Pars Distalis and Pars Tuberalis Thyroid-Stimulating Hormones and Their Roles in Macro-Thyroid-Stimulating Hormone Formation
Abstract
1. Introduction
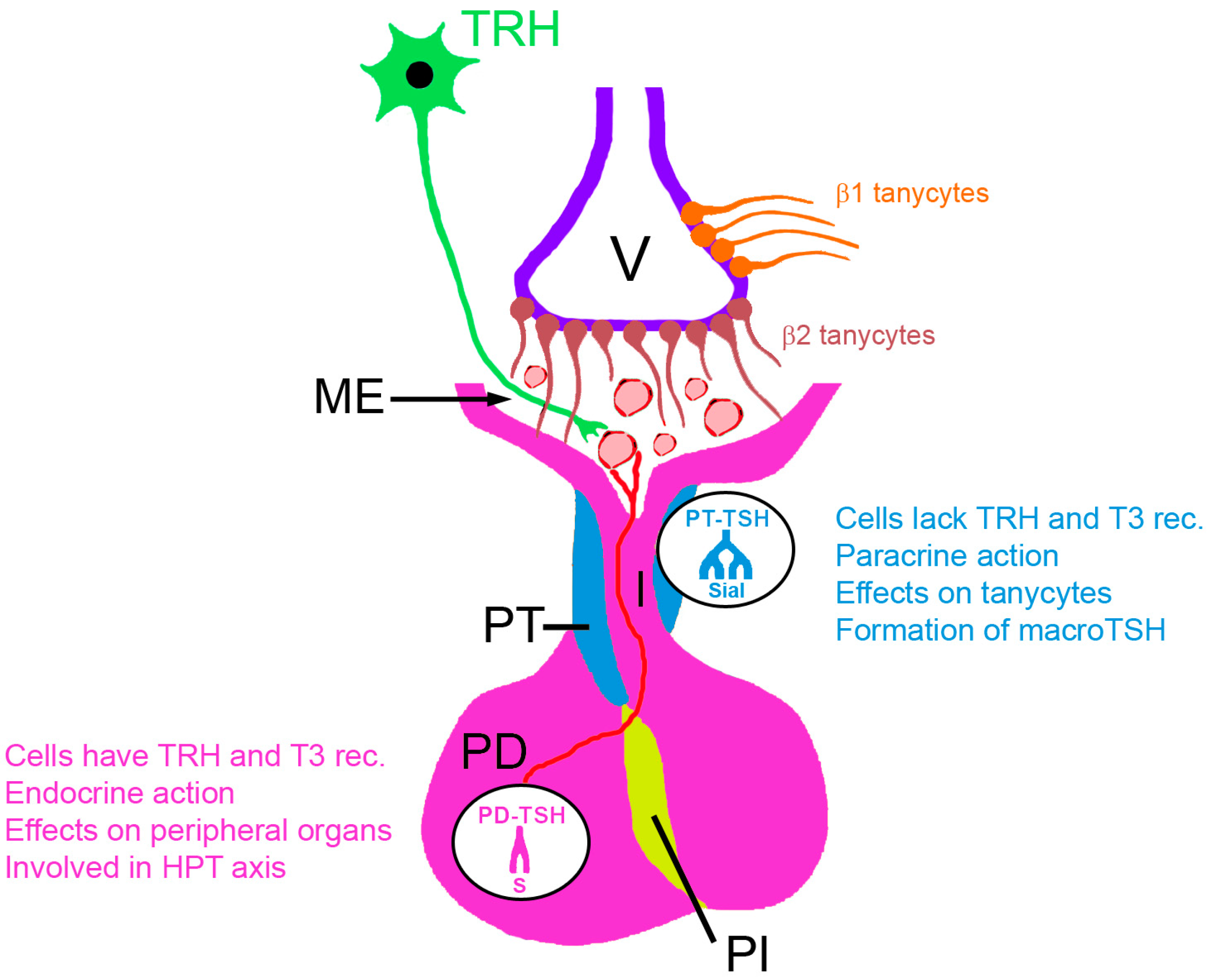
2. The Two Sources of Pituitary TSH
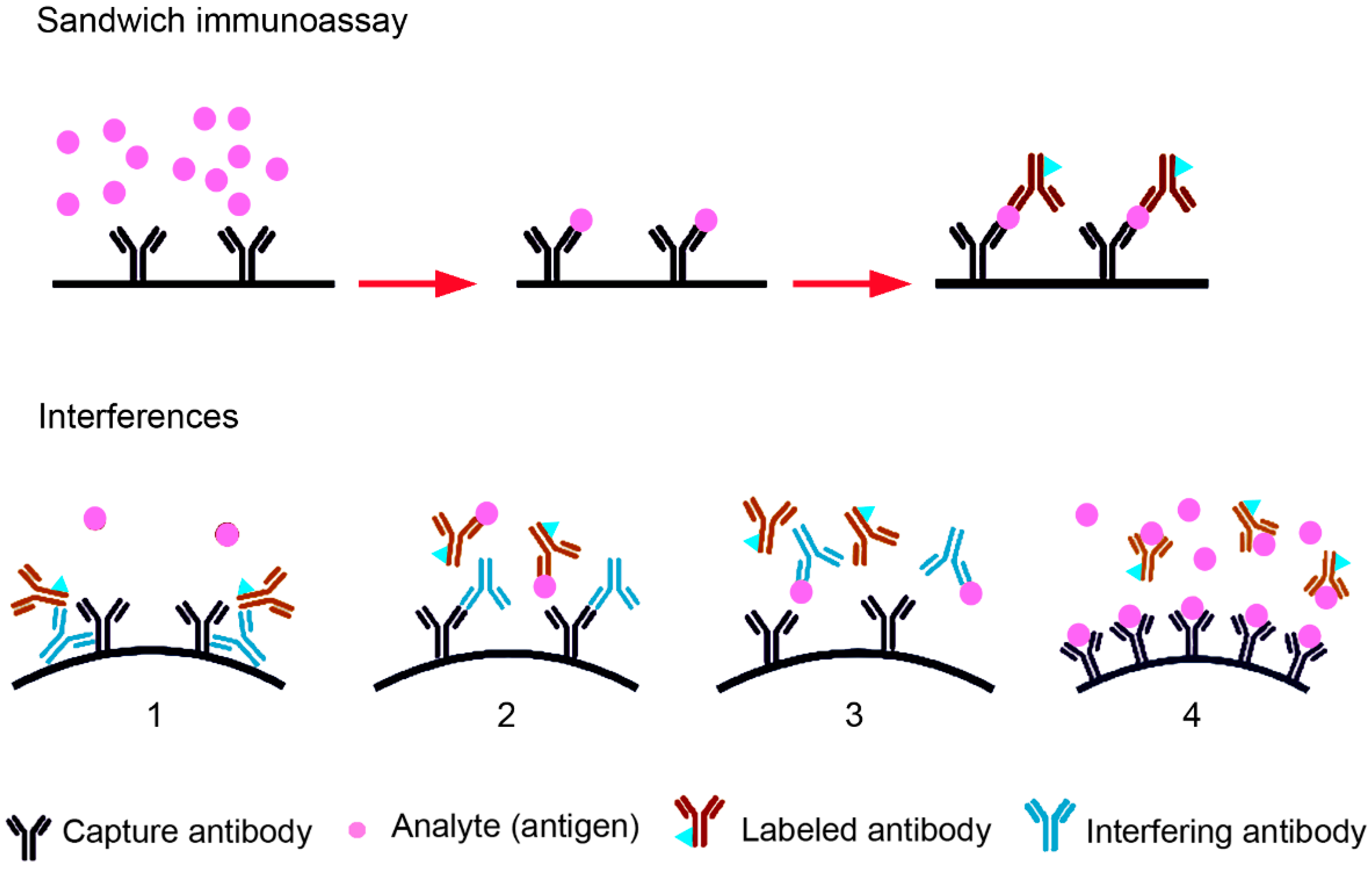
3. Routine TSH Determination in the Laboratory and Interference with the Assays Used
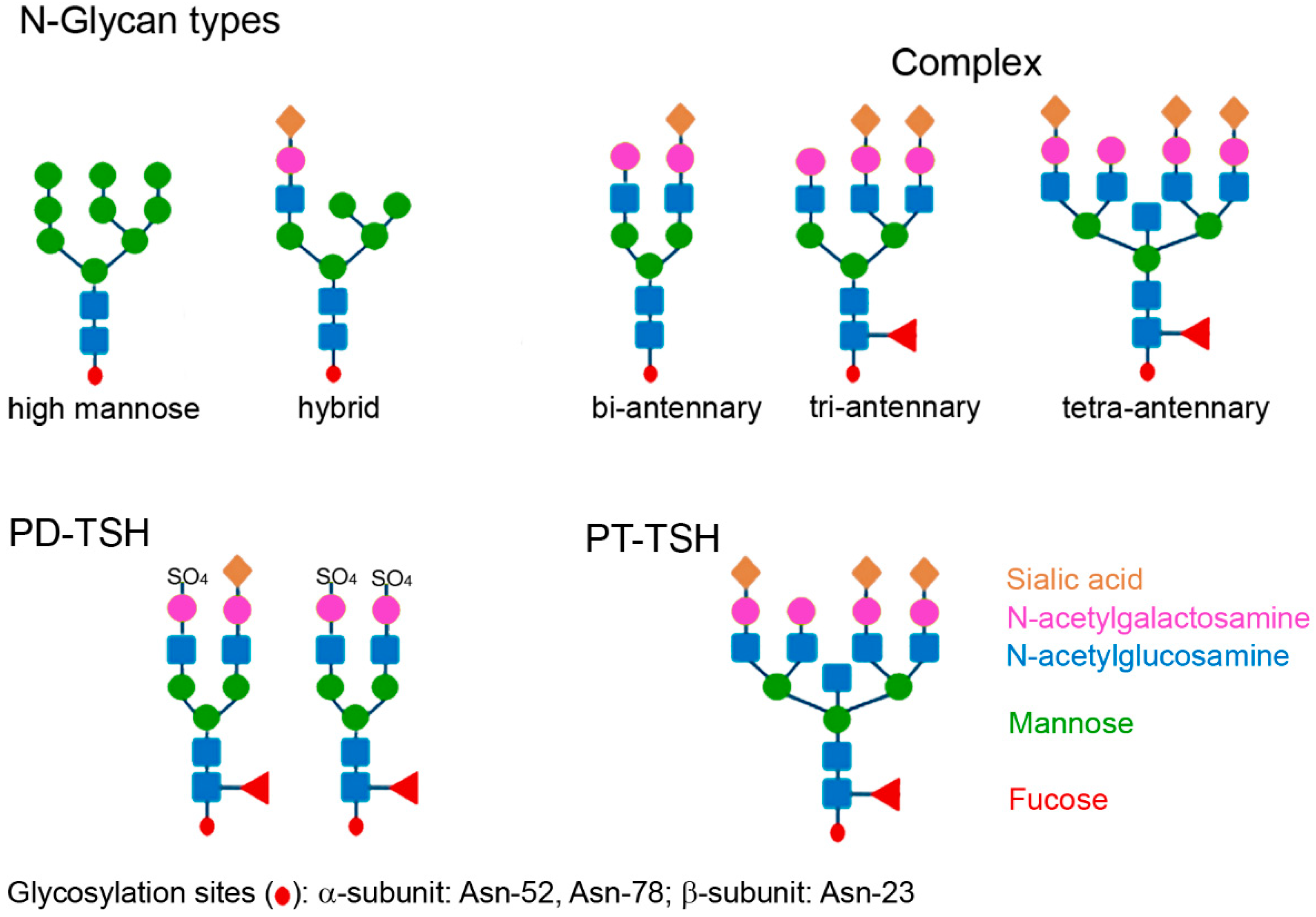
4. The Role of Glycosylation in Macro-TSH Formation
5. Identification and Prevalence of Macro-TSH
6. Characteristics of Macro-TSH and Hypotheses concerning Its Role in Humans
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abs | Antibodies |
| ACTH | Adrenocorticotropic hormone |
| BBB | Blood–brain barrier |
| CGH | Corticotroph-derived glycoprotein hormone |
| CSF | Cerebrospinal fluid |
| DiO2 | Deiodinase type 2 |
| DiO3 | Deiodinase type 3 |
| EEG | Electroencephalogram |
| FSC | Follicle stellate cell |
| FSH | Follicle stimulating hormone |
| fT3, fT4 | Free triiodothyronine, free thyroxine |
| GFAP | Glial fibrillary acidic protein |
| GFC | Gel filtration chromatography |
| HAMA | Human anti-mouse Antibodies |
| hCG | Human chorionic gonadotropin |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| HPT | Hypothalamus pituitary thyroid |
| IP3 | Inositol trisphosphate |
| IST | Inappropriate secretion of TSH |
| LH | Luteinizing hormone |
| MT1a | Melatonin receptor 1a |
| ME | Median eminence |
| MSH | Melanocyte-stimulating hormone |
| NACB | National academy of clinical biochemistry |
| PD | Pars distalis |
| PEG | Polyethylene glycol |
| PFA | Perifornical area |
| RMP | Reference measurement procedure |
| RI | Reference interval |
| PT | Pars tuberalis |
| PRL | Prolactin |
| SCH | Subclinical hypothyroidism |
| SCN | Suprachiasmatic nucleus |
| SI | Système international d’unités |
| SITSH | Syndrome of inappropriate secretion of TSH |
| SPINA-GT | Thyroid homeostasis parameter structure parameter inference approach-Gain of Thyroid |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| T2DM | Type 2 diabetes mellitus |
| TRH | Thyrotropin releasing hormone |
| TSH | Thyroid-stimulating hormone |
| V | Ventricle |
References
- Ikegami, K.; Liao, X.H.; Hoshino, Y.; Ono, H.; Ota, W.; Ito, Y.; Nishiwaki-Ohkawa, T.; Sato, C.; Kitajima, K.; Iigo, M.; et al. Tissue-specific posttranslational modification allows functional targeting of thyrotropin. Cell Rep. 2014, 9, 801–810. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Fliers, E.; Franke, A.N.; Wortel, J.; Buijs, R.M. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology 2000, 141, 3832–3841. [Google Scholar] [CrossRef]
- Ikegami, K.; Refetoff, S.; Van Cauter, E.; Yoshimura, T. Interconnection between circadian clocks and thyroid function. Nat. Rev. Endocrinol. 2019, 15, 590–600. [Google Scholar] [CrossRef]
- Chiamolera, M.I.; Wondisford, F.E. Thyrotropin-Releasing Hormone and the Thyroid Hormone Feedback Mechanism. Endocrinology 2009, 150, 1091–1096. [Google Scholar] [CrossRef]
- Hanon, E.A.; Lincoln, G.A.; Fustin, J.M.; Dardente, H.; Masson-Pévet, M.; Morgan, P.J.; Hazlerigg, D.G. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr. Biol. 2008, 18, 1147–1152. [Google Scholar] [CrossRef]
- Dash, P. Blood Brain Barrier and Cerebral Metabolism. In Homeostasis and Higher Brain Functions; Update October 2020; Department of Neurobiology and Anatomy, McGovern Medical School: Houston, TX, USA, 2020; Available online: https://nba.uth.tmc.edu/neuroscience/m/s4/chapter11.html#:~:text=Areas%20of%20brain%20without%20a,Area%20postrema (accessed on 2 May 2023).
- Korf, H.W.; Møller, M. Arcuate nucleus, median eminence, and hypophysial pars tuberalis. Handb. Clin. Neurol. 2021, 180, 227–251. [Google Scholar] [CrossRef]
- Loh, T.P.; Kao, S.L.; Halsall, D.J.; Toh, S.A.; Chan, E.; Ho, S.C.; Tai, E.S.; Khoo, C.M. Macro-thyrotropin: A case report and review of literature. J. Clin. Endocrinol. Metab. 2012, 97, 1823–1828. [Google Scholar] [CrossRef]
- Kennaway, D.; Voultsios, A.; Varcoe, T.; Moyer, R. Melatonin in mice: Rhythms, response to light, adrenergic stimulation, and metabolism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R358–R365. [Google Scholar] [CrossRef]
- Weaver, D.R.; Stehle, J.H.; Stopa, E.G.; Reppert, S.M. Melatonin receptors in human hypothalamus and pituitary: Implications for circadian and reproductive responses to melatonin. J. Clin. Endocrinol. Metab. 1993, 76, 295–301. [Google Scholar] [CrossRef]
- Wu, Y.H.; Zhou, J.N.; Balesar, R.; Unmehopa, U.; Bao, A.; Jockers, R.; Van Heerikhuize, J.; Swaab, D.F. Distribution of MT1 melatonin receptor immunoreactivity in the human hypothalamus and pituitary gland: Colocalization of MT1 with vasopressin, oxytocin, and corticotropin-releasing hormone. J. Comp. Neurol. 2006, 499, 897–910. [Google Scholar] [CrossRef]
- Yamada, S.; Horiguchi, K.; Akuzawa, M.; Sakamaki, K.; Shimomura, Y.; Kobayashi, I.; Andou, Y.; Yamada, M. Seasonal Variation in Thyroid Function in Over 7,000 Healthy Subjects in an Iodine-sufficient Area and Literature Review. J. Endocr. Soc. 2022, 6, bvac054. [Google Scholar] [CrossRef] [PubMed]
- Kuzmenko, N.V.; Tsyrlin, V.A.; Pliss, M.G.; Galagudza, M.M. Seasonal variations in levels of human thyroid-stimulating hormone and thyroid hormones: A meta-analysis. Chronobiol. Int. 2021, 38, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhang, G.; Xu, P.; Guo, R.; Li, J.; Guan, H.; Li, Y. Seasonal Changes of Thyroid Function Parameters in Women of Reproductive Age Between 2012 and 2018: A Retrospective, Observational, Single-Center Study. Front. Endocrinol. 2021, 12, 719225. [Google Scholar] [CrossRef]
- Santi, D.; Spaggiari, G.; Brigante, G.; Setti, M.; Tagliavini, S.; Trenti, T.; Simoni, M. Semi-annual seasonal pattern of serum thyrotropin in adults. Sci. Rep. 2019, 9, 10786. [Google Scholar] [CrossRef] [PubMed]
- Contet, C.; Goulding, S.P.; Kuljis, D.A.; Barth, A.L. International Review of Neurobiology; Candice, C., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 128, pp. 281–342. [Google Scholar]
- Wittmann, G.; Farkas, E.; Szilvásy-Szabó, A.; Gereben, B.; Fekete, C.; Lechan, R.M. Variable proopiomelanocortin expression in tanycytes of the adult rat hypothalamus and pituitary stalk. J. Comp. Neurol. 2017, 525, 411–441. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, A.; Lazcano, I.; Sánchez-Jaramillo, E.; Uribe, R.M.; Jaimes-Hoy, L.; Joseph-Bravo, P.; Charli, J.L. Tanycytes and the Control of Thyrotropin-Releasing Hormone Flux Into Portal Capillaries. Front. Endocrinol. 2019, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Farkas, E.; Varga, E.; Kovács, B.; Szilvásy-Szabó, A.; Cote-Vélez, A.; Péterfi, Z.; Matziari, M.; Tóth, M.; Zelena, D.; Mezriczky, Z.; et al. A Glial-Neuronal Circuit in the Median Eminence Regulates Thyrotropin-Releasing Hormone-Release via the Endocannabinoid System. iScience 2020, 23, 100921. [Google Scholar] [CrossRef]
- Nakayama, T.; Yoshimura, T. Seasonal Rhythms: The Role of Thyrotropin and Thyroid Hormones. Thyroid 2018, 28, 4–10. [Google Scholar] [CrossRef]
- Bolborea, M.; Helfer, G.; Ebling, F.J.; Barrett, P. Dual signal transduction pathways activated by TSH receptors in rat primary tanycyte cultures. J. Mol. Endocrinol. 2015, 54, 241–250. [Google Scholar] [CrossRef]
- Wood, S.; Loudon, A. The pars tuberalis: The site of the circannual clock in mammals? Gen. Comp. Endocrinol. 2018, 258, 222–235. [Google Scholar] [CrossRef]
- Prummel, M.F.; Brokken, L.J.; Wiersinga, W.M. Ultra short-loop feedback control of thyrotropin secretion. Thyroid 2004, 14, 825–829. [Google Scholar] [CrossRef]
- Pires, M.; Tortosa, F. Update on Pituitary Folliculo-Stellate Cells. Int. Arch. Endocrinol. Clin. Res. 2006, 2, 006. [Google Scholar] [CrossRef]
- Fliers, E.; Unmehopa, U.A.; Alkemade, A. Functional neuroanatomy of thyroid hormone feedback in the human hypothalamus and pituitary gland. Mol. Cell. Endocrinol. 2006, 251, 1–8. [Google Scholar] [CrossRef]
- Pfaff, D.W.; Rubin, R.T.; Schneider, J.E.; Head, G.A. Principles of Hormone/Behavior Relations, 2nd ed.; Pfaff, D.W., Rubin, R.T., Schneider, J.E., Head, G.A., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 293–314. [Google Scholar]
