Inducing Magnetic Properties with Ferrite Nanoparticles in Resins for Additive Manufacturing
Abstract
1. Introduction
2. Results and Discussion
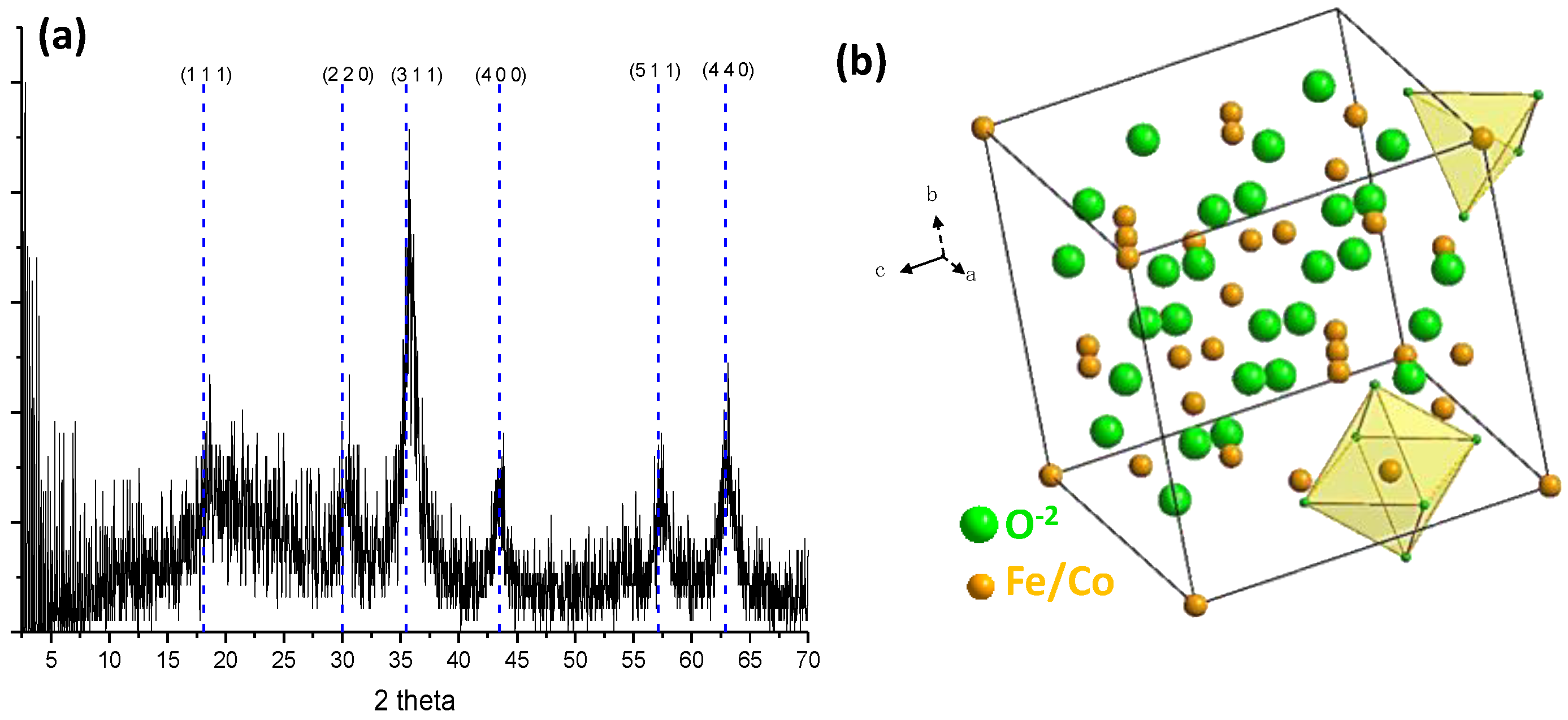
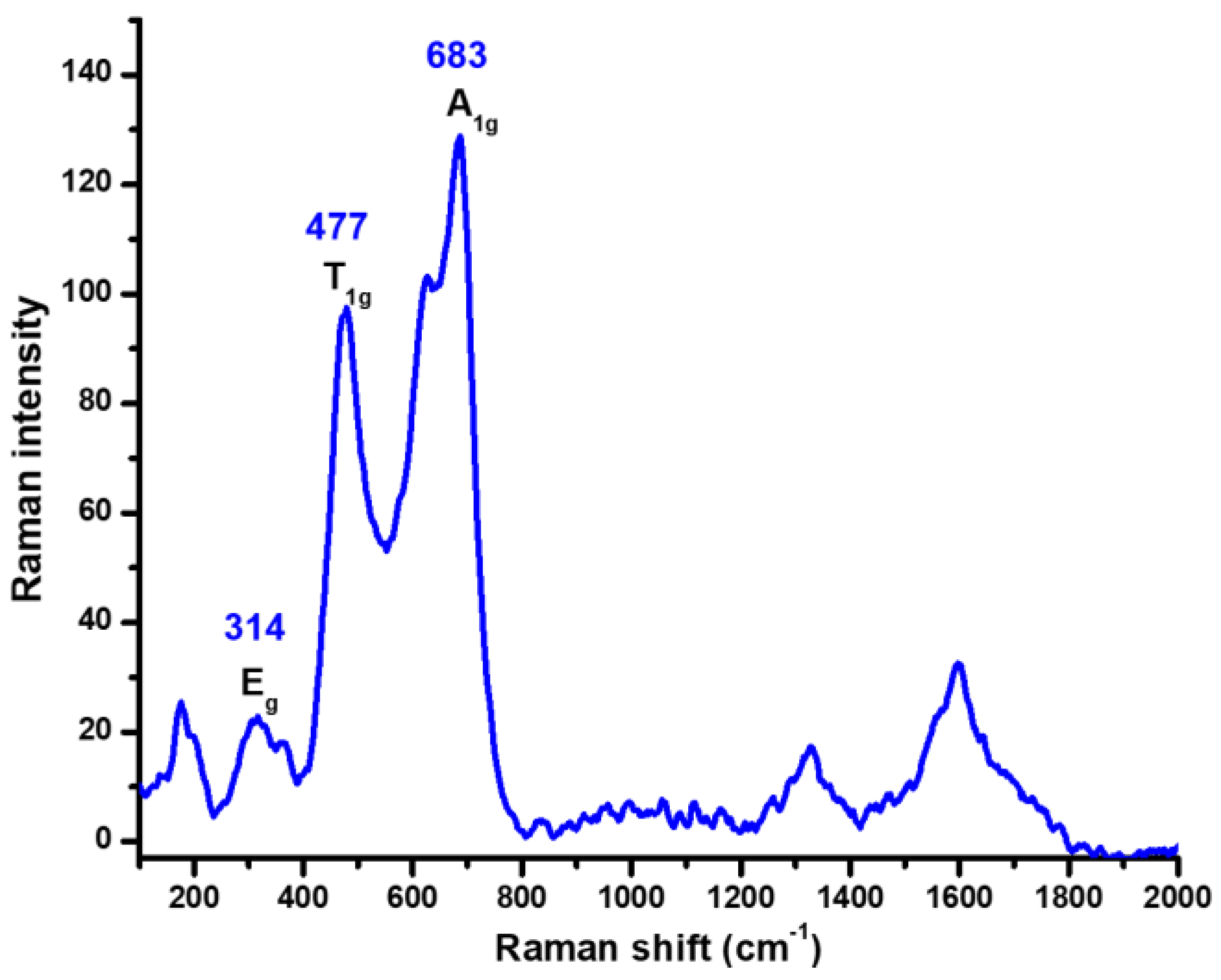
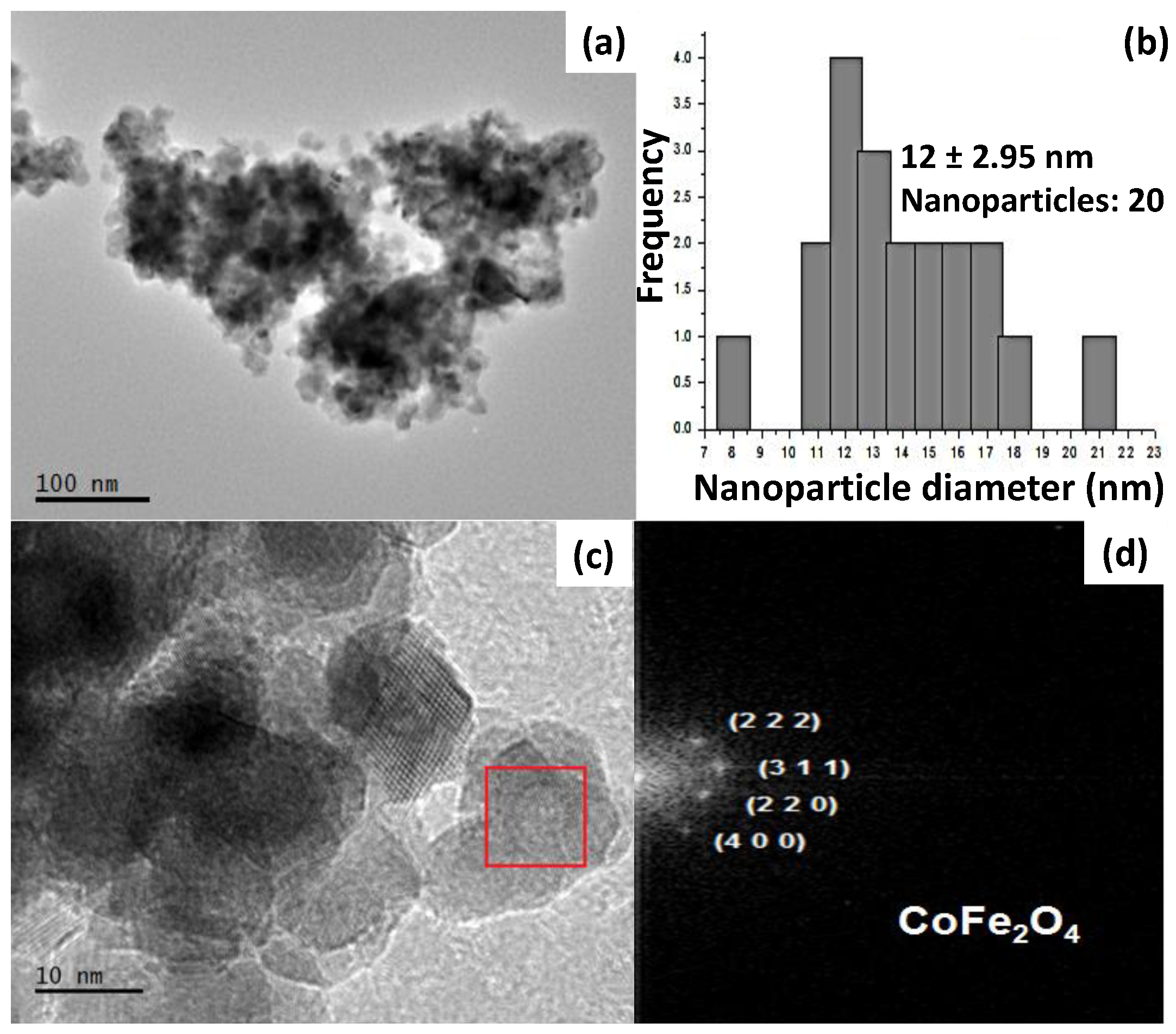
2.1. Characterization of CoFe2O4
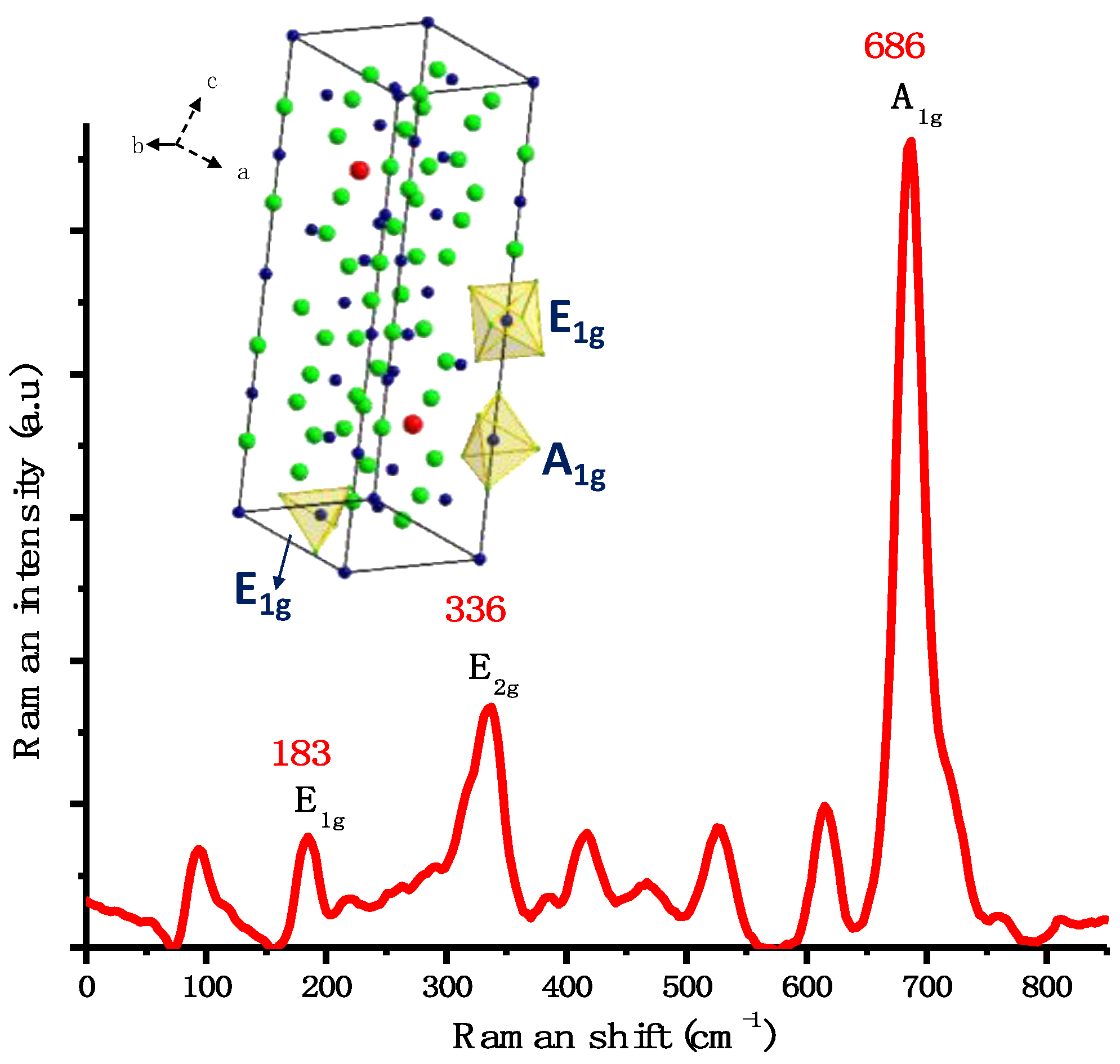
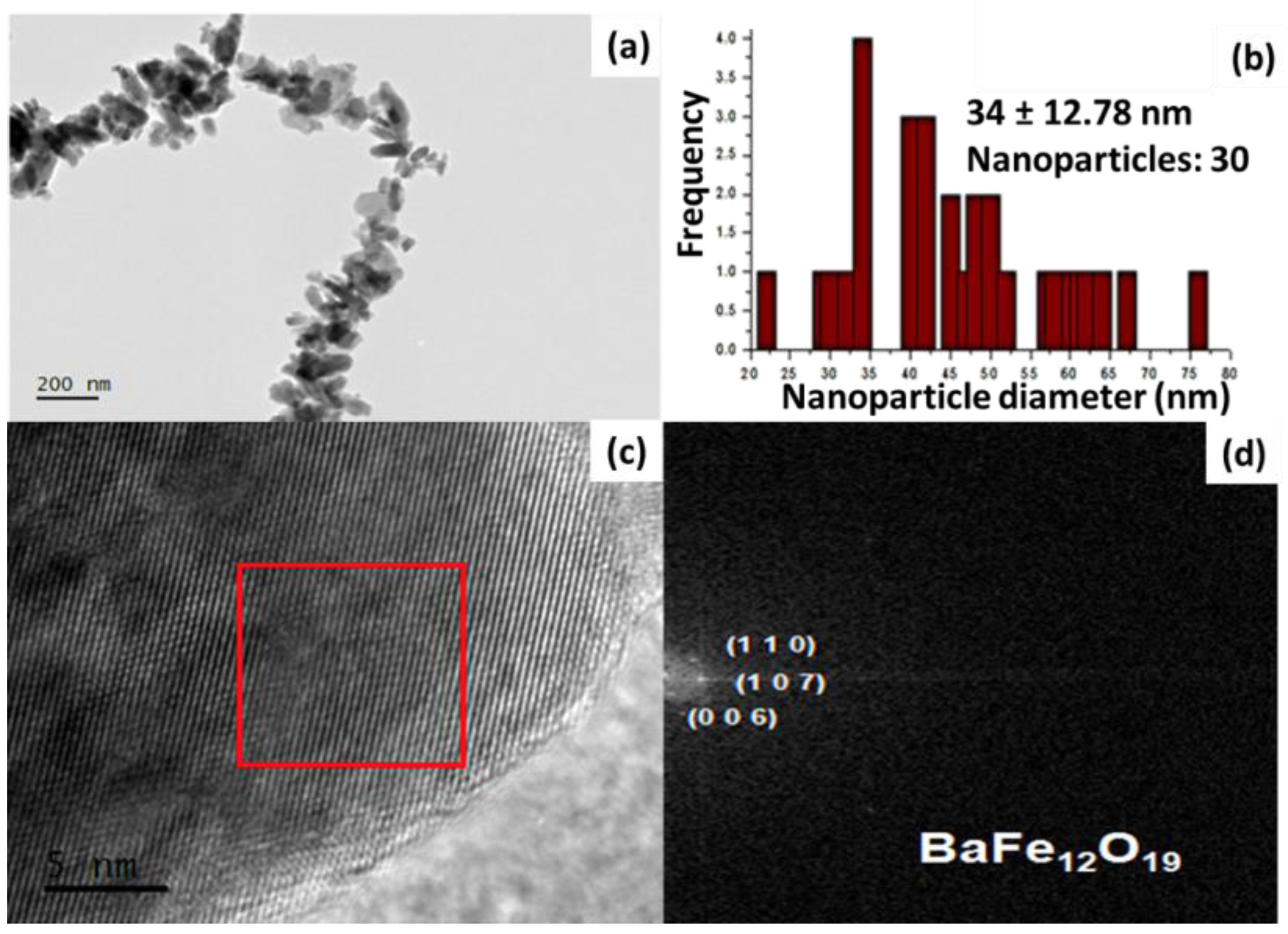
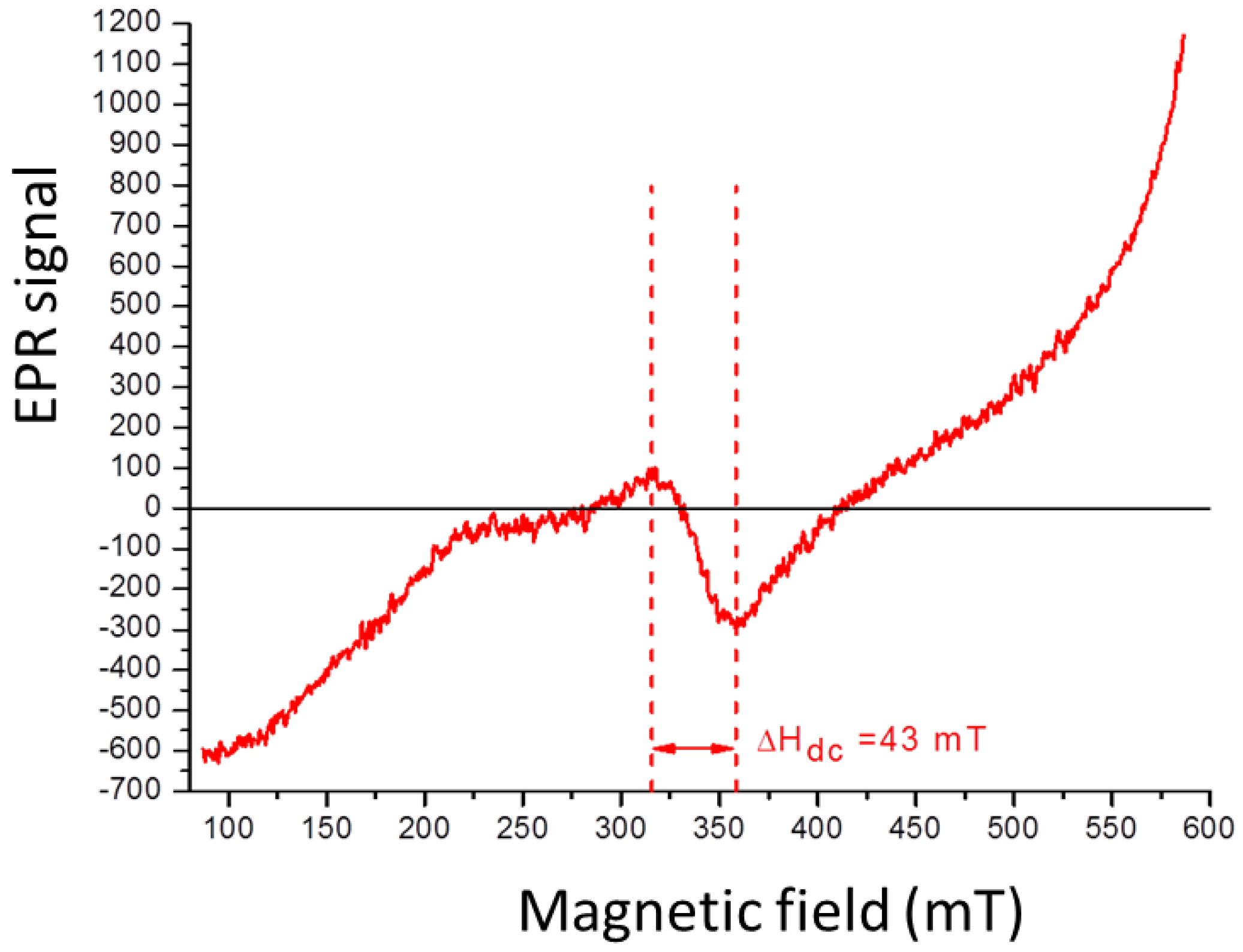
2.2. Characterization of BaFe12O19 NPs
2.3. Additive Manufacturing Process
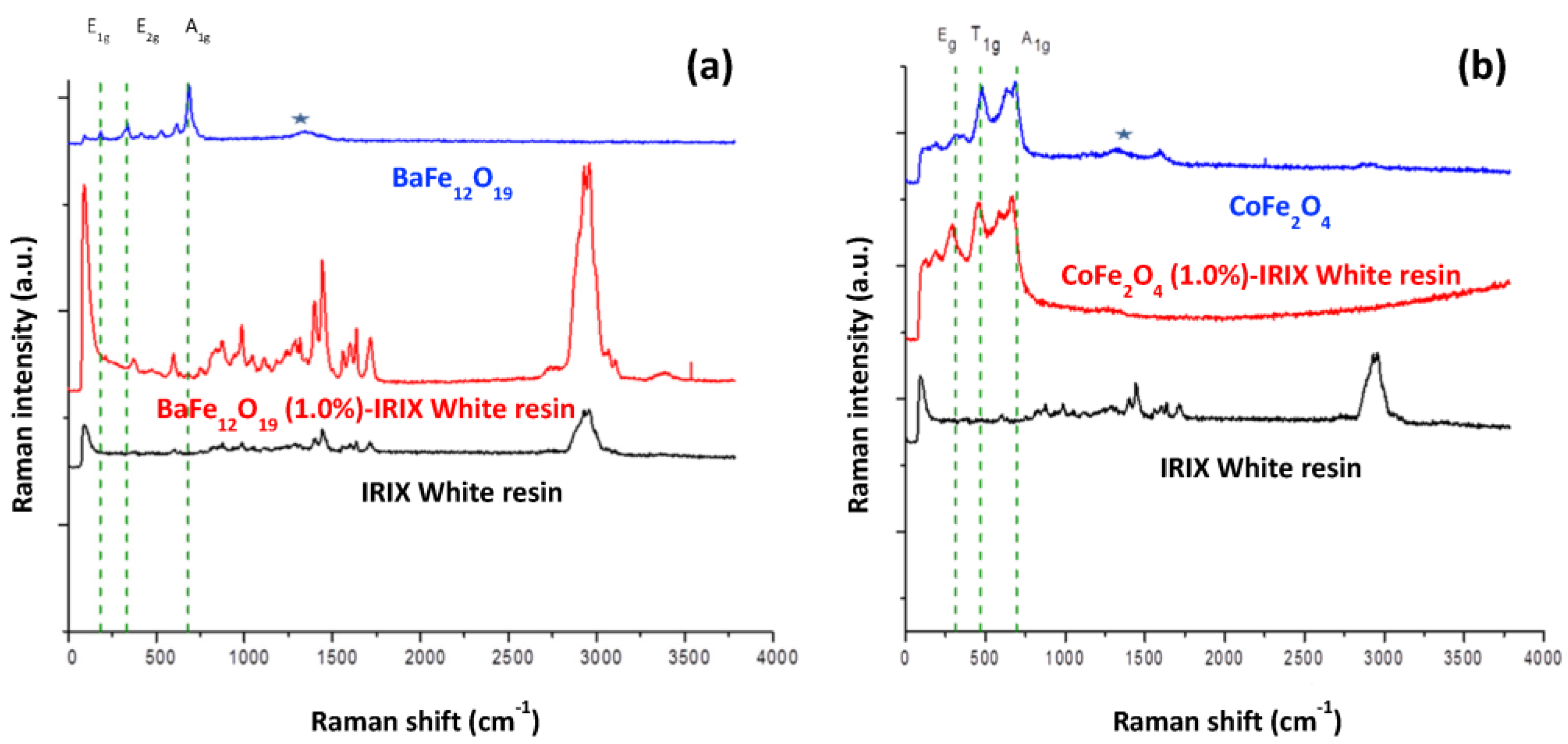
2.3.1. Ferrite–IRIX White Resin
2.3.2. Ferrite–Anycubic Green Resin
