2-DG Regulates Immune Imbalance on the Titanium Surface after Debridement
Abstract
1. Introduction
2. Results
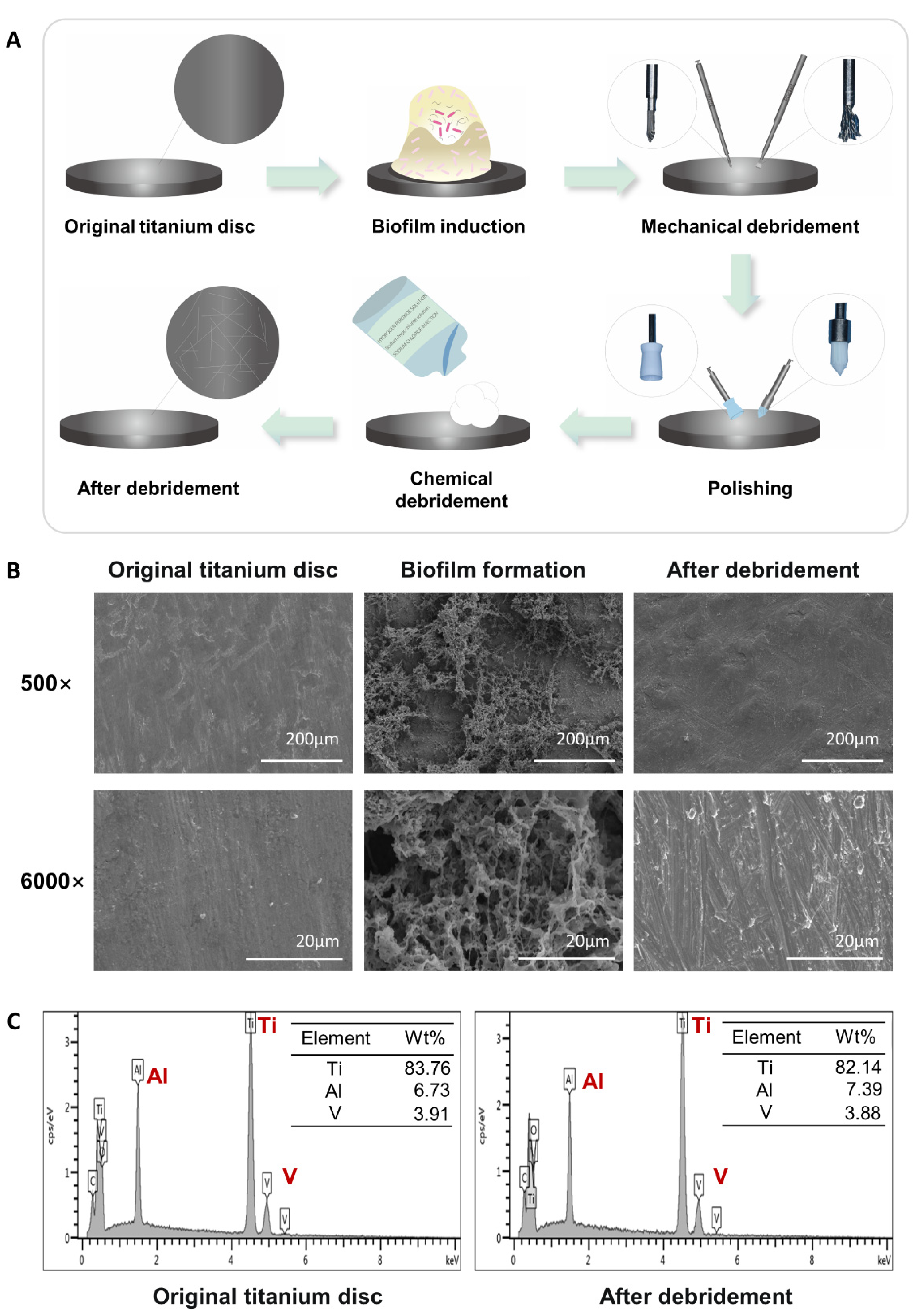
2.1. Construction and Characterization of the Titanium Surface after Debridement
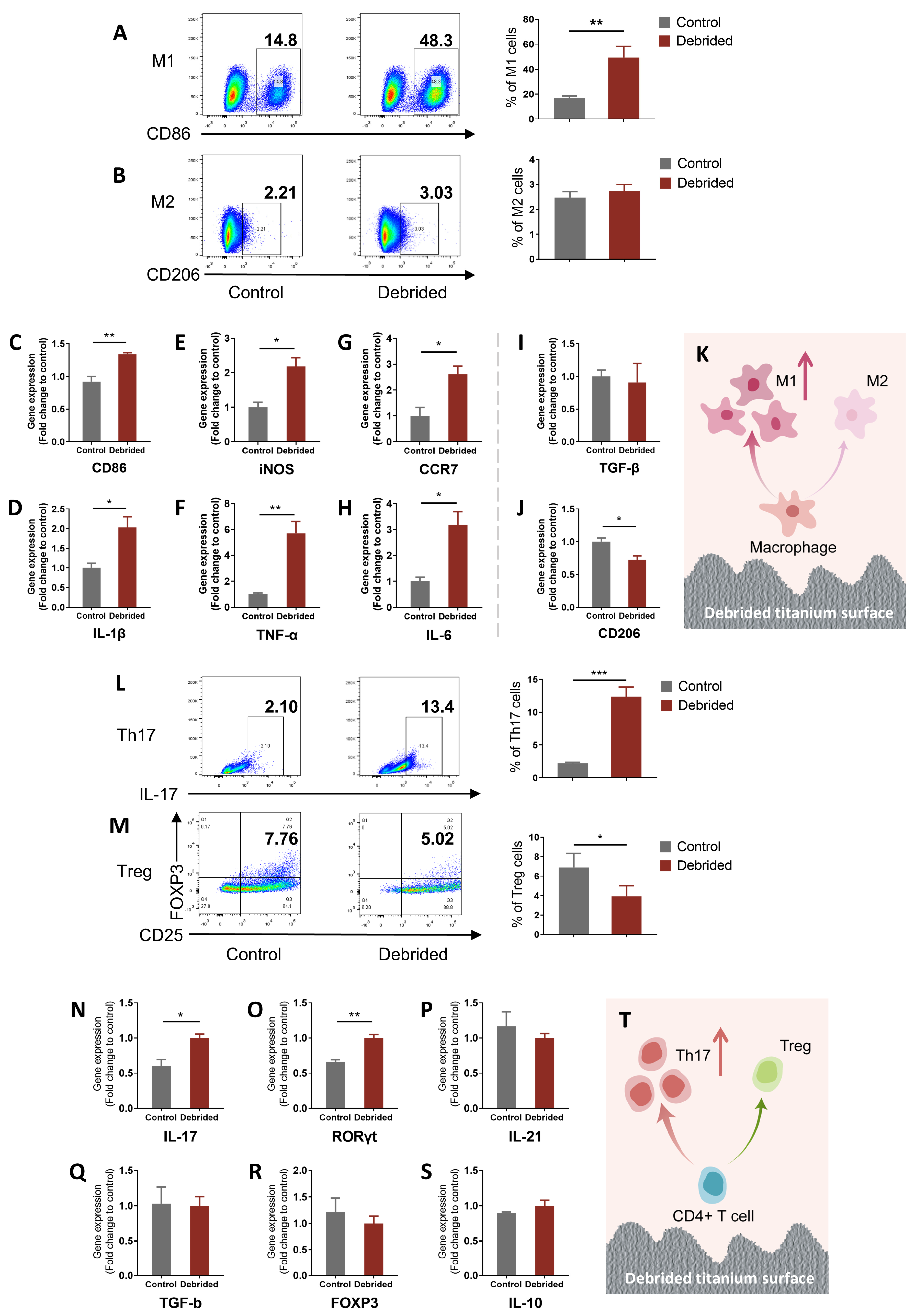
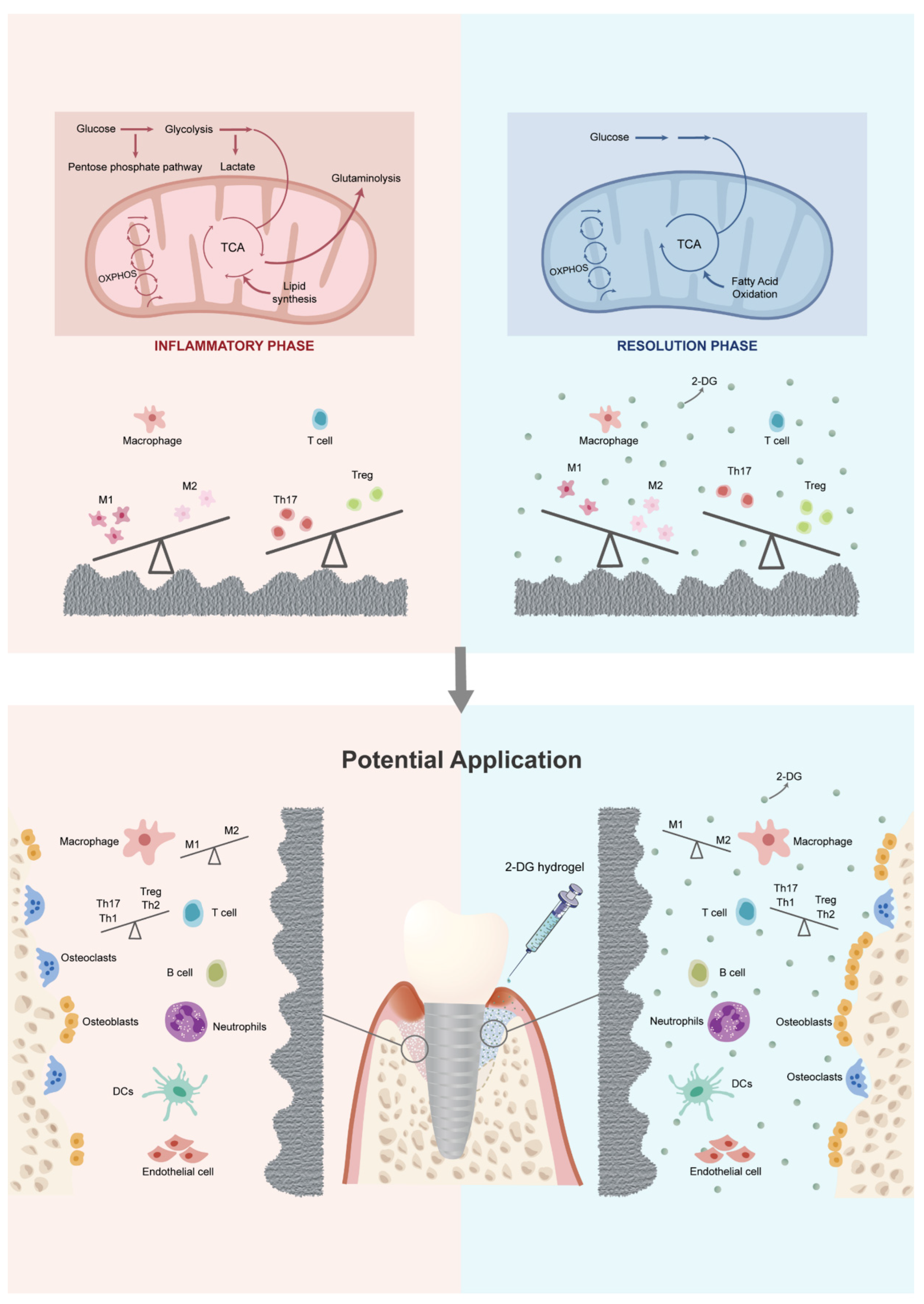
2.2. Titanium Surface after Debridement Results in Immune Imbalance
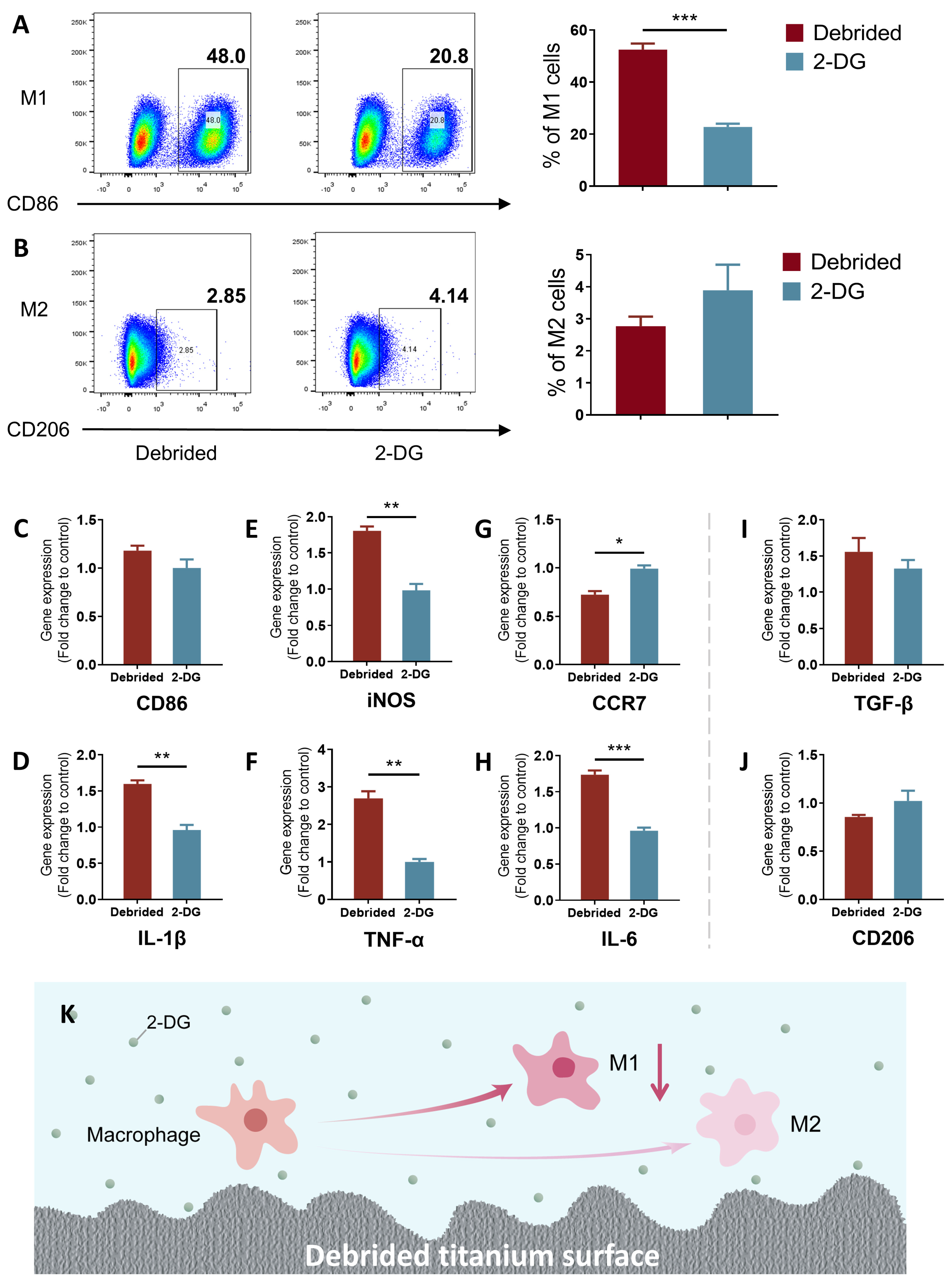
2.3. 2-DG Could Regulate the Pro-Inflammatory Immune Imbalance of Macrophages Induced by Titanium Surface after Debridement
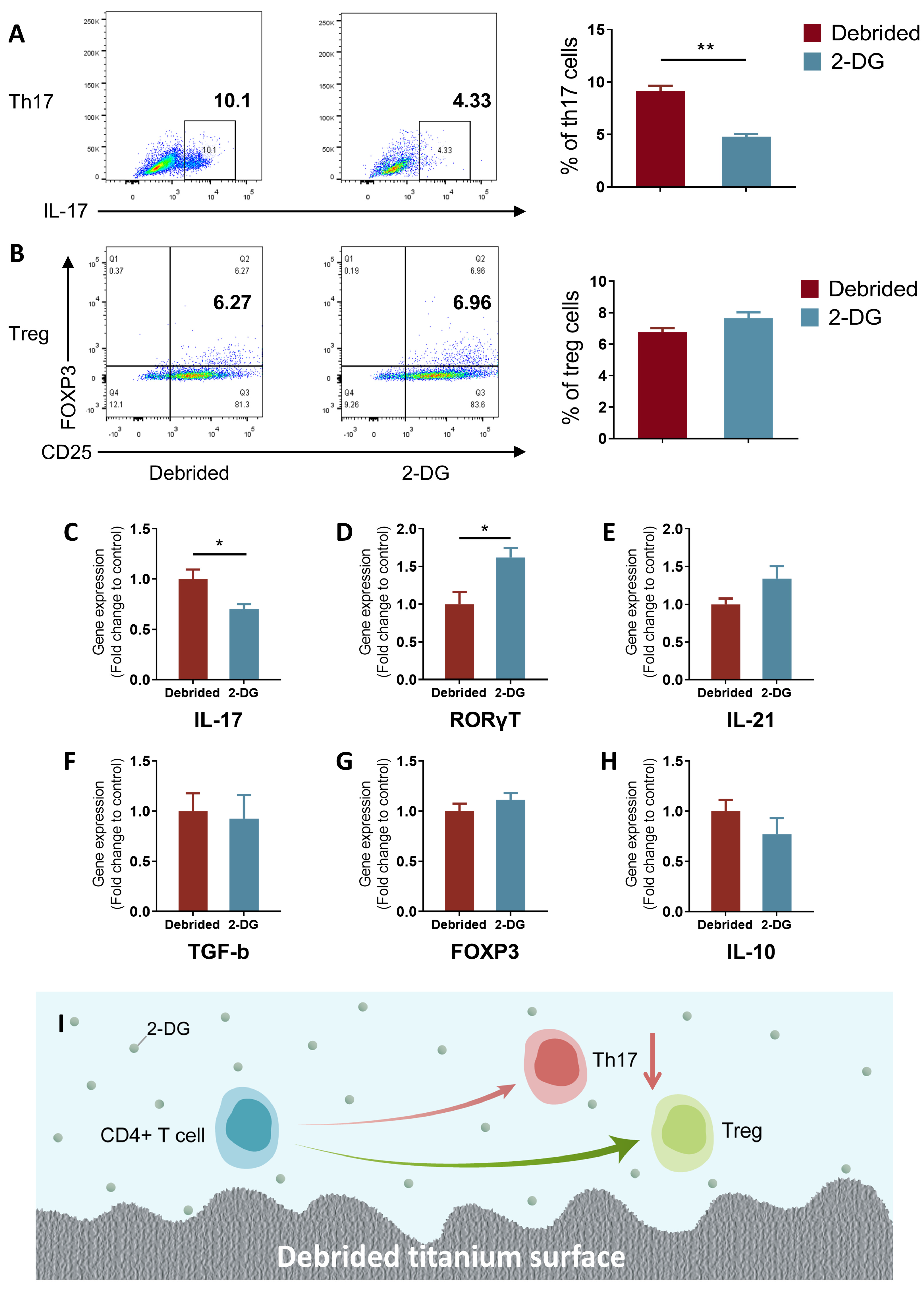
2.4. 2-DG Could Regulate the Pro-Inflammatory Immune Imbalance of T Cells Induced by Titanium Surface after Debridement
3. Discussion
4. Materials and Methods
4.1. Titanium Discs
4.2. Bacterial Culture and Biofilm Formation
4.3. Debridement on Bacterial-Colonized Titanium Discs
4.4. Scanning Electron Microscopy (SEM) and Chemical Analyses
4.5. Cell Preparation and Culture
4.6. Flow Cytometry
4.7. RNA Extraction and qRT-PCR
