 × 0.16 × 0.13 | 0.22 × 0.13 × 0.11 | 0.19 × 0.16 × 0.11 | 0.11 × 0.09 × 0.06 |
| Tmin, Tmax | 0.93, 0.95 | 0.95, 0.96 | 0.95, 0.97 | 0.97, 0.98 |
| No. of reflections | ||||
| measured | 69,918 | 63,319 | 13,087 | 28,368 |
| independent | 9847 | 9628 | 2392 | 5231 |
| observed [I > 2.0σ(I)] | 8419 | 6040 | 1927 | 4113 |
| No. of parameters | 499 | 568 | 151 | 334 |
| No. of restraints | 0 | 0 | 7 | 18 |
| Rint | 0.015 | 0.057 | 0.056 | 0.018 |
| (sinθ/λ)max (Å−1) | 0.643 | 0.613 | 0.625 | 0.623 |
| Refinement | ||||
| R[F2 > 2σ(F2)] | 0.041 | 0.052 | 0.053 | 0.042 |
| Rw(F2) | 0.062 | 0.088 | 0.137 | 0.082 |
| S | 1.00 | 1.00 | 1.00 | 1.00 |
| Δρmax, Δρmin (e Å−3) | 0.26, −0.34 | 0.53, −0.39 | 0.49, −0.29 | 0.65, −0.53 |
| Absolute structure parameter | −0.02 (6) | |||
| Bond Length (Å) | |||||||
| 1 | 2 | 3 * | 4 | ||||
| Ti(1)—O(1) | 2.0378 (16) | Ti(1)—O(1) | 2.065 (2) | Ti(1)—O(1) | 2.039 (3) | Ti(1)—O(1) | 2.0605 (16) |
| Ti(1)—O(3) | 1.8742 (15) | Ti(1)—O(3) | 1.856 (2) | Ti(1)—O(3) | 1.866 (2) | Ti(1)—O(3) | 1.8675 (16) |
| Ti(1)—O(4) | 2.0644 (16) | Ti(1)—O(4) | 2.070 (2) | Ti(1)—O(4) | 2.0340 (16) | ||
| Ti(1)—O(6) | 1.8639 (15) | Ti(1)—O(6) | 1.861 (2) | Ti(1)—O(6) | 1.8582 (16) | ||
| Ti(1)—O(7) | 2.0363 (17) | Ti(1)—O(7) | 2.052 (2) | Ti(1)—O(7) | 2.0445 (16) | ||
| Ti(1)—O(9) | 1.8703 (15) | Ti(1)—O(9) | 1.860 (2) | Ti(1)—O(9) | 1.8493 (16) | ||
| Ti(2)—O(10) | 2.0546 (17) | Ti(2)—O(10) | 2.048 (2) | ||||
| Ti(2)—O(12) | 1.8679 (15) | Ti(2)—O(12) | 1.878 (2) | ||||
| Ti(2)—O(13) | 2.0414 (17) | Ti(2)—O(13) | 2.061 (2) | ||||
| Ti(2)—O(15) | 1.8666 (15) | Ti(2)—O(15) | 1.874 (2) | ||||
| Ti(2)—O(16) | 2.0474 (17) | Ti(2)—O(16) | 2.072 (2) | ||||
| Ti(2)—O(18) | 1.8817 (15) | Ti(2)—O(18) | 1.827 (2) | ||||
| Angles (°) | |||||||
| O(1)—Ti(1)—O(3) | 78.95 (6) | O(1)—Ti(1)—O(3) | 79.17 (9) | O(1) i—Ti(1)—O(1) ii | 83.76 (13) | O(1)—Ti(1)—O(3) | 77.99 (6) |
| O(1)—Ti(1)—O(4) | 80.72 (7) | O(1)—Ti(1)—O(4) | 80.80 (8) | O(1) i—Ti(1)—O(3) i | 78.57 (11) | O(1)—Ti(1)—O(4) | 84.76 (7) |
| O(3)—Ti(1)—O(4) | 158.42 (6) | O(3)—Ti(1)—O(4) | 159.23 (9) | O(1) ii—Ti(1)—O(3) i | 159.59 (11) | O(3)—Ti(1)—O(4) | 103.72 (7) |
| O(1)—Ti(1)—O(6) | 101.93 (7) | O(1)—Ti(1)—O(6) | 100.37 (10) | O(1) i—Ti(1)—O(3) ii | 104.19 (11) | O(1)—Ti(1)—O(6) | 160.50 (7) |
| O(3)—Ti(1)—O(6) | 98.05 (7) | O(3)—Ti(1)—O(6) | 99.60 (10) | O(3) i—Ti(1)—O(3) ii | 95.87 (11) | O(3)—Ti(1)—O(6) | 95.93 (7) |
| O(4)—Ti(1)—O6) | 79.15 (7) | O(4)—Ti(1)—O(6) | 78.67 (9) | O(4)—Ti(1)—O(6) | 78.66 (7) | ||
| O(1)—Ti(1)—O(7) | 83.54 (7) | O(1)—Ti(1)—O(7) | 81.86 (9) | O(1)—Ti(1)—O(7) | 85.08 (7) | ||
| O(3)—Ti(1)—O(7) | 103.48 (8) | O(3)—Ti(1)—O(7) | 98.51 (9) | O(3)—Ti(1)—O(7) | 161.19 (7) | ||
| O(4)—Ti(1)—O(7) | 81.22 (8) | O(4)—Ti(1)—O(7) | 83.97 (8) | O(4)—Ti(1)—O(7) | 82.60 (7) | ||
| O(6)—Ti(1)—O(7) | 158.44 (6) | O(6)—Ti(1)—O(7) | 161.85 (9) | O(6)—Ti(1)—O(7) | 102.71 (7) | ||
| O(1)—Ti(1)—O(9) | 160.26 (5) | O(1)—Ti(1)—O(9) | 160.92 (9) | O(1)—Ti(1)—O(9) | 103.53 (7) | ||
| O(3)—Ti(1)—O(9) | 95.52 (8) | O(3)—Ti(1)—O(9) | 101.20 (9) | O(3)—Ti(1)—O(9) | 97.60 (7) | ||
| O(4)—Ti(1)—O(9) | 106.06 (6) | O(4)—Ti(1)—O(9) | 99.53 (9) | O(4)—Ti(1)—O(9) | 158.35 (7) | ||
| O(6)—Ti(1)—O(9) | 97.59 (7) | O(6)—Ti(1)—O(9) | 98.37 (10) | O(6)—Ti(1)—O(9) | 95.60 (7) | ||
| O(7)—Ti(1)—O(9) | 79.33 (6) | O(7)—Ti(1)—O(9) | 79.22 (9) | O(7)—Ti(1)—O(9) | 78.31 (7) | ||
| O(10)—Ti(2)—O(12) | 78.46 (6) | O(10)—Ti(2)—O(12) | 78.78 (9) | ||||
| O(10)—Ti(2)—O(13) | 81.99 (8) | O(10)—Ti(2)—O(13) | 80.55 (9) | ||||
| O(12)—Ti(2)—O(13) | 158.67 (6) | O(12)—Ti(2)—O(13) | 100.63 (10) | ||||
| O(10)—Ti(2)—O(15) | 104.53 (7) | O(10)—Ti(2)—O(15) | 158.26 (10) | ||||
| O(12)—Ti(2)—O(15) | 96.88 (7) | O(12)—Ti(2)—O(15) | 99.05 (10) | ||||
| O(13)—Ti(2)—O(15) | 79.81 (7) | O(13)—Ti(2)—O(15) | 78.60 (9) | ||||
| O(10)—Ti(2)—O(16) | 83.22 (7) | O(10)—Ti(2)—O(16) | 82.84 (9) | ||||
| O(12)—Ti(2)—O16) | 101.00 (7) | O(12)—Ti(2)—O(16) | 160.96 (9) | ||||
| O(13)—Ti(2)—O(16) | 84.96 (8) | O(13)—Ti(2)—O(16) | 81.08 (9) | ||||
| O(15)—Ti(2)—O(16) | 161.65 (6) | O(15)—Ti(2)—O(16) | 99.87 (9) | ||||
| O(10)—Ti(2)—O(18) | 160.75 (6) | O(10)—Ti(2)—O(18) | 99.39 (10) | ||||
| O(12)—Ti(2)—O(18) | 98.69 (8) | O(12)—Ti(2)—O(18) | 99.36 (10) | ||||
| O(13)—Ti(2)—O(18) | 102.58 (7) | O(13)—Ti(2)—O(18) | 159.58 (9) | ||||
| O(15)—Ti(2)—O(18) | 94.70 (7) | O(15)—Ti(2)—O(18) | 102.29 (10) | ||||
| O(16)—Ti(2)—O(18) | 78.62 (6) | O(16)—Ti(2)—O(18) | 78.67 (9) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsave, O.; Iordanidou, C.; Hatzidimitriou, A.; Yavropoulou, M.P.; Kassi, E.N.; Nasiri-Ansari, N.; Gabriel, C.; Salifoglou, A. Structural Speciation of Ti(IV)-(α-Hydroxycarboxylic Acid) Complexes in Metabolism-Related (Patho)Physiology—In Vitro Approaches to (Pre)Adipocyte Differentiation and Mineralization. Int. J. Mol. Sci. 2023, 24, 11865. https://doi.org/10.3390/ijms241411865
Tsave O, Iordanidou C, Hatzidimitriou A, Yavropoulou MP, Kassi EN, Nasiri-Ansari N, Gabriel C, Salifoglou A. Structural Speciation of Ti(IV)-(α-Hydroxycarboxylic Acid) Complexes in Metabolism-Related (Patho)Physiology—In Vitro Approaches to (Pre)Adipocyte Differentiation and Mineralization. International Journal of Molecular Sciences. 2023; 24(14):11865. https://doi.org/10.3390/ijms241411865
Chicago/Turabian StyleTsave, Olga, Catherine Iordanidou, Antonios Hatzidimitriou, Maria P. Yavropoulou, Eva N. Kassi, Narjes Nasiri-Ansari, Catherine Gabriel, and Athanasios Salifoglou. 2023. "Structural Speciation of Ti(IV)-(α-Hydroxycarboxylic Acid) Complexes in Metabolism-Related (Patho)Physiology—In Vitro Approaches to (Pre)Adipocyte Differentiation and Mineralization" International Journal of Molecular Sciences 24, no. 14: 11865. https://doi.org/10.3390/ijms241411865
APA StyleTsave, O., Iordanidou, C., Hatzidimitriou, A., Yavropoulou, M. P., Kassi, E. N., Nasiri-Ansari, N., Gabriel, C., & Salifoglou, A. (2023). Structural Speciation of Ti(IV)-(α-Hydroxycarboxylic Acid) Complexes in Metabolism-Related (Patho)Physiology—In Vitro Approaches to (Pre)Adipocyte Differentiation and Mineralization. International Journal of Molecular Sciences, 24(14), 11865. https://doi.org/10.3390/ijms241411865








