NanoLC-EI-MS: Perspectives in Biochemical Analysis
Abstract
1. Introduction
2. The Evolution of LC-EI-MS Interfacing
2.1. From Direct Liquid Introduction (DLI) to Modern Liquid Electron Ionization (LEI)
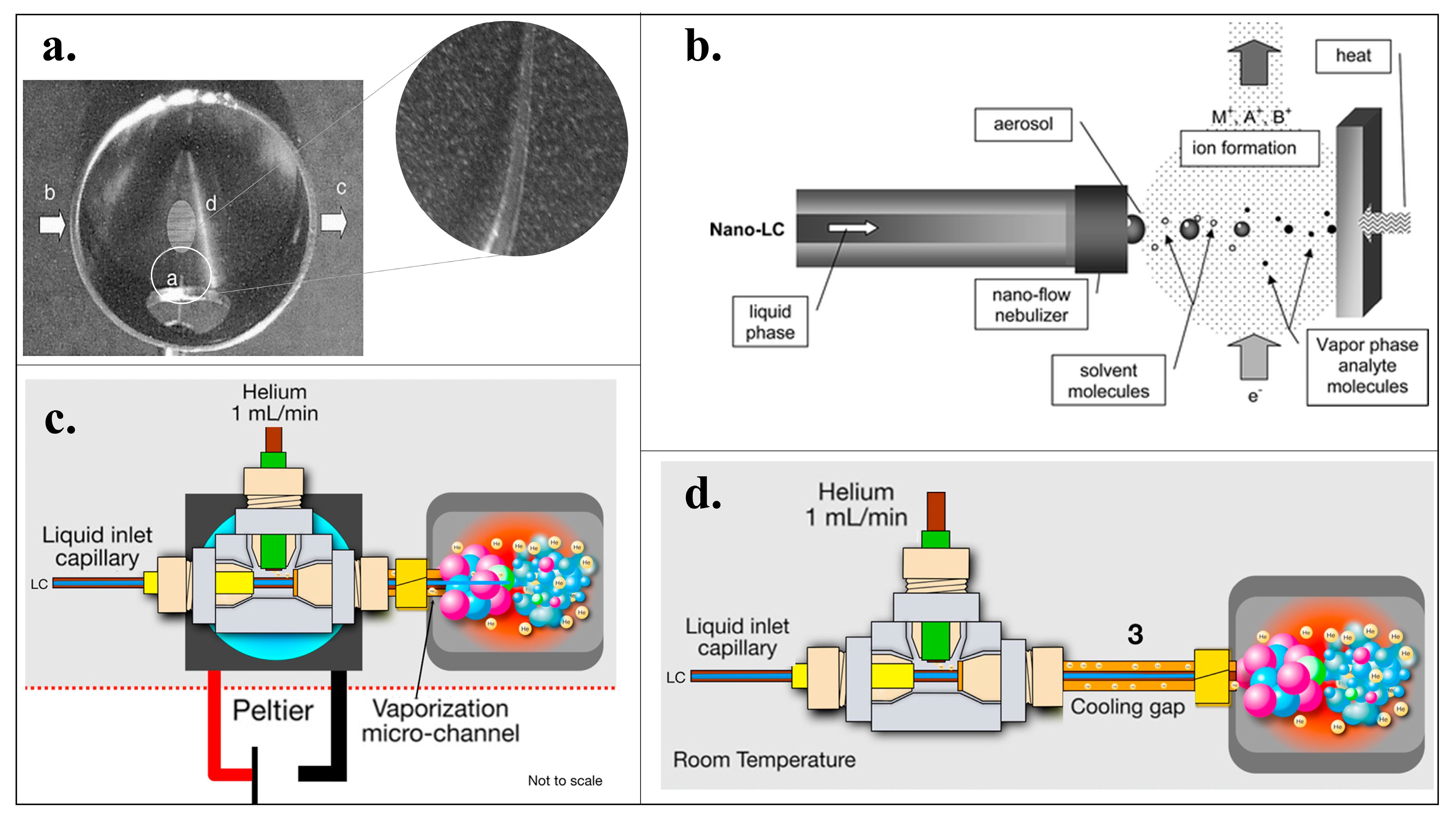
2.2. Cold EI with Supersonic Molecular Beams
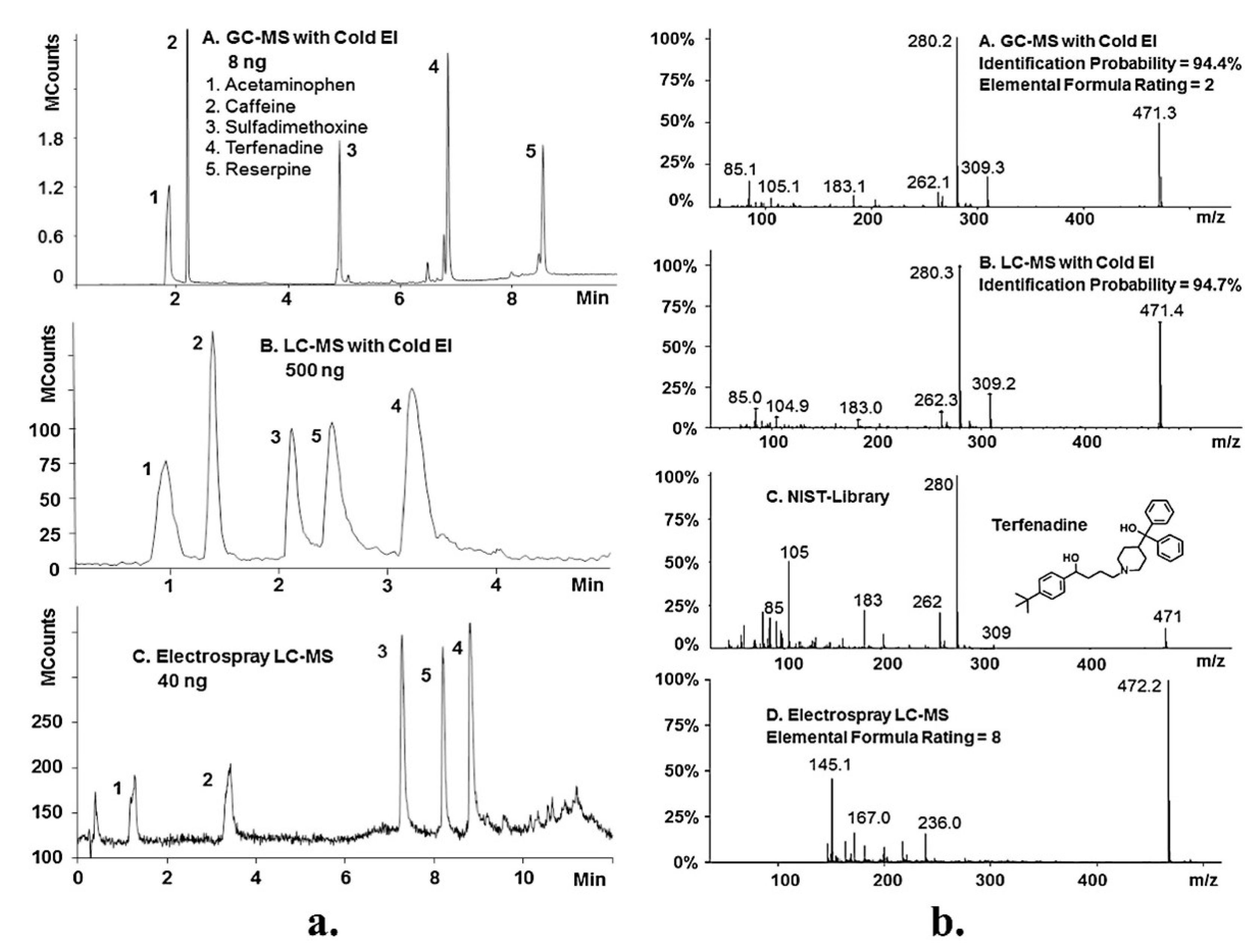
3. Potential of NanoLC-EI-MS in Biochemical Analysis
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APCI | Atmosphere pressure chemical ionization |
| APPI | Atmosphere pressure photoionization |
| Cap-EI | Capillary-EI interface |
| CRM | charge residue model |
| CID | Collision-induced dissociation |
| Direct-EI | Direct-EI interface |
| DLI | Direct liquid introduction interface |
| EI | Electron ionization |
| ESI | Electrospray ionization |
| FFAs | free-fatty acids |
| GC | Gas chromatography |
| HPLC | High-performance liquid chromatography |
| HRMS | High-resolution mass spectrometry |
| IEM | Ion-evaporation model |
| LC | Liquid chromatography |
| MAGIC | Monodispersive aerosol generation interface |
| MB | Mobile belt interface |
| MS | Mass spectrometry |
| MS/MS | Tandem mass spectrometry |
| m/z | Mass-to-charge ratio |
| NEFAs | non-esterified fatty acids |
| NIST | National Institute of standards and technology |
| PGIs | Potential genotoxic impurities |
| PB | Particle beam interface |
| SMB | Supersonic molecular beam |
| UV-vis | Ultraviolet detector |
References
- Banerjee, S. Empowering Clinical Diagnostics with Mass Spectrometry. ACS Omega 2020, 5, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Seger, C.; Salzmann, L. After Another Decade: LC–MS/MS Became Routine in Clinical Diagnostics. Clin. Biochem. 2020, 82, 2–11. [Google Scholar] [CrossRef]
- Adaway, J.E.; Keevil, B.G.; Owen, L.J. Liquid Chromatography Tandem Mass Spectrometry in the Clinical Laboratory. Ann. Clin. Biochem. 2015, 52, 18–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.B.; French, D. Implementation of Liquid Chromatography/Mass Spectrometry into the Clinical Laboratory. Clin. Chim. Acta 2013, 420, 4–10. [Google Scholar] [CrossRef]
- Feider, C.L.; Krieger, A.; Dehoog, R.J.; Eberlin, L.S. Ambient Ionization Mass Spectrometry: Recent Developments and Applications. Anal. Chem. 2019, 91, 4266–4290. [Google Scholar] [CrossRef]
