Protective Effect of Electroacupuncture on Chemotherapy-Induced Salivary Gland Hypofunction in a Mouse Model
Abstract
1. Introduction
2. Results
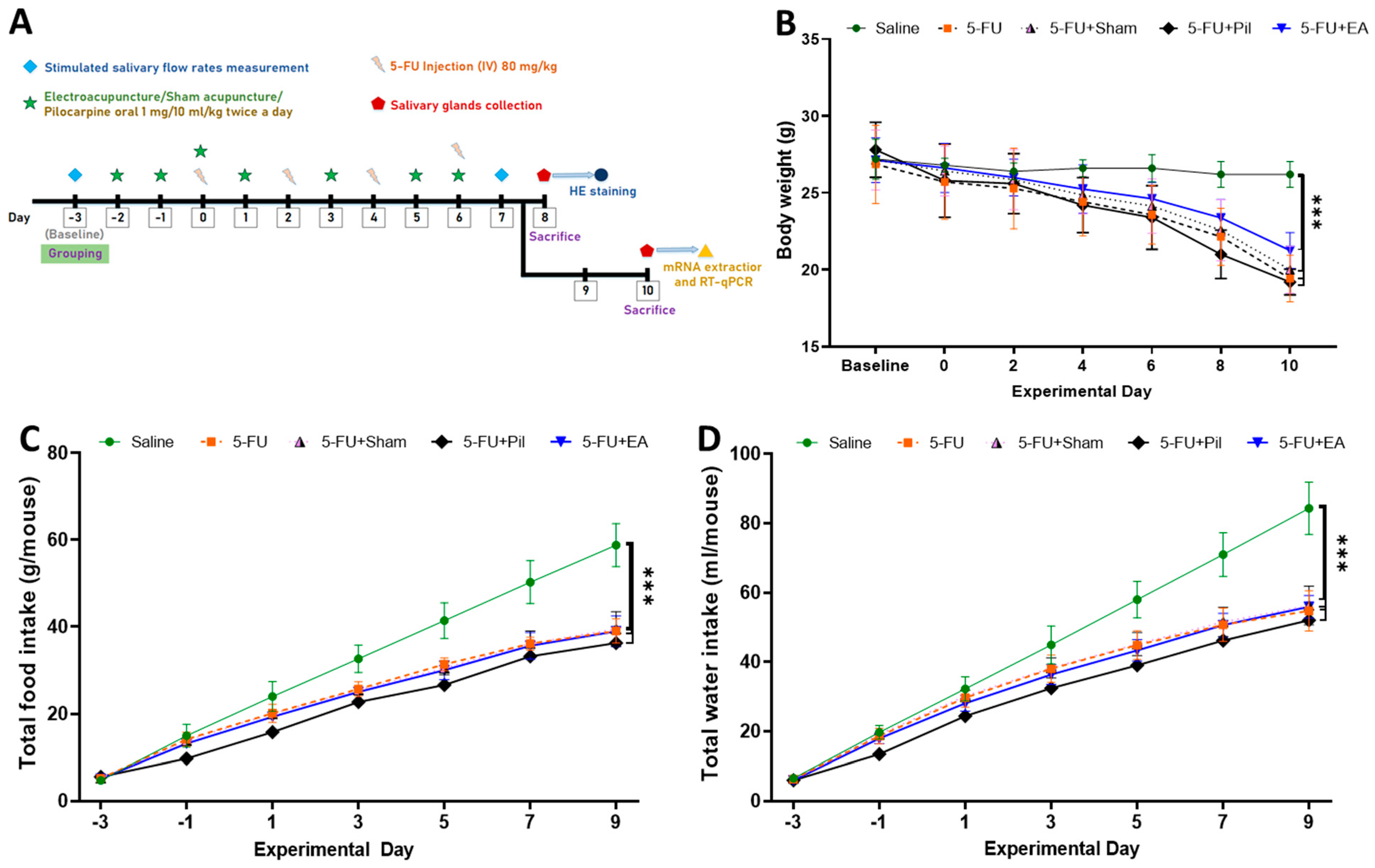
2.1. Body Weight, Accumulated Food, and Water Consumption Were Decreased in 5−FU-Injected Mice
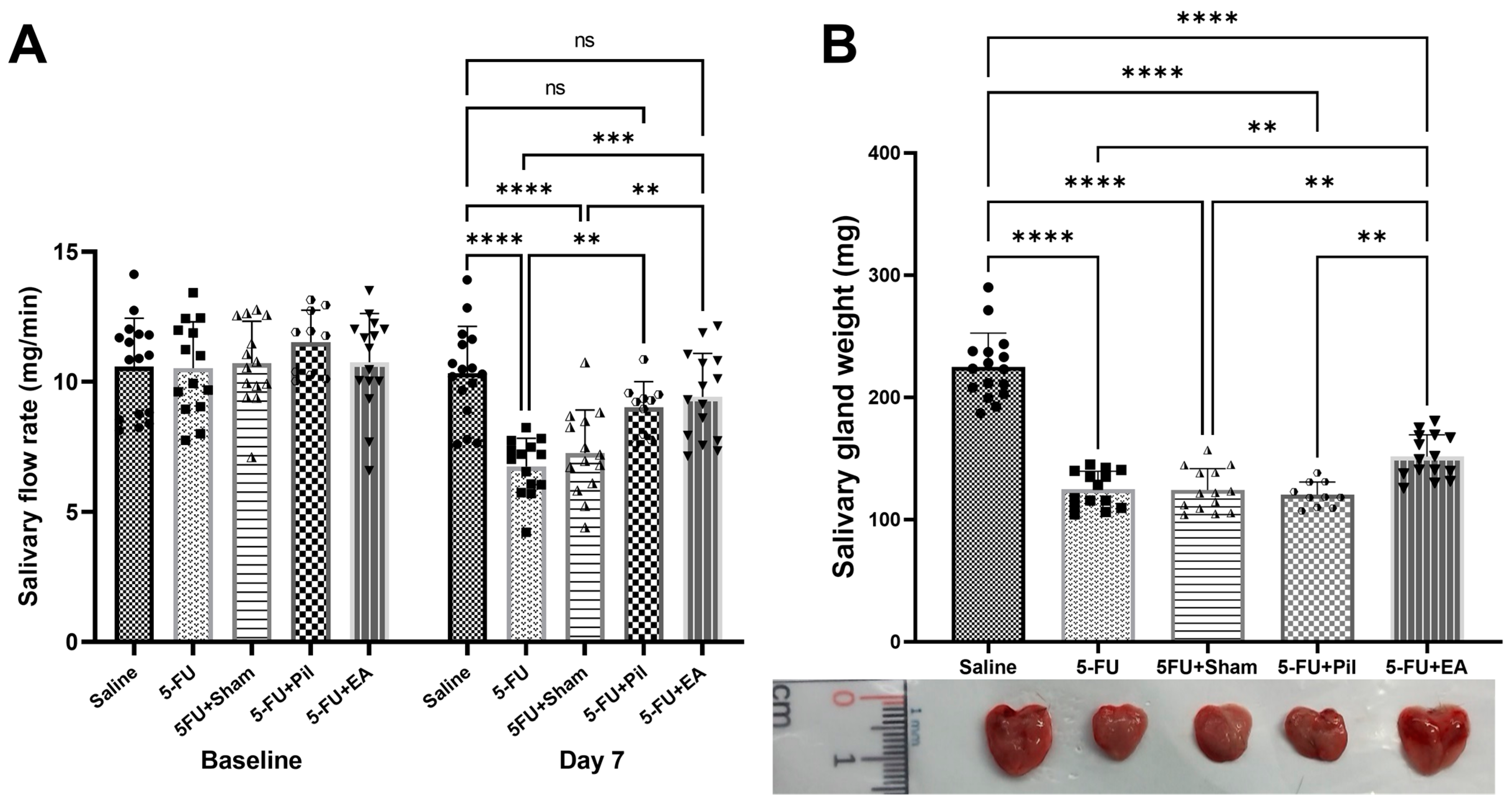
2.2. EA and Pilocarpine Prevented the Stimulated Salivary Flow Rate Decrease, but Only