Amoebiasis: Advances in Diagnosis, Treatment, Immunology Features and the Interaction with the Intestinal Ecosystem
Abstract
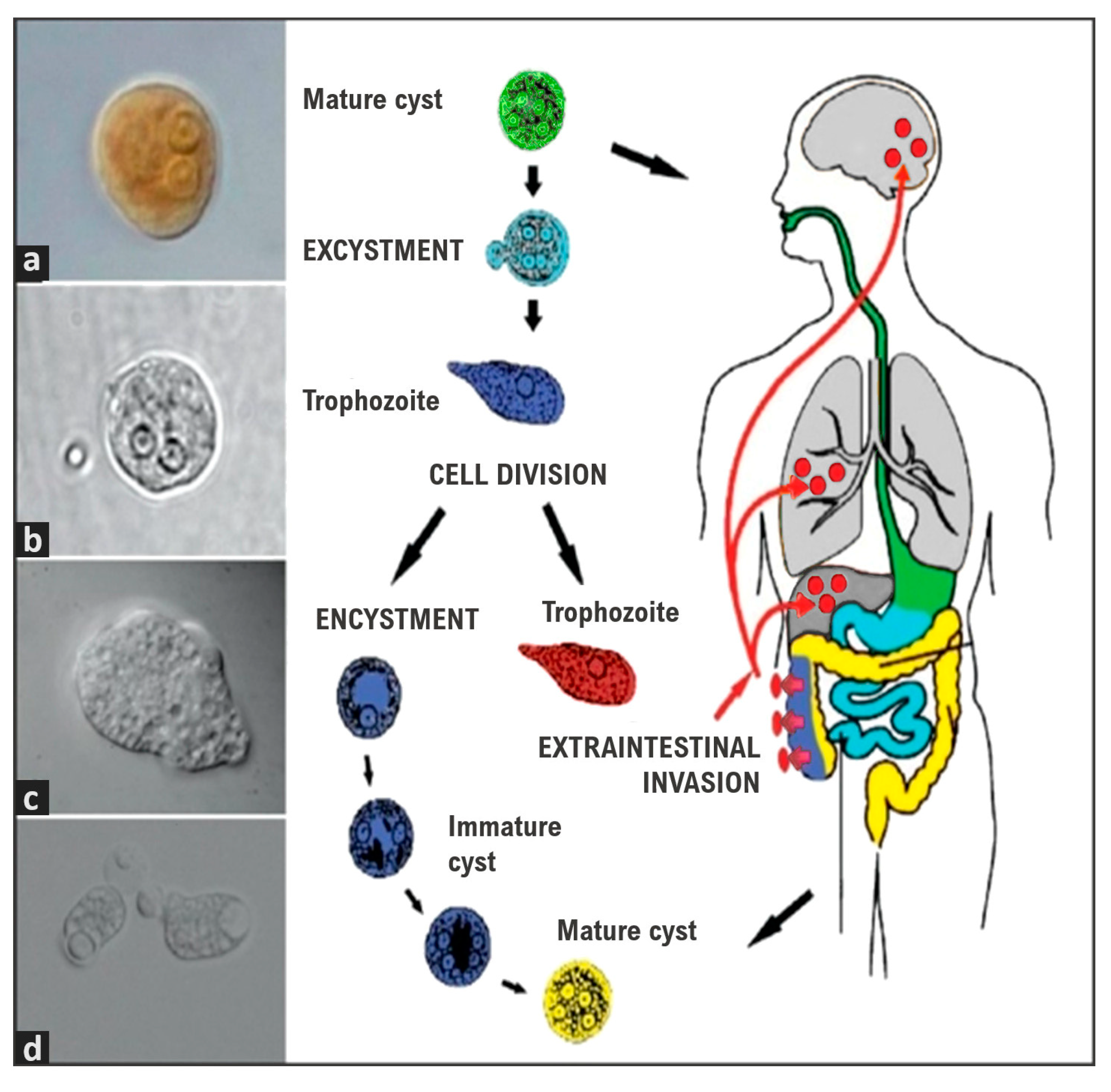
1. Introduction
2. Clinical Features
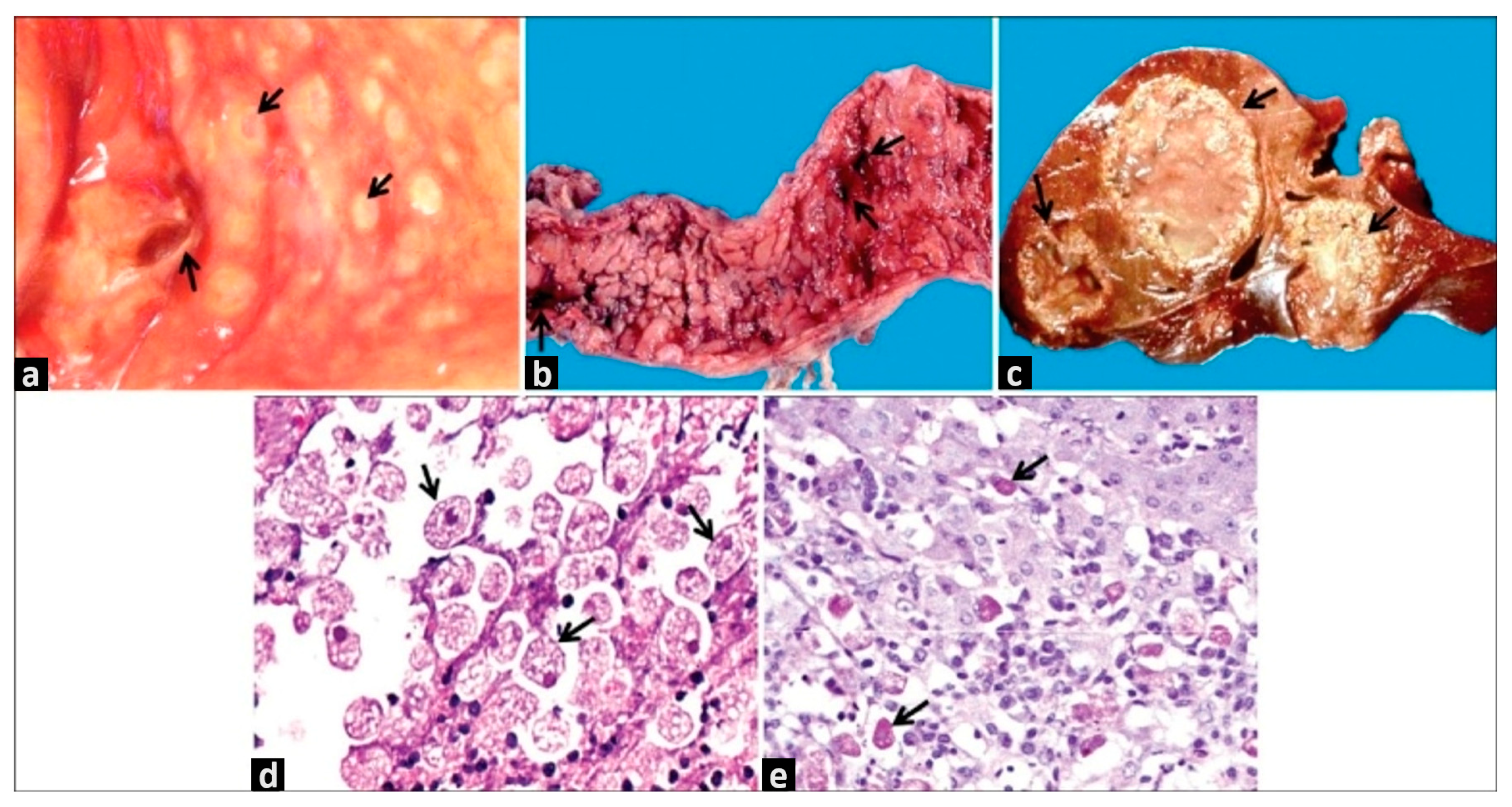
2.1. Amoebic Liver Abscess
2.2. The Severe Forms of Amoebiasis
3. Diagnosis
3.1. Macroscopy and Microscopy Analyses
3.2. Imagen Diagnosis
3.3. Immunological and Molecular Diagnosis
4. Treatment and Prevention
5. Pathogenesis
6. Intestinal Ecosystem and Entamoeba Interactions
7. Discussion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Niu, Q.; Fan, W.; Huang, F.; He, H. Oral Microbiota and Gastrointestinal Cancer. OTT 2019, 12, 4721–4728. [Google Scholar] [CrossRef]
- WHO/PAHO/UNESCO Report. A Consultation with Experts on Amoebiasis. Mexico City, Mexico, 28–29 January 1997. Epidemiol. Bull. 1997, 18, 13–14.
- Ximénez, C.; Morán, P.; Rojas, L.; Valadez, A.; Gómez, A. Reassessment of the Epidemiology of Amebiasis: State of the Art. Infect. Genet. Evol. 2009, 9, 1023–1032. [Google Scholar] [CrossRef]
- Carrero, J.C.; Reyes-López, M.; Serrano-Luna, J.; Shibayama, M.; Unzueta, J.; León-Sicairos, N.; De La Garza, M. Intestinal Amoebiasis: 160 Years of Its First Detection and Still Remains as a Health Problem in Developing Countries. Int. J. Med. Microbiol. 2020, 310, 151358. [Google Scholar] [CrossRef]
- Nath, J.; Ghosh, S.K.; Singha, B.; Paul, J. Molecular Epidemiology of Amoebiasis: A Cross-Sectional Study among North East Indian Population. PLoS Negl. Trop. Dis. 2015, 9, e0004225. [Google Scholar] [CrossRef]
- Cui, Z.; Li, J.; Chen, Y.; Zhang, L. Molecular Epidemiology, Evolution, and Phylogeny of Entamoeba spp. Infect. Genet. Evol. 2019, 75, 104018. [Google Scholar] [CrossRef]
- Tharmaratnam, T.; Kumanan, T.; Iskandar, M.A.; D’Urzo, K.; Gopee-Ramanan, P.; Loganathan, M.; Tabobondung, T.; Tabobondung, T.A.; Sivagurunathan, S.; Patel, M.; et al. Entamoeba histolytica and Amoebic Liver Abscess in Northern Sri Lanka: A Public Health Problem. Trop. Med. Health 2020, 48, 2. [Google Scholar] [CrossRef]
- Shirley, D.-A.; Moonah, S. Fulminant Amebic Colitis after Corticosteroid Therapy: A Systematic Review. PLoS Negl. Trop. Dis. 2016, 10, e0004879. [Google Scholar] [CrossRef]
- Ankri, S. Entamoeba histolytica—Gut Microbiota Interaction: More Than Meets the Eye. Microorganisms 2021, 9, 581. [Google Scholar] [CrossRef]
- Nagaraja, S.; Ankri, S. Target Identification and Intervention Strategies against Amebiasis. Drug Resist. Updates 2019, 44, 1–14. [Google Scholar] [CrossRef]
- Ximénez, C.; Morán, P.; Rojas, L.; Valadez, A.; Gómez, A.; Ramiro, M.; Cerritos, R.; González, E.; Hernández, E.; Oswaldo, P. Novelties on Amoebiasis: A Neglected Tropical Disease. J. Glob. Infect. Dis. 2011, 3, 166. [Google Scholar] [CrossRef]
- Mi-ichi, F.; Ikeda, K.; Tsugawa, H.; Deloer, S.; Yoshida, H.; Arita, M. Stage-Specific De Novo Synthesis of Very-Long-Chain Dihydroceramides Confers Dormancy to Entamoeba Parasites. mSphere 2021, 6, e00174-21. [Google Scholar] [CrossRef] [PubMed]
- Vaisusuk, K.; Saijuntha, W. Intestinal Protozoa: Their Role as Human Pathogens and Zoonoses. In Biodiversity of Southeast Asian Parasites and Vectors Causing Human Disease; Petney, T.N., Saijuntha, W., Mehlhorn, H., Eds.; Parasitology Research Monographs; Springer Nature Switzerland AG: Cham, Switzerland, 2021; pp. 35–61. ISBN 978-3-030-71161-0. [Google Scholar]
- Nakada-Tsukui, K.; Nozaki, T. Immune Response of Amebiasis and Immune Evasion by Entamoeba histolytica. Front. Immunol. 2016, 7, 175. [Google Scholar] [CrossRef]
- Shirley, D.-A.T.; Farr, L.; Watanabe, K.; Moonah, S. A Review of the Global Burden, New Diagnostics, and Current Therapeutics for Amebiasis. Open Forum Infect. Dis. 2018, 5, ofy161. [Google Scholar] [CrossRef]