- Sarkar, P.K.; Prajapati, P.K.; Shukla, V.J.; Ravishankar, B.; Choudhary, A.K. A Timeline of Stable of Isotopes and Mass Spectrometry. Indian J. Exp. Biol. 2009, 47, 987–992. [Google Scholar]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Antignac, J.-P.; de Wasch, K.; Monteau, F.; De Brabander, H.; Andre, F.; Le Bizec, B. The Ion Suppression Phenomenon in Liquid Chromatography–Mass Spectrometry and Its Consequences in the Field of Residue Analysis. Anal. Chim. Acta 2005, 529, 129–136. [Google Scholar] [CrossRef]
- Heemskerk, A.A.M.; Busnel, J.-M.; Schoenmaker, B.; Derks, R.J.E.; Klychnikov, O.; Hensbergen, P.J.; Deelder, A.M.; Mayboroda, O.A. Ultra-Low Flow Electrospray Ionization-Mass Spectrometry for Improved Ionization Efficiency in Phosphoproteomics. Anal. Chem. 2012, 84, 4552–4559. [Google Scholar] [CrossRef]
- Vargas Medina, D.A.; Pereira dos Santos, N.G.; da Silva Burato, J.S.; Borsatto, J.V.B.; Lanças, F.M. An Overview of Open Tubular Liquid Chromatography with a Focus on the Coupling with Mass Spectrometry for the Analysis of Small Molecules. J. Chromatogr. A 2021, 1641, 461989. [Google Scholar] [CrossRef]
- Maciel, E.V.S.; Pereira dos Santos, N.G.; Vargas Medina, D.A.; Lanças, F.M. Electron Ionization Mass Spectrometry: Quo Vadis? Electrophoresis 2022, 43, 1587–1600. [Google Scholar] [CrossRef] [PubMed]
- Vargas Medina, D.A.; Maciel, E.V.S.; Lanças, F.M. Mass Spectrometric Detection, Instrumentation, and Ionization Methods. In Liquid Chromatography; Elsevier: Amsterdam, The Netherlands, 2023; pp. 679–706. ISBN 9780323999687. [Google Scholar]
- Tsizin, S.; Fialkov, A.B.; Amirav, A. Electron Ionization Mass Spectrometry for Both Liquid and Gas Chromatography in One System without the Need for Hardware Adjustments. J. Am. Soc. Mass Spectrom. 2020, 31, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Termopoli, V.; Famiglini, G.; Palma, P.; Piergiovanni, M.; Rocio-Bautista, P.; Ottaviani, M.F.; Cappiello, A.; Saeed, M.; Perry, S. Evaluation of a Liquid Electron Ionization Liquid Chromatography–Mass Spectrometry Interface. J. Chromatogr. A 2019, 1591, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, M.; Gadelha, L.; Steinbeck, C.; Sorokina, M.; Peters, K. MAW: The Reproducible Metabolome Annotation Workflow for Untargeted Tandem Mass Spectrometry. J. Cheminform. 2023, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Hopley, C.; Bristow, T.; Lubben, A.; Simpson, A.; Bull, E.; Klagkou, K.; Herniman, J.; Langley, J. Towards a Universal Product Ion Mass Spectral Library–Reproducibility of Product Ion Spectra across Eleven Different Mass Spectrometers. Rapid Commun. Mass Spectrom. 2008, 22, 1779–1786. [Google Scholar] [CrossRef]