2.3.3. Characterization of Composite Nanostructured Samples
3. Materials and Methods
3.1. Experimental Procedures
3.1.1. Materials
3.1.2. Methods
3.1.3. Synthesis of BaFe12O19 NPs
3.1.4. Synthesis of CoFe2O4 NPs
3.1.5. Obtaining Nanostructured Materials
3.1.6. AM Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gharagozlou, M.; Ramezanzadeh, B.; Baradaran, Z. Synthesize and Characterization of a Novel Anticorrosive Cobalt Ferrite Nanoparticles Dispersed in Silica Matrix (CoFe2O4-SiO2) to Improve the Corrosion Protection Performance of Epoxy Coating. Appl. Surf. Sci. 2016, 377, 86–98. [Google Scholar] [CrossRef]
- Stoll, J.A.; Lachowicz, D.; Kmita, A.; Gajewska, M.; Sikora, M.; Berent, K.; Przybylski, M.; Russek, S.E.; Celinski, Z.J.; Hankiewicz, J.H. Synthesis of Manganese Zinc Ferrite Nanoparticles in Medical-Grade Silicone for MRI Applications. Int. J. Mol. Sci. 2023, 24, 5685. [Google Scholar] [CrossRef] [PubMed]
- Erdem, D.; Bingham, N.S.; Heiligtag, F.J.; Pilet, N.; Warnicke, P.; Heyderman, L.J.; Niederberger, M. CoFe2O4 and CoFe2O4-SiO2 Nanoparticle Thin Films with Perpendicular Magnetic Anisotropy for Magnetic and Magneto-Optical Applications. Adv. Funct. Mater. 