Decreased Levels of Soluble Developmental Endothelial Locus-1 Are Associated with Thrombotic Microangiopathy in Pregnancy
Abstract
1. Introduction
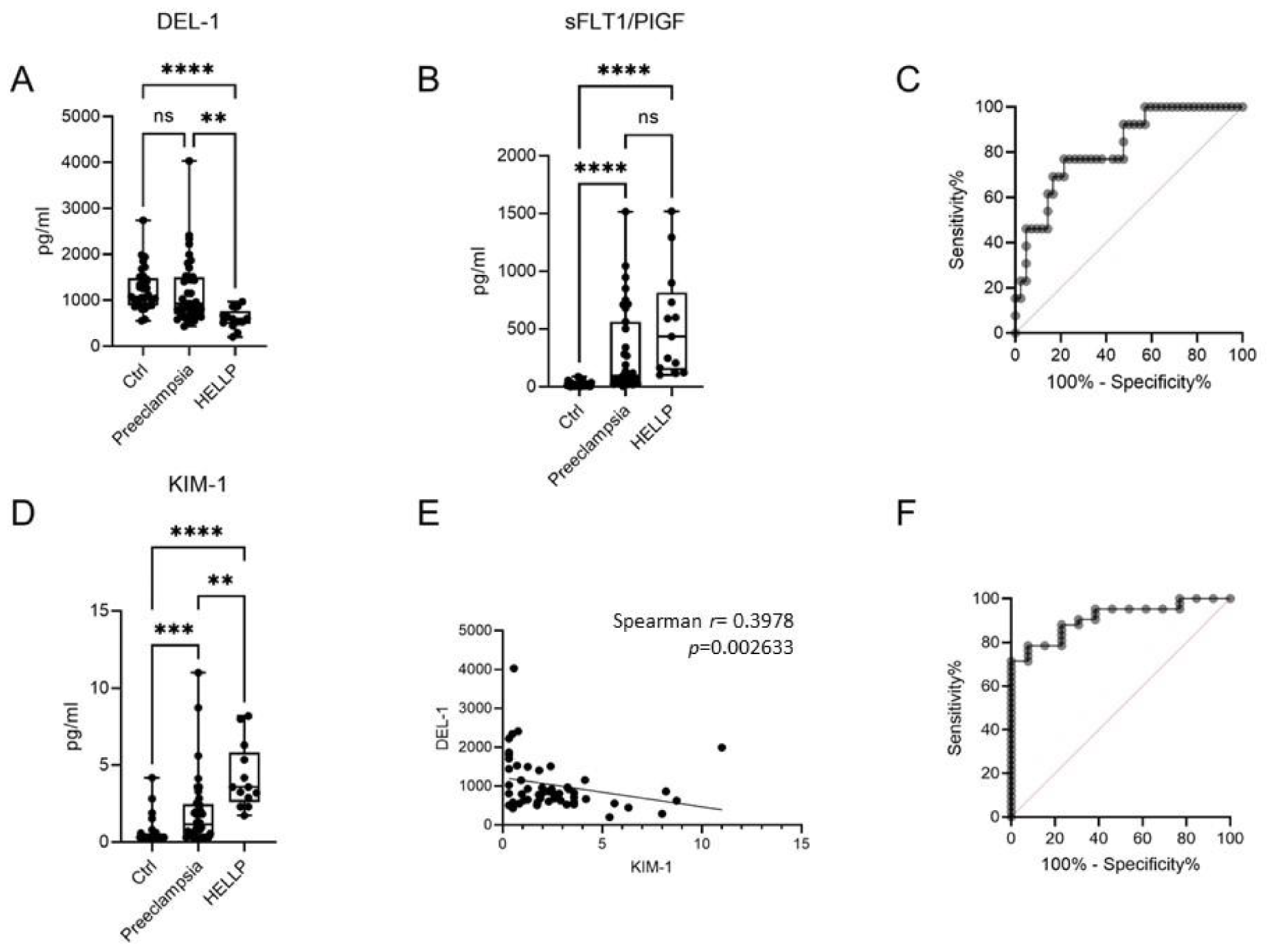
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Immunoassays
4.3. Immunohistochemical Staining
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haram, K.; Svendsen, E.; Abildgaard, U. The HELLP syndrome: Clinical issues and management. A Review. BMC Pregnancy Childbirth 2009, 9, 8. [Google Scholar] [CrossRef]
- Karumanchi, S.A.; Maynard, S.E.; Stillman, I.E.; Epstein, F.H.; Sukhatme, V.P. Preeclampsia: A renal perspective. Kidney Int. 2005, 67, 2101–2113. [Google Scholar] [CrossRef]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.-H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef]
- Van Lieshout, L.C.E.W.; Koek, G.H.; Spaanderman, M.A.; van Runnard Heimel, P.J. Placenta derived factors involved in the pathogenesis of the liver in the syndrome of haemolysis, elevated liver enzymes and low platelets (HELLP): A review. Pregnancy Hypertens. 2019, 18, 42–48. [Google Scholar] [CrossRef]
- Lokki, A.I.; Heikkinen-Eloranta, J.; Jarva, H.; Saisto, T.; Lokki, M.-L.; Laivuori, H.; Meri, S. Complement Activation and Regulation in Preeclamptic Placenta. Front. Immunol. 2014, 5, 312. [Google Scholar] [CrossRef]
- Lokki, A.I.; Heikkinen-Eloranta, J. Pregnancy induced TMA in severe preeclampsia results from complement-mediated throboinflammation. Hum. Immunol. 2021, 82, 371–378. [Google Scholar] [CrossRef]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicola, M. Kidney Injury Molecule-1 (KIM-1), a Putative Epithelial Cell Adhesion Molecule Containing a Novel Immunoglobulin Domain, Is Up-regulated in Renal Cells after Injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef]
- Vaidya, V.S.; Ozer, J.S.; Dieterle, F.; Collings, F.B.; Ramirez, V.; Troth, S.; Muniappa, N.; Thudium, D.; Gerhold, D.; Holder, D.J.; et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat. Biotechnol. 2010, 28, 478–485. [Google Scholar] [CrossRef]
- Sabbisetti, V.S.; Waikar, S.S.; Antoine, D.J.; Smiles, A.; Wang, C.; Ravisankar, A.; Ito, K.; Sharma, S.; Ramadesikan, S.; Lee, M.; et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J. Am. Soc. Nephrol. 2014, 25, 2177–2186. [Google Scholar] [CrossRef]