- Ali, I.K.M.; Clark, C.G.; Petri, W.A. Molecular Epidemiology of Amebiasis. Infect. Genet. Evol. 2008, 8, 698–707. [Google Scholar] [CrossRef]
- Castellanos-Castro, S.; Bolaños, J.; Orozco, E. Lipids in Entamoeba histolytica: Host-Dependence and Virulence Factors. Front. Cell. Infect. Microbiol. 2020, 10, 75. [Google Scholar] [CrossRef]
- Shirley, D.-A.; Hung, C.-C.; Moonah, S. Entamoeba histolytica (Amebiasis). In Hunter’s Tropical Medicine and Emerging Infectious Disease; Ryan, E.T., Hunter, G.W., Hill, D.R., Solomon, T., Endy, T.P., Aronson, N., Ryan, E.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 699–706. ISBN 978-0-323-55512-8. [Google Scholar]
- Roediger, R.; Lisker-Melman, M. Pyogenic and Amebic Infections of the Liver. Gastroenterol. Clin. N. Am. 2020, 49, 361–377. [Google Scholar] [CrossRef]
- Kantor, M.; Abrantes, A.; Estevez, A.; Schiller, A.; Torrent, J.; Gascon, J.; Hernandez, R.; Ochner, C. Entamoeba histolytica: Updates in Clinical Manifestation, Pathogenesis, and Vaccine Development. Can. J. Gastroenterol. Hepatol. 2018, 2018, 4601420. [Google Scholar] [CrossRef]
- Victoria-Hernández, J.A.; Ventura-Saucedo, A.; López-Morones, A.; Martínez-Hernández, S.L.; Medina-Rosales, M.N.; Muñoz-Ortega, M.; Ávila-Blanco, M.E.; Cervantes-García, D.; Barba-Gallardo, L.F.; Ventura-Juárez, J. Case Report: Multiple and Atypical Amoebic Cerebral Abscesses Resistant to Treatment. BMC Infect. Dis. 2020, 20, 669. [Google Scholar] [CrossRef]
- El-Radhi, A.S. Fever in Common Infectious Diseases. In Clinical Manual of Fever in Children; El-Radhi, A.S., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 85–140. ISBN 978-3-319-92335-2. [Google Scholar]
- Martínez-Palomo, A.; Espinosa-Cantellano, M.; Tsutsumi, G.R.; Tsutsumi, V. Amebic Liver Abscess. In Liver Pathophysiology: Therapies and Antioxidants; Muriel, P., Ed.; Academic Press: London, UK, 2017; pp. 181–186. ISBN 978-0-12-804321-9. [Google Scholar]
- Tanaka, E.; Tashiro, Y.; Kotake, A.; Takeyama, N.; Umemoto, T.; Nagahama, M.; Hashimoto, T. Spectrum of CT Findings in Amebic Colitis. Jpn. J. Radiol. 2021, 39, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.; Morán, P.; Valadez, A.; Gómez, A.; González, E.; Hernández, E.; Partida, O.; Nieves, M.; Gudiño, M.; Magaña, U.; et al. Entamoeba histolytica and Entamoeba dispar Infection in Mexican School Children: Genotyping and Phylogenetic Relationship. BMC Infect. Dis. 2016, 16, 485. [Google Scholar] [CrossRef]
- Ximenez, C.; Partida, O.; Nieves, M.; Hernandez, E.; Moran, P.; Valadez, A.; Gonzalez, E.; Cerritos, R.; Rojas, L. Immune Response in Human Amebiasis: A Protective Response? In Amebiasis: Biology and Pathogenesis of Entamoeba; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Ximénez, C.; Cerritos, R.; Rojas, L.; Dolabella, S.; Morán, P.; Shibayama, M.; González, E.; Valadez, A.; Hernández, E.; Valenzuela, O.; et al. Human Amebiasis: Breaking the Paradigm? Int. J. Environ. Res. Public Health 2010, 7, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Guarner, V. Treatment and Prevention of Amebiasis. In Amebiasis: Infection and Disease by Entamoeba Histolytica; Kretschmer, R.R., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 221–238. ISBN 978-0-429-28253-9. [Google Scholar]
- Proctor, E.M. Laboratory Diagnosis of Amebiasis. Clin. Lab. Med. 1991, 11, 829–859. [Google Scholar] [CrossRef]
- Tanyuksel, M.; Petri, W.A. Laboratory Diagnosis of Amebiasis. Clin. Microbiol. Rev. 2003, 16, 713–729. [Google Scholar] [CrossRef] [PubMed]
- Saidin, S.; Othman, N.; Noordin, R. Update on Laboratory Diagnosis of Amoebiasis. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 15–38. [Google Scholar] [CrossRef]
- Juwita, P.M.; Yudhawati, R. Secondary Pleuropulmonary Amoebiasis Due to Liver Abscess Rupture: A Complication Case Report in Low Resource Setting. Int. J. Surg. Case Rep. 2021, 85, 106231. [Google Scholar] [CrossRef]