2016, 26, 1954–1963. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Tishkevich, D.I.; Podgornaya, S.V.; Kaniukov, E.; Darwish, M.A.; Zubar, T.I.; Timofeev, A.V.; Trukhanova, E.L.; Kostishin, V.G.; Trukhanov, S.V. Impact of the Nanocarbon on Magnetic and Electrodynamic Properties of the Ferrite/Polymer Composites. Nanomaterials 2022, 12, 868. [Google Scholar] [CrossRef] [PubMed]
- Houbi, A.; Aldashevich, Z.A.; Atassi, Y.; Bagasharova Telmanovna, Z.; Saule, M.; Kubanych, K. Microwave Absorbing Properties of Ferrites and Their Composites: A Review. J. Magn. Magn. Mater. 2021, 529, 167839. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization Behavior of Poly(ε-Caprolactone)/Layered Double Hydroxide Nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Zhou, B.; Xu, W.; Syed, A.A.; Chau, Y.; Chen, L.; Chew, B.; Yassine, O.; Wu, X.; Gao, Y.; Zhang, J.; et al. Design and Fabrication of Magnetically Functionalized Flexible Micropillar Arrays for Rapid and Controllable Microfluidic Mixing. Lab. Chip 2015, 15, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Kesner, S.B.; Howe, R.D. Design Principles for Rapid Prototyping Forces Sensors Using 3-D Printing. IEEE/ASME Trans. Mechatron. 