- Scheubert, K.; Hufsky, F.; Rasche, F.; Böcker, S. Computing Fragmentation Trees from Metabolite Multiple Mass Spectrometry Data. J. Comput. Biol. 2011, 18, 1383–1397. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Rigano, F.; Tranchida, P.Q.; Dugo, P.; Mondello, L. High-Performance Liquid Chromatography Combined with Electron Ionization Mass Spectrometry: A Review. TrAC-Trends Anal. Chem. 2019, 118, 112–122. [Google Scholar] [CrossRef]
- Demarque, D.P.; Crotti, A.E.M.; Vessecchi, R.; Lopes, J.L.C.; Lopes, N.P. Fragmentation Reactions Using Electrospray Ionization Mass Spectrometry: An Important Tool for the Structural Elucidation and Characterization of Synthetic and Natural Products. Nat. Prod. Rep. 2016, 33, 432–455. [Google Scholar] [CrossRef]
- Carazzone, C.; Rodríguez, J.P.G.; Gonzalez, M.; López, G.-D. Volatilomics of Natural Products: Whispers from Nature. In Metabolomics-Methodology and Applications in Medical Sciences and Life Sciences; IntechOpen: London, UK, 2021; Volume 11, p. 13. ISBN 0000957720. [Google Scholar]
- Stone, J.A.; Fitzgerald, R.L. Liquid Chromatography–Mass Spectrometry Education for Clinical Laboratory Scientists. Clin. Lab. Med. 2018, 38, 527–537. [Google Scholar] [CrossRef]
- Gross, J.H. Mass Spectrometry; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-10709-2. [Google Scholar]
- Vargas Medina, D.A.; Maciel, E.V.S.; de Toffoli, A.L.; Lanças, F.M. Miniaturization of Liquid Chromatography Coupled to Mass Spectrometry. TrAC-Trends Anal. Chem. 2020, 128, 115910. [Google Scholar] [CrossRef]
- Palma, P.; Famiglini, G.; Trufelli, H.; Pierini, E.; Termopoli, V.; Cappiello, A. Electron Ionization in LC-MS: Recent Developments and Applications of the Direct-EI LC-MS Interface. Anal. Bioanal. Chem. 2011, 399, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Vargas Medina, D.A.; Pereira dos Santos, N.G.; Maciel, E.V.S.; Lanças, F.M. Current Prospects on Nano Liquid Chromatography Coupled to Electron Ionization Mass Spectrometry (NanoLC-EI-MS). J. Liq. Chromatogr. Relat. Technol. 2022, 44, 862–871. [Google Scholar] [CrossRef]
- Palma, P.; Pierini, E.; Cappiello, A. LC-MS Interfaces. In Analytical Separation Science; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 87–110. [Google Scholar]