4.8. Statistic Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vignoletti, F.; Di Domenico, G.L.; Di Martino, M.; Montero, E.; de Sanctis, M. Prevalence and risk indicators of peri-implantitis in a sample of university-based dental patients in Italy: A cross-sectional study. J. Clin. Periodontol. 2019, 46, 597–605. [Google Scholar] [CrossRef]
- Krebs, M.; Kesar, N.; Begic, A.; von Krockow, N.; Nentwig, G.H.; Weigl, P. Incidence and prevalence of peri-implantitis and peri-implant mucositis 17 to 23 (18.9) years postimplant placement. Clin. Implant. Dent. Relat. Res. 2019, 21, 1116–1123. [Google Scholar] [CrossRef]
- Matarazzo, F.; Saboia-Gomes, R.; Alves, B.E.S.; de Oliveira, R.P.; Araujo, M.G. Prevalence, extent and severity of peri-implant diseases. A cross-sectional study based on a university setting in Brazil. J. Periodontal Res. 2018, 53, 910–915. [Google Scholar] [CrossRef]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S158–S171. [Google Scholar] [CrossRef]
- Atieh, M.A.; Alsabeeha, N.H.; Faggion, C.M., Jr.; Duncan, W.J. The frequency of peri-implant diseases: A systematic review and meta-analysis. J. Periodontol. 2013, 84, 1586–1598. [Google Scholar] [CrossRef]