EA Treatment Diminished the Salivary Gland Weight Decrease Caused by 5−FU
2.3. Observation of SMG Hematoxylin and Eosin Staining: EA Treatment Prevented the Decrease in Acini Number and the Increase in Acinar Cell Size Induced by 5−FU
2.4. EA Diminished the Decrease in Salivary IgA Secretion Rates and Lysozyme Activity Caused by 5−FU
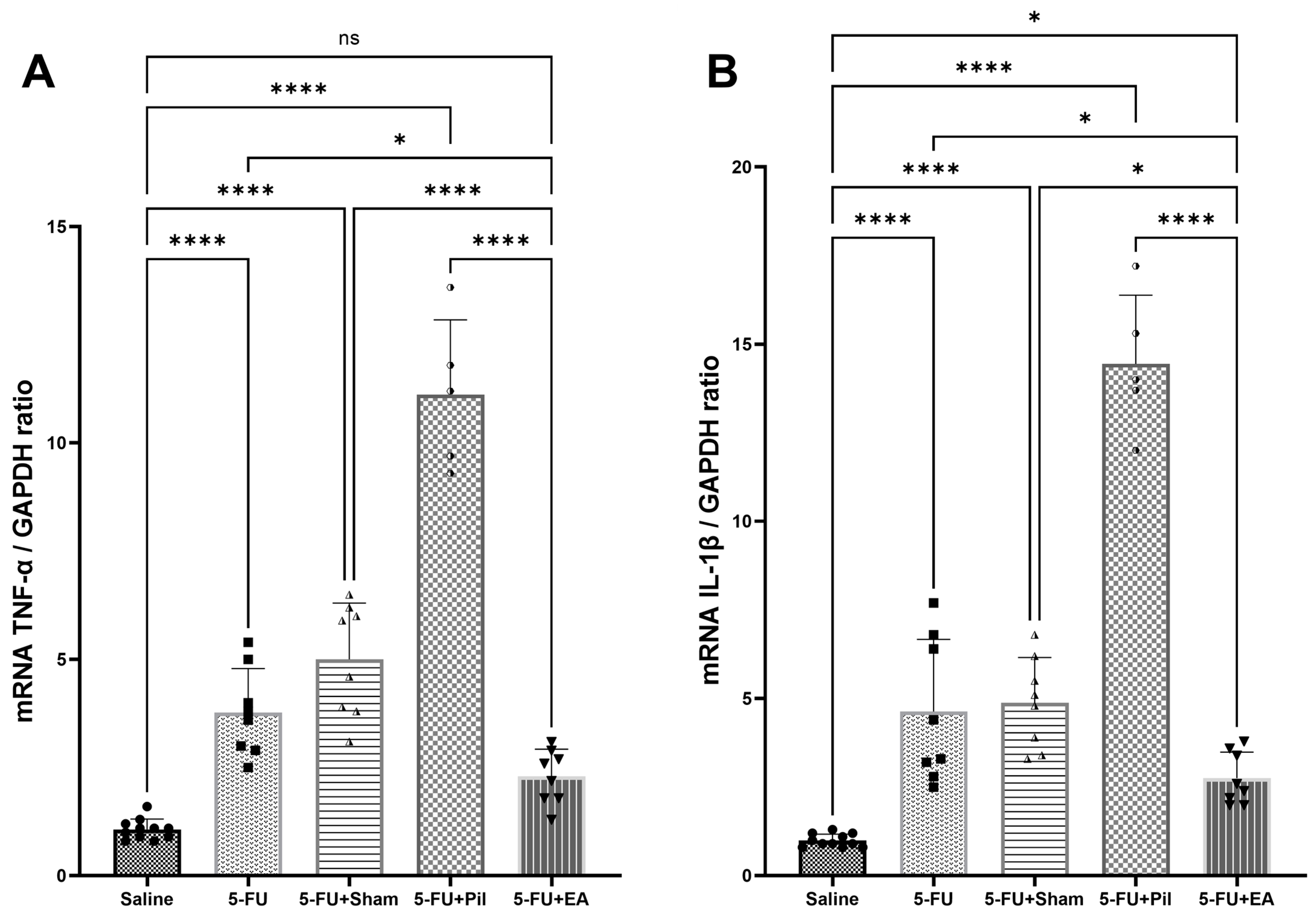
2.5. EA Attenuated the 5−FU-Induced Pro-Inflammatory Cytokines (Tumor Necrosis Factor-α and Interleukin-1β) Expressions in the Salivary Gland
2.6. Pilocarpine and EA Treatment Reversed the Decrease in AQP5 mRNA Expression Induced by 5−FU in the Salivary Glands
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Study Design and Experimental Groups
- (1)
- 5−FU group: 5−FU injection, and anesthesia was only given on treatment days.