- Famiglini, G.; Palma, P.; Termopoli, V.; Cappiello, A.; Tsizin, S.; Seemann, B.; Alon, T.; Fialkov, A.B.; Amirav, A. Electron Ionization LC-MS. In Comprehensive Analytical Chemistry; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 79, pp. 1–28. [Google Scholar]
- Famiglini, G.; Palma, P.; Termopoli, V.; Cappiello, A. The History of Electron Ionization in LC-MS, from the Early Days to Modern Technologies: A Review. Anal. Chim. Acta 2021, 1167, 338350. [Google Scholar] [CrossRef]
- Cappiello, A.; Balogh, M.; Famiglini, G.; Mangani, F.; Palma, P. An Efficient Liquid Chromatography-Mass Spectrometry Interface for the Generation of Electron Ionization Spectra. Anal. Chem. 2000, 72, 3841–3846. [Google Scholar] [CrossRef]
- Cappiello, A.; Famiglini, G.; Mangani, F.; Palma, P. A Simple Approach for Coupling Liquid Chromatography and Electron Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2002, 13, 265–273. [Google Scholar] [CrossRef]
- Cappiello, A.; Famiglini, G.; Mangani, F.; Palma, P. New Trends in the Application of Electron Ionization to Liquid Chromatography-Mass Spectrometry Interfacing. Mass Spectrom. Rev. 2001, 20, 88–104. [Google Scholar] [CrossRef] [PubMed]
- Flender, C.; Wolf, C.; Leonhard, P.; Karas, M. Nano-Liquid Chromatography-Direct Electron Ionization Mass Spectrometry: Improving Performance by a New Ion Source Adapter. J. Mass Spectrom. 2011, 46, 1004–1010. [Google Scholar] [CrossRef]
- Rigano, F.; Albergamo, A.; Sciarrone, D.; Beccaria, M.; Purcaro, G.; Mondello, L. Nano Liquid Chromatography Directly Coupled to Electron Ionization Mass Spectrometry for Free Fatty Acid Elucidation in Mussel. Anal. Chem. 2016, 88, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- Termopoli, V.; Famiglini, G.; Palma, P.; Piergiovanni, M.; Cappiello, A. Atmospheric Pressure Vaporization Mechanism for Coupling a Liquid Phase with Electron Ionization Mass Spectrometry. Anal. Chem. 2017, 89, 2049–2056. [Google Scholar] [CrossRef]
- Cappiello, A.; Famiglini, G.; Pierini, E.; Palma, P.; Trufelli, H. Advanced Liquid Chromatography-Mass Spectrometry Interface Based on Electron Ionization. Anal. Chem. 2007, 79, 5364–5372. [Google Scholar] [CrossRef] [PubMed]
- Granot, O.; Amirav, A. LC-MS with Electron Ionization of Cold Molecules in Supersonic Molecular Beams. Int. J. Mass Spectrom. 2005, 244, 15–28. [Google Scholar] [CrossRef]
- Seemann, B.; Alon, T.; Tsizin, S.; Fialkov, A.B.; Amirav, A. Electron Ionization LC-MS with Supersonic Molecular Beams-the New Concept, Benefits and Applications. J. Mass Spectrom. 2015, 50, 1252–1263. [Google Scholar] [CrossRef]
- Alon, T.; Amirav, A. Isotope Abundance Analysis Methods and Software for Improved Sample Identification with Supersonic Gas Chromatography/Mass Spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 2579–2588. [Google Scholar] [CrossRef]
- Tsizin, S.; Bokka, R.; Keshet, U.; Alon, T.; Fialkov, A.B.; Tal, N.; Amirav, A. Comparison of Electrospray LC–MS, LC–MS with Cold EI and GC–MS with Cold EI for Sample Identification. Int. J. Mass Spectrom. 2017, 422, 119–125. [Google Scholar] [CrossRef]
- Amirav, A.; Fialkov, A.B.; Gordin, A.; Elkabets, O.; Margolin Eren, K.J. Cold Electron Ionization (EI) Is Not a Supplementary Ion Source to Standard EI. It Is a Highly Superior Replacement Ion Source. J. Am. Soc. Mass Spectrom. 2021, 32, 2631–2635. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Pregibon, T.; Chumanov, K.; Marcus, R.K. Determination of Catechins and Caffeine in Proposed Green Tea Standard Reference Materials by Liquid Chromatography-Particle Beam/Electron Ionization Mass Spectrometry (LC-PB/EIMS). Talanta 2010, 82, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Krishna, M.V.B.; Marcus, R.K. Liquid Chromatography-Particle Beam Electron Ionization Mass Spectrometry Method for Analysis of Botanical Extracts: Evaluation of Ephedrine Alkaloids in Standard Reference Materials. J. AOAC Int. 2010, 93, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Burdette, C.Q.; Phillips, M.M.; Rimmer, C.A.; Marcus, R.K. Determination of Isoflavone Content in SRM 3238 Using Liquid Chromatography-Particle Beam/Electron Ionization Mass Spectrometry. J. AOAC Int. 2015, 98, 1483–1490. [Google Scholar] [CrossRef]
- Trufelli, H.; Famiglini, G.; Termopoli, V.; Cappiello, A. Profiling of Non-Esterified Fatty Acids in Human Plasma Using Liquid Chromatography-Electron Ionization Mass Spectrometry. Anal. Bioanal. Chem. 2011, 400, 2933–2941. [Google Scholar] [CrossRef]