- Pritt, B.S.; Clark, C.G. Amebiasis. Mayo Clin. Proc. 2008, 83, 1154–1160. [Google Scholar] [CrossRef]
- Dolabella, S.S.; Serrano-Luna, J.; Navarro-García, F.; Cerritos, R.; Ximénez, C.; Galván-Moroyoqui, J.M.; Silva, E.F.; Tsutsumi, V.; Shibayama, M. Amoebic Liver Abscess Production by Entamoeba dispar. Ann. Hepatol. 2012, 11, 107–117. [Google Scholar] [CrossRef]
- Reyna-Fabián, M.E.; Zermeño, V.; Ximénez, C.; Flores, J.; Romero, M.F.; Diaz, D.; Argueta, J.; Moran, P.; Valadez, A.; Cerritos, R. Analysis of the Bacterial Diversity in Liver Abscess: Differences Between Pyogenic and Amebic Abscesses. Am. J. Trop. Med. Hyg. 2016, 94, 147–155. [Google Scholar] [CrossRef]
- Sayek, I.; Onat, D. Pyogenic and Amebic Liver Abscess. In Surgical treatment: Evidence-Based and Problem-Oriented; Holzheimer, R., Mannick, J.A., Eds.; Zuckschwerdt: München, Germeny, 2001; ISBN 978-3-88603-714-8. [Google Scholar]
- Rigothier, M.C.; Khun, H.; Tavares, P.; Carmona, A.; Huerre, M.; Guillén, N. Fate of Entamoeba histolytica during Establishment of Amoebic Liver Abscess Analyzed by Quantitative Radioimaging and Histology. Infect. Immun. 2002, 2002 70, 3208–3215. [Google Scholar] [CrossRef]
- Pandak, N.; Golchinheydari, S.; Mahdi, A.S.; Al Majrafi, A.; Deenadayalan, S.S.; Khamis, F. Amebic Liver Abscess: A Disease Native to Oman? Sultan. Qaboos. Univ. Med. J. 2021, 22, 253. [Google Scholar] [CrossRef]
- Moran, P.; Gómez, A.; Valadez, A.; Ramos, F.; García, G.; Limón, A.; Valenzuela, O.; Ramiro, M.; Melendro, E.I.; Ximenez, C. Amebic and Pyogenic Liver Abscess: Importance of Differential Diagnosis in Endemic Areas of Amebiasis. Trop. Med. Int. Health 2007, 12, 153–154. [Google Scholar] [CrossRef]
- Sánchez-Guillén, M.D.C.; Pérez-Fuentes, R.; Salgado-Rosas, H.; Ruiz-Argüelles, A.; Ackers, J.; Shire, A.; Talamas-Rohana, P. Differentiation of Entamoeba histolytica/Entamoeba dispar by PCR and Their Correlation with Humoral and Cellular Immunity in Individuals with Clinical Variants of Amoebiasis. Am. J. Trop. Med. Hyg. 2002, 66, 731–737. [Google Scholar] [CrossRef]
- López Revilla, R.; Navarro-García, F.; Valadez-Sánchez, M.; López Vidal, Y.; Calva Mercado, J. Dot-Enzyme-Linked Immunosorbent Assay (Dot-ELISA) of Anti-Entamoeba histolytica Antibodies in Human Serum and Colostrum. Arch. Invest. Med. Mex 1991, 22, 249–253. [Google Scholar]
- Schouten, W.J.; Oostburg, B.F.J.; Noordpool, H.; Makbin, M.; Bos, H.J. A Seroepidemiological Study of Amebiasis in Surinam by the Enzyme-Linked Immunosorbent Assay (Elisa)*. Am. J. Trop. Med. Hyg. 1980, 29, 358–363. [Google Scholar] [CrossRef]
- Salvatierra, B.; Tapia-Conyer, R.; Sepulveda-Amor, J.; Gutierrez, G.; Ortiz-Ortiz, L.; Caballero-Salcedo, A.; Viveros-Rogel, M. Seroepidemiology of Amebiasis in Mexico. Am. J. Trop. Med. Hyg. 1994, 50, 412–419. [Google Scholar] [CrossRef]
- Haque, R.; Neville, L.M.; Hahn, P.; Petri, W.A. Rapid Diagnosis of Entamoeba Infection by Using Entamoeba and Entamoeba histolytica Stool Antigen Detection Kits. J. Clin. Microbiol. 1995, 33, 2558–2561. [Google Scholar] [CrossRef]
- Haque, R.; Kress, K.; Wood, S.; Jackson, T.F.H.G.; Lyerly, D.; Wilkins, T.; Petri, W.A. Diagnosis of Pathogenic Entamoeba histolytica Infection Using a Stool ELISA Based on Monoclonal Antibodies to the Galactose-Specific Adhesin. J. Infect. Dis. 1993, 167, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.; Petri, W.A. Diagnosis of Amebiasis in Bangladesh. Arch. Med. Res. 2006, 37, 272–275. [Google Scholar] [CrossRef]
- Spadafora, L.J.; Kearney, M.R.; Siddique, A.; Ali, I.K.; Gilchrist, C.A.; Arju, T.; Hoffstrom, B.; Nguyen, F.K.; Petri, W.A.; Haque, R.; et al. Species-Specific Immunodetection of an Entamoeba histolytica Cyst Wall Protein. PLoS Negl. Trop. Dis. 2016, 10, e0004697. [Google Scholar] [CrossRef]
