Sertindole, an Antipsychotic Drug, Curbs the STAT3/BCL-xL Axis to Elicit Human Bladder Cancer Cell Apoptosis In Vitro
Abstract
1. Introduction
2. Results
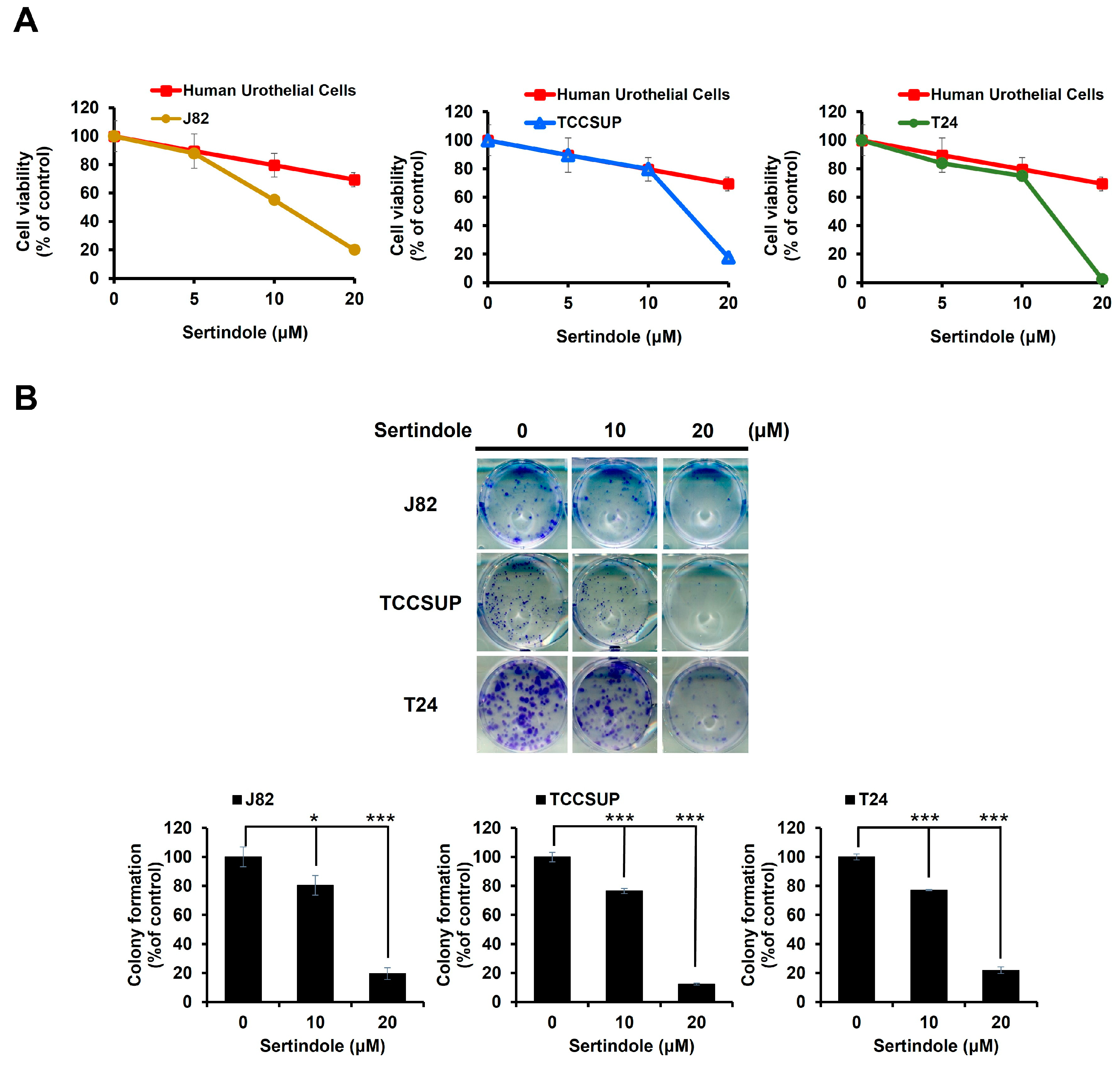
2.1. Sertindole Is Cytotoxic to Various Human Urinary Bladder TCC Cell Lines While Showing Less Cytotoxicity to Normal Human Urothelial Cells
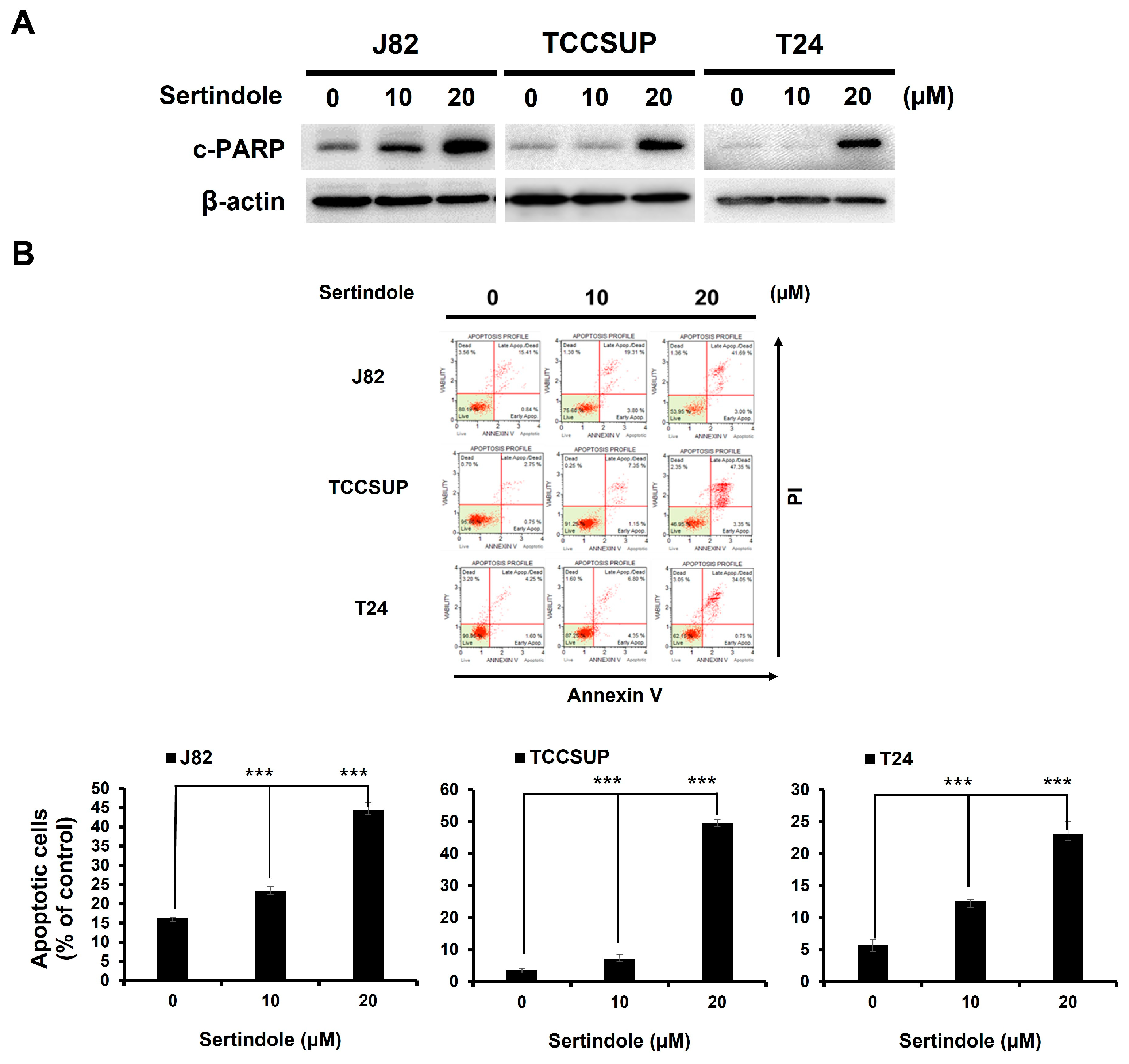
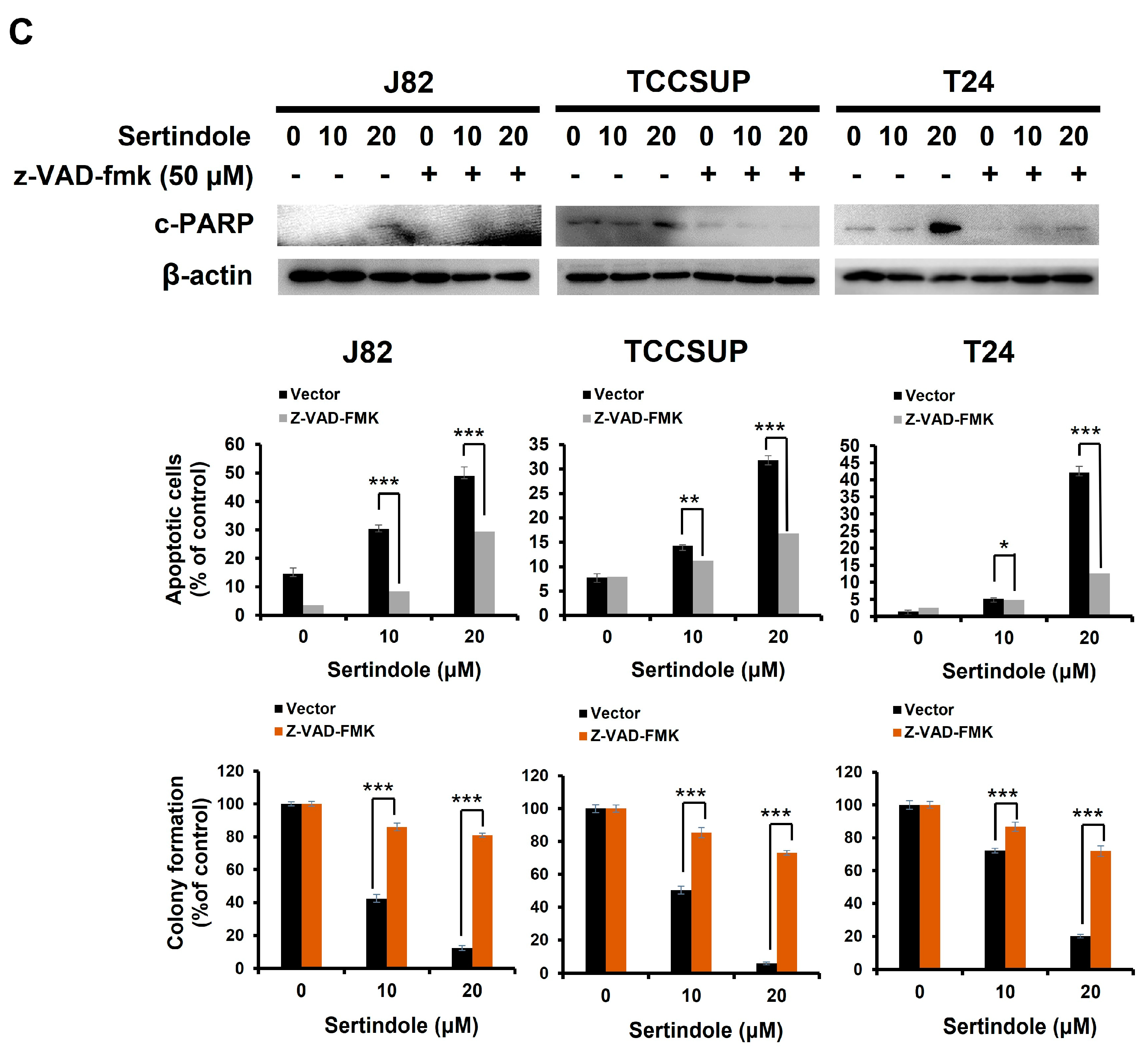
2.2. Sertindole-Induced Bladder Cancer Cytotoxicity Depends on Apoptosis Induction
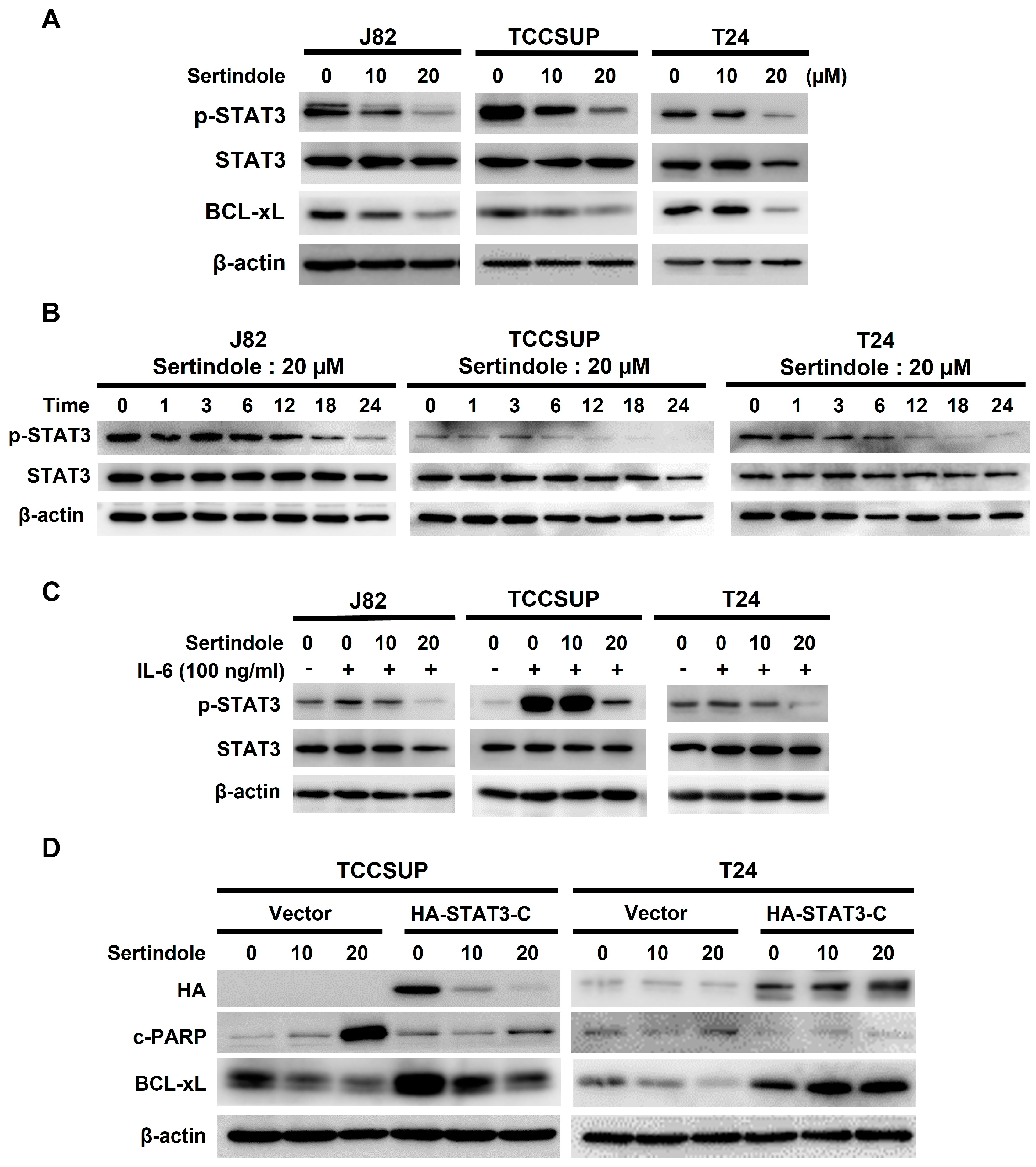
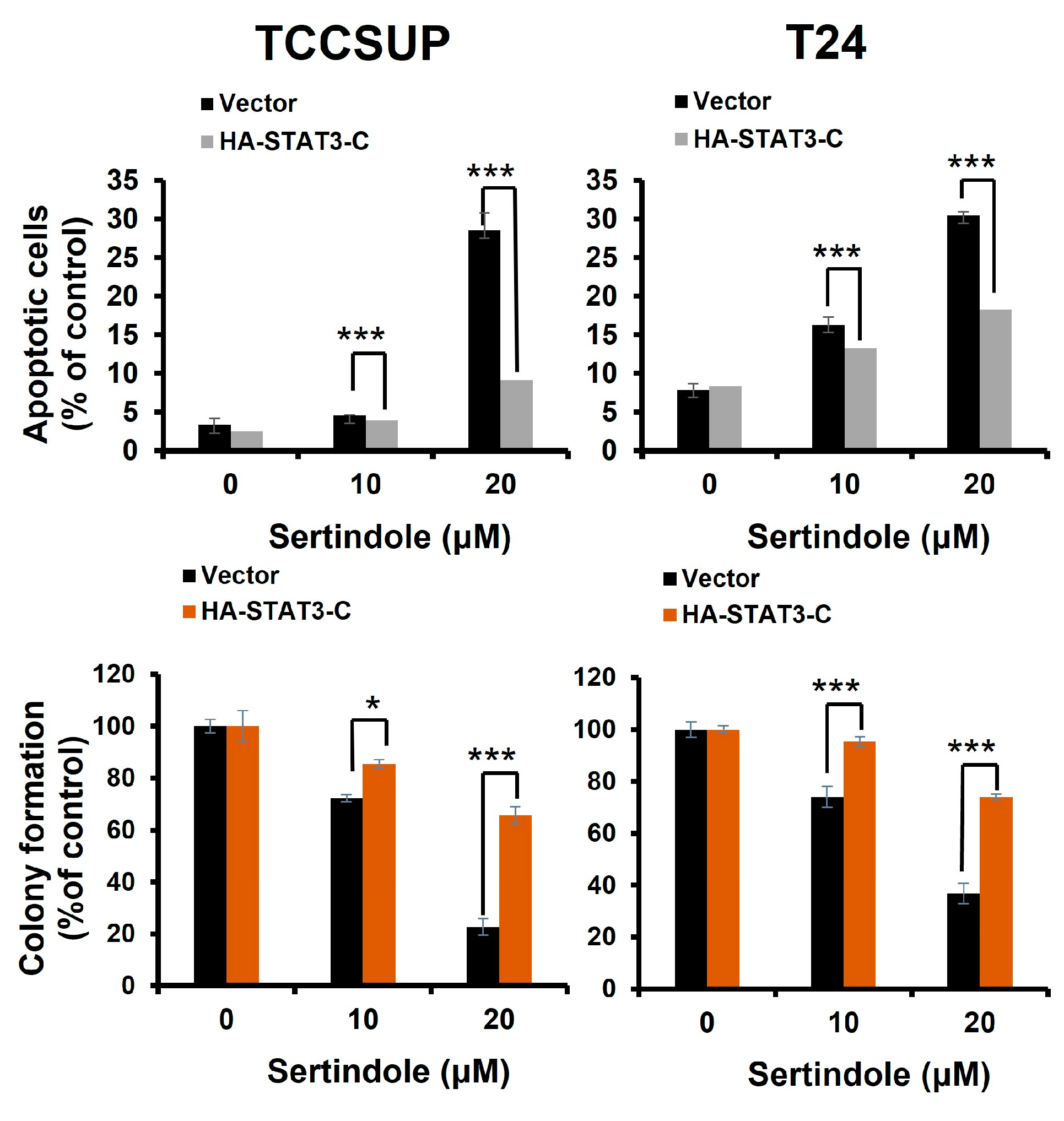
2.3. Suppression of STAT3 Activation Is Pivotal to Sertindole-Induced Human Bladder Cancer Apoptosis
2.4. BCL-xL Downregulation Is Responsible for Human Bladder Cell Apoptosis Resulting from Sertindole-Induced STAT3 Inhibition
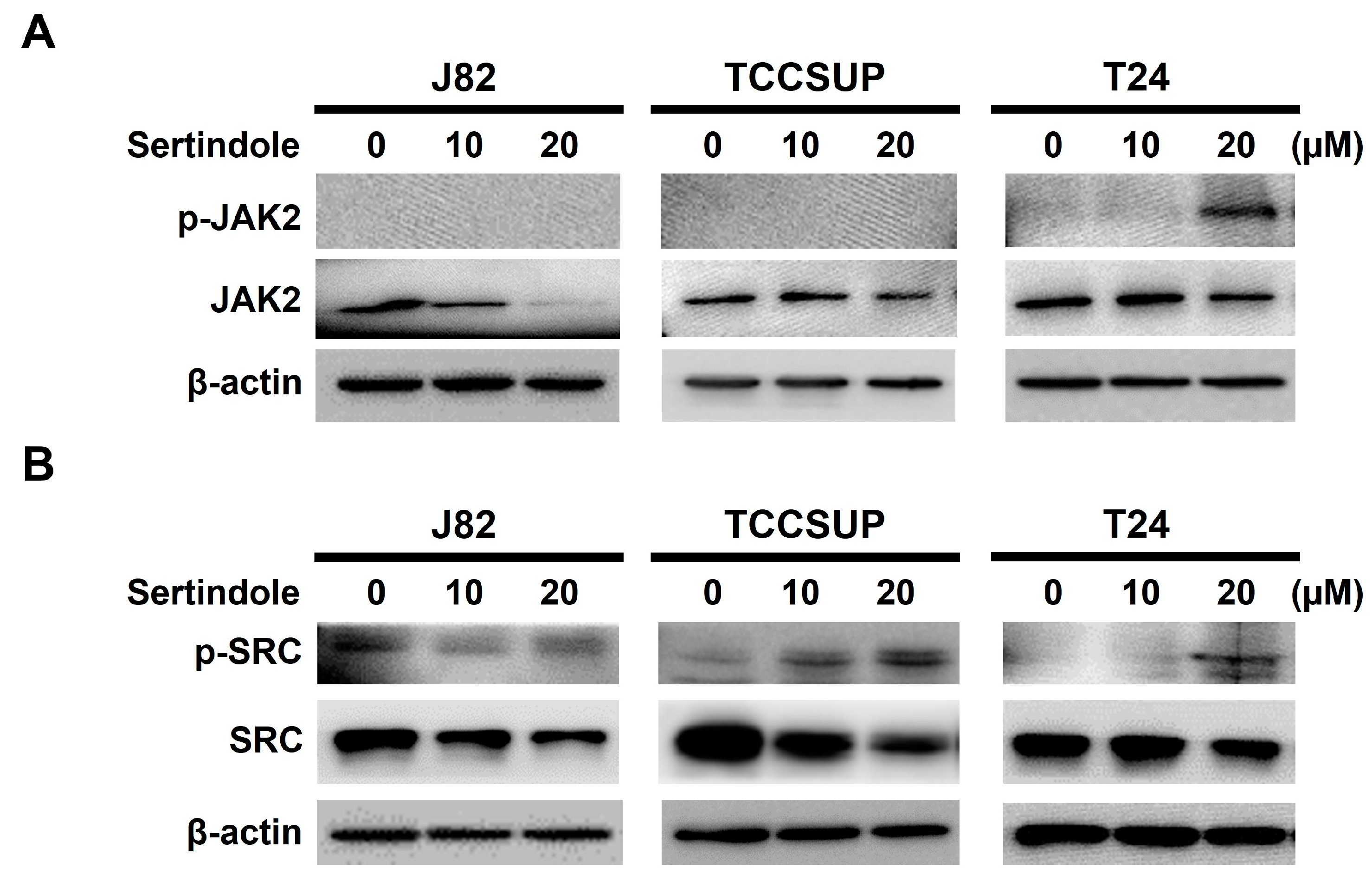
2.5. Neither JAK2 nor SRC Appears to Involve in Sertindole-Inhibited STAT3 Activation
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plasmids
4.3. Cell Culture
4.4. Cytotoxicity Assay
4.5. Immunoblotting
4.6. Apoptosis Assay
4.7. Establishment of HA-STAT3-C and HA-BCL-xL Stable Clones in TCCSUP and T24 Cells
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dobruch, J.; Oszczudłowski, M. Bladder cancer: Current challenges and future directions. Medicina 2021, 57, 749. [Google Scholar] [CrossRef]
- Crocetto, F.; Buonerba, C.; Caputo, V.; Ferro, M.; Persico, F.; Trama, F.; Iliano, E.; Rapisarda, S.; Bada, M.; Facchini, G.; et al. Urologic malignancies: Advances in the analysis and interpretation of clinical findings. Future Sci. OA 2021, 7, FSO674. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Fung, F.D.H.; Leung, C.; Cheung, W.W.L.; Goggins, W.B.; Ng, C.F. The global epidemiology of bladder cancer: A joinpoint regression analysis of its incidence and mortality trends and projection. Sci. Rep. 2018, 8, 1129. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, M.G.; Rota, M.; Catto, J.W.; La Vecchia, C. The role of tobacco smoke in bladder and kidney carcinogenesis: A comparison of exposures and meta-analysis of incidence and mortality risks. Eur. Urol. 2016, 70, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jee, S.H.; Shin, H.R.; Park, E.H.; Shin, A.; Jung, K.W.; Hwang, S.S.; Cha, E.S.; Yun, Y.H.; Park, S.K.; et al. Attributable fraction of tobacco smoking on cancer using population-based nationwide cancer incidence and mortality data in Korea. BMC Cancer 2014, 14, 406. [Google Scholar] [CrossRef]
- Burger, M.; Catto, J.W.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef]
- Aveta, A.; Cacciapuoti, C.; Barone, B.; Di Zazzo, E.; Del Giudice, F.; Maggi, M.; Ferro, M.; Terracciano, D.; Busetto, G.M.; Lucarelli, G.; et al. The impact of meat intake on bladder cancer incidence: Is it really a relevant risk? Cancers 2022, 14, 4775. [Google Scholar] [CrossRef]
- Ferro, M.; Chiujdea, S.; Musi, G.; Lucarelli, G.; Del Giudice, F.; Hurle, R.; Damiano, R.; Cantiello, F.; Mari, A.; Minervini, A.; et al. Impact of age on outcomes of patients with pure carcinoma in situ of the bladder: Multi-institutional cohort analysis. Clin. Genitourin. Cancer 2022, 20, e166–e172. [Google Scholar] [CrossRef]
- Piyathilake, C. Dietary factors associated with bladder cancer. Investig. Clin. Urol. 2016, 57 (Suppl. 1), S14–S25. [Google Scholar] [CrossRef]
- Czene, K.; Lichtenstein, P.; Hemminki, K. Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish Family-Cancer Database. Int. J. Cancer 2002, 99, 260–266. [Google Scholar] [CrossRef]
- Charlton, M.E.; Adamo, M.P.; Sun, L.; Deorah, S. Bladder cancer collaborative stage variables and their data quality, usage, and clinical implications: A review of SEER data, 2004–2010. Cancer 2014, 120 (Suppl. 23), 3815–3825. [Google Scholar] [CrossRef]