- Cappiello, A.; Famiglini, G.; Palma, P.; Termopoli, V.; Trufelli, H. A New Liquid Chromatography-Mass Spectrometry Approach for Generic Screening and Quantitation of Potential Genotoxic Alkylation Compounds without Derivatization. J. Chromatogr. A 2012, 1255, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Cappiello, A.; Famiglini, G.; Termopoli, V.; Trufelli, H.; Zazzeroni, R.; Jacquoilleot, S.; Radici, L.; Saib, O. Application of Liquid Chromatography-Direct-Electron Ionization-MS in an in vitro Dermal Absorption Study: Quantitative Determination of Trans -Cinnamaldehyde. Anal. Chem. 2011, 83, 8537–8542. [Google Scholar] [CrossRef] [PubMed]
- Rigano, F.; Russo, M.; Arigò, A.; Dugo, P.; Mondello, L. Combining Linear Retention Index and Electron Ionization Mass Spectrometry for a Reliable Identification in Nano Liquid Chromatography. J. Chromatogr. A 2020, 1610, 460581. [Google Scholar] [CrossRef]
- Famiglini, G.; Termopoli, V.; Palma, P.; Cappiello, A. Liquid Chromatography-Electron Ionization Tandem Mass Spectrometry with the Direct-EI Interface in the Fast Determination of Diazepam and Flunitrazepam in Alcoholic Beverages. Electrophoresis 2016, 37, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Riboni, N.; Magrini, L.; Bianchi, F.; Careri, M.; Cappiello, A. Sol-Gel Coated Ion Sources for Liquid Chromatography-Direct Electron Ionization Mass Spectrometry. Anal. Chim. Acta 2017, 978, 35–41. [Google Scholar] [CrossRef]
- Vargas Medina, D.A.; da Burato, J.S.S.; Borsatto, J.V.B.; Lanças, F.M. Porous Layer Open Tubular Nano Liquid Chromatography Directly Coupled to Electron Ionization Mass Spectrometry. J. Chromatogr. A 2022, 1674, 463143. [Google Scholar] [CrossRef]
- Abonamah, J.V.; Eckenrode, B.A.; Moini, M. On-Site Detection of Fentanyl and Its Derivatives by Field Portable Nano-Liquid Chromatography-Electron Lonization-Mass Spectrometry (NLC-EI-MS). Forensic Chem. 2019, 16, 100180. [Google Scholar] [CrossRef]
- Burdette, C.Q.; Marcus, R.K. Determination of Isoflavone Content in Soy, Red Clover, and Kudzu Dietary Supplement Materials by Liquid Chromatography-Particle Beam/Electron Ionization Mass Spectrometry. J. AOAC Int. 2013, 96, 925–932. [Google Scholar] [CrossRef]
- Castro, J.; Krishna, M.V.B.; Ojeda, G.; Marcus, R.K. Selenium Speciation by Liquid Chromatography-Particle Beam/Mass Spectrometry (LC-PB/MS): Application to a Yeast Reference Material and Synthetic Urine. Anal. Methods 2013, 5, 4053. [Google Scholar] [CrossRef]
- Xiang, P.; Yang, Y.; Zhao, Z.; Chen, M.; Liu, S. Ultrafast Gradient Separation with Narrow Open Tubular Liquid Chromatography. Anal. Chem. 2019, 91, 10738–10743. [Google Scholar] [CrossRef]