- Rivera, W.L.; Tachibana, H.; Silva-Tahat, M.R.A.; Uemura, H.; Kanbara, H. Differentiation of Entamoeba histolytica and E. dispar DNA from Cysts Present in Stool Specimens by Polymerase Chain Reaction: Its Field Application in the Philippines. Parasitol. Res. 1996, 82, 585–589. [Google Scholar] [CrossRef]
- Clark, C.G.; Diamond, L.S. Intraspecific Variation and Phylogenetic Relationships in the Genus Entamoeba as Revealed by Riboprinting. J. Eukaryot. Microbiol. 1997, 44, 142–154. [Google Scholar] [CrossRef]
- Fotedar, R.; Stark, D.; Beebe, N.; Marriott, D.; Ellis, J.; Harkness, J. PCR Detection of Entamoeba histolytica, Entamoeba dispar and Entamoeba moshkovskii in Stool Samples from Sydney, Australia. J. Clin. Microbiol. 2007, 45, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Troll, H.; Marti, H.; Weiss, N. Simple Differential Detection of Entamoeba histolytica and Entamoeba dispar in Fresh Stool Specimens by Sodium Acetate- Acetic Acid-Formalin Concentration and PCR. J. Clin. Microbiol. 1997, 35, 1701–1705. [Google Scholar] [CrossRef]
- Ramos, F.; Morán, P.; González, E.; García, G.; Ramiro, M.; Gómez, A.; De León, M.D.C.G.; Melendro, E.I.; Valadez, A.; Ximénez, C. Entamoeba histolytica and Entamoeba dispar: Prevalence Infection in a Rural Mexican Community. Exp. Parasitol. 2005, 110, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, Z.; Petmitr, S.; Mungthin, M.; Leelayoova, S.; Chavalitshewinkoon-Petmitr, P. Differential Detection of Entamoeba histolytica, Entamoeba dispar and Entamoeba moshkovskii by a Single-Round PCR Assay. J. Clin. Microbiol. 2006, 44, 3196–3200. [Google Scholar] [CrossRef]
- Fonte, L.; Montano, I.; Núñez, Y.O.; Maestre, J.L.; Fernández, M.A.; Torres-Núñez, D.; Silva, J.A. Multiplex Polymerase Chain Reaction Amplification and Differentiation of Entamoeba histolytica and Entamoeba dispar DNA from Stool Samples. Am. J. Trop. Med. Hyg. 2001, 64, 293–297. [Google Scholar] [CrossRef]
- Khairnar, K.; Parija, S.C. A Novel Nested Multiplex Polymerase Chain Reaction (PCR) Assay for Differential Detection of Entamoeba histolytica, E. Moshkovskii and E. Dispar DNA in Stool Samples. BMC Microbiol. 2007, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, D.; Nuchamowitz, Y.; Stolarsky, T. Comparison of Use of Enzyme-Linked Immunosorbent Assay-Based Kits and PCR Amplification of RRNA Genes for Simultaneous Detection of Entamoeba histolytica and E. dispar. J. Clin. Microbiol. 1997, 35, 2405–2407. [Google Scholar] [CrossRef] [PubMed]
- Millan-Velasco, F.; Schoolnick, G.; Wirth, D.; Acuna-Soto, R.; Samuelson, J.; De Girolami, P.; Zarate, L. Application of the Polymerase Chain Reaction to the Epidemiology of Pathogenic and Nonpathogenic Entamoeba histolytica. Am. J. Trop. Med. Hyg. 1993, 48, 58–70. [Google Scholar] [CrossRef]
- Evangelopoulos, G.; Spanakos, E.; Pat, A. A Nested, Multiplex, PCR Assay for the Simultaneous Detection and Differentiation of Entamoeba histolytica and Entamoeba dispar in Faeces. Ann. Trop. Med. And. Parasitol. 2000, 94, 233–240. [Google Scholar] [CrossRef]
- Myjak, P.; Kur, J.; Pietkiewicz, H. Usefulness of New DNA Extraction Procedure for PCR Technique in Species Identification of Entamoeba Isolates. Wiad. Parazytol. 1997, 43, 163–170. [Google Scholar]
- Verweij, J.J.; Blotkamp, J.; Brienen, E.A.T.; Aguirre, A.; Polderman, A.M. Differentiation of Entamoeba histolytica and Entamoeba dispar Cysts Using Polymerase Chain Reaction on DNA Isolated from Faeces with Spin Columns. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Verweij, J.J.; Blangé, R.A.; Templeton, K.; Schinkel, J.; Brienen, E.A.T.; Van Rooyen, M.A.A.; Van Lieshout, L.; Polderman, A.M. Simultaneous Detection of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum in Fecal Samples by Using Multiplex Real-Time PCR. J. Clin. Microbiol. 2004, 42, 1220–1223. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kabir, M.; Mondal, D.; Ali, I.K.M.; Petri, W.A.; Haque, R. Real-Time-PCR Assay for Diagnosis of Entamoeba histolytica Infection. J. Clin. Microbiol. 2005, 43, 2168–2172. [Google Scholar] [CrossRef]
