Sex-Specific Protection of Endothelial Function after Vascular Ischemia/Reperfusion Injury by the Senomorphic Agent Ruxolitinib
Abstract
1. Introduction
2. Results
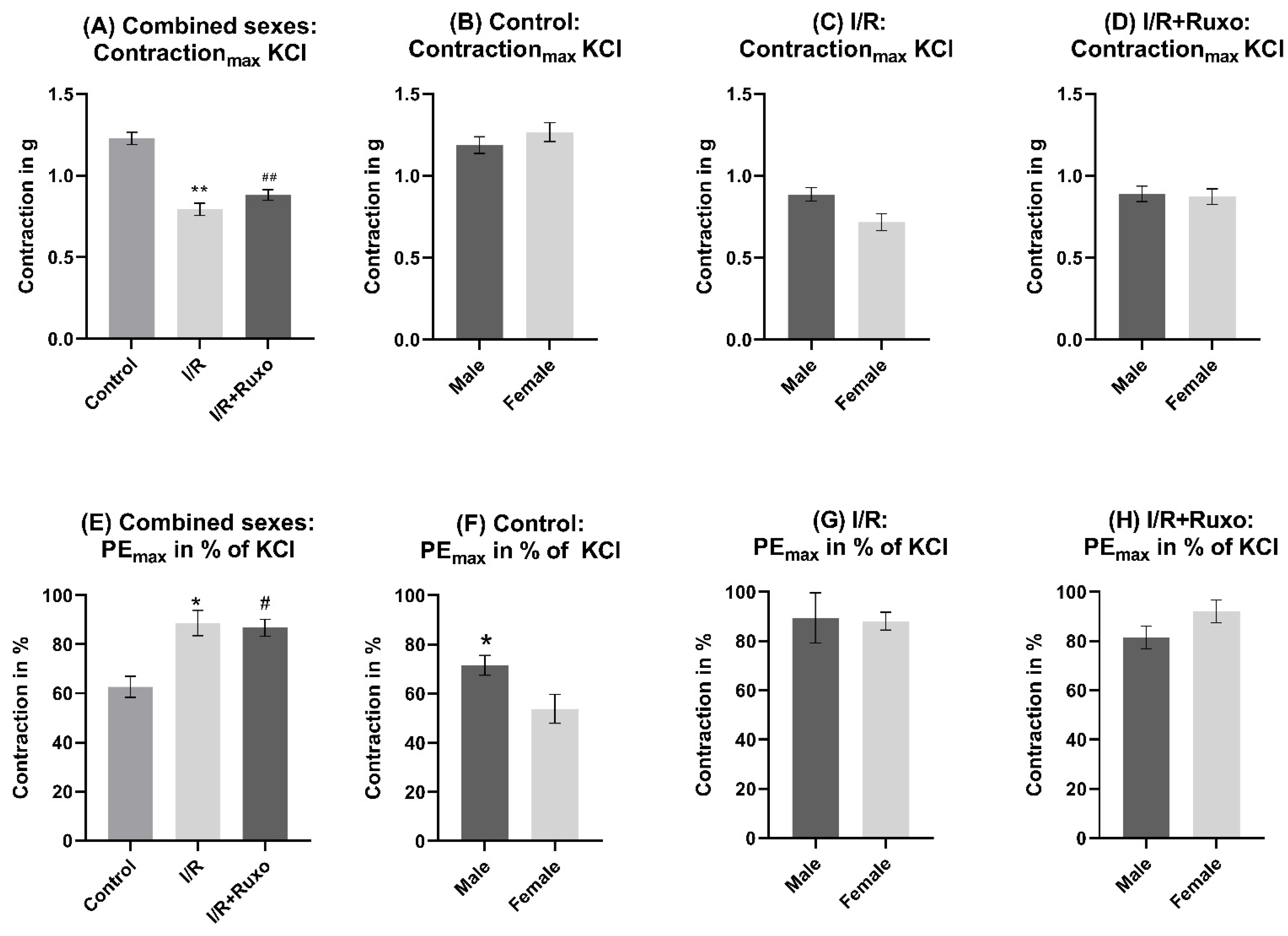
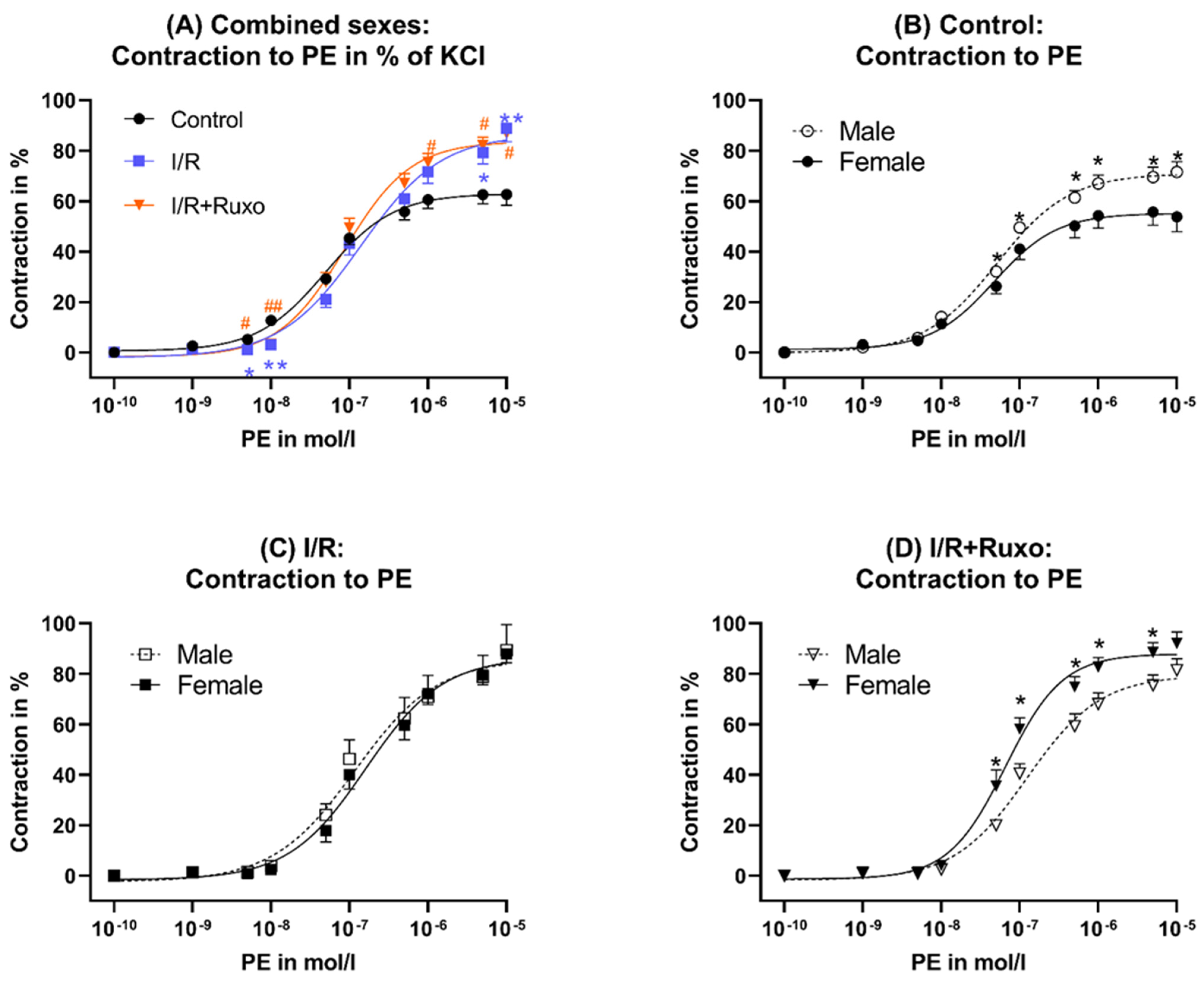
2.1. Vascular Contractility
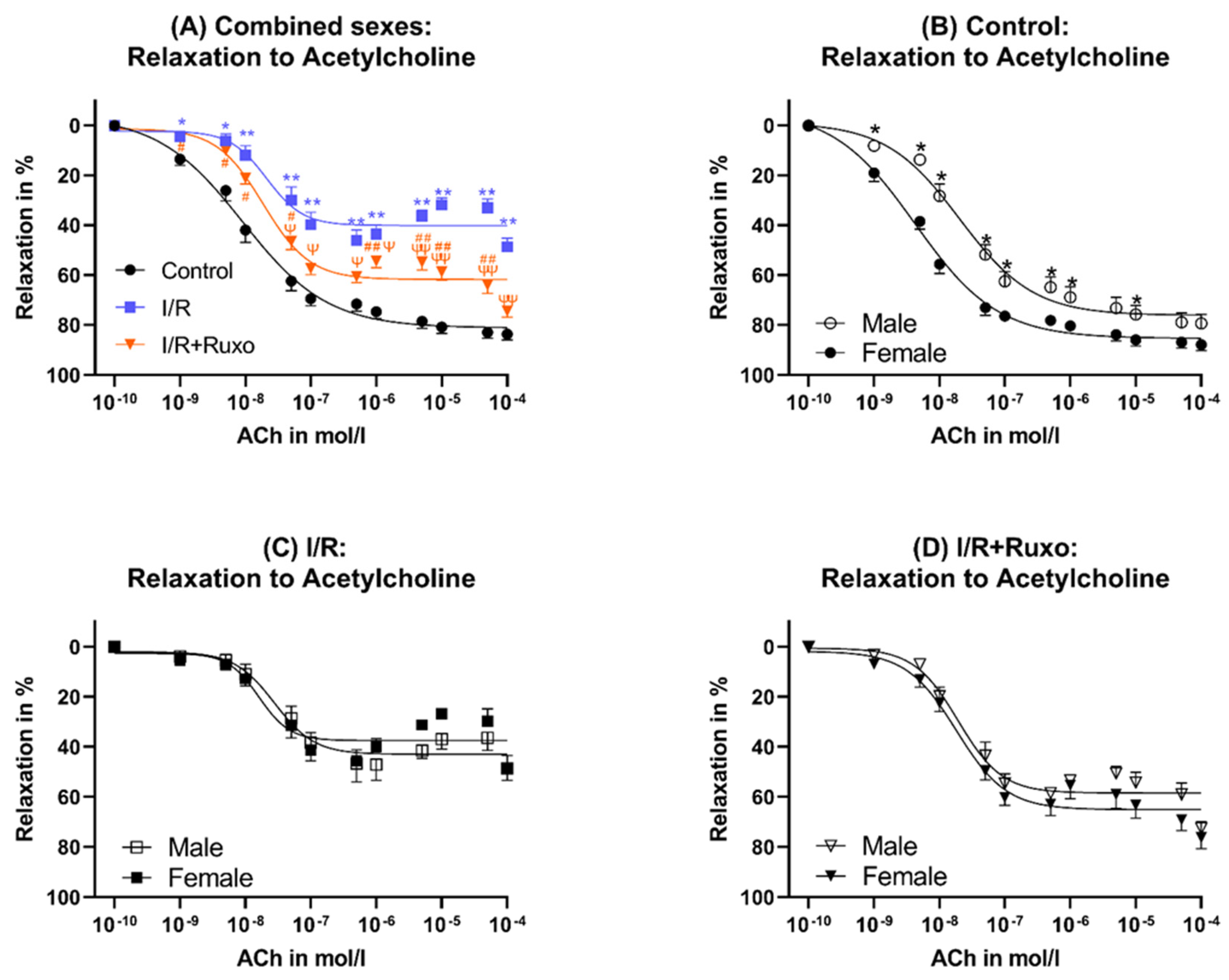
2.2. Endothelial-Dependent Relaxation
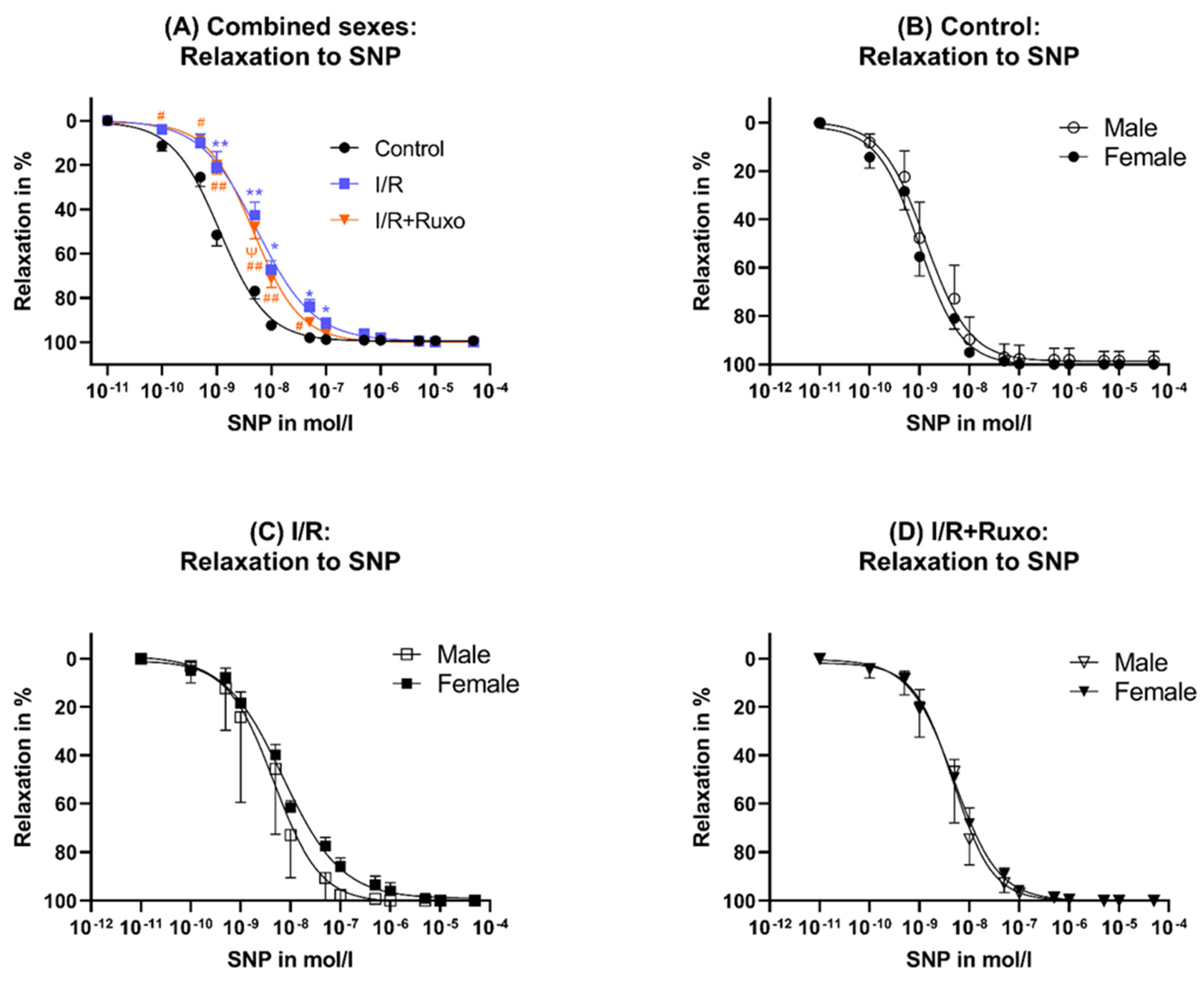
2.3. Endothelial-Independent Relaxation
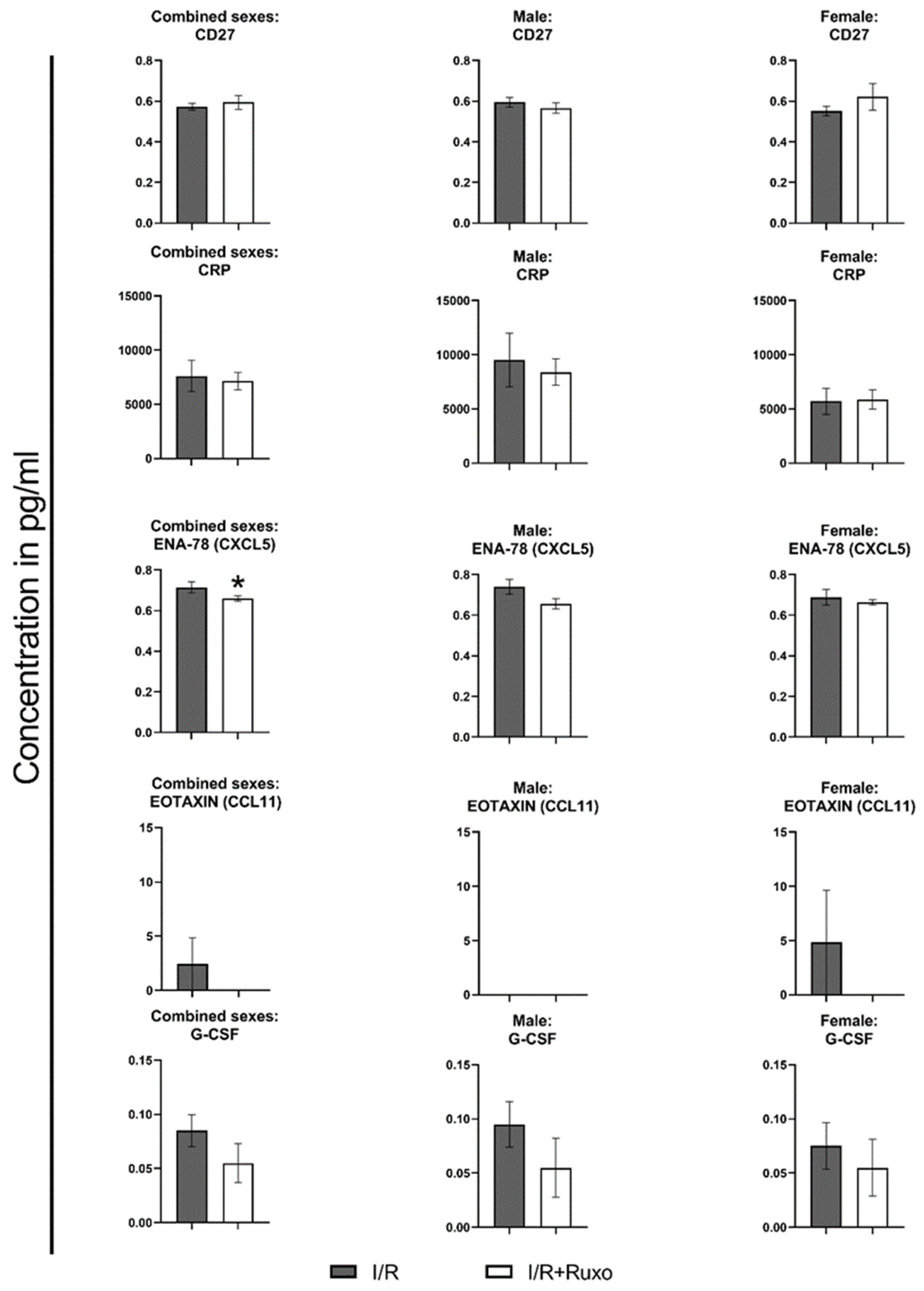
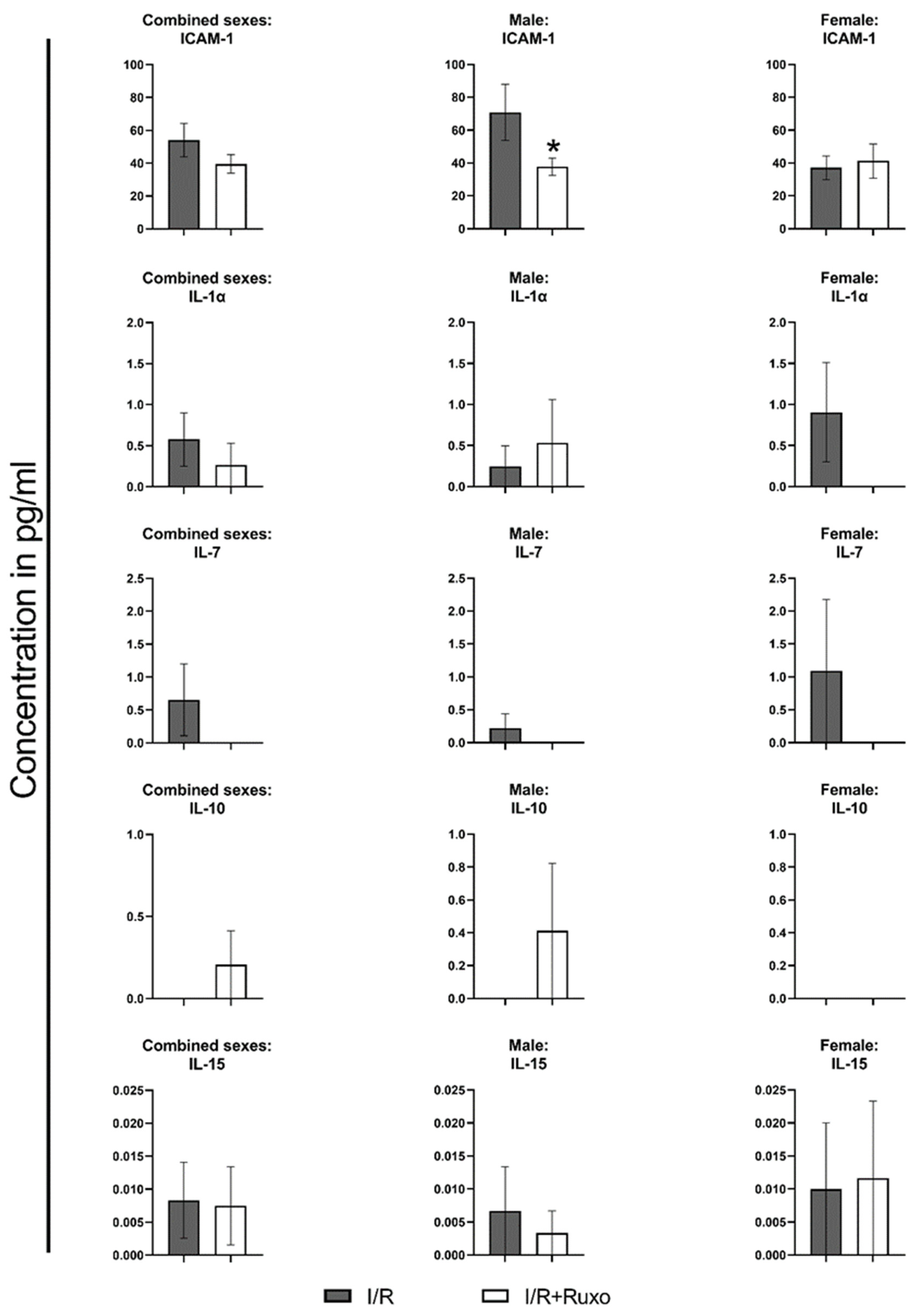
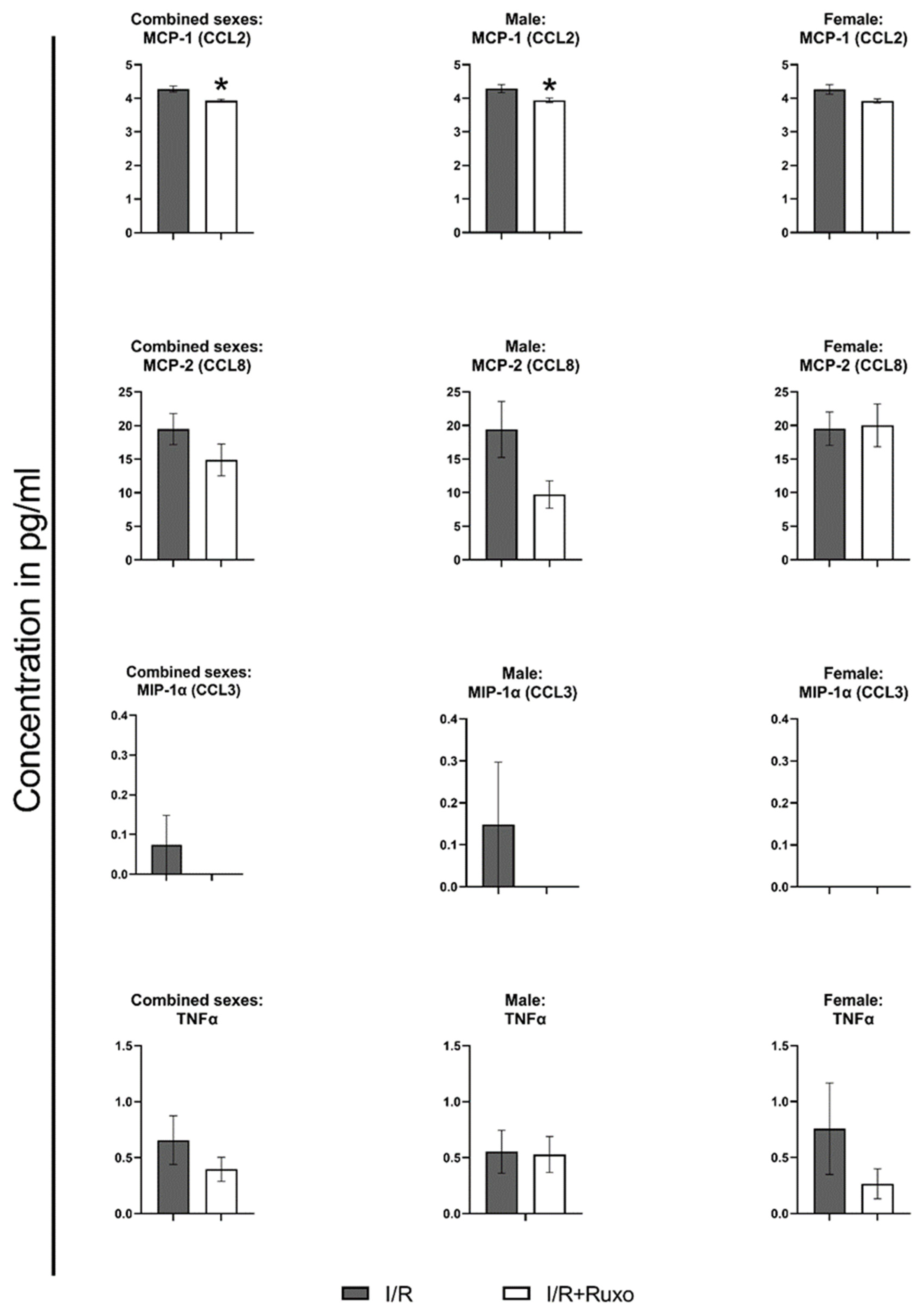
2.4. Cytokine Release
3. Discussion
4. Materials and Methods
4.1. Animals and Anesthesia
4.2. Harvesting and Preparation of the Aorta
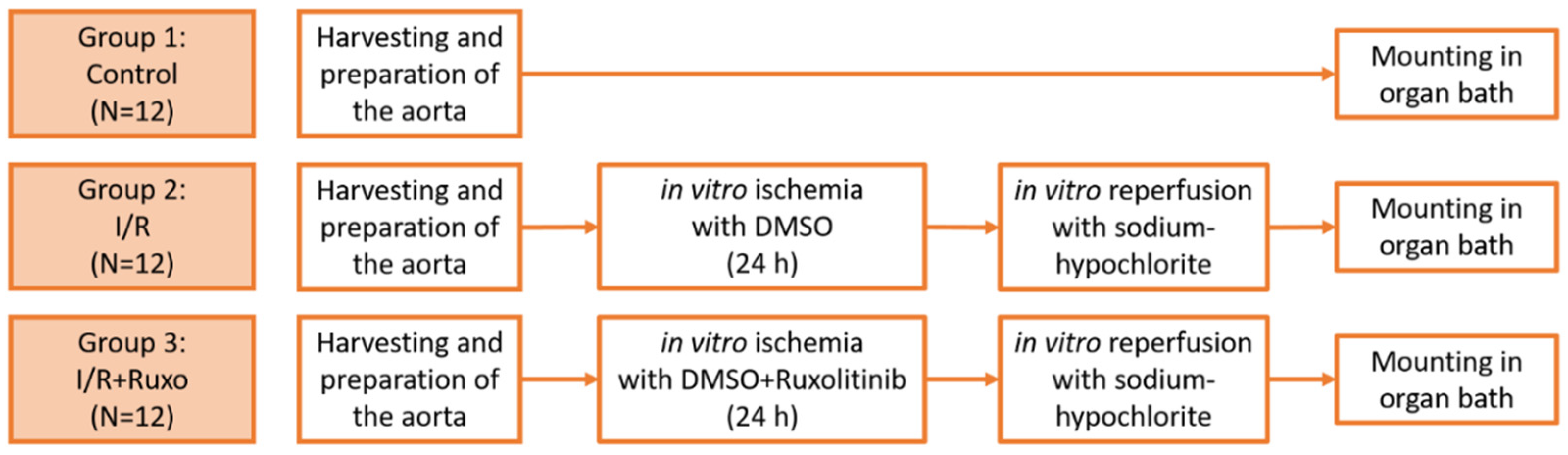
4.3. Groups and In Vitro Ischemia/Reperfusion Model
4.4. Organ Bath Functional Experiments
4.5. Quantification of Cytokine Release
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACh | acetylcholine |
| CABG | coronary artery bypass grafting |
| CCL | CC-chemokine ligand |
| CD | cluster of differentiation |
| CRP | c-reactive protein |
| GRO | growth-regulated-oncogene |
| CXCL | chemokine (C-X-C motif) ligand |
| DMSO | dimethyl sulfoxide |
| ENA | epithelial neutrophil-activating peptide |
| G-CSF | granulocyte-colony-stimulating factor |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| M-CSF | macrophage colony-stimulating factor |
| KCl | potassium chloride |
| KHS | Krebs–Henseleit solution |
| ICAM | intercellular adhesion molecule |
| IL | interleukin |
| IP | interferon-gamma-induced protein |
| JAK | Janus kinase |
| I/R | ischemia/reperfusion |
| MCP | monocyte chemoattractant protein |
| MIG | monokine induced by gamma-interferon |
| MIP | macrophage inflammatory proteins |
| PCI | percutaneous coronary intervention |
| PE | phenylephrine |
| ROS | reactive oxygen species |
| SASP | senescence-associated secretory phenotype |
| SC | senescence cell |
| SEM | standard error of the mean |
| SNP | sodium nitroprusside |
| TNFα | tumor necrosis factor α |
| VEGF-A | vascular endothelial growth factor A |
References
- Lagoumtzi, S.M.; Chondrogianni, N. Senolytics and senomorphics: Natural and synthetic therapeutics in the treatment of aging and chronic diseases. Free Radic. Biol. Med. 2021, 171, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.-Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y.; Li, C.-J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Wilbring, M.; Tugtekin, S.M.; Zatschler, B.; Ebner, A.; Reichenspurner, H.; Matschke, K.; Deussen, A. Even short-time storage in physiological saline solution impairs endothelial vascular function of saphenous vein grafts. Eur. J. Cardiothorac. Surg. 2011, 40, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed]