- de Waal, Y.C.M.; van Winkelhoff, A.J.; Meijer, H.J.A.; Raghoebar, G.M.; Winkel, E.G. Differences in peri-implant conditions between fully and partially edentulous subjects: A systematic review. J. Clin. Periodontol. 2013, 40, 266–286. [Google Scholar] [CrossRef]
- Tarnow, D.P. Increasing Prevalence of Peri-implantitis: How Will We Manage? J. Dent. Res. 2016, 95, 7–8. [Google Scholar] [CrossRef]
- Cha, J.K.; Lee, J.S.; Kim, C.S. Surgical Therapy of Peri-Implantitis with Local Minocycline: A 6-Month Randomized Controlled Clinical Trial. J. Dent. Res. 2019, 98, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Cosgarea, R.; Sculean, A. Prevalence and Mechanisms of Peri-implant Diseases. J. Dent. Res. 2017, 96, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Keim, D.; Nickles, K.; Dannewitz, B.; Ratka, C.; Eickholz, P.; Petsos, H. In vitro efficacy of three different implant surface decontamination methods in three different defect configurations. Clin. Oral Implants Res. 2019, 30, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Kotsakis, G.A.; Lan, C.; Barbosa, J.; Lill, K.; Chen, R.; Rudney, J.; Aparicio, C. Antimicrobial Agents Used in the Treatment of Peri-Implantitis Alter the Physicochemistry and Cytocompatibility of Titanium Surfaces. J. Periodontol. 2016, 87, 809–819. [Google Scholar] [CrossRef]
- Chen, Z.; Klein, T.; Murray, R.Z.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater. Today 2016, 19, 304–321. [Google Scholar] [CrossRef]
- Jiang, F.; Qi, X.; Wu, X.; Lin, S.; Shi, J.; Zhang, W.; Jiang, X. Regulating macrophage-MSC interaction to optimize BMP-2-induced osteogenesis in the local microenvironment. Bioact. Mater. 2023, 25, 307–318. [Google Scholar] [CrossRef]
- Mestres, G.; Carter, S.D.; Hailer, N.P.; Diez-Escudero, A. A practical guide for evaluating the osteoimmunomodulatory properties of biomaterials. Acta Biomater. 2021, 130, 115–137. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Wong, K.H.M.; Shen, J.; Wang, W.; Wu, J.; Li, J.; Lin, Z.; Chen, Z.; Matinlinna, J.P.; Zheng, Y.; et al. TRPM7 kinase-mediated immunomodulation in macrophage plays a central role in magnesium ion-induced bone regeneration. Nat. Commun. 