- Babjuk, M.; Oosterlinck, W.; Sylvester, R.; Kaasinen, E.; Böhle, A.; Palou-Redorta, J.; Rouprêt, M.; European Association of Urology (EAU). EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur. Urol. 2011, 59, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in cancer immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Man, Q.W.; Huo, F.Y.; Gao, X.; Lin, H.; Li, S.R.; Wang, J.; Su, F.C.; Cai, L.; Shi, Y.; et al. STAT3 pathway in cancers: Past, present, and future. MedComm 2022, 3, e124. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Guan, X.; Qin, J.J. Inhibiting STAT3 signaling pathway by natural products for cancer prevention and therapy: In vitro and in vivo activity and mechanisms of action. Pharmacol. Res. 2022, 182, 106357. [Google Scholar] [CrossRef]
- Turkson, J.; Ryan, D.; Kim, J.S.; Zhang, Y.; Chen, Z.; Haura, E.; Laudano, A.; Sebti, S.; Hamilton, A.D.; Jove, R. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J. Biol. Chem. 2001, 276, 45443–45455. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Bromberg, J.; Darnell, J.E., Jr. The role of STATs in transcriptional control and their impact on cellular function. Oncogene 2000, 19, 2468–2473. [Google Scholar] [CrossRef]
- Kim, B.H.; Yi, E.H.; Ye, S.K. Signal transducer and activator of transcription 3 as a therapeutic target for cancer and the tumor microenvironment. Arch. Pharm. Res. 2016, 39, 1085–1099. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Mahabady, M.K.; Nabavi, N.; Zabolian, A.; Banihashemi, S.M.; Haddadi, A.; Entezari, M.; Hushmandi, K.; Makvandi, P.; et al. Pre-clinical investigation of STAT3 pathway in bladder cancer: Paving the way for clinical translation. Biomed. Pharmacother. 2021, 133, 111077. [Google Scholar] [CrossRef]
- Murray, P.J. The JAK-STAT signaling pathway: Input and output integration. J. Immunol. 2007, 178, 2623–2629. [Google Scholar] [CrossRef] [PubMed]
- Korac-Prlic, J.; Degoricija, M.; Vilović, K.; Haupt, B.; Ivanišević, T.; Franković, L.; Grivennikov, S.; Terzić, J. Targeting Stat3 signaling impairs the progression of bladder cancer in a mouse model. Cancer Lett. 2020, 490, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Grinshpoon, A.; Barchana, M.; Ponizovsky, A.; Lipshitz, I.; Nahon, D.; Tal, O.; Weizman, A.; Levav, I. Cancer in schizophrenia: Is the risk higher or lower? Schizophr. Res. 2005, 73, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Tabarés-Seisdedos, R.; Dumont, N.; Baudot, A.; Valderas, J.M.; Climent, J.; Valencia, A.; Crespo-Facorro, B.; Vieta, E.; Gómez-Beneyto, M.; Martínez, S.; et al. No paradox, no progress: Inverse cancer comorbidity in people with other complex diseases. Lancet Oncol. 2011, 12, 604–608. [Google Scholar] [CrossRef]
- Tabarés-Seisdedos, R.; Rubenstein, J.L. Inverse cancer comorbidity: A serendipitous opportunity to gain insight into CNS disorders. Nat. Rev. Neurosci. 2013, 14, 293–304. [Google Scholar] [CrossRef]
- Moore, N.; Hall, G.; Sturkenboom, M.; Mann, R.; Lagnaoui, R.; Begaud, B. Biases affecting the proportional reporting ratio (PPR) in spontaneous reports pharmacovigilance databases: The example of sertindole. Pharmacoepidemiol. Drug Saf. 2003, 12, 271–281. [Google Scholar] [CrossRef]
- Spina, E.; Zoccali, R. Sertindole: Pharmacological and clinical profile and role in the treatment of schizophrenia. Expert Opin. Drug Metab. Toxicol. 2008, 4, 629–638. [Google Scholar] [CrossRef]
- Kasper, S.; Möller, H.J.; Hale, A. The European post-marketing observational sertindole study: An investigation of the safety of antipsychotic drug treatment. Eur. Arch. Psychiatry Clin. Neurosci. 2010, 260, 59–68. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, S.J.; Kim, E.S.; Jo, Y.K.; Hong, J.; Cho, D.H. Sertindole, a potent antagonist at dopamine D₂ receptors, induces autophagy by increasing reactive oxygen species in SH-SY5Y neuroblastoma cells. Biol. Pharm. Bull. 2012, 35, 1069–1075. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, C.; Liu, F.; Mao, Y.; Xu, W.; Fan, T.; Sun, Q.; He, S.; Chen, Y.; Guo, W.; et al. Antiproliferative activities of the second-generation antipsychotic drug sertindole against breast cancers with a potential application for treatment of breast-to-brain metastases. Sci. Rep. 2018, 8, 15753. [Google Scholar] [CrossRef]