2011, 16, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Löwa, N.; Fabert, J.-M.; Gutkelch, D.; Paysen, H.; Kosch, O.; Wiekhorst, F. 3D-Printing of Novel Magnetic Composites Based on Magnetic Nanoparticles and Photopolymers. J. Magn. Magn. Mater. 2019, 469, 456–460. [Google Scholar] [CrossRef]
- Chaudhary, V.; Mantri, S.A.; Ramanujan, R.V.; Banerjee, R. Additive Manufacturing of Magnetic Materials. Prog. Mater. Sci. 2020, 114, 100688. [Google Scholar] [CrossRef]
- Peng, E.; Wei, X.; Herng, T.S.; Garbe, U.; Yu, D.; Ding, J. Ferrite-Based Soft and Hard Magnetic Structures by Extrusion Free-Forming. RSC Adv. 2017, 7, 27128–27138. [Google Scholar] [CrossRef]
- Fedorov, I.; Barhanko, D.; Hallberg, M.; Lindbaeck, M. Hot Turbine Guide Vane Performance Improvement with Metal Additive Manufacturing at Siemens Energy. In Volume 2C: Turbomachinery—Design Methods and CFD Modeling for Turbomachinery; Ducts, Noise, and Component Interactions; American Society of Mechanical Engineers: New York, NY, USA, 2021. [Google Scholar]
- SIEMENS Industrialize Additive Manufacturing. Available online: https://www.siemens.com/global/en/markets/machinebuilding/additivemanufacturing.html (accessed on 18 May 2023).
- GE Research Technology Domain: Additive Manufacturing. Available online: https://www.ge.com/research/technology-domains/additive-manufacturing (accessed on 18 May 2023).
- Stansbury, J.W.; Idacavage, M.J. 3D Printing with Polymers: Challenges among Expanding Options and Opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Flores Arias, Q.Y. Síntesis y Caracterización de Materiales Nanoestructurados de Ferrita; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2014. [Google Scholar]
- Ivanova, O.; Williams, C.; Campbell, T. Additive Manufacturing (AM) and Nanotechnology: Promises and Challenges. Rapid Prototyp. J. 2013, 19, 353–364. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Ramanujan, D.; Ramani, K.; Chen, Y.; Williams, C.B.; Wang, C.C.L.; Shin, Y.C.; Zhang, S.; Zavattieri, P.D. The Status, Challenges, and Future of Additive Manufacturing in Engineering. Comput.-Aided Des. 2015, 69, 65–89. [Google Scholar] [CrossRef]
- Chandramohan, P.; Srinivasan, M.P.; Velmurugan, S.; Narasimhan, S.V. Cation Distribution and Particle Size Effect on Raman Spectrum of CoFe2O4. J. Solid State Chem. 2011, 184, 89–96. [Google Scholar] [CrossRef]
- Morales-Morales, J.A. Síntesis de Nano Polvos de Hematita A-Fe2O3 Por El Método de Precipitación Controlada. Cienc. Desarro. 2017, 8. [Google Scholar] [CrossRef]
- Widodo, R.D.; Manaf, A. Physical Characteristics and Magnetic Properties of BaFe12O19/SrTiO3 Based Composites Derived from Mechanical Alloying. AIP Conf. Proc. 2016, 1725, 20098. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redón, R.; Aviles-Avila, M.D.; Ruiz-Huerta, L.; Montiel, H.; Elías-Zúñiga, A.; Daza-Gómez, L.-C.; Martínez-Romero, O. Inducing Magnetic Properties with Ferrite Nanoparticles in Resins for Additive Manufacturing. Int. J. Mol. Sci. 2023, 24, 11838. https://doi.org/10.3390/ijms241411838
Redón R, Aviles-Avila MD, Ruiz-Huerta L, Montiel H, Elías-Zúñiga A, Daza-Gómez L-C, Martínez-Romero O. Inducing Magnetic Properties with Ferrite Nanoparticles in Resins for Additive Manufacturing. International Journal of Molecular Sciences. 2023; 24(14):11838. https://doi.org/10.3390/ijms241411838
Chicago/Turabian StyleRedón, Rocío, Miriam D. Aviles-Avila, Leopoldo Ruiz-Huerta, Herlinda Montiel, Alex Elías-Zúñiga, Lucy-Caterine Daza-Gómez, and Oscar Martínez-Romero. 2023. "Inducing Magnetic Properties with Ferrite Nanoparticles in Resins for Additive Manufacturing" International Journal of Molecular Sciences 24, no. 14: 11838. https://doi.org/10.3390/ijms241411838
APA StyleRedón, R., Aviles-Avila, M. D., Ruiz-Huerta, L., Montiel, H., Elías-Zúñiga, A., Daza-Gómez, L.-C., & Martínez-Romero, O. (2023). Inducing Magnetic Properties with Ferrite Nanoparticles in Resins for Additive Manufacturing. International Journal of Molecular Sciences, 24(14), 11838. https://doi.org/10.3390/ijms241411838