- Donna, L.; Carden, D. Neil Granger. Pathophysiology of ischaemia–reperfusion injury. Economica 2003, 70, 691–697. [Google Scholar] [CrossRef]
- Shawky, A.M.; Almalki, F.A.; Abdalla, A.N.; Abdelazeem, A.H.; Gouda, A.M. A Comprehensive Overview of Globally Approved JAK Inhibitors. Pharmaceutics 2022, 14, 1001. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Martens, U.M. (Ed.) Small Molecules in Hematology, 3rd ed.; Springer International Publishing: Cham, Switzerland, 2018; ISBN 9783319914398. [Google Scholar]
- Xu, M.; Tchkonia, T.; Ding, H.; Ogrodnik, M.; Lubbers, E.R.; Pirtskhalava, T.; White, T.A.; Johnson, K.O.; Stout, M.B.; Mezera, V.; et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl. Acad. Sci. USA 2015, 112, E6301–E6310. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Maranini, B.; Bortoluzzi, A.; Silvagni, E.; Govoni, M. Focus on Sex and Gender: What We Need to Know in the Management of Rheumatoid Arthritis. J. Pers. Med. 2022, 12, 499. [Google Scholar] [CrossRef]
- Erkens, R.; Totzeck, M.; Brum, A.; Duse, D.; Bøtker, H.E.; Rassaf, T.; Kelm, M. Endothelium-dependent remote signaling in ischemia and reperfusion: Alterations in the cardiometabolic continuum. Free Radic. Biol. Med. 2021, 165, 265–281. [Google Scholar] [CrossRef]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Haque, N.S.; Fallon, J.T.; Taubman, M.B.; Harpel, P.C. The chemokine receptor CCR8 mediates human endothelial cell chemotaxis induced by I-309 and Kaposi sarcoma herpesvirus-encoded vMIP-I and by lipoprotein(a)-stimulated endothelial cell conditioned medium. Blood 2001, 97, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Reutershan, J.; Morris, M.A.; Burcin, T.L.; Smith, D.F.; Chang, D.; Saprito, M.S.; Ley, K. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J. Clin. Investig. 2006, 116, 695–702. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, T.; Chen, L.; Luo, W.; Chao, J. MCP-1 mediates ischemia-reperfusion-induced cardiomyocyte apoptosis via MCPIP1 and CaSR. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H59–H71. [Google Scholar] [CrossRef]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Holbrook, J.; Lara-Reyna, S.; Jarosz-Griffiths, H.; McDermott, M. Tumour necrosis factor signalling in health and disease. F1000Research 2019, 8, 111. [Google Scholar] [CrossRef]
- Lawson, C.; Wolf, S. ICAM-1 signaling in endothelial cells. Pharmacol. Rep. 2009, 61, 22–32. [Google Scholar] [CrossRef]
- Korkmaz-Icöz, S.; Ballikaya, B.; Soethoff, J.; Kraft, P.; Sayour, A.A.; Radovits, T.; Loganathan, S.; Karck, M.; Szabó, G.; Veres, G. Graft Preservation Solution DuraGraft® Alleviates Vascular Dysfunction Following In Vitro Ischemia/Reperfusion Injury in Rats. Pharmaceuticals 2021, 14, 1028. [Google Scholar] [CrossRef]
- Wiessner, R.; Eisold, S.; Linnebacher, M.; Bünger, C.; Nizze, H.; Wacke, R.; Benz, S.; Schareck, W.; Klar, E. Up-regulation of ICAM-1 during cold ischemia triggers early neutrophil infiltration in human pancreas allograft reperfusion. Transplant. Proc. 2009, 41, 3622–3627. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Y.; Tu, Y.-F.; Lin, Y.-C.; Huang, C.-C. CXCL5 signaling is a shared pathway of neuroinflammation and blood-brain barrier injury contributing to white matter injury in the immature brain. J. Neuroinflam. 2016, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Cuollo, L.; Antonangeli, F.; Santoni, A.; Soriani, A. The Senescence-Associated Secretory Phenotype (SASP) in the Challenging Future of Cancer Therapy and Age-Related Diseases. Biology 2020, 9, 485. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2022, 290, 1362–1383. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Wu, C.-Y.; Peng, H.-H.; Voisin, L.; Perfettini, J.-L.; Ko, Y.-F.; Young, J.D. Emerging use of senolytics and senomorphics against aging and chronic diseases. Med. Res. Rev. 2020, 40, 2114–2131. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zingarelli, B.; Szabó, C. Crucial role of endogenous interleukin-10 production in myocardial ischemia/reperfusion injury. Circulation 2000, 101, 1019–1026. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion--from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Wang, M.; Crisostomo, P.; Wairiuko, G.M.; Meldrum, D.R. Estrogen receptor-alpha mediates acute myocardial protection in females. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2204–H2209. [Google Scholar] [CrossRef]
- Melo, Z.; Gutierrez-Mercado, Y.K.; Garcia-Martínez, D.; Portilla-de-Buen, E.; Canales-Aguirre, A.A.; Gonzalez-Gonzalez, R.; Franco-Acevedo, A.; Palomino, J.; Echavarria, R. Sex-dependent mechanisms involved in renal tolerance to ischemia-reperfusion: Role of inflammation and histone H3 citrullination. Transpl. Immunol. 2020, 63, 101331. [Google Scholar] [CrossRef]
- Rajasimhan, S.; Pamuk, O.; Katz, J.D. Safety of Janus Kinase Inhibitors in Older Patients: A Focus on the Thromboembolic Risk. Drugs Aging 2020, 37, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jian, Z.; Zhong, Y.; Ye, Y.; Zhang, Y.; Hu, X.; Pu, B.; Gu, L.; Xiong, X. Janus Kinase Inhibition Ameliorates Ischemic Stroke Injury and Neuroinflammation Through Reducing NLRP3 Inflammasome Activation via JAK2/STAT3 Pathway Inhibition. Front. Immunol. 2021, 12, 714943. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Luo, M.; Li, R.; Huang, Y.; Zhang, R.; Wu, Q.; Wang, F.; Li, Y.; Yu, X. Blockage of JAK/STAT signalling attenuates renal ischaemia-reperfusion injury in rat. Nephrol. Dial. Transpl. 2008, 23, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Khashab, F.; Al-Saleh, F.; Al-Kandari, N.; Fadel, F.; Al-Maghrebi, M. JAK Inhibition Prevents DNA Damage and Apoptosis in Testicular Ischemia-Reperfusion Injury via Modulation of the ATM/ATR/Chk Pathway. Int. J. Mol. Sci. 2021, 22, 13390. [Google Scholar] [CrossRef]
- Benhamú, B.; Martín-Fontecha, M.; Vázquez-Villa, H.; López-Rodríguez, M.L.; Ortega-Gutiérrez, S. New Trends in Aging Drug Discovery. Biomedicines 2022, 10, 2006. [Google Scholar] [CrossRef]
- Radovits, T.; Lin, L.; Zotkina, J.; Koch, A.; Rauen, U.; Köhler, G.; Karck, M.; Szabó, G. Endothelial Dysfunction After Long-term Cold Storage in HTK Organ Preservation Solutions: Effects of Iron Chelators and N-α-acetyl-l-histidine. J. Heart Lung Transplant. 2008, 27, 208–216. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saemann, L.; Naujoks, P.; Hartrumpf, L.; Pohl, S.; Simm, A.; Szabó, G. Sex-Specific Protection of Endothelial Function after Vascular Ischemia/Reperfusion Injury by the Senomorphic Agent Ruxolitinib. Int. J. Mol. Sci. 2023, 24, 11727. https://doi.org/10.3390/ijms241411727
Saemann L, Naujoks P, Hartrumpf L, Pohl S, Simm A, Szabó G. Sex-Specific Protection of Endothelial Function after Vascular Ischemia/Reperfusion Injury by the Senomorphic Agent Ruxolitinib. International Journal of Molecular Sciences. 2023; 24(14):11727. https://doi.org/10.3390/ijms241411727
Chicago/Turabian StyleSaemann, Lars, Paula Naujoks, Lotta Hartrumpf, Sabine Pohl, Andreas Simm, and Gábor Szabó. 2023. "Sex-Specific Protection of Endothelial Function after Vascular Ischemia/Reperfusion Injury by the Senomorphic Agent Ruxolitinib" International Journal of Molecular Sciences 24, no. 14: 11727. https://doi.org/10.3390/ijms241411727
APA StyleSaemann, L., Naujoks, P., Hartrumpf, L., Pohl, S., Simm, A., & Szabó, G. (2023). Sex-Specific Protection of Endothelial Function after Vascular Ischemia/Reperfusion Injury by the Senomorphic Agent Ruxolitinib. International Journal of Molecular Sciences, 24(14), 11727. https://doi.org/10.3390/ijms241411727