- Stark, D.; Van Hal, S.; Fotedar, R.; Butcher, A.; Marriott, D.; Ellis, J.; Harkness, J. Comparison of Stool Antigen Detection Kits to PCR for Diagnosis of Amebiasis. J. Clin. Microbiol. 2008, 46, 1678–1681. [Google Scholar] [CrossRef]
- Stark, D.; Fotedar, R.; van Hal, S.; Beebe, N.; Marriott, D.; Ellis, J.T.; Harkness, J. Prevalence of Enteric Protozoa in Human Immunodeficiency Virus (HIV)-Positive and HIV-Negative Men Who Have Sex with Men from Sydney, Australia. Am. J. Trop. Med. Hyg. 2007, 76, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H.; Kobayashi, S.; Okuzawa, E.; Masuda, G. Detection of Pathogenic Entamoeba histolytica DNA in Liver Abscess Fluid by Polymerase Chain Reaction. Int. J. Parasitol. 1992, 22, 1193–1196. [Google Scholar] [CrossRef]
- Zaman, S.; Khan, M.A. Direct Ampli®cation of Entamoeba histolytica DNA from Amoebic Liver Abscess Pus Using Polymerase Chain Reaction. Parasitol. Res. 2000, 86, 724–728. [Google Scholar] [CrossRef]
- Martínez-Castillo, M.; Pacheco-Yepez, J.; Flores-Huerta, N.; Guzmán-Téllez, P.; Jarillo-Luna, R.A.; Cárdenas-Jaramillo, L.M.; Campos-Rodríguez, R.; Shibayama, M. Flavonoids as a Natural Treatment Against Entamoeba histolytica. Front. Cell. Infect. Microbiol. 2018, 8, 209. [Google Scholar] [CrossRef]
- Gonzales, M.L.M.; Dans, L.F.; Sio-Aguilar, J. Antiamoebic Drugs for Treating Amoebic Colitis. Cochrane Database Syst. Rev. 2019, 1, CD006085. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.; Huston, C.D.; Hughes, M.; Houpt, E.; Petri, W.A. Amebiasis. N. Engl. J. Med. 2003, 348, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Marie, C.; Petri, W.A. Amoebic Dysentery. BMJ Clin. Evid. 2013, 2013, 0918. [Google Scholar] [PubMed]
- Ansari, M.F.; Siddiqui, S.M.; Agarwal, S.M.; Vikramdeo, K.S.; Mondal, N.; Azam, A. Metronidazole Hydrazone Conjugates: Design, Synthesis, Antiamoebic and Molecular Docking Studies. Bioorg. Med. Chem. Lett. 2015, 25, 3545–3549. [Google Scholar] [CrossRef]
- Adagu, I.S. In Vitro Activity of Nitazoxanide and Related Compounds against Isolates of Giardia intestinalis, Entamoeba histolytica and Trichomonas vaginalis. J. Antimicrob. Chemother. 2002, 49, 103–111. [Google Scholar] [CrossRef]
- Bansal, D.; Sehgal, R.; Chawla, Y.; Mahajan, R.C.; Malla, N. In Vitro Activity of Antiamoebic Drugs against Clinical Isolates of Entamoeba histolytica and Entamoeba dispar. Ann. Clin. Microbiol. Antimicrob. 2004, 3, 27. [Google Scholar] [CrossRef]
- Farthing, M.J. Treatment Options for the Eradication of Intestinal Protozoa. Nat. Rev. Gastroenterol. Hepatol. 2006, 3, 436–445. [Google Scholar] [CrossRef]
- Souza Passos, A.; Da Silva Santos, R.; Dos Santos Andrade, R.; Ribeiro Santos Junior, E.; Santos Bomfim, E.; Sousa Ribeiro, L.; Andrade Dos Santos, G.; Araujo De Albuquerque, W.; Amor, A.L.M. Enteric Parasites and Socio-Epidemiological Variables in an Academic Community. Rev. Patol. Trop. 2021, 50, 163–178. [Google Scholar] [CrossRef]
- Shahi, P.; Moreau, F.; Chadee, K. Entamoeba histolytica Cyclooxygenase-Like Protein Regulates Cysteine Protease Expression and Virulence. Front. Cell. Infect. Microbiol. 2019, 8, 447. [Google Scholar] [CrossRef]
- Betanzos, A.; Javier-Reyna, R.; García-Rivera, G.; Bañuelos, C.; González-Mariscal, L.; Schnoor, M.; Orozco, E. The EhCPADH112 Complex of Entamoeba histolytica Interacts with Tight Junction Proteins Occludin and Claudin-1 to Produce Epithelial Damage. PLoS ONE 2013, 8, e65100. [Google Scholar] [CrossRef]
- Betanzos; Bañuelos; Orozco Host Invasion by Pathogenic Amoebae: Epithelial Disruption by Parasite Proteins. Genes. 2019, 10, 618. [CrossRef]
- Begum, S.; Gorman, H.; Chadha, A.; Chadee, K. Role of Inflammasomes in Innate Host Defense against Entamoeba histolytica. J. Leukoc. Biol. 2020, 108, 801–812. [Google Scholar] [CrossRef]
- Que, X.; Reed, S.L. Cysteine Proteinases and the Pathogenesis of Amebiasis. Clin. Microbiol. Rev. 2000, 13, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Rawat, A.; Roy, M.; Jyoti, A.; Kaushik, S.; Verma, K.; Srivastava, V.K. Cysteine Proteases: Battling Pathogenic Parasitic Protozoans with Omnipresent Enzymes. Microbiol. Res. 2021, 249, 126784. [Google Scholar] [CrossRef]
- Loftus, B.; Anderson, I.; Davies, R.; Alsmark, U.C.M.; Samuelson, J.; Amedeo, P.; Roncaglia, P.; Berriman, M.; Hirt, R.P.; Mann, B.J.; et al. The Genome of the Protist Parasite Entamoeba histolytica. Nature 2005, 433, 865–868. [Google Scholar] [CrossRef]
- Clark, C.G.; Alsmark, U.C.M.; Tazreiter, M.; Saito-Nakano, Y.; Ali, V.; Marion, S.; Weber, C.; Mukherjee, C.; Bruchhaus, I.; Tannich, E.; et al. Structure and Content of the Entamoeba histolytica Genome. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 65, pp. 51–190. ISBN 978-0-12-374166-0. [Google Scholar]
- Lorenzi, H.A.; Puiu, D.; Miller, J.R.; Brinkac, L.M.; Amedeo, P.; Hall, N.; Caler, E.V. New Assembly, Reannotation and Analysis of the Entamoeba histolytica Genome Reveal New Genomic Features and Protein Content Information. PLoS Negl. Trop. Dis. 2010, 4, e716. [Google Scholar] [CrossRef]
- Weedall, G.D.; Hall, N. Evolutionary Genomics of Entamoeba. Res. Microbiol. 2011, 162, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Naiyer, S.; Kaur, D.; Ahamad, J.; Singh, S.S.; Singh, Y.P.; Thakur, V.; Bhattacharya, A.; Bhattacharya, S. Transcriptomic Analysis Reveals Novel Downstream Regulatory Motifs and Highly Transcribed Virulence Factor Genes of Entamoeba histolytica. BMC Genom. 2019, 20, 206. [Google Scholar] [CrossRef]
- Bhattacharya, S. Episomal and Chromosomal DNA Replication and Recombination in Entamoeba histolytica. Front. Mol. Biosci. 2023, 10, 1212082. [Google Scholar] [CrossRef]
- Singh, N.; Bhattacharya, A.; Bhattacharya, S. Homologous Recombination Occurs in Entamoeba and Is Enhanced during Growth Stress and Stage Conversion. PLoS ONE 2013, 8, e74465. [Google Scholar] [CrossRef]
- Romero, M.; Cerritos, R.; Ximenez, C. Horizontal Gene Transfers from Bacteria to Entamoeba Complex: A Strategy for Dating Events along Species Divergence. J. Parasitol. Res. 2016, 2016, 3241027. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, C.A.; Houpt, E.; Trapaidze, N.; Fei, Z.; Crasta, O.; Asgharpour, A.; Evans, C.; Martino-Catt, S.; Baba, D.J.; Stroup, S.; et al. Impact of Intestinal Colonization and Invasion on the Entamoeba histolytica Transcriptome. Mol. Biochem. Parasitol. 2006, 147, 163–176. [Google Scholar] [CrossRef]
- Davis, P.H.; Schulze, J.; Stanley, S.L. Transcriptomic Comparison of Two Entamoeba histolytica Strains with Defined Virulence Phenotypes Identifies New Virulence Factor Candidates and Key Differences in the Expression Patterns of Cysteine Proteases, Lectin Light Chains, and Calmodulin. Mol. Biochem. Parasitol. 2007, 151, 118–128. [Google Scholar] [CrossRef]
- Davis, P.H.; Zhang, Z.; Chen, M.; Zhang, X.; Chakraborty, S.; Stanley, S.L. Identification of a Family of BspA like Surface Proteins of Entamoeba histolytica with Novel Leucine Rich Repeats. Mol. Biochem. Parasitol. 2006, 145, 111–116. [Google Scholar] [CrossRef]
- König, C.; Honecker, B.; Wilson, I.W.; Weedall, G.D.; Hall, N.; Roeder, T.; Metwally, N.G.; Bruchhaus, I. Taxon-Specific Proteins of the Pathogenic Entamoeba Species E. histolytica and E. nuttalli. Front. Cell. Infect. Microbiol. 2021, 11, 641472. [Google Scholar] [CrossRef]