- Coomans, C.P.; Ramkisoensing, A.; Meijer, J.H. The suprachiasmatic nuclei as a seasonal clock. Front. Neuroendocrinol. 2015, 37, 29–42. [Google Scholar] [CrossRef]
- Hu, K.; Scheer, F.A.; Ivanov, P.; Buijs, R.M.; Shea, S.A. The suprachiasmatic nucleus functions beyond circadian rhythm generation. Neuroscience 2007, 149, 508–517. [Google Scholar] [CrossRef]
- Sheehan, M.T. Biochemical Testing of the Thyroid: TSH is the Best and, Oftentimes, Only Test Needed—A Review for Primary Care. Clin. Med. Res. 2016, 14, 83–92. [Google Scholar] [CrossRef]
- Gronfier, C.; Brandenberger, G. Ultradian rhythms in pituitary and adrenal hormones: Their relations to sleep. Sleep Med. Rev. 1998, 2, 17–29. [Google Scholar] [CrossRef]
- Coppeta, L.; Di Giampaolo, L.; Rizza, S.; Balbi, O.; Baldi, S.; Pietroiusti, A.; Magrini, A. Relationship between the night shift work and thyroid disorders: A systematic review and meta-analysis. Endocr. Regul. 2020, 54, 64–70. [Google Scholar] [CrossRef]
- Wu, K.; Zhou, Y.; Ke, S.; Huang, J.; Gao, X.; Li, B.; Lin, X.; Liu, X.; Liu, X.; Ma, L.; et al. Lifestyle is associated with thyroid function in subclinical hypothyroidism: A cross-sectional study. BMC Endocr. Disord. 2021, 21, 112. [Google Scholar] [CrossRef]
- Kim, W.; Lee, J.; Ha, J.; Jo, K.; Lim, D.J.; Lee, J.M.; Chang, S.A.; Kang, M.I.; Kim, M.H. Association between Sleep Duration and Subclinical Thyroid Dysfunction Based on Nationally Representative Data. J. Clin. Med. 2019, 8, 2010. [Google Scholar] [CrossRef]
- Van der Spoel, E.; Roelfsema, F.; Van Heemst, D. Within-Person Variation in Serum Thyrotropin Concentrations: Main Sources, Potential Underlying Biological Mechanisms, and Clinical Implications. Front. Endocrinol. 2021, 12, 619568. [Google Scholar] [CrossRef]
- Samuels, M.H.; Henry, P.; Luther, M.; Ridgway, E.C. Pulsatile TSH secretion during 48-hour continuous TRH infusions. Thyroid 1993, 3, 201–206. [Google Scholar] [CrossRef]
- Okada, S.L.; Ellsworth, J.L.; Durnam, D.M.; Haugen, H.S.; Holloway, J.L.; Kelley, M.L.; Lewis, K.E.; Ren, H.; Sheppard, P.O.; Storey, H.M.; et al. A glycoprotein hormone expressed in corticotrophs exhibits unique binding properties on thyroid-stimulating hormone receptor. Mol. Endocrinol. 2006, 20, 414–425. [Google Scholar] [CrossRef]
- Karponis, D.; Ananth, S. The role of thyrostimulin and its potential clinical significance. Endocr. Regul. 2017, 51, 117–128. [Google Scholar] [CrossRef]
- Ghazal, K.; Brabant, S.; Prie, D.; Piketty, M.L. Hormone Immunoassay Interference: A 2021 Update. Ann. Lab. Med. 2022, 42, 3–23. [Google Scholar] [CrossRef]
- Favresse, J.; Burlacu, M.C.; Maiter, D.; Gruson, D. Interferences With Thyroid Function Immunoassays: Clinical Implications and Detection Algorithm. Endocr. Rev. 2018, 39, 830–850. [Google Scholar] [CrossRef]
- Morton, A. When lab tests lie … heterophile antibodies. Aust. Fam. Physician 2014, 43, 391–393. [Google Scholar]
- Rulander, N.J.; Cardamone, D.; Senior, M.; Snyder, P.J.; Master, S.R. Interference from anti-streptavidin antibody. Arch. Pathol. Lab. Med. 2013, 137, 1141–1146. [Google Scholar] [CrossRef]
- Vos, M.J.; Rondeel, J.M.M.; Mijnhout, G.S.; Endert, E. Immunoassay interference caused by heterophilic antibodies interacting with biotin. Clin. Chem. Lab. Med. 2017, 55, e122–e126. [Google Scholar] [CrossRef]
- Gessl, A.; Blueml, S.; Bieglmayer, C.; Marculescu, R. Anti-ruthenium antibodies mimic macro-TSH in electrochemiluminescent immunoassay. Clin. Chem. Lab. Med. 2014, 52, 1589–1594. [Google Scholar] [CrossRef]
- Buijs, M.M.; Gorgels, J.P.; Endert, E. Interference by antiruthenium antibodies in the Roche thyroid-stimulating hormone assay. Ann. Clin. Biochem. 2011, 48, 276–281. [Google Scholar] [CrossRef]
- Saleem, M.; Martin, H.; Coates, P. Prolactin Biology and Laboratory Measurement: An Update on Physiology and Current Analytical Issues. Clin. Biochem. Rev. 2018, 39, 3–16. [Google Scholar]
- De Oliveira Andrade, L.J.; Matos de Oliveira, G.C. “Incidentalormones”—Macro-hormones. SciELO 2021. preprints. [Google Scholar] [CrossRef]
- Kasum, M.; Oreskovic, S.; Zec, I.; Jezek, D.; Tomic, V.; Gall, V.; Adzic, G. Macroprolactinemia: New insights in hyperprolactinemia. Biochem. Med. 2012, 22, 171–179. [Google Scholar] [CrossRef]
- Day, R. Encyclopedia of the Neuroscience; Squire, L.R., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 1139–1141. [Google Scholar]
- Varki, A.; Cummings, R.; Esko, J.; Stanly, P.; Hart, G.; Aebi, M.; Mohnen, D.; Kinoshita, T.; Packer, N.; Prestegard, J.; et al. Essentials of Glycobiology, 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022. [Google Scholar]
- Ruiz-Canada, C.; Kelleher, D.J.; Gilmore, R. Cotranslational and posttranslational N-glycosylation of polypeptides by distinct mammalian OST isoforms. Cell 2009, 136, 272–283. [Google Scholar] [CrossRef]
- Ząbczyńska, M.; Kozłowska, K.; Pocheć, E. Glycosylation in the Thyroid Gland: Vital Aspects of Glycoprotein Function in Thyrocyte Physiology and Thyroid Disorders. Int. J. Mol. Sci. 2018, 19, 2792. [Google Scholar] [CrossRef]
- Fahie-Wilson, M. In hyperprolactinemia, testing for macroprolactin is essential. Clin. Chem. 2003, 49, 1434–1436. [Google Scholar] [CrossRef]
- Wide, L.; Eriksson, K. Thyrotropin N-glycosylation and Glycan Composition in Severe Primary Hypothyroidism. J. Endocr. Soc. 2021, 5, bvab006. [Google Scholar] [CrossRef]
- Donadio, S.; Pascual, A.; Thijssen, J.H.; Ronin, C. Feasibility study of new calibrators for thyroid-stimulating hormone (TSH) immunoprocedures based on remodeling of recombinant TSH to mimic glycoforms circulating in patients with thyroid disorders. Clin. Chem. 2006, 52, 286–297. [Google Scholar] [CrossRef]
- Gesundheit, N.; Magner, J.A.; Chen, T.; Weintraub, B.D. Differential sulfation and sialylation of secreted mouse thyrotropin (TSH) subunits: Regulation by TSH-releasing hormone. Endocrinology 1986, 119, 455–463. [Google Scholar] [CrossRef]
- Szkudlinski, M.W.; Fremont, V.; Ronin, C.; Weintraub, B.D. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiol. Rev. 2002, 82, 473–502. [Google Scholar] [CrossRef]
- Trojan, J.; Theodoropoulou, M.; Usadel, K.H.; Stalla, G.K.; Schaaf, L. Modulation of human thyrotropin oligosaccharide structures—Enhanced proportion of sialylated and terminally galactosylated serum thyrotropin isoforms in subclinical and overt primary hypothyroidism. J. Endocrinol. 1998, 158, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Wide, L.; Eriksson, K. Unique Pattern of N-Glycosylation, Sialylation, and Sulfonation on TSH Molecules in Serum of Children Up to 18 Months. J. Clin. Endocrinol. Metab. 2019, 104, 4651–4659. [Google Scholar] [CrossRef] [PubMed]
- Beck-Peccoz, P.; Persani, L. Variable biological activity of thyroid-stimulating hormone. Eur. J. Endocrinol. 1994, 131, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Persani, L.; Ferretti, E.; Borgato, S.; Faglia, G.; Beck-Peccoz, P. Circulating thyrotropin bioactivity in sporadic central hypothyroidism. J. Clin. Endocrinol. Metab. 2000, 85, 3631–3635. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.M.; Soldin, D.; Buckey, T.M.; Burman, K.D.; Soldin, O.P. Thyrotropin isoforms: Implications for thyrotropin analysis and clinical practice. Thyroid 2014, 24, 411–423. [Google Scholar] [CrossRef]
- Persani, L.; Borgato, S.; Romoli, R.; Asteria, C.; Pizzocaro, A.; Beck-Peccoz, P. Changes in the degree of sialylation of carbohydrate chains modify the biological properties of circulating thyrotropin isoforms in various physiological and pathological states. J. Clin. Endocrinol. Metab. 1998, 83, 2486–2492. [Google Scholar] [CrossRef]
- Horimoto, M.; Nishikawa, M.; Ishihara, T.; Yoshikawa, N.; Yoshimura, M.; Inada, M. Bioactivity of thyrotropin (TSH) in patients with central hypothyroidism: Comparison between in vivo 3,5,3′-triiodothyronine response to TSH and in vitro bioactivity of TSH. J. Clin. Endocrinol. Metab. 1995, 80, 1124–1128. [Google Scholar] [CrossRef]
- Ertek, S. Molecular economy of nature with two thyrotropins from different parts of the pituitary: Pars tuberalis thyroid-stimulating hormone and pars distalis thyroid-stimulating hormone. Arch. Med. Sci. 2021, 17, 189–195. [Google Scholar] [CrossRef]
- Peters, T.J. All about Albumin: Biochemistry, Genetics, and Medical Applications; Academic Press: Cambridge, MA, USA, 1996. [Google Scholar]
- Fukushita, M.; Watanabe, N.; Yoshimura Noh, J.; Yoshihara, A.; Matsumoto, M.; Suzuki, N.; Yoshimura, R.; Sugino, K.; Ito, K. A case of macro-TSH consisting of IgA-bound TSH. Endocr. J. 2021, 68, 1241–1246. [Google Scholar] [CrossRef]
- Orgiazzi, J. The Concept of Macro-TSH Revisited. Clin. Thyroidol. 2021, 27, 26–29. [Google Scholar] [CrossRef]