- Choi, E.Y.; Chavakis, E.; Czabanka, M.A.; Langer, H.F.; Fraemohs, L.; Economopoulou, M.; Kundu, R.K.; Orlandi, A.; Zheng, Y.Y.; Prieto, D.A.; et al. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science 2008, 322, 1101–1104. [Google Scholar] [CrossRef]
- Kourtzelis, I.; Li, X.; Mitroulis, I.; Grosser, D.; Kajikawa, T.; Wang, B.; Grzybek, M.; von Renesse, J.; Czogalla, A.; Troullinaki, M.; et al. DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nat. Immunol. 2019, 20, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Chavakis, T. DEL-1-Regulated Immune Plasticity and Inflammatory Disorders. Trends Mol. Med. 2019, 25, 444–459. [Google Scholar] [CrossRef]
- Roberts, J.M. Endothelial Dysfunction in Preeclampsia. Semin. Reprod. Med. 1998, 16, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.K.; Yemeni, E.A.; Blann, A.D.; Lip, G.Y.H. Thrombomodulin, von Willebrand factor and E-selectin as plasma markers of endothelial damage/dysfunction and activation in pregnancy induced hypertension. Thromb. Res. 2004, 113, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Hulstein, J.J.J.; Heimel, P.J.V.R.; Franx, A.; Lenting, P.J.; Bruinse, H.W.; Silence, K.; DE Groot, P.G.; Fijnheer, R. Acute activation of the endothelium results in increased levels of active von Willebrand factor in hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome. J. Thromb. Haemost. 2006, 4, 2569–2575. [Google Scholar] [CrossRef]
- Hidai, C.; Zupancic, T.; Penta, K.; Mikhail, A.; Kawana, M.; Quertermous, E.E.; Aoka, Y.; Fukagawa, M.; Matsui, Y.; Platika, D.; et al. Cloning and characterization of developmental endothelial locus-1: An embryonic endothelial cell protein that binds the αvβ3 integrin receptor. Genes Dev. 1998, 12, 21–33. [Google Scholar] [CrossRef]
- Ho, H.-K.V.; Jang, J.J.; Kaji, S.; Spektor, G.; Fong, A.; Yang, P.; Hu, B.S.; Schatzman, R.; Quertermous, T.; Cooke, J.P. Developmental endothelial locus-1 (Del-1), a novel angiogenic protein: Its role in ischemia. Circulation 2004, 109, 1314–1319. [Google Scholar] [CrossRef]
- Eskan, M.A.; Jotwani, R.; Abe, T.; Chmelar, J.; Lim, J.-H.; Liang, S.; Ciero, P.A.; Krauss, J.L.; Li, F.; Rauner, M.; et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat. Immunol. 2012, 13, 465–473. [Google Scholar] [CrossRef]
- Kim, W.-Y.; Lee, S.-H.; Kim, D.-Y.; Ryu, H.J.; Chon, G.R.; Park, Y.Y.; Fu, Y.; Huh, J.W.; Lim, C.-M.; Koh, Y.; et al. Serum developmental endothelial locus-1 is associated with severity of sepsis in animals and humans. Sci. Rep. 2019, 9, 13005. [Google Scholar] [CrossRef]
- Hyun, Y.-M.; Seo, S.-U.; Choi, W.S.; Kwon, H.-J.; Kim, D.-Y.; Jeong, S.; Kang, G.-Y.; Yi, E.; Kim, M.; Ryu, H.J.; et al. Endogenous DEL-1 restrains melanoma lung metastasis by limiting myeloid cell-associated lung inflammation. Sci. Adv. 2020, 6, eabc4882. [Google Scholar] [CrossRef]
- Mitroulis, I.; Chen, L.-S.; Singh, R.P.; Kourtzelis, I.; Economopoulou, M.; Kajikawa, T.; Troullinaki, M.; Ziogas, A.; Ruppova, K.; Hosur, K.; et al. Secreted protein Del-1 regulates myelopoiesis in the hematopoietic stem cell niche. J. Clin. Investig. 2017, 127, 3624–3639. [Google Scholar] [CrossRef]
- Mitroulis, I.; Kang, Y.-Y.; Gahmberg, C.G.; Siegert, G.; Hajishengallis, G.; Chavakis, T.; Choi, E.-Y. Developmental endothelial locus-1 attenuates complement-dependent phagocytosis through inhibition of Mac-1-integrin. Thromb. Haemost. 2014, 111, 1004–1006. [Google Scholar] [CrossRef]
- Von Ameln, A.K.; Cremer, S.; Hoffmann, J.; Schuster, P.; Khedr, S.; Korovina, I.; Troullinaki, M.; Neuwirth, A.; Sprott, D.; Chatzigeorgiou, A.; et al. Endogenous developmental endothelial locus-1 limits ischaemia-related angiogenesis by blocking inflammation. Thromb. Haemost. 2017, 117, 1150–1163. [Google Scholar] [CrossRef]
- Failer, T.; Amponsah-Offeh, M.; Neuwirth, A.; Kourtzelis, I.; Subramanian, P.; Mirtschink, P.; Peitzsch, M.; Matschke, K.; Tugtekin, S.M.; Kajikawa, T.; et al. Developmental endothelial locus-1 protects from hypertension-induced cardiovascular remodeling via immunomodulation. J. Clin. Investig. 2022, 132, e126155. [Google Scholar] [CrossRef]
- Tranquilli, A.; Dekker, G.; Magee, L.; Roberts, J.; Sibai, B.; Steyn, W.; Zeeman, G.; Brown, M. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2014, 4, 97–104. [Google Scholar] [CrossRef]

| Preeclampsia n = 44 | HELLP n = 13 | Controls n = 35 | p Value | |
|---|---|---|---|---|
| Age (years), mean (±SD), | 33.2 ± 2.8 | 34.6 ± 5.2 | 29.9 ± 4.3 | 0.13 |
| Systolic BP,mean (±SD), mm Hg | 153.04 ± 7.6 | 175.3 ± 9.9 | 128.3 ± 7.1 | 0.001 |
| Diastolic BP,mean (±SD), mm Hg | 88.6 ± 3.4 | 96.7 ± 8.05 | 74.7 ± 7.5 | 0.001 |
| Gestational age (weeks) | 34.07 ± 1.8 | 32.6 ± 3.8 | 38.09 ± 2.7 | 0.05 |
| Laboratory findings | ||||
| WBC (±SD) K/μl | 13,242.6 ± 4915.3 | 11,963.0 ± 28,635.3 | 11,237.8 ± 1477.8 | 0.21 |
| Hb (±SD) g/dL | 11.1 ± 0.91 | 11.7 ± 1.18 | 12.2 ± 0.5 | 0.047 |