- Das, K.; Ganguly, S. Evolutionary Genomics and Population Structure of Entamoeba histolytica. Comput. Struct. Biotechnol. J. 2014, 12, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V.; Iyer, L.R.; Roy, R.; Bhargava, V.; Panda, S.; Paul, J.; Verweij, J.J.; Clark, C.G.; Bhattacharya, A.; Bhattacharya, S. Genomic Distribution of SINEs in Entamoeba histolytica Strains: Implication for Genotyping. BMC Genom. 2013, 14, 432. [Google Scholar] [CrossRef]
- Urquieta-Ramírez, L.; Ramírez-Montiel, F.; Andrade-Guillén, S.; Páramo-Pérez, I.; Rangel-Serrano, Á.; Reyes-Cortes, R.; Franco, B.; Mendoza-Macías, C.L.; Anaya-Velázquez, F.; Padilla-Vaca, F. Contribution of Neutral Sphingomyelinases to in Vitro Virulence of Entamoeba histolytica. Exp. Parasitol. 2018, 194, 38–44. [Google Scholar] [CrossRef]
- Wilson, I.W.; Weedall, G.D.; Lorenzi, H.; Howcroft, T.; Hon, C.-C.; Deloger, M.; Guillén, N.; Paterson, S.; Clark, C.G.; Hall, N. Genetic Diversity and Gene Family Expansions in Members of the Genus Entamoeba. Genome Biol. Evol. 2019, 11, 688–705. [Google Scholar] [CrossRef]
- Nakada-Tsukui, K.; Sekizuka, T.; Sato-Ebine, E.; Escueta-de Cadiz, A.; Ji, D.; Tomii, K.; Kuroda, M.; Nozaki, T. AIG1 Affects in Vitro and in Vivo Virulence in Clinical Isolates of Entamoeba histolytica. PLoS Pathog. 2018, 14, e1006882. [Google Scholar] [CrossRef]
- Tabernero, L.; Aricescu, A.R.; Jones, E.Y.; Szedlacsek, S.E. Protein Tyrosine Phosphatases: Structure-Function Relationships: PTP Structure-Function Relationship. FEBS J. 2008, 275, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Linford, A.S.; Jiang, N.M.; Edwards, T.E.; Sherman, N.E.; Van Voorhis, W.C.; Stewart, L.J.; Myler, P.J.; Staker, B.L.; Petri, W.A. Crystal Structure and Putative Substrate Identification for the Entamoeba histolytica Low Molecular Weight Tyrosine Phosphatase. Mol. Biochem. Parasitol. 2014, 193, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Torres-Cifuentes, D.M.; Espíritu-Gordillo, P.; Baylón-Pacheco, L.; Rosales-Encína, J.L. Low Molecular Weight Protein Tyrosine Phosphatase (LMW-PTP2) Protein Can Potentially Modulate Virulence of the Parasite Entamoeba histolytica. Mol. Biochem. Parasitol. 2021, 242, 111360. [Google Scholar] [CrossRef] [PubMed]
- Gastelum-Martínez, A.; León-Sicairos, C.; Plata-Guzmán, L.; Soto-Castro, L.; León-Sicairos, N.; De La Garza, M. Iron-Modulated Virulence Factors of Entamoeba histolytica. Future Microbiol. 2018, 13, 1329–1341. [Google Scholar] [CrossRef] [PubMed]
- Rath, P.P.; Gourinath, S. The Actin Cytoskeleton Orchestra in Entamoeba histolytica. Proteins 2020, 88, 1361–1375. [Google Scholar] [CrossRef]
- Medina-Rosales, M.N.; Muñoz-Ortega, M.H.; García-Hernández, M.H.; Talamás-Rohana, P.; Medina-Ramírez, I.E.; Salas-Morón, L.G.; Martínez-Hernández, S.L.; Ávila-Blanco, M.E.; Medina-Rosales, B.; Ventura-Juárez, J. Acetylcholine Upregulates Entamoeba histolytica Virulence Factors, Enhancing Parasite Pathogenicity in Experimental Liver Amebiasis. Front. Cell. Infect. Microbiol. 2021, 10, 586354. [Google Scholar] [CrossRef]
- Leon-Coria, A.; Kumar, M.; Chadee, K. The Delicate Balance between Entamoeba histolytica, Mucus and Microbiota. Gut Microbes. 2020, 11, 118–125. [Google Scholar] [CrossRef]
- Iyer, L.R.; Verma, A.K.; Paul, J.; Bhattacharya, A. Phagocytosis of Gut Bacteria by Entamoeba histolytica. Front. Cell. Infect. Microbiol. 2019, 9, 34. [Google Scholar] [CrossRef]
- Partida-Rodríguez, O.; Serrano-Vázquez, A.; Nieves-Ramírez, M.E.; Moran, P.; Rojas, L.; Portillo, T.; González, E.; Hernández, E.; Finlay, B.B.; Ximenez, C. Human Intestinal Microbiota: Interaction between Parasites and the Host Immune Response. Arch. Med. Res. 2017, 48, 690–700. [Google Scholar] [CrossRef]