2021, 12, 2885. [Google Scholar] [CrossRef]
- Zhu, L.; Hua, F.; Ding, W.; Ding, K.; Zhang, Y.; Xu, C. The correlation between the Th17/Treg cell balance and bone health. Immun. Ageing 2020, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Vantucci, C.E.; Ahn, H.; Fulton, T.; Schenker, M.L.; Pradhan, P.; Wood, L.B.; Guldberg, R.E.; Roy, K.; Willett, N.J. Development of systemic immune dysregulation in a rat trauma model of biomaterial-associated infection. Biomaterials 2021, 264, 120405. [Google Scholar] [CrossRef]
- Shen, P.C.; Wu, C.L.; Jou, I.M.; Lee, C.H.; Juan, H.Y.; Lee, P.J.; Chen, S.H.; Hsieh, J.L. T helper cells promote disease progression of osteoarthritis by inducing macrophage inflammatory protein-1gamma. Osteoarthr. Cartil. 2011, 19, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.H.; Leone, R.D.; Horton, M.R.; Powell, J.D. Targeting metabolism to regulate immune responses in autoimmunity and cancer. Nat. Rev. Drug Discov. 2019, 18, 669–688. [Google Scholar] [CrossRef]
- Pajak, B.; Siwiak, E.; Sołtyka, M.; Priebe, A.; Zieliński, R.; Fokt, I.; Ziemniak, M.; Jaśkiewicz, A.; Borowski, R.; Domoradzki, T.; et al. 2-Deoxy-d-Glucose and Its Analogs: From Diagnostic to Therapeutic Agents. Int. J. Mol. Sci. 2019, 21, 234. [Google Scholar] [CrossRef]
- Zhang, D.; Li, J.; Wang, F.; Hu, J.; Wang, S.; Sun, Y. 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett. 2014, 355, 176–183. [Google Scholar] [CrossRef]
- Cheong, J.-H.; Park, E.S.; Liang, J.; Dennison, J.B.; Tsavachidou, D.; Nguyen-Charles, C.; Wa Cheng, K.; Hall, H.; Zhang, D.; Lu, Y.; et al. Dual inhibition of tumor energy pathway by 2-deoxyglucose and metformin is effective against a broad spectrum of preclinical cancer models. Mol. Cancer Ther. 2011, 10, 2350–2362. [Google Scholar] [CrossRef]
- Soto-Heredero, G.; Gomez de Las Heras, M.M.; Gabande-Rodriguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, S.; Vuckovic, I.; Jeon, R.; Lerman, A.; Folmes, C.D.; Dzeja, P.P.; Herrmann, J. Glycolytic Stimulation Is Not a Requirement for M2 Macrophage Differentiation. Cell Metab. 2018, 28, 463–475. [Google Scholar] [CrossRef]
- Angelin, A.; Gil-de-Gomez, L.; Dahiya, S.; Jiao, J.; Guo, L.; Levine, M.H.; Wang, Z.; Quinn, W.J., 3rd; Kopinski, P.K.; Wang, L.; et al. Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments. Cell Metab. 2017, 25, 1282–1293 e1287. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jia, A.; Wang, Y.; Dong, L.; Wang, Y.; He, Y.; Wang, S.; Cao, Y.; Yang, H.; Bi, Y.; et al. Immune effects of glycolysis or oxidative phosphorylation metabolic pathway in protecting against bacterial infection. J. Cell Physiol. 2019, 234, 20298–20309. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.T.; Zhang, M.; Yang, C.L.; Zhang, P.; Zhang, N.; Du, T.; Ge, M.R.; Yue, L.T.; Li, X.L.; Li, H.; et al. Enhanced glycolysis contributes to the pathogenesis of experimental autoimmune neuritis. J. Neuroinflamm. 2018, 15, 51. [Google Scholar] [CrossRef]
- Umar, S.; Palasiewicz, K.; Volin, M.V.; Romay, B.; Rahat, R.; Tetali, C.; Arami, S.; Guma, M.; Ascoli, