Antioxidant Defense Capacity Is Reduced in Thyroid Stem/Precursor Cells Compared to Differentiated Thyrocytes
Abstract
1. Introduction
2. Results
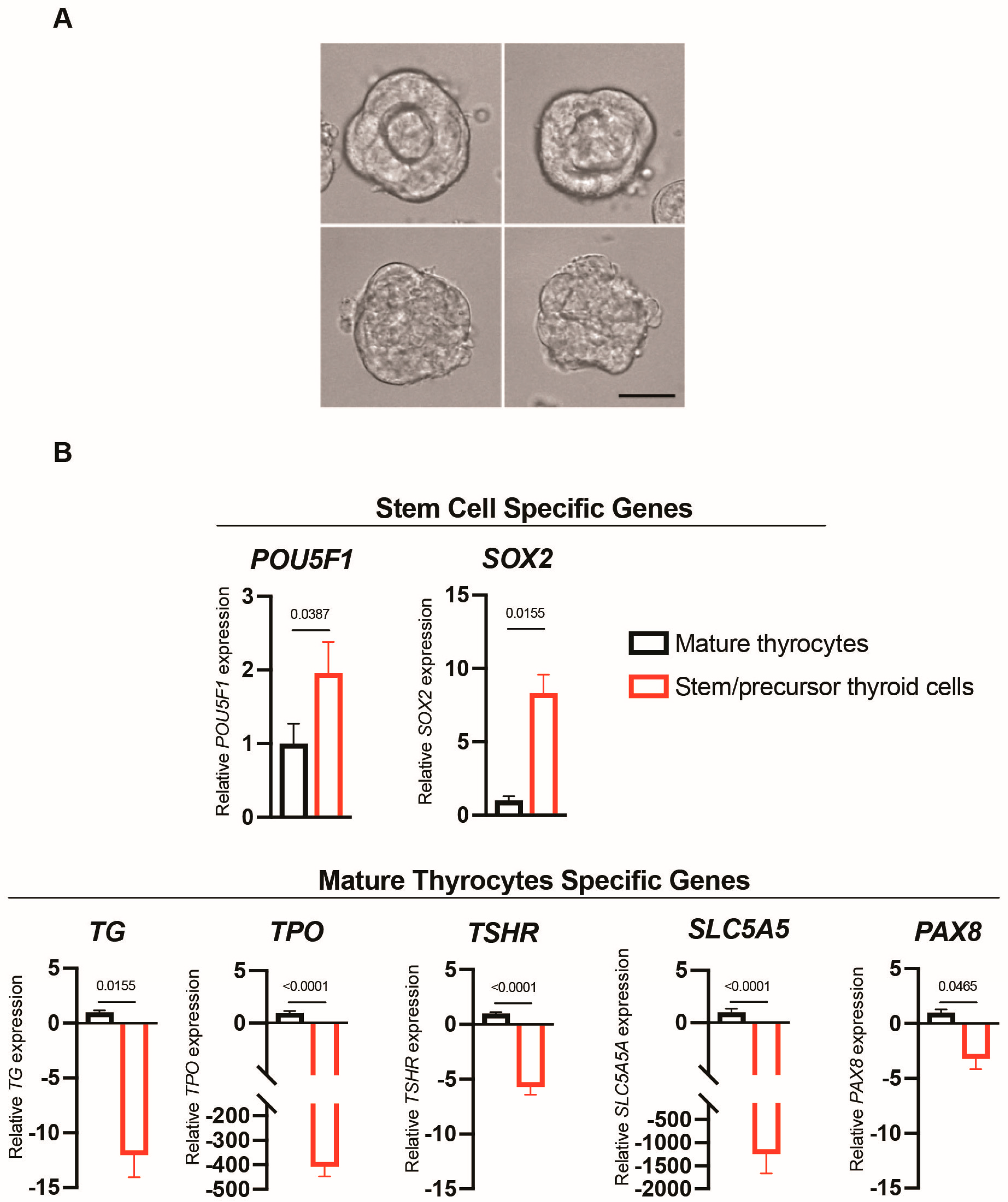
2.1. Differences in Morphology and Gene Markers in the Two Cell Models Used
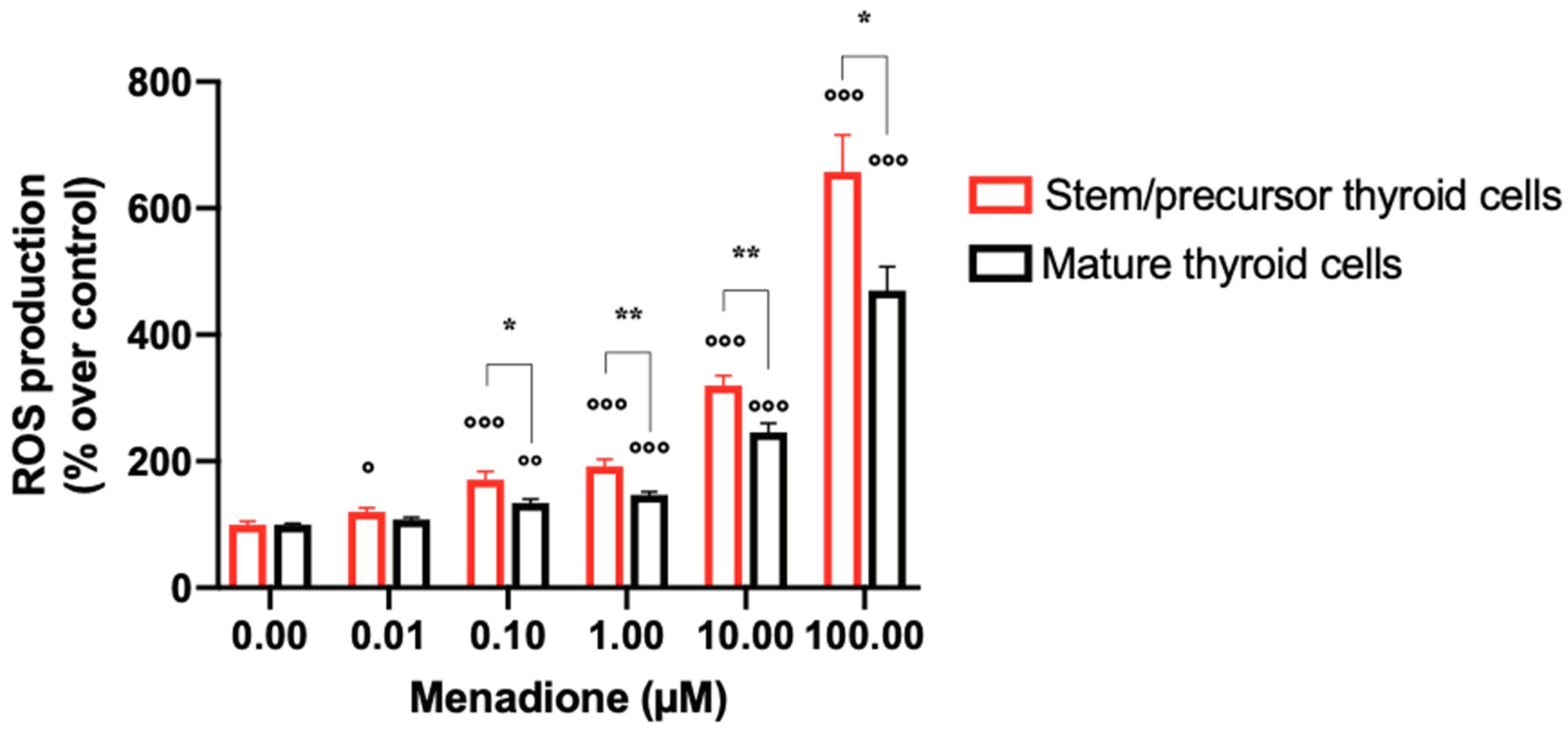
2.2. Menadione Effect on ROS Generation
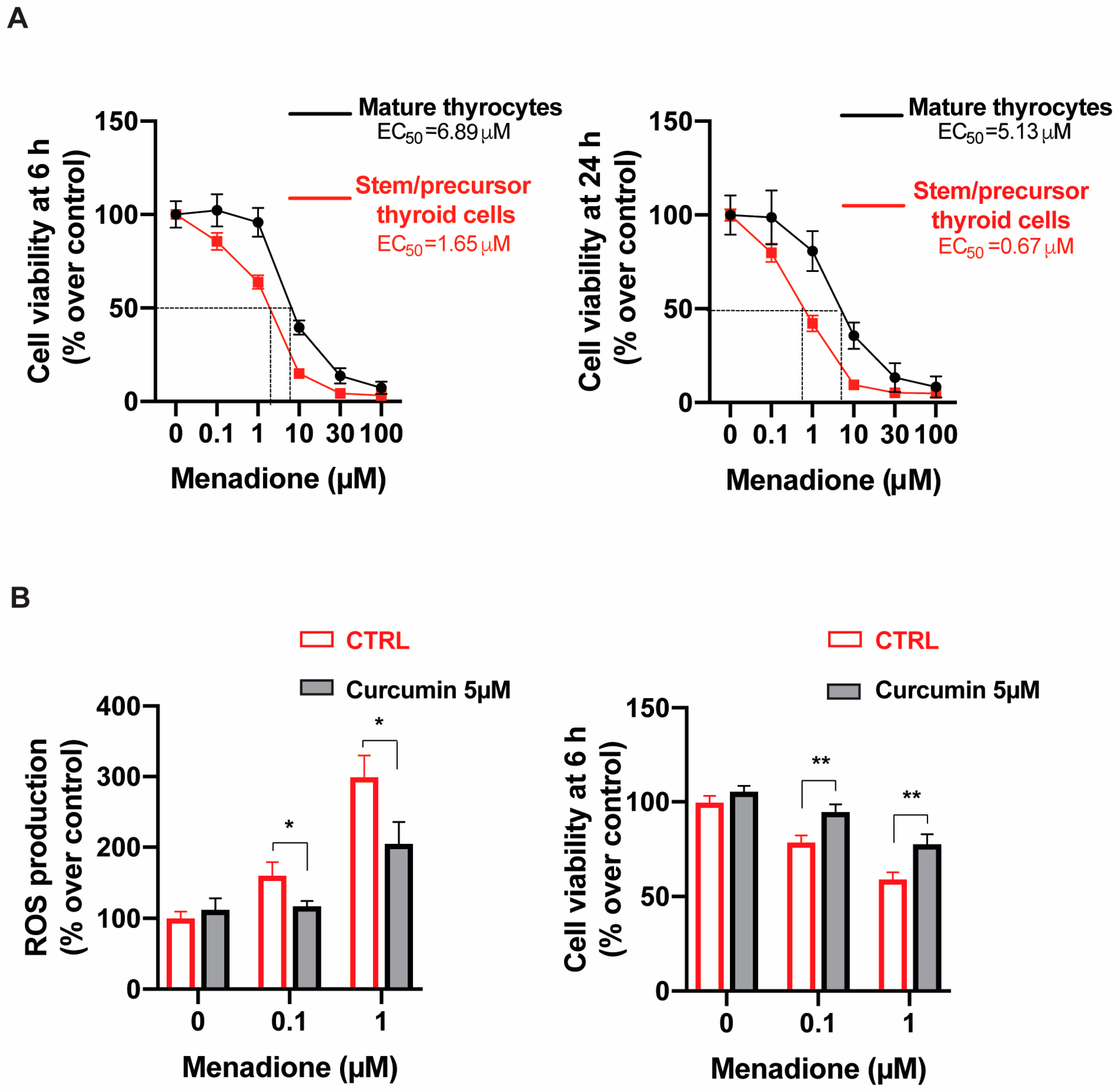
2.3. Menadione Effect on Thyroid Cell Viability
2.4. The Antioxidant Curcumin Reduces Menadione-Induced ROS Production and Menadione Toxicity in Stem/Precursor Thyroid Cells
2.5. Antioxidant Gene Expression Is Reduced in Stem/Precursor Thyroid Cells Relative to Differentiated Thyrocytes
2.5.1. Master Regulators of the Antioxidant Defense
2.5.2. Hydrogen Peroxide Producing Enzymes
2.5.3. Antioxidant Enzymes and Glutathione
2.5.4. NADPH-Generating Enzymes
2.5.5. Detoxification Systems
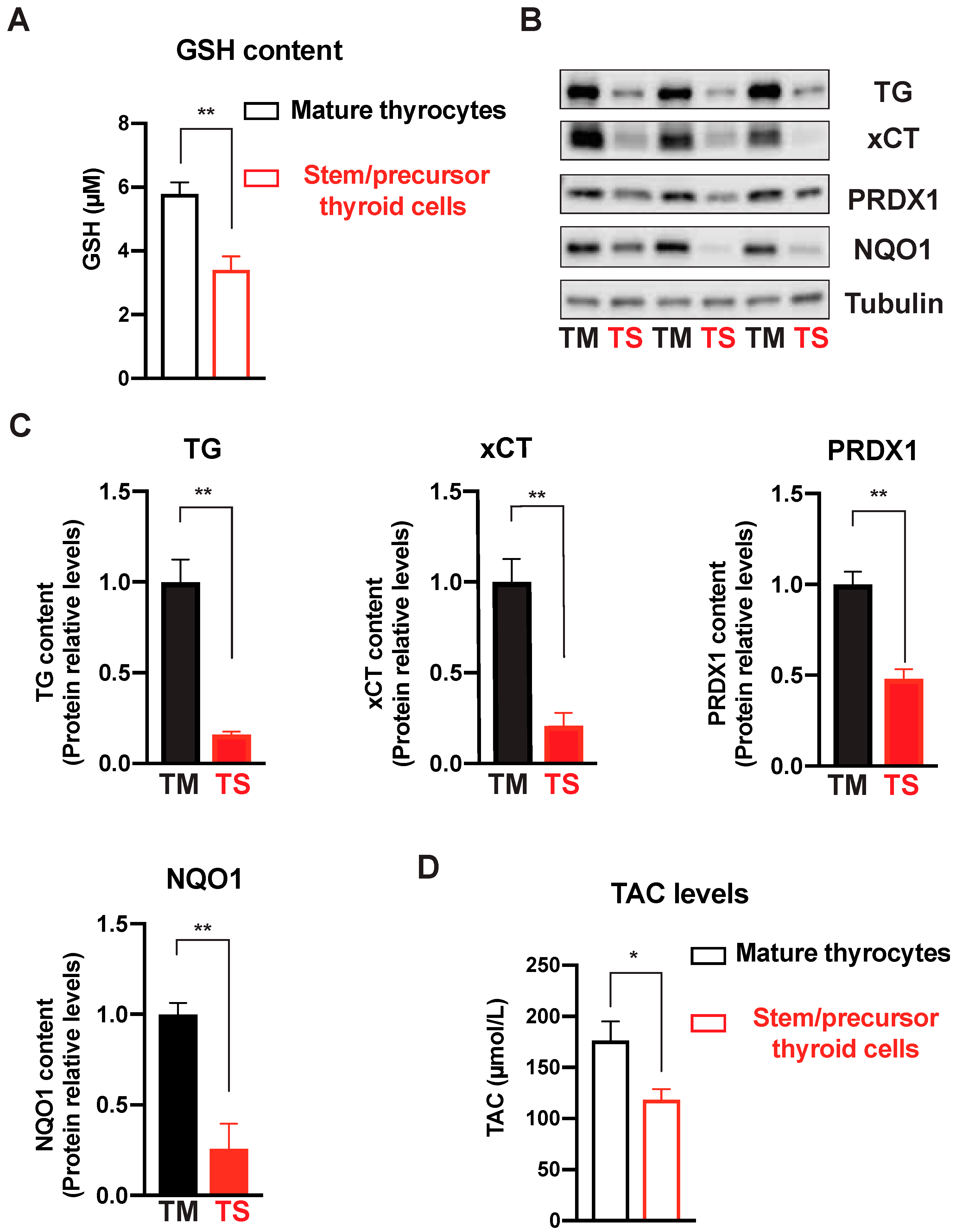
2.6. Antioxidant Enzymatic Protein Measurements and Total Antioxidant Capacity
3. Discussion
4. Materials and Methods
4.1. Human Thyroid Cells
4.2. Cell Exposure to Oxidative Stress
4.3. ROS Measurement
4.4. Cell Viability
4.5. Gene Expression
4.6. Western Immunoblotting
4.7. Glutathione Measurement
4.8. Total Antioxidant Capacity (TAC)
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E. Oxidative Stress and Cancer. CPD 2019, 24, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.; Mandic, A.; Maricic, S.; Srdjenovic, B. Oxidative Stress and Its Role in Cancer. J. Can. Res. Ther. 2021, 17, 22. [Google Scholar] [CrossRef]
- Wang, R.; Liang, L.; Matsumoto, M.; Iwata, K.; Umemura, A.; He, F. Reactive Oxygen Species and NRF2 Signaling, Friends or Foes in Cancer? Biomolecules 2023, 13, 353. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Driessens, N.; Costa, M.; De Deken, X.; Detours, V.; Corvilain, B.; Maenhaut, C.; Miot, F.; Van Sande, J.; Many, M.-C.; et al. Roles of Hydrogen Peroxide in Thyroid Physiology and Disease. J. Clin. Endocrinol. Metab. 2007, 92, 3764–3773. [Google Scholar] [CrossRef]
- Szanto, I.; Pusztaszeri, M.; Mavromati, M. H2O2 Metabolism in Normal Thyroid Cells and in Thyroid Tumorigenesis: Focus on NADPH Oxidases. Antioxidants 2019, 8, 126. [Google Scholar] [CrossRef]
- Ohye, H.; Sugawara, M. Dual Oxidase, Hydrogen Peroxide and Thyroid Diseases. Exp. Biol. Med. 2010, 235, 424–433. [Google Scholar] [CrossRef]
- Renaud, C.O.; Ziros, P.G.; Chartoumpekis, D.V.; Bongiovanni, M.; Sykiotis, G.P. Keap1/Nrf2 Signaling: A New Player in Thyroid Pathophysiology and Thyroid Cancer. Front. Endocrinol. 2019, 10, 510. [Google Scholar] [CrossRef]
- Thanas, C.; Ziros, P.G.; Chartoumpekis, D.V.; Renaud, C.O.; Sykiotis, G.P. The Keap1/Nrf2 Signaling Pathway in the Thyroid—2020 Update. Antioxidants 2020, 9, 1082. [Google Scholar] [CrossRef]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef]
- Chartoumpekis, D.V.; Ziros, P.G.; Habeos, I.G.; Sykiotis, G.P. Emerging Roles of Keap1/Nrf2 Signaling in the Thyroid Gland and Perspectives for Bench-to-Bedside Translation. Free Radic. Biol. Med. 2022, 190, 276–283. [Google Scholar] [CrossRef]
- Ziros, P.G.; Habeos, I.G.; Chartoumpekis, D.V.; Ntalampyra, E.; Somm, E.; Renaud, C.O.; Bongiovanni, M.; Trougakos, I.P.; Yamamoto, M.; Kensler, T.W.; et al. NFE2-Related Transcription Factor 2 Coordinates Antioxidant Defense with Thyroglobulin Production and Iodination in the Thyroid Gland. Thyroid 2018, 28, 780–798. [Google Scholar] [CrossRef]
- Kim, H.; Lee, T.-H.; Park, E.S.; Suh, J.M.; Park, S.J.; Chung, H.K.; Kwon, O.-Y.; Kim, Y.K.; Ro, H.K.; Shong, M. Role of Peroxiredoxins in Regulating Intracellular Hydrogen Peroxide and Hydrogen Peroxide-Induced Apoptosis in Thyroid Cells. J. Biol. Chem. 2000, 275, 18266–18270. [Google Scholar] [CrossRef]
- Jaganjac, M.; Milkovic, L.; Sunjic, S.B.; Zarkovic, N. The NRF2, Thioredoxin, and Glutathione System in Tumorigenesis and Anticancer Therapies. Antioxidants 2020, 9, 1151. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Cazarin, J.; Dupuy, C.; Pires de Carvalho, D. Redox Homeostasis in Thyroid Cancer: Implications in Na+/I− Symporter (NIS) Regulation. Int. J. Mol. Sci. 2022, 23, 6129. [Google Scholar] [CrossRef]
- Suvorova, E.S.; Lucas, O.; Weisend, C.M.; Rollins, M.F.; Merrill, G.F.; Capecchi, M.R.; Schmidt, E.E. Cytoprotective Nrf2 Pathway Is Induced In Chronically Txnrd 1-Deficient Hepatocytes. PLoS ONE 2009, 4, e6158. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Ohno, T.; Igarashi, S.; Yoshimura, T.; Yamashiro, K.; Sakai, M. Aldehyde Oxidase 1 Gene Is Regulated by Nrf2 Pathway. Gene 2012, 505, 374–378. [Google Scholar] [CrossRef]
- Driessens, N.; Versteyhe, S.; Ghaddhab, C.; Burniat, A.; De Deken, X.; Van Sande, J.; Dumont, J.-E.; Miot, F.; Corvilain, B. Hydrogen Peroxide Induces DNA Single- and Double-Strand Breaks in Thyroid Cells and Is Therefore a Potential Mutagen for This Organ. Endocr. -Relat. Cancer 2009, 16, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. Oxidative Stress: A New Risk Factor for Thyroid Cancer. Endocr. Relat. Cancer 2012, 19, C7–C11. [Google Scholar] [CrossRef]
- Ameziane El Hassani, R.; Buffet, C.; Leboulleux, S.; Dupuy, C. Oxidative Stress in Thyroid Carcinomas: Biological and Clinical Significance. Endocr. Relat. Cancer 2019, 26, R131–R143. [Google Scholar] [CrossRef] [PubMed]
- Kochman, J.; Jakubczyk, K.; Bargiel, P.; Janda-Milczarek, K. The Influence of Oxidative Stress on Thyroid Diseases. Antioxidants 2021, 10, 1442. [Google Scholar] [CrossRef] [PubMed]
- Krohn, K.; Maier, J.; Paschke, R. Mechanisms of Disease: Hydrogen Peroxide, DNA Damage and Mutagenesis in the Development of Thyroid Tumors. Nat. Rev. Endocrinol. 2007, 3, 713–720. [Google Scholar] [CrossRef]
- Maier, J.; van Steeg, H.; van Oostrom, C.; Karger, S.; Paschke, R.; Krohn, K. Deoxyribonucleic Acid Damage and Spontaneous Mutagenesis in the Thyroid Gland of Rats and Mice. Endocrinology 2006, 147, 3391–3397. [Google Scholar] [CrossRef] [PubMed]
- Coclet, J.; Foureau, F.; Ketelbant, P.; Galand, P.; Dumont, J.E. Cell Population Kinetics in Dog and Human Adult Thyroid. Clin. Endocrinol. 1989, 31, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. Molecular Pathogenesis and Mechanisms of Thyroid Cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef]
- Heydarzadeh, S.; Kia, S.K.; Zarkesh, M.; Pakizehkar, S.; Hosseinzadeh, S.; Hedayati, M. The Cross-Talk between Polyphenols and the Target Enzymes Related to Oxidative Stress-Induced Thyroid Cancer. Oxidative Med. Cell. Longev. 2022, 2022, 2724324. [Google Scholar] [CrossRef]
- Muzza, M.; Pogliaghi, G.; Colombo, C.; Carbone, E.; Cirello, V.; Palazzo, S.; Frattini, F.; Gentilini, D.; Gazzano, G.; Persani, L.; et al. Oxidative Stress Correlates with More Aggressive Features in Thyroid Cancer. Cancers 2022, 14, 5857. [Google Scholar] [CrossRef]
- Pervaiz, S.; Taneja, R.; Ghaffari, S. Oxidative Stress Regulation of Stem and Progenitor Cells. Antioxid. Redox Signal. 2009, 11, 2777–2789. [Google Scholar] [CrossRef]
- Perales-Clemente, E.; Folmes, C.D.L.; Terzic, A. Metabolic Regulation of Redox Status in Stem Cells. Antioxid. Redox Signal. 2014, 21, 1648–1659. [Google Scholar] [CrossRef]
- Samimi, A.; Khodayar, M.J.; Alidadi, H.; Khodadi, E. The Dual Role of ROS in Hematological Malignancies: Stem Cell Protection and Cancer Cell Metastasis. Stem Cell Rev. Rep. 2020, 16, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Adusumilli, V.S.; Walker, T.L.; Overall, R.W.; Klatt, G.M.; Zeidan, S.A.; Zocher, S.; Kirova, D.G.; Ntitsias, K.; Fischer, T.J.; Sykes, A.M.; et al. ROS Dynamics Delineate Functional States of Hippocampal Neural Stem Cells and Link to Their Activity-Dependent Exit from Quiescence. Cell Stem Cell 2021, 28, 300–314.e6. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, M.; Sugawara, Y.; Wen, K.; Giulivi, C. Generation of Oxygen Free Radicals in Thyroid Cells and Inhibition of Thyroid Peroxidase. Exp. Biol. Med. 2002, 227, 141–146. [Google Scholar] [CrossRef]
- Criddle, D.N.; Gillies, S.; Baumgartner-Wilson, H.K.; Jaffar, M.; Chinje, E.C.; Passmore, S.; Chvanov, M.; Barrow, S.; Gerasimenko, O.V.; Tepikin, A.V.; et al. Menadione-Induced Reactive Oxygen Species Generation via Redox Cycling Promotes Apoptosis of Murine Pancreatic Acinar Cells. J. Biol. Chem. 2006, 281, 40485–40492. [Google Scholar] [CrossRef]
- Thomas, N.O.; Shay, K.P.; Kelley, A.R.; Butler, J.A.; Hagen, T.M. Glutathione Maintenance Mitigates Age-Related Susceptibility to Redox Cycling Agents. Redox Biol. 2016, 10, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dou, W.; Zheng, Y.; Wen, Q.; Qin, M.; Wang, X.; Tang, H.; Zhang, R.; Lv, D.; Wang, J.; et al. Curcumin Upregulates Nrf2 Nuclear Translocation and Protects Rat Hepatic Stellate Cells against Oxidative Stress. Mol. Med. Rep. 2016, 13, 1717–1724. [Google Scholar] [CrossRef]
- Shin, J.W.; Chun, K.-S.; Kim, D.-H.; Kim, S.-J.; Kim, S.H.; Cho, N.-C.; Na, H.-K.; Surh, Y.-J. Curcumin Induces Stabilization of Nrf2 Protein through Keap1 Cysteine Modification. Biochem. Pharmacol. 2020, 173, 113820. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection Against Oxidative Injury. Curr. Mol. Med. 2020, 20, 116–133. [Google Scholar] [CrossRef]
- Serrander, L.; Cartier, L.; Bedard, K.; Banfi, B.; Lardy, B.; Plastre, O.; Sienkiewicz, A.; Fórró, L.; Schlegel, W.; Krause, K.-H. NOX4 Activity Is Determined by MRNA Levels and Reveals a Unique Pattern of ROS Generation. Biochem. J. 2007, 406, 105–114. [Google Scholar] [CrossRef]
- Nisimoto, Y.; Diebold, B.A.; Cosentino-Gomes, D.; Lambeth, J.D. Nox4: A Hydrogen Peroxide-Generating Oxygen Sensor. Biochemistry 2014, 53, 5111–5120. [Google Scholar] [CrossRef]
- Nishida, S.; Nakano, T.; Kimoto, S.; Kusunoki, T.; Suzuki, K.; Taniguchi, N.; Murata, K.; Tomura, T.T. Induction of Manganese Superoxide Dismutase by Thyroid Stimulating Hormone in Rat Thyroid Cells. FEBS Lett. 1997, 416, 69–71. [Google Scholar] [CrossRef]
- Laatikainen, L.E.; Castellone, M.D.; Hebrant, A.; Hoste, C.; Cantisani, M.C.; Laurila, J.P.; Salvatore, G.; Salerno, P.; Basolo, F.; Näsman, J.; et al. Extracellular Superoxide Dismutase Is a Thyroid Differentiation Marker Down-Regulated in Cancer. Endocr. -Relat. Cancer 2010, 17, 785–796. [Google Scholar] [CrossRef]
- Schweizer, U.; Chiu, J.; Köhrle, J. Peroxides and Peroxide-Degrading Enzymes in the Thyroid. Antioxid. Redox Signal. 2008, 10, 1577–1592. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; He, S.; Liu, Y.; Liu, Y.; Feng, F.; Guo, Q.; Zhao, L.; Sun, H. Overview of Human 20 Alpha-Hydroxysteroid Dehydrogenase (AKR1C1): Functions, Regulation, and Structural Insights of Inhibitors. Chem. Biol. Interact. 2022, 351, 109746. [Google Scholar] [CrossRef]
- Boas, M.; Feldt-Rasmussen, U.; Main, K.M. Thyroid Effects of Endocrine Disrupting Chemicals. Mol. Cell. Endocrinol. 2012, 355, 240–248. [Google Scholar] [CrossRef]
- Noble, M.; Smith, J.; Power, J.; Mayer-Pröschel, M. Redox State as a Central Modulator of Precursor Cell Function. Ann. N. Y. Acad. Sci. 2006, 991, 251–271. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, K.; Dakik, P.; Medkour, Y.; Mitrofanova, D.; Titorenko, V.I. Quiescence Entry, Maintenance, and Exit in Adult Stem Cells. Int. J. Mol. Sci. 2019, 20, 2158. [Google Scholar] [CrossRef] [PubMed]
- Gianì, F.; Masto, R.; Trovato, M.A.; Franco, A.; Pandini, G.; Vigneri, R. Thyroid Stem Cells But Not Differentiated Thyrocytes Are Sensitive to Slightly Increased Concentrations of Heavy Metals. Front. Endocrinol. 2021, 12, 652675. [Google Scholar] [CrossRef]
- Gianì, F.; Masto, R.; Trovato, M.A.; Malandrino, P.; Russo, M.; Pellegriti, G.; Vigneri, P.; Vigneri, R. Heavy Metals in the Environment and Thyroid Cancer. Cancers 2021, 13, 4052. [Google Scholar] [CrossRef]
- Thomas, D.; Friedman, S.; Lin, R.-Y. Thyroid Stem Cells: Lessons from Normal Development and Thyroid Cancer. Endocr. Relat. Cancer 2008, 15, 51–58. [Google Scholar] [CrossRef]
- Goffart, S.; Tikkanen, P.; Michell, C.; Wilson, T.; Pohjoismäki, J.L.O. The Type and Source of Reactive Oxygen Species Influences the Outcome of Oxidative Stress in Cultured Cells. Cells 2021, 10, 1075. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.F.; Latif, R.; Sachidanandam, R.; Ma, R. The Transient Human Thyroid Progenitor Cell: Examining the Thyroid Continuum from Stem Cell to Follicular Cell. Thyroid 2021, 31, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Gianì, F.; Pandini, G.; Scalisi, N.M.; Vigneri, P.; Fazzari, C.; Malandrino, P.; Russo, M.; Masucci, R.; Belfiore, A.; Pellegriti, G.; et al. Effect of Low-Dose Tungsten on Human Thyroid Stem/Precursor Cells and Their Progeny. Endocr. Relat. Cancer 2019, 26, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Antonchuk, J. Formation of Embryoid Bodies from Human Pluripotent Stem Cells Using AggreWellTM Plates. In Basic Cell Culture Protocols; Helgason, C.D., Miller, C.L., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 946, pp. 523–533. ISBN 978-1-62703-127-1. [Google Scholar]
- Gianì, F.; Russo, G.; Pennisi, M.; Sciacca, L.; Frasca, F.; Pappalardo, F. Computational Modeling Reveals MAP3K8 as Mediator of Resistance to Vemurafenib in Thyroid Cancer Stem Cells. Bioinformatics 2019, 35, 2267–2275. [Google Scholar] [CrossRef]
| Gene Symbol and Extended Name | Fold Regulation (Mean ± S.E.M.) | p Value | |
|---|---|---|---|
| Master Regulators of the Antioxidant Defense | |||
| NFE2L2 | Nuclear factor, erythroid-2-like 2 | −1.40 ± 0.08 | 0.0159 |
| KEAP1 | Kelch-like ECH-associated protein 1 | −1.45 ± 0.12 | 0.0289 |
| Hydrogen Peroxide Producing Enzymes | |||
| DUOX1 | Dual oxidase 1 | −1.65 ± 0.31 | 0.1259 |
| DUOX2 | Dual oxidase 2 | −2.65 ± 0.43 | 0.0374 |
| NOX4 | NADPH oxidase 4 | 2.04 ± 0.11 | 0.0093 |
| Antioxidant Enzymes | |||
| SOD1 | Superoxide dismutase 1 | −1.58 ± 0.11 | 0.0019 |
| SOD2 | Superoxide dismutase 2 | −2.35 ± 0.26 | <0.0001 |
| SOD3 | Superoxide dismutase 3 | −3.50 ± 0.94 | 0.0127 |
| CAT | Catalase | −1.42 ± 0.05 | 0.0013 |
| PRDX1 | Peroxiredoxin 1 | −2.71 ± 0.35 | 0.0040 |
| TXNRD1 | Thioredoxin Reductase 1 | −4.31 ± 0.99 | 0.0099 |
| Glutathione antioxidant system | |||
| GPX3 | Glutathione peroxidase 3 | −6.79 ± 0.97 | 0.0040 |
| SLC7A11 | Solute carrier family 7 member 11 | −7.78 ± 0.97 | <0.0001 |
| GSR | Glutathione-disulfide reductase | −1.96 ± 0.25 | 0.0450 |
| NADPH-generating enzymes | |||
| G6PD | Glucose-6-phosphate dehydrogenase | −3.17 ± 0.48 | 0.0003 |
| PGD | Phosphogluconate dehydrogenase | −3.49 ± 0.76 | 0.0003 |
| ME1 | Malic enzyme 1 | −5.57 ± 1.21 | 0.0002 |
| Detoxification Systems | |||
| Phase I | |||
| EPHX1 | Epoxide hydrolase 1 | −3.75 ± 0.49 | <0.0001 |
| AKR1C1 | Aldo-keto reductase family 1 member C1 | −11.91 ± 3.17 | 0.0003 |
| AOX1 | Aldehyde oxidase 1 | −15.91 ± 3.35 | <0.0001 |
| Phase II | |||
| MGST1 | Microsomal glutathione S-transferase 1 | −1.51 ± 0.10 | 0.0014 |
| NQO1 | NAD(P)H quinone dehydrogenase 1 | −2.24 ± 0.27 | 0.0038 |
| Phase III | |||
| ABCC1 | ATP-binding cassette subfamily C member 1 | −1.57 ± 0.24 | 0.0039 |
| Category | Gene | Primer Direction | Primer Sequence | Amplicon |
|---|---|---|---|---|
| Master Regulators of the Antioxidant Defense | NFE2L2 | For | TCCATTCCTGAGTTACAGTGTC | 228 |
| Rev | CACTGTCAACTGGTTGGGGT | |||
| KEAP1 | For | TGCGTCCTGCACAACTGTAT | 199 | |
| Rev | CCAGGAACGTGTGACCATCA | |||
| Hydrogen Peroxide-Producing Enzymes | DUOX1 | For | ACGTGCTGGTCGCTGTTATC | 204 |
| Rev | AAGGGAAGCAACAGAGGGTC | |||
| DUOX2 | For | TTAGTTCTGAAGAGGAACGGGG | 199 | |
| Rev | TCGGCCTGGTTGATGTCCA | |||
| NOX4 | For | TCCGGAGCAATAAGCCAGTC | 199 | |
| Rev | ACCCCAAATGTTGCTTTGGT | |||
| Antioxidant Enzymes | SOD1 | For | ACAAAGATGGTGTGGCCGAT | 162 |
| Rev | AACGACTTCCAGCGTTTCCT | |||
| SOD2 | For | TCCGGTTTTGGGGTATCTGG | 155 | |
| Rev | CGGTGACGTTCAGGTTGTTC | |||
| SOD3 | For | AGCTGGAAAGGTGCCCGA | 149 | |
| Rev | CTTGGCGTACATGTCTCGGAT | |||
| CAT | For | CTGACTACGGGAGCCACATC | 192 | |
| Rev | CATCCAGTGATGAGCGGGTT | |||
| PRDX1 | For | CAAAGCCACAGCTGTTATGCC | 186 | |
| Rev | GAAGCACCAATCACTTGGCAG | |||
| TXNRD1 | For | TGGAGTGCGCTGGATTTCTT | 187 | |
| Rev | CCTGGTGTCCCTGCTTCAAT | |||
| Glutathione Antioxidant System | GPX3 | For | TACGAGTACGGAGCCCTCAC | 160 |
| Rev | GACCGAATGGTGCAAGCTCT | |||
| SLC7A11 | For | ACAGGGATTGGCTTCGTCAT | 190 | |
| Rev | GGCAGATTGCCAAGATCTCAA | |||
| GSR | For | TGGCACTTGCGTGAATGTTG | 225 | |
| Rev | GCATGGCCACGGATGATTTC | |||
| NADPH-Generating Enzymes | G6PD | For | GGCCGTGTACACCAAGATGA | 212 |
| Rev | GCAGTGGGGTGAAAATACGC | |||
| PGD | For | AAGATGGTGCACAACGGGAT | 218 | |