- Hattori, N.; Ishihara, T.; Matsuoka, N.; Saito, T.; Shimatsu, A. Anti-thyrotropin autoantibodies in patients with macro-thyrotropin and long-term changes in macro-thyrotropin and serum thyrotropin levels. Thyroid 2017, 27, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Richa, V.; Rahul, G.; Sarika, A. Macroprolactin; a frequent cause of misdiagnosed hyperprolactinemia in clinical practice. J. Reprod. Infertil. 2010, 11, 161–167. [Google Scholar] [PubMed]
- Larsen, C.B.; Petersen, E.R.B.; Overgaard, M.; Bonnema, S.J. Macro-TSH: A Diagnostic Challenge. Eur. Thyroid J. 2021, 10, 93–97. [Google Scholar] [CrossRef]
- Hattori, N.; Ishihara, T.; Shimatsu, A. Variability in the detection of macro TSH in different immunoassay systems. Eur. J. Endocrinol. 2016, 174, 9–15. [Google Scholar] [CrossRef]
- Mendoza, H.; Connacher, A.; Srivastava, R. Unexplained high thyroid stimulating hormone: A “BIG” problem. BMJ Case Rep. 2009, 2009, bcr0120091474. [Google Scholar] [CrossRef]
- Rix, M.; Laurberg, P.; Porzig, C.; Kristensen, S.R. Elevated thyroid-stimulating hormone level in a euthyroid neonate caused by macro thyrotropin-IgG complex. Acta Paediatr. 2011, 100, e135–e137. [Google Scholar] [CrossRef] [PubMed]
- Mills, F.; Jeffery, J.; Mackenzie, P.; Cranfield, A.; Ayling, R.M. An immunoglobulin G complexed form of thyroid-stimulating hormone (macro thyroid-stimulating hormone) is a cause of elevated serum thyroid-stimulating hormone concentration. Ann. Clin. Biochem. 2013, 50, 416–420. [Google Scholar] [CrossRef]
- Picazo-Perea, M.; Ruiz-Gines, M.; Ruiz-Gines, J.; Sastre-Marcos, J.; Agudo-Macazaga, M.; Lorenzo-Lozano, M. Macro-TSH in COVID-19 Patients with an Underlying Thyroid Condition: A Case Series and Literature Review. Ann. Thyroid Res. 2021, 7, 312–319. [Google Scholar]
- Giusti, M.; Conte, L.; Repetto, A.M.; Gay, S.; Marroni, P.; Mittica, M.; Mussap, M. Detection of Polyethylene Glycol Thyrotropin (TSH) Precipitable Percentage (Macro-TSH) in Patients with a History of Thyroid Cancer. Endocrinol. Metab. 2017, 32, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Ohba, K.; Maekawa, M.; Iwahara, K.; Suzuki, Y.; Matsushita, A.; Sasaki, S.; Oki, Y.; Nakamura, H. Abnormal thyroid hormone response to TRH in a case of macro-TSH and the cut-off value for screening cases of inappropriate TSH elevation. Endocr. J. 2020, 67, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Peynirci, H.; Ersoy, C.; Sahin, A.; Imamoglu, S. Macro-TSH Can be a Rare Cause of Elevated Serum Thyroid Stimulating Hormone Concentration: A Case Report. Med. Sci. 2014, 3, 1691–1696. [Google Scholar] [CrossRef]
- D’Arcy, R.; Hunter, S.; Spence, K.; McDonnell, M. A Case of macro-TSH masquerading as subclinical hypothyroidism. BMJ Case Rep. 2021, 14, e243436. [Google Scholar] [CrossRef]
- Kirac, C.O.; Abusoglu, S.; Paydas Hataysal, E.; Kebapcilar, A.; Ipekci, S.H.; Ünlü, A.; Kebapcilar, L. A rare cause of subclinical hypothyroidism: Macro-thyroid-stimulating hormone. Diagnosis 2020, 7, 75–77. [Google Scholar] [CrossRef]
- Hattori, N.; Ishihara, T.; Yamagami, K.; Shimatsu, A. Macro TSH in patients with subclinical hypothyroidism. Clin. Endocrinol. 2015, 83, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, H.; Takeoka, K.; Nishi, I.; Shindoh, Y.; Tsukada, Y.; Amino, N. Novel thyrotropin (TSH)-TSH antibody complex in a healthy woman and her neonates. Thyroid 1995, 5, 299–303. [Google Scholar] [CrossRef]
- Verhoye, E.; Van den Bruel, A.; Delanghe, J.R.; Debruyne, E.; Langlois, M.R. Spuriously high thyrotropin values due to anti-thyrotropin antibodies in adult patients. Clin. Chem. Lab. Med. 2009, 47, 604–606. [Google Scholar] [CrossRef]
- Biktagirova, E.M.; Vagapova, G.R.; Semakov, G.P.; Zolotoverkchova, N.I.; Nevzorova, T.A.; Andrianova, I.A.; Evtyugina, N.G.; Akberova, N.I.; Khisamutdinov, A.N.; Abramova, Z.I. Detection of macro-thyrotropinaemia in patients with Hashimotos thyroiditis and subclinical hypothyroidism. Med. Immunol. 2019, 21, 1063–1072. [Google Scholar] [CrossRef]
- Lewis, E.; Lim, R.; Joseph, F.; Ewins, D.; Goenka, N.; Bowles, S.; Faye, S.; Kertesz, G. Recognising macro-TSH: A rare cause of inappropriately high TSH values. Clin. Chem. Lab. Med. 2011, 49, S421. [Google Scholar]
- Ni, J.; Yu, L.; Li, J.; Zhang, L.; Yang, Q.; Kou, C.; Li, S.; Tian, G.; Wang, Y.; Liu, X.; et al. Interference Due to Heterophilic Antibody, Biotin and Thyroid Hormone Autoantibody. Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- The American Thyroid Association. Standardization and Harmonization. 2019. Available online: https://www.thyroid.org/wp-content/uploads/publications/lab-services/ata-harmonization-standardization.pdf (accessed on 10 May 2023).
- Baloch, Z.; Carayon, P.; Conte-Devolx, B.; Demers, L.M.; Feldt-Rasmussen, U.; Henry, J.F.; LiVosli, V.A.; Niccoli-Sire, P.; John, R.; Ruf, J.; et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 2003, 13, 3–126. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Clerico, A.; Zaninotto, M.; Trenti, T.; Tozzoli, R.; Aloe, R.; Alfano, A.; Rizzardi, S.; Dittadi, R.; Migliardi, M.; et al. Percentile transformation and recalibration functions allow harmonization of thyroid-stimulating hormone (TSH) immunoassay results. Clin. Chem. Lab. Med. 2020, 58, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Mankarious, S.; Lee, M.; Fischer, S.; Pyun, K.H.; Ochs, H.D.; Oxelius, V.A.; Wedgwood, R.J. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J. Lab. Clin. Med. 1988, 112, 634–640. [Google Scholar]
- Sakai, H.; Fukuda, G.; Suzuki, N.; Watanabe, C.; Odawara, M. Falsely elevated thyroid-stimulating hormone (TSH) level due to macro-TSH. Endocr. J. 2009, 56, 435–440. [Google Scholar] [CrossRef]
- Shimatsu, A.; Hattori, N. Macroprolactinemia: Diagnostic, clinical, and pathogenic significance. Clin. Dev. Immunol. 2012, 2012, 167132. [Google Scholar] [CrossRef]
- Sheikhi, V.; Heidari, Z. Increase in Thyrotropin Is Associated with an Increase in Serum Prolactin in Euthyroid Subjects and Patients with Subclinical Hypothyroidism. Med. J. Islam. Repub. Iran 2021, 35, 167. [Google Scholar] [CrossRef]
- Elenkova, A.; Petrossians, P.; Zacharieva, S.; Beckers, A. High prevalence of autoimmune thyroid diseases in patients with prolactinomas: A cross-sectional retrospective study in a single tertiary referral centre. Ann. Endocrinol. 2016, 77, 37–42. [Google Scholar] [CrossRef]
- Onal, E.D.; Saglam, F.; Sacikara, M.; Ersoy, R.; Cakir, B. Thyroid autoimmunity in patients with hyperprolactinemia: An observational study. Arq. Bras. Endocrinol. Metabol. 2014, 58, 48–52. [Google Scholar] [CrossRef]
- Hekimsoy, Z.; Kafesçiler, S.; Güçlü, F.; Ozmen, B. The prevalence of hyperprolactinaemia in overt and subclinical hypothyroidism. Endocr. J. 2010, 57, 1011–1015. [Google Scholar] [CrossRef]
- Kadoya, M.; Koyama, S.; Morimoto, A.; Miyoshi, A.; Kakutani, M.; Hamamoto, K.; Kurajoh, M.; Shoji, T.; Moriwaki, Y.; Koshiba, M.; et al. Serum Macro TSH Level is Associated with Sleep Quality in Patients with Cardiovascular Risks—HSCAA Study. Sci. Rep. 2017, 7, 44387. [Google Scholar] [CrossRef] [PubMed]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, T.V.; Succurro, E.; Arturi, F.; Giancotti, A.; Peronace, C.; Quirino, A.; Sesti, F.; Andreozzi, F.; Hribal, M.L.; Perticone, F.; et al. Serum IgG2 levels are specifically associated with whole-body insulin-mediated glucose disposal in non-diabetic offspring of type 2 diabetic individuals: A cross-sectional study. Sci. Rep. 2018, 8, 13616. [Google Scholar] [CrossRef] [PubMed]
- Gulcelik, N.E.; Usman, A. Macroprolactinaemia in diabetic patients. Neuro Endocrinol. Lett. 2010, 31, 270–274. [Google Scholar] [PubMed]
- Hattori, N.; Aisaka, K.; Yamada, A.; Matsuda, T.; Shimatsu, A. Prevalence and pathogenesis of macro-TSH in neonates: Analysis of umbilical cord blood from 939 neonates and their mothers. Thyroid 2022, 33, 45–52. [Google Scholar] [CrossRef]
- McCarthy, A.; Moran, C. A grave interference: TSH interference due to macro-TSH post-thyroidectomy for graves” disease. Endocr. Abstr. 2022, 82, WC4. [Google Scholar] [CrossRef]
- Ohba, K. An Update on the Pathophysiology and Diagnosis of Inappropriate Secretion of Thyroid-Stimulating Hormone. Int. J. Mol. Sci. 2021, 22, 6611. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fröhlich, E.; Wahl, R. Pars Distalis and Pars Tuberalis Thyroid-Stimulating Hormones and Their Roles in Macro-Thyroid-Stimulating Hormone Formation. Int. J. Mol. Sci. 2023, 24, 11699. https://doi.org/10.3390/ijms241411699
Fröhlich E, Wahl R. Pars Distalis and Pars Tuberalis Thyroid-Stimulating Hormones and Their Roles in Macro-Thyroid-Stimulating Hormone Formation. International Journal of Molecular Sciences. 2023; 24(14):11699. https://doi.org/10.3390/ijms241411699
Chicago/Turabian StyleFröhlich, Eleonore, and Richard Wahl. 2023. "Pars Distalis and Pars Tuberalis Thyroid-Stimulating Hormones and Their Roles in Macro-Thyroid-Stimulating Hormone Formation" International Journal of Molecular Sciences 24, no. 14: 11699. https://doi.org/10.3390/ijms241411699
APA StyleFröhlich, E., & Wahl, R. (2023). Pars Distalis and Pars Tuberalis Thyroid-Stimulating Hormones and Their Roles in Macro-Thyroid-Stimulating Hormone Formation. International Journal of Molecular Sciences, 24(14), 11699. https://doi.org/10.3390/ijms241411699