- Burgess, S.L.; Petri, W.A. The Intestinal Bacterial Microbiome and E. histolytica Infection. Curr. Trop. Med. Rep. 2016, 3, 71–74. [Google Scholar] [CrossRef]
- Guillén, N. The Interaction between Entamoeba histolytica and Enterobacteria Shed Light on an Ancient Antibacterial Response. Cell. Microbiol. 2019, 21, 13039. [Google Scholar] [CrossRef] [PubMed]
- Varet, H.; Shaulov, Y.; Sismeiro, O.; Trebicz-Geffen, M.; Legendre, R.; Coppée, J.-Y.; Ankri, S.; Guillen, N. Enteric Bacteria Boost Defences against Oxidative Stress in Entamoeba histolytica. Sci. Rep. 2018, 8, 9042. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, L.A.; Gil-Becerril, K.; Galindo-Gómez, S.; Estrada-García, T.; Ximénez, C.; Leon-Coria, A.; Moreau, F.; Chadee, K.; Tsutsumi, V. Entamoeba histolytica Interaction with Enteropathogenic Escherichia coli Increases Parasite Virulence and Inflammation in Amebiasis. Infect. Immun. 2019, 87, e00279-19. [Google Scholar] [CrossRef] [PubMed]
- Silva Oliveira, F.M.; Claúdia De Carvalho Fernandes, A.; Henrique De Cicco Sandes, S.; Prata, L.O.; Junior, M.A.; Vilela Da Silva, C.A.; Campolina-Silva, G.H.; Lorenzo De Jesus Oliveira, M.; Oliveira, C.A.; Neumann, E.; et al. Co-Infection by Salmonella enterica Subsp. Enterica Serovar Typhimurium and Entamoeba dispar Pathogenic Strains Enhances Colitis and the Expression of Amoebic Virulence Factors. Microb. Pathog. 2021, 158, 105010. [Google Scholar] [CrossRef]
- Verma, A.K.; Verma, R.; Ahuja, V.; Paul, J. Real-Time Analysis of Gut Flora in Entamoeba histolytica Infected Patients of Northern India. BMC Microbiol. 2012, 12, 183. [Google Scholar] [CrossRef]
- Morton, E.R.; Lynch, J.; Froment, A.; Lafosse, S.; Heyer, E.; Przeworski, M.; Blekhman, R.; Ségurel, L. Variation in Rural African Gut Microbiota Is Strongly Correlated with Colonization by Entamoeba and Subsistence. PLoS Genet. 2015, 11, e1005658. [Google Scholar] [CrossRef]
- Serrano-Luna, J.; Piña-Vázquez, C.; Reyes-López, M.; Ortiz-Estrada, G.; De La Garza, M. Proteases from Entamoeba spp. and Pathogenic Free-Living Amoebae as Virulence Factors. J. Trop. Med. 2013, 2013, 1–32. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morán, P.; Serrano-Vázquez, A.; Rojas-Velázquez, L.; González, E.; Pérez-Juárez, H.; Hernández, E.G.; Padilla, M.d.l.A.; Zaragoza, M.E.; Portillo-Bobadilla, T.; Ramiro, M.; et al. Amoebiasis: Advances in Diagnosis, Treatment, Immunology Features and the Interaction with the Intestinal Ecosystem. Int. J. Mol. Sci. 2023, 24, 11755. https://doi.org/10.3390/ijms241411755
Morán P, Serrano-Vázquez A, Rojas-Velázquez L, González E, Pérez-Juárez H, Hernández EG, Padilla MdlA, Zaragoza ME, Portillo-Bobadilla T, Ramiro M, et al. Amoebiasis: Advances in Diagnosis, Treatment, Immunology Features and the Interaction with the Intestinal Ecosystem. International Journal of Molecular Sciences. 2023; 24(14):11755. https://doi.org/10.3390/ijms241411755
Chicago/Turabian StyleMorán, Patricia, Angélica Serrano-Vázquez, Liliana Rojas-Velázquez, Enrique González, Horacio Pérez-Juárez, Eric G. Hernández, Maria de los Angeles Padilla, Martha E. Zaragoza, Tobías Portillo-Bobadilla, Manuel Ramiro, and et al. 2023. "Amoebiasis: Advances in Diagnosis, Treatment, Immunology Features and the Interaction with the Intestinal Ecosystem" International Journal of Molecular Sciences 24, no. 14: 11755. https://doi.org/10.3390/ijms241411755
APA StyleMorán, P., Serrano-Vázquez, A., Rojas-Velázquez, L., González, E., Pérez-Juárez, H., Hernández, E. G., Padilla, M. d. l. A., Zaragoza, M. E., Portillo-Bobadilla, T., Ramiro, M., & Ximénez, C. (2023). Amoebiasis: Advances in Diagnosis, Treatment, Immunology Features and the Interaction with the Intestinal Ecosystem. International Journal of Molecular Sciences, 24(14), 11755. https://doi.org/10.3390/ijms241411755