- (2)
- Sham acupuncture group: 5−FU injection with sham acupuncture treatment (5−FU + Sham).
- (3)
- Electroacupuncture (EA) group: 5−FU injection with EA treatment (5−FU + EA).
- (4)
- Pilocarpine—positive control group: 5−FU injection and 1 mg/10 mL/kg pilocarpine (Sigma-Aldrich, St. Louis, MO, USA) given orally twice per day on the same days as EA sessions (5−FU + Pil); the dose was chosen based on a published study [52], and other groups were instead given distilled water. The use of pilocarpine as an effective prophylactic and treatment medication for xerostomia was reported in the previous study [53].
- (5)
- Control group: administered PBS only, and anesthesia was only given on treatment days (Saline)
4.3. Mouse Model of 5−FU-Induced Xerostomia
4.4. Electro- and Sham Acupuncture Treatment Procedures
4.5. Pilocarpine-Stimulated Salivary Flow Rate Measurement
4.6. Quantification of Salivary Lysozyme Activity and IgA Secretion Rates via ELISA
4.7. Hematoxylin and Eosin Staining and Histological Analysis
4.8. Immunohistochemistry (IHC)
4.9. Evaluation of Pro-Inflammatory Cytokines TNF-α, IL-1β, and AQP5 mRNA Expression via Quantitative PCR
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plemons, J.M.; Al-Hashimi, I.; Marek, C.L. Managing xerostomia and salivary gland hypofunction: Executive summary of a report from the American Dental Association Council on Scientific Affairs. J. Am. Dent. Assoc. 2014, 145, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Pedersen, A.M.; Vissink, A.; Andersen, E.; Brown, C.G.; Davies, A.N.; Dutilh, J.; Fulton, J.S.; Jankovic, L.; Lopes, N.N.; et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Prevalence, severity and impact on quality of life. Support. Care Cancer 2010, 18, 1039–1060. [Google Scholar] [CrossRef] [PubMed]
- Wilberg, P.; Hjermstad, M.J.; Ottesen, S.; Herlofson, B.B. Chemotherapy-Associated Oral Sequelae in Patients with Cancers Outside the Head and Neck Region. J. Pain. Symptom Manag. 2014, 48, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, S.; Ujihara, I.; Sago-Ito, M.; Nodai, T.; Shikayama, T.; Inenaga, K.; Ono, K. Hyposalivation due to chemotherapy exacerbates oral ulcerative mucositis and delays its healing. Arch. Oral Biol. 2019, 105, 20–26. [Google Scholar] [CrossRef]
- Al-Rudayni, A.H.M.; Gopinath, D.; Maharajan, M.K.; Menon, R.K. Impact of oral mucositis on quality of life in patients undergoing oncological treatment: A systematic review. Transl. Cancer Res. 2020, 9, 3126–3134. [Google Scholar] [CrossRef]
- Harrison, J.D. 5—Salivary Gland Histology. In Surgery of the Salivary Glands; Witt, R.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 37–42. [Google Scholar] [CrossRef]
- Amano, O.; Mizobe, K.; Bando, Y.; Sakiyama, K. Anatomy and Histology of Rodent and Human Major Salivary Glands—Overview of the Japan Salivary Gland Society-Sponsored Workshop—. Acta Histochem. Cytochem. 2012, 45, 241–250. [Google Scholar] [CrossRef]
- de Paula, F.; Teshima, T.H.N.; Hsieh, R.; Souza, M.M.; Nico, M.M.S.; Lourenco, S.V. Overview of Human Salivary Glands: Highlights of Morphology and Developing Processes. Anat. Rec. 2017, 300, 1180–1188. [Google Scholar] [CrossRef]
- Tamborrini, G.; Hricko, P.; Beier, K. Salivary Glands: Basic Anatomy and Histology. In Sjögren’s Syndrome and the Salivary Glands: Novel Techniques in Diagnosis, Management and Treatment; Bruyn, G.A.W., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 105–116. [Google Scholar] [CrossRef]