- Dai, C.; Liu, P.; Wang, X.; Yin, Y.; Jin, W.; Shen, L.; Chen, Y.; Chen, Z.; Wang, Y. The antipsychotic agent sertindole exhibited antiproliferative activities by inhibiting the stat3 signaling pathway in human gastric cancer cells. J. Cancer 2020, 11, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.; Desnoyers, S.; Ottaviano, Y.; Davidson, N.E.; Poirier, G.G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: An early marker of chemotherapy-induced apoptosis. Cancer Res. 1993, 53, 3976–3985. [Google Scholar] [PubMed]
- Bromberg, J.F.; Wrzeszczynska, M.H.; Devgan, G.; Zhao, Y.; Pestell, R.G.; Albanese, C.; Darnell, J.E., Jr. Stat3 as an oncogene. Cell 1999, 98, 295–303. [Google Scholar] [CrossRef]
- Catlett-Falcone, R.; Landowski, T.H.; Oshiro, M.M.; Turkson, J.; Levitzki, A.; Savino, R.; Ciliberto, G.; Moscinski, L.; Fernández-Luna, J.L.; Nuñez, G.; et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 1999, 10, 105–115. [Google Scholar] [CrossRef]
- Dong, J.; Cheng, X.D.; Zhang, W.D.; Qin, J.J. Recent update on development of small-molecule STAT3 inhibitors for cancer therapy: From phosphorylation inhibition to protein degradation. J. Med. Chem. 2021, 64, 8884–8915. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef]
- Hua, Y.; Dai, X.; Xu, Y.; Xing, G.; Liu, H.; Lu, T.; Chen, Y.; Zhang, Y. Drug repositioning: Progress and challenges in drug discovery for various diseases. Eur. J. Med. Chem. 2022, 234, 114239. [Google Scholar] [CrossRef]
- Vlachos, N.; Lampros, M.; Voulgaris, S.; Alexiou, G.A. Repurposing antipsychotics for cancer treatment. Biomedicines 2021, 9, 1785. [Google Scholar] [CrossRef]
- Matteoni, S.; Matarrese, P.; Ascione, B.; Buccarelli, M.; Ricci-Vitiani, L.; Pallini, R.; Villani, V.; Pace, A.; Paggi, M.G.; Abbruzzese, C. Anticancer properties of the antipsychotic drug chlorpromazine and its synergism with temozolomide in restraining human glioblastoma proliferation in vitro. Front. Oncol. 2021, 11, 635472. [Google Scholar] [CrossRef]
- Dong, Y.; Furuta, T.; Sabit, H.; Kitabayashi, T.; Jiapaer, S.; Kobayashi, M.; Ino, Y.; Todo, T.; Teng, L.; Hirao, A.; et al. Identification of antipsychotic drug fluspirilene as a potential anti-glioma stem cell drug. Oncotarget 2017, 8, 111728–111741. [Google Scholar] [CrossRef]
- Papadopoulos, F.; Isihou, R.; Alexiou, G.A.; Tsalios, T.; Vartholomatos, E.; Markopoulos, G.S.; Sioka, C.; Tsekeris, P.; Kyritsis, A.P.; Galani, V. Haloperidol induced cell cycle arrest and apoptosis in glioblastoma cells. Biomedicines 2020, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Srivastava, S.K. Penfluridol suppresses glioblastoma tumor growth by Akt-mediated inhibition of GLI1. Oncotarget 2017, 8, 32960–32976. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Kaushik, I.; Srivastava, S.K. Pimozide suppresses the growth of brain tumors by targeting STAT3-mediated autophagy. Cells 2020, 9, 2141. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.; Saki, M.; Vlashi, E.; Cheng, F.; Duhachek-Muggy, S.; Alli, C.; Yu, G.; Medina, P.; He, L.; Damoiseaux, R.; et al. The dopamine receptor antagonist trifluoperazine prevents phenotype conversion and improves survival in mouse models of glioblastoma. Proc. Natl. Acad. Sci. USA 2020, 117, 11085–11096. [Google Scholar] [CrossRef]
- Nielsen, J.; Matz, J.; Mittoux, A.; Polcwiartek, C.; Struijk, J.J.; Toft, E.; Kanters, J.K.; Graff, C. Cardiac effects of sertindole and quetiapine: Analysis of ECGs from a randomized double-blind study in patients with schizophrenia. Eur. Neuropsychopharmacol. 2015, 25, 303–311. [Google Scholar] [CrossRef]
- Dou, Z.; Zhao, D.; Chen, X.; Xu, C.; Jin, X.; Zhang, X.; Wang, Y.; Xie, X.; Li, Q.; Di, C.; et al. Aberrant Bcl-x splicing in cancer: From molecular mechanism to therapeutic modulation. J. Exp. Clin. Cancer Res. 2021, 40, 194. [Google Scholar] [CrossRef]
- Yoshimine, S.; Kikuchi, E.; Kosaka, T.; Mikami, S.; Miyajima, A.; Okada, Y.; Oya, M. Prognostic significance of Bcl-xL expression and efficacy of Bcl-xL targeting therapy in urothelial carcinoma. Br. J. Cancer 2013, 108, 2312–2320. [Google Scholar] [CrossRef]
- Kunze, D.; Wuttig, D.; Fuessel, S.; Kraemer, K.; Kotzsch, M.; Meye, A.; Grimm, M.O.; Hakenberg, O.W.; Wirth, M.P. Multitarget siRNA inhibition of antiapoptotic genes (XIAP, BCL2, BCL-X(L)) in bladder cancer cells. Anticancer Res. 2008, 28, 2259–2263. [Google Scholar]
- Rieger, C.; Huebner, D.; Temme, A.; Wirth, M.P.; Fuessel, S. Antisense- and siRNA-mediated inhibition of the anti-apoptotic gene Bcl-xL for chemosensitization of bladder cancer cells. Int. J. Oncol. 2015, 47, 1121–1130. [Google Scholar] [CrossRef]
- Kim, M.; Morales, L.D.; Jang, I.S.; Cho, Y.Y.; Kim, D.J. Protein tyrosine phosphatases as potential regulators of STAT3 signaling. Int. J. Mol. Sci. 2018, 19, 2708. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.C.; Teng, H.W.; Shiau, C.W.; Tai, W.T.; Hung, M.H.; Yang, S.H.; Jiang, J.K.; Chen, K.F. Pharmacological targeting SHP-1-STAT3 signaling is a promising therapeutic approach for the treatment of colorectal cancer. Neoplasia 2015, 17, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Song, H.; Yoon, Y.J.; Park, S.J.; Kim, S.Y.; Han, D.C.; Kwon, B.M. Ethacrynic acid inhibits STAT3 activity through the modulation of SHP2 and PTP1B tyrosine phosphatases in DU145 prostate carcinoma cells. Biochem. Pharmacol. 2020, 175, 113920. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.Y.; Yang, W.T.; Ho, Y.J.; Chang, G.M.; Chang, H.H.; Hsu, C.Y.; Chang, C.C.; Chen, Y.H. Hispolon methyl ether, a hispolon analog, suppresses the SRC/STAT3/Survivin signaling axis to induce cytotoxicity in human urinary bladder transitional carcinoma cell lines. Int. J. Mol. Sci. 2023, 24, 138. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.P.; Li, S.; Chuang, W.L.; Li, C.H.; Chen, G.J.; Chang, C.C.; Or, C.R.; Lin, P.Y.; Chang, C.C. Blockade of STAT3 signaling contributes to anticancer effect of 5-acetyloxy-6,7,8,4′-tetra-methoxyflavone, a tangeretin derivative, on human glioblastoma multiforme cells. Int. J. Mol. Sci. 2019, 20, 3366. [Google Scholar] [CrossRef]
- Or, C.R.; Huang, C.W.; Chang, C.C.; Lai, Y.C.; Chen, Y.J.; Chang, C.C. Obatoclax, a pan-BCL-2 inhibitor, downregulates survivin to induce apoptosis in human colorectal carcinoma cells via suppressing WNT/β-catenin signaling. Int. J. Mol. Sci. 2020, 21, 1773. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-Y.; Yang, W.-T.; Lin, J.-H.; Lu, C.-H.; Hu, K.-C.; Lan, T.-H.; Chang, C.-C. Sertindole, an Antipsychotic Drug, Curbs the STAT3/BCL-xL Axis to Elicit Human Bladder Cancer Cell Apoptosis In Vitro. Int. J. Mol. Sci. 2023, 24, 11852. https://doi.org/10.3390/ijms241411852
Hsu C-Y, Yang W-T, Lin J-H, Lu C-H, Hu K-C, Lan T-H, Chang C-C. Sertindole, an Antipsychotic Drug, Curbs the STAT3/BCL-xL Axis to Elicit Human Bladder Cancer Cell Apoptosis In Vitro. International Journal of Molecular Sciences. 2023; 24(14):11852. https://doi.org/10.3390/ijms241411852
Chicago/Turabian StyleHsu, Chao-Yu, Wei-Ting Yang, Ju-Hwa Lin, Chien-Hsing Lu, Kai-Cheng Hu, Tsuo-Hung Lan, and Chia-Che Chang. 2023. "Sertindole, an Antipsychotic Drug, Curbs the STAT3/BCL-xL Axis to Elicit Human Bladder Cancer Cell Apoptosis In Vitro" International Journal of Molecular Sciences 24, no. 14: 11852. https://doi.org/10.3390/ijms241411852
APA StyleHsu, C.-Y., Yang, W.-T., Lin, J.-H., Lu, C.-H., Hu, K.-C., Lan, T.-H., & Chang, C.-C. (2023). Sertindole, an Antipsychotic Drug, Curbs the STAT3/BCL-xL Axis to Elicit Human Bladder Cancer Cell Apoptosis In Vitro. International Journal of Molecular Sciences, 24(14), 11852. https://doi.org/10.3390/ijms241411852