- Chen, A.; Liu, S.; Yang, Y.; Xiang, P. Liquid Chromatographic Separation Using a 2 Μm i.d. Open Tubular Column at Elevated Temperature. Anal. Chem. 2021, 93, 4361–4364. [Google Scholar] [CrossRef]
| Compound | Summary of the Experimental Conditions | ME ± RSD (%) | ||
|---|---|---|---|---|
| Matrix Investigated | Extraction Method | ESI-MS | Direct-EI LC-MS | |
| Ibuprofen | Human plasma | LLE | 64 ± 22 | 101 ± 5 |
| Human plasma | SPE | 52 ± 14 | 97 ± 4 | |
| Phenacetin | Human plasma | LLE | 135 ± 14 | 99 ± 2 |
| Human plasma | SPE | 123 ± 13 | 101 ± 2 | |
| Matrix | Analytes | Mobile Phase Flow Rate (µL min−1) | Interface | Acquisition Mode | LODs | Ref. |
|---|---|---|---|---|---|---|
| Green tea NIST standard reference | catechins | 900 | PB | TIC and SIM | 5.8–74 ng mL−1 | [42] |
| Botanical extracts | ephedrine alkaloids | 1000 | PB | TIC and SIM | <1.0 ng mL−1 | [43] |
| Botanical extracts | Isoflavones | 1200 | PB | TIC | -- | [44] |
| Human plasma | fatty acids | 0.4 | Direct-EI | TIC and SIM | 1–12 pmol | [45] |
| Mussel tissues | fatty acids | 0.15 | Direct-EI | 0.19–2.25 µg mg−1 | [34] | |
| Acetaminophen 500 mg tablets | potential genotoxic impurities | 0.4 | Direct-EI | SIM | 0.13 to 1.5 μg g−1 | [46] |
| in Vitro Skin Penetration Samples | trans-Cinnamaldehyde | 0.4 | Direct-EI | SIM | 0.1 ng μL−1 | [47] |
| Human Brain | Untargeted | 0.1 | LEI | TIC | -- | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira dos Santos, N.G.; Maciel, E.V.S.; Vargas Medina, D.A.; Lanças, F.M. NanoLC-EI-MS: Perspectives in Biochemical Analysis. Int. J. Mol. Sci. 2023, 24, 11746. https://doi.org/10.3390/ijms241411746
Pereira dos Santos NG, Maciel EVS, Vargas Medina DA, Lanças FM. NanoLC-EI-MS: Perspectives in Biochemical Analysis. International Journal of Molecular Sciences. 2023; 24(14):11746. https://doi.org/10.3390/ijms241411746
Chicago/Turabian StylePereira dos Santos, Natalia Gabrielly, Edvaldo Vasconcelos Soares Maciel, Deyber Arley Vargas Medina, and Fernando Mauro Lanças. 2023. "NanoLC-EI-MS: Perspectives in Biochemical Analysis" International Journal of Molecular Sciences 24, no. 14: 11746. https://doi.org/10.3390/ijms241411746
APA StylePereira dos Santos, N. G., Maciel, E. V. S., Vargas Medina, D. A., & Lanças, F. M. (2023). NanoLC-EI-MS: Perspectives in Biochemical Analysis. International Journal of Molecular Sciences, 24(14), 11746. https://doi.org/10.3390/ijms241411746