| Rev | TCCCTGATCTTTGGCAGCAG | |||
| ME1 | For | ACCCTCACCTCAACAAGGACT | 87 | |
| Rev | TGTTGAAGGAAGGTGGCAACA | |||
| Detoxification System | EPHX1 | For | GCTGACCAACGTCATGCTCT | 120 |
| Rev | ACATAGACCTTCATCCGCTCA | |||
| AKR1C1 | For | GAAGCTGGCTTCCGCCAT | 158 | |
| Rev | ACCAACTCTGGTCGATGGGA | |||
| AOX1 | For | AAACGCCTCGAACCCATCAT | 222 | |
| Rev | CTTATGATCCCCCGTCAGGC | |||
| MGST1 | For | GACCTCACCCAGGTAATGGA | 214 | |
| Rev | TGCGTACACGTTCTACTCTGTC | |||
| NQO1 | For | GAGCACTGATCGTACTGGCT | 185 | |
| Rev | AAAGTTCGCAGGGTCCTTCA | |||
| ABCC1 | For | GAGGACACGTCGGAACAAGT | 141 | |
| Rev | CGCATCCACCTTGGAACTCT | |||
| Mature-Thyrocyte-Specific Genes | TG | Hs00174974_m1 | ||
| TPO | Hs00174927_m1 | |||
| TSHR | Hs01053846_m1 | |||
| NIS | Hs00166567_m1 | |||
| PAX8 | For | GGCCTTTGTGAATGGCAGAC | 243 | |
| Rev | TTCTGGCGTTTGTAGTCCCC | |||
| Stem-Cell-Specific Genes | POU5F1 | Hs01654807_s1 | ||
| SOX2 | Hs04234836_s1 | |||
| Housekeeping Genes | RPS3 | For | CCACTAGAGGTCTGTGTGCC | 157 |
| Rev | CCTCGGAGTTTCCCAGACAC | |||
| RPS6 | For | TGTTACTCCACGTGTCCTGC | 166 | |
| Rev | AAGTCTGCGTCTCTTCGCAA | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gianì, F.; Allia, F.; Trovato, M.A.; Masto, R.; Pellegriti, G.; Vigneri, R. Antioxidant Defense Capacity Is Reduced in Thyroid Stem/Precursor Cells Compared to Differentiated Thyrocytes. Int. J. Mol. Sci. 2023, 24, 11509. https://doi.org/10.3390/ijms241411509
Gianì F, Allia F, Trovato MA, Masto R, Pellegriti G, Vigneri R. Antioxidant Defense Capacity Is Reduced in Thyroid Stem/Precursor Cells Compared to Differentiated Thyrocytes. International Journal of Molecular Sciences. 2023; 24(14):11509. https://doi.org/10.3390/ijms241411509
Chicago/Turabian StyleGianì, Fiorenza, Fabio Allia, Maria Antonietta Trovato, Roberta Masto, Gabriella Pellegriti, and Riccardo Vigneri. 2023. "Antioxidant Defense Capacity Is Reduced in Thyroid Stem/Precursor Cells Compared to Differentiated Thyrocytes" International Journal of Molecular Sciences 24, no. 14: 11509. https://doi.org/10.3390/ijms241411509
APA StyleGianì, F., Allia, F., Trovato, M. A., Masto, R., Pellegriti, G., & Vigneri, R. (2023). Antioxidant Defense Capacity Is Reduced in Thyroid Stem/Precursor Cells Compared to Differentiated Thyrocytes. International Journal of Molecular Sciences, 24(14), 11509. https://doi.org/10.3390/ijms241411509