| Platelets (±SD) K/μl | 208.9 ± 24.6 | 92.3 ± 27.8 | 231.9 ± 26.9 | <0.0001 |
| MPV (±SD) | 11.12 ± 0.78 | 11.6 ± 1.04 | 8.8 ± 0.82 | <0.0001 |
| Glucose (±SD) mg/dL | 97.1 ± 12.05 | 107.1 ± 21.15 | 95.01 ± 16.3 | 0.4 |
| eGFR | 112.5 ± 12.3 | 110. ± 23.8 | 106.3 ± 26.9 | 0.75 |
| Creatinine mean (±SD) mg/dL | 0.68 ± 0.008 | 0.66 ± 0.013 | 0.56 ± 0.005 | 0.64 |
| Urea mean (±SD) mg/dL | 23.38 ± 6.7 | 28.7 ± 13.7 | 19.72 ± 4.04 | 0.087 |
| SGOT (±SD) U/L | 22.8 ± 8.1 | 240 ± 98.4 | 16.4 ± 2.3 | <0.0001 |
| SGPT (±SD) U/L | 18.8 ± 8.6 | 182.2 ± 121.4 | 14.3 ± 3.5 | <0.0001 |
| Uric acid (±SD) mg/dL | 6.13 ± 0.8 | 6.8 ± 1.9 | 4.6 ± 1.04 | <0.0001 |
| CRP (±SD) mg/dL | 1.5 ± 1.04 | 1.2 ± 1.4 | 0.67 ± 0.5 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanidou, G.; Konstantinidis, T.G.; Natsi, A.-M.; Kantartzi, K.; Panopoulou, M.; Kontomanolis, E.; Tsigalou, C.; Lambropoulou, M.; Gavriilaki, E.; Panagoutsos, S.; et al. Decreased Levels of Soluble Developmental Endothelial Locus-1 Are Associated with Thrombotic Microangiopathy in Pregnancy. Int. J. Mol. Sci. 2023, 24, 11762. https://doi.org/10.3390/ijms241411762
Romanidou G, Konstantinidis TG, Natsi A-M, Kantartzi K, Panopoulou M, Kontomanolis E, Tsigalou C, Lambropoulou M, Gavriilaki E, Panagoutsos S, et al. Decreased Levels of Soluble Developmental Endothelial Locus-1 Are Associated with Thrombotic Microangiopathy in Pregnancy. International Journal of Molecular Sciences. 2023; 24(14):11762. https://doi.org/10.3390/ijms241411762
Chicago/Turabian StyleRomanidou, Gioulia, Theocharis G. Konstantinidis, Anastasia-Maria Natsi, Konstantia Kantartzi, Maria Panopoulou, Emmanouil Kontomanolis, Christina Tsigalou, Maria Lambropoulou, Eleni Gavriilaki, Stylianos Panagoutsos, and et al. 2023. "Decreased Levels of Soluble Developmental Endothelial Locus-1 Are Associated with Thrombotic Microangiopathy in Pregnancy" International Journal of Molecular Sciences 24, no. 14: 11762. https://doi.org/10.3390/ijms241411762
APA StyleRomanidou, G., Konstantinidis, T. G., Natsi, A.-M., Kantartzi, K., Panopoulou, M., Kontomanolis, E., Tsigalou, C., Lambropoulou, M., Gavriilaki, E., Panagoutsos, S., Pasadakis, P., & Mitroulis, I. (2023). Decreased Levels of Soluble Developmental Endothelial Locus-1 Are Associated with Thrombotic Microangiopathy in Pregnancy. International Journal of Molecular Sciences, 24(14), 11762. https://doi.org/10.3390/ijms241411762