- Barbosa, S.C.M.; Pereira, V.B.M.; Wong, D.V.T.; Santana, A.P.M.; Lucetti, L.T.; Carvalho, L.L.; Barbosa, C.R.N.; Callado, R.B.; Silva, C.A.A.; Lopes, C.D.H.; et al. Amifostine reduces inflammation and protects against 5-fluorouracil-induced oral mucositis and hyposalivation. Braz. J. Med. Biol. Res. 2019, 52, e8251. [Google Scholar] [CrossRef]
- Kojima, T.; Kanemaru, S.-i.; Hirano, S.; Tateya, I.; Suehiro, A.; Kitani, Y.; Kishimoto, Y.; Ohno, S.; Nakamura, T.; Ito, J. The protective efficacy of basic fibroblast growth factor in radiation-induced salivary gland dysfunction in mice. Laryngoscope 2011, 121, 1870–1875. [Google Scholar] [CrossRef]
- Mafra, C.A.d.C.C.; Vasconcelos, R.C.; Medeiros, C.A.C.X.d.; Leitão, R.F.d.C.; Brito, G.A.d.C.; Costa, D.V.d.S.; Guerra, G.C.B.; Araújo, R.F.d.; Medeiros, A.C.; Araújo, A.A.d. Gliclazide Prevents 5-FU-Induced Oral Mucositis by Reducing Oxidative Stress, Inflammation, and P-Selectin Adhesion Molecules. Front. Physiol. 2019, 10, 327. [Google Scholar] [CrossRef]
- Jensen, S.B.; Pedersen, A.M.L.; Vissink, A.; Andersen, E.; Brown, C.G.; Davies, A.N.; Dutilh, J.; Fulton, J.S.; Jankovic, L.; Lopes, N.N.F.; et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Management strategies and economic impact. Support. Care Cancer 2010, 18, 1061–1079. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, V.; Zadik, Y.; Elad, S. Photobiomodulation Therapy for Cancer Treatment-Related Salivary Gland Dysfunction: A Systematic Review. Photobiomodul. Photomed. Laser Surg. 2020, 38, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.K.; Meng, Z.; Rosenthal, D.I.; Shen, Y.; Chambers, M.; Yang, P.; Wei, Q.; Hu, C.; Wu, C.; Bei, W.; et al. Effect of True and Sham Acupuncture on Radiation-Induced Xerostomia among Patients with Head and Neck Cancer: A Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e1916910. [Google Scholar] [CrossRef]
- Assy, Z.; Brand, H.S. A systematic review of the effects of acupuncture on xerostomia and hyposalivation. BMC Complement. Altern. Med. 2018, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Hou, B.L.; Holodny, A.I.; Cassileth, B.R. Functional magnetic resonance imaging (fMRI) changes and saliva production associated with acupuncture at LI-2 acupuncture point: A randomized controlled study. BMC Complement. Altern. Med. 2008, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Kukucka, M.; Krajcuskova, Z. Automatized Multi-Electrode Voltage Map Measurement of Active Points on Skin. Commun. Sci. Lett. Univ. Zilina 2011, 13, 51–55. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Chen, H.-B.; Liu, J.; Dai, W.-J.; Lu, Q.-Q.; Li, J.-C. Research status and prospects of acupuncture for prevention and treatment of chemo- and radiotherapy-induced salivary gland dysfunction in head and neck cancer. Anatom. Rec. 2021, 304, 2381–2396. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Su, Y.; Qi, L.; Yang, W.; Fu, M.; Jing, X.; Wang, Y.; Ma, Q. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature 2021, 598, 641–645. [Google Scholar] [CrossRef]
- Bomfin, L.E.; Braga, C.M.; Oliveira, T.A.; Martins, C.S.; Foschetti, D.A.; Santos, A.; Costa, D.V.S.; Leitao, R.F.C.; Brito, G.A.C. 5-Fluorouracil induces inflammation and oxidative stress in the major salivary glands affecting salivary flow and saliva composition. Biochem. Pharmacol. 2017, 145, 34–45. [Google Scholar] [CrossRef]
- Mach, P.S.; Amor, B.; Messing, B.; Chicault, P.; Ghozlan, R.; Delbarre, F. Salivary immunoglobulin determinations: Their diagnostic value in Sjogren’s syndrome. Biomedicine 1976, 25, 31–35. [Google Scholar]
- Yao, C.; Li, X.; Murdiastuti, K.; Kosugi-Tanaka, C.; Akamatsu, T.; Kanamori, N.; Hosoi, K. Lipopolysaccharide-induced elevation and secretion of interleukin-1beta in the submandibular gland of male mice. Immunology 2005, 116, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Song, Y.; Gillespie, A.; Carlson, E.J.; Epstein, C.J.; Verkman, A.S. Defective Secretion of Saliva in Transgenic Mice Lacking Aquaporin-5 Water Channels. J. Biol. Chem. 1999, 274, 20071–20074. [Google Scholar] [CrossRef] [PubMed]
- Krane, C.M.; Melvin, J.E.; Nguyen, H.-V.; Richardson, L.; Towne, J.E.; Doetschman, T.; Menon, A.G. Salivary Acinar Cells from Aquaporin 5-deficient Mice Have Decreased Membrane Water Permeability and Altered Cell Volume Regulation. J. Biol. Chem. 2001, 276, 23413–23420. [Google Scholar] [CrossRef]
- Kim, R.; Hahn, S.; Shin, J.; Ock, C.-Y.; Kim, M.; Keam, B.; Kim, T.M.; Kim, D.-W.; Heo, D.S. The Effect of Induction Chemotherapy Using Docetaxel, Cisplatin, and Fluorouracil on Survival in Locally Advanced Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Cancer Res. Treat. 2016, 48, 907–916. [Google Scholar] [CrossRef]
- Jensen, S.B.; Mouridsen, H.T.; Reibel, J.; Brünner, N.; Nauntofte, B. Adjuvant chemotherapy in breast cancer patients induces temporary salivary gland hypofunction. Oral Oncol. 2008, 44, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, R.; Fujimaki, M.; Ikenoue, Y.; Danjo, K.; Koizumi, W.; Ichikawa, T. Influence of an elemental diet on 5-fluorouracil-induced morphological changes in the mouse salivary gland and colon. Support. Care Cancer 2016, 24, 1609–1616. [Google Scholar] [CrossRef]
- Bertolini, M.; Sobue, T.; Thompson, A.; Dongari-Bagtzoglou, A. Chemotherapy Induces Oral Mucositis in Mice Without Additional Noxious Stimuli. Transl. Oncol. 2017, 10, 612–620. [Google Scholar] [CrossRef]
- Ni, X.; Tian, T.; Chen, D.; Liu, L.; Li, X.; Li, F.; Liang, F.; Zhao, L. Acupuncture for Radiation-Induced Xerostomia in Cancer Patients: A Systematic Review and Meta-Analysis. Integr. Cancer Ther. 2020, 19, 1534735420980825. [Google Scholar] [CrossRef]
- Mihardja, H.; Susworo, R.; Srilestari, A.; Umri, H. An evaluation of the effect of acupuncture on salivary pH and the Xerostomia Inventory score innasopharyngeal carcinoma patients with chemoradiation-induced xerostomia. J. Phys. Conf. Ser. 2017, 884, 012127. [Google Scholar] [CrossRef]
- Hideaki, W.; Tatsuya, H.; Shogo, M.; Naruto, Y.; Hideaki, T.; Yoichi, M.; Yoshihiro, O.; Kazuo, U.; Hidenori, T. Effect of 100 Hz electroacupuncture on salivary immunoglobulin A and the autonomic nervous system. Acupunct. Med. 2015, 33, 451–456. [Google Scholar] [CrossRef]
- Salimi, F.; Saavedra, F.; Andrews, B.; FitzGerald, J.; Winter, S.C. Trans-cutaneous electrical nerve stimulation to treat dry mouth (xerostomia) following radiotherapy for head and neck cancer. A systematic review. Ann. Med. Surg. 2021, 63, 102146. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, R.; Harada, K.; Ferdous, T.; Mishima, K. Amino Acids May Have Protective Effects on Salivary Glands of 5-FU-administered Mice. In Vivo 2022, 36, 198–205. [Google Scholar] [CrossRef]
- Susa, T.; Sawai, N.; Aoki, T.; Iizuka-Kogo, A.; Kogo, H.; Negishi, A.; Yokoo, S.; Takata, K.; Matsuzaki, T. Effects of repeated administration of pilocarpine and isoproterenol on aquaporin-5 expression in rat salivary glands. Acta Histochem. Cytochem. 2013, 46, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Burlage, F.R.; Faber, H.; Kampinga, H.H.; Langendijk, J.A.; Vissink, A.; Coppes, R.P. Enhanced proliferation of acinar and progenitor cells by prophylactic pilocarpine treatment underlies the observed amelioration of radiation injury to parotid glands. Radiother. Oncol. 2009, 90, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, T.M.F.; Alanis, L.R.A.; Sapelli, S.d.S.; de Lima, A.A.S.; de Noronha, L.; Rosa, E.A.R.; Althobaiti, Y.S.; Almalki, A.H.; Sari, Y.; Ignacio, S.A.; et al. Effects of Benzodiazepines on Acinar and Myoepithelial Cells. Front. Pharmacol. 2016, 7, 173. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Susa, T.; Shimizu, K.; Sawai, N.; Suzuki, T.; Aoki, T.; Yokoo, S.; Takata, K. Function of the membrane water channel aquaporin-5 in the salivary gland. Acta Histochem. Cytochem. 2012, 45, 251–259. [Google Scholar] [CrossRef]
- Yao, Q.-T.; Wu, Y.-H.; Liu, S.-H.; Song, X.-B.; Xu, H.; Li, J.; Shi, L. Pilocarpine improves submandibular gland dysfunction in irradiated rats by downregulating the tight junction protein claudin-4. Oral Dis. 2022, 28, 1528–1538. [Google Scholar] [CrossRef]
- Sakai, A.; Sugawara, Y.; Kuroishi, T.; Sasano, T.; Sugawara, S. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjögren’s syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J. Immunol. 2008, 181, 2898–2906. [Google Scholar] [CrossRef]
- Hishida, S.; Ozaki, N.; Honda, T.; Shigetomi, T.; Ueda, M.; Hibi, H.; Sugiura, Y. Atrophy of submandibular gland by the duct ligation and a blockade of SP receptor in rats. Nagoya J. Med. Sci. 2016, 78, 215–227. [Google Scholar]
- Proctor, G.B.; Shaalan, A.M. Disease-Induced Changes in Salivary Gland Function and the Composition of Saliva. J. Dent. Res. 2021, 100, 1201–1209. [Google Scholar] [CrossRef]
- Chen, Y.; Lei, Y.; Mo, L.Q.; Li, J.; Wang, M.H.; Wei, J.C.; Zhou, J. Electroacupuncture pretreatment with different waveforms prevents brain injury in rats subjected to cecal ligation and puncture via inhibiting microglial activation, and attenuating inflammation, oxidative stress and apoptosis. Brain Res. Bull. 2016, 127, 248–259. [Google Scholar] [CrossRef]
- Yu, M.L.; Wei, R.D.; Zhang, T.; Wang, J.M.; Cheng, Y.; Qin, F.F.; Fu, S.P.; Lu, Z.G.; Lu, S.F. Electroacupuncture Relieves Pain and Attenuates Inflammation Progression Through Inducing IL-10 Production in CFA-Induced Mice. Inflammation 2020, 43, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Dhar, R.; Zhang, L.; Li, Y.; Rana, M.N.; Hu, Z.; Li, Z.; Cui, H.; Tang, H. Electroacupuncture ameliorates cardiopulmonary bypass induced apoptosis in lung via ROS/Nrf2/NLRP3 inflammasome pathway. Life Sci. 2019, 238, 116962. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, X.; Yang, S.; Huang, J.; Xue, X.; Zheng, Y.; Shang, G.; Tao, J.; Chen, L. Electroacupunctre improves motor impairment via inhibition of microglia-mediated neuroinflammation in the sensorimotor cortex after ischemic stroke. Life Sci. 2016, 151, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Guo, Y.; Gong, Y.; Zhang, Y.; Fan, W.; Yao, K.; Chen, Z.; Dou, B.; Lin, X.; Chen, B.; et al. The Anti-Inflammatory Actions and Mechanisms of Acupuncture from Acupoint to Target Organs via Neuro-Immune Regulation. J. Inflamm. Res. 2021, 14, 7191–7224. [Google Scholar] [CrossRef]
- Farag, A.M.; Holliday, C.; Cimmino, J.; Roomian, T.; Papas, A. Comparing the effectiveness and adverse effects of pilocarpine and cevimeline in patients with hyposalivation. Oral Dis. 2019, 25, 1937–1944. [Google Scholar] [CrossRef]
- Rades, D.; Fehlauer, F.; Bajrovic, A.; Mahlmann, B.; Richter, E.; Alberti, W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother. Oncol. 2004, 70, 261–264. [Google Scholar] [CrossRef]
- Anné, P.R.; Machtay, M.; Rosenthal, D.I.; Brizel, D.M.; Morrison, W.H.; Irwin, D.H.; Chougule, P.B.; Estopinal, N.C.; Berson, A.; Curran, W.J., Jr. A Phase II trial of subcutaneous amifostine and radiation therapy in patients with head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 445–452. [Google Scholar] [CrossRef]
- Pfister, D.G.; Cassileth, B.R.; Deng, G.E.; Yeung, K.S.; Lee, J.S.; Garrity, D.; Cronin, A.; Lee, N.; Kraus, D.; Shaha, A.R.; et al. Acupuncture for pain and dysfunction after neck dissection: Results of a randomized controlled trial. J. Clin. Oncol. 2010, 28, 2565–2570. [Google Scholar] [CrossRef]
- Minagi, H.O.; Ikai, K.; Araie, T.; Sakai, M.; Sakai, T. Benefits of long-term pilocarpine due to increased muscarinic acetylcholine receptor 3 in salivary glands. Biochem. Biophys. Res. Commun. 2018, 503, 1098–1102. [Google Scholar] [CrossRef]
- Yang, W.-f.; Liao, G.-q.; Hakim, S.G.; Ouyang, D.-q.; Ringash, J.; Su, Y.-x. Is Pilocarpine Effective in Preventing Radiation-Induced Xerostomia? A Systematic Review and Meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.P.; Lemos Junior, C.A.; Alves, F.A.; Migliari, D.A. Acupuncture for the prevention of radiation-induced xerostomia in patients with head and neck cancer. Braz. Oral Res. 2011, 25, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.K.; James, J.L.; Sagar, S.; Wyatt, G.; Nguyen-Tân, P.F.; Singh, A.K.; Lukaszczyk, B.; Cardinale, F.; Yeh, A.M.; Berk, L. Phase 2 results from Radiation Therapy Oncology Group Study 0537: A phase 2/3 study comparing acupuncture-like transcutaneous electrical nerve stimulation versus pilocarpine in treating early radiation-induced xerostomia. Cancer 2012, 118, 4244–4252. [Google Scholar] [CrossRef] [PubMed]
- Bagavant, H.; Trzeciak, M.; Papinska, J.; Biswas, I.; Dunkleberger, M.L.; Sosnowska, A.; Deshmukh, U.S. A Method for the Measurement of Salivary Gland Function in Mice. J. Vis. Exp. 2018, 131, e57203. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.-H.V.; Chiu, K.-C.; Shih, Y.-H.; Liu, C.-J.; Bao Quach, T.V.; Hsia, S.-M.; Chen, Y.-H.; Shieh, T.-M. Protective Effect of Electroacupuncture on Chemotherapy-Induced Salivary Gland Hypofunction in a Mouse Model. Int. J. Mol. Sci. 2023, 24, 11654. https://doi.org/10.3390/ijms241411654
Nguyen T-HV, Chiu K-C, Shih Y-H, Liu C-J, Bao Quach TV, Hsia S-M, Chen Y-H, Shieh T-M. Protective Effect of Electroacupuncture on Chemotherapy-Induced Salivary Gland Hypofunction in a Mouse Model. International Journal of Molecular Sciences. 2023; 24(14):11654. https://doi.org/10.3390/ijms241411654
Chicago/Turabian StyleNguyen, Thanh-Hien Vu, Kuo-Chou Chiu, Yin-Hwa Shih, Chung-Ji Liu, Tran Van Bao Quach, Shih-Min Hsia, Yi-Hung Chen, and Tzong-Ming Shieh. 2023. "Protective Effect of Electroacupuncture on Chemotherapy-Induced Salivary Gland Hypofunction in a Mouse Model" International Journal of Molecular Sciences 24, no. 14: 11654. https://doi.org/10.3390/ijms241411654
APA StyleNguyen, T.-H. V., Chiu, K.-C., Shih, Y.-H., Liu, C.-J., Bao Quach, T. V., Hsia, S.-M., Chen, Y.-H., & Shieh, T.-M. (2023). Protective Effect of Electroacupuncture on Chemotherapy-Induced Salivary Gland Hypofunction in a Mouse Model. International Journal of Molecular Sciences, 24(14), 11654. https://doi.org/10.3390/ijms241411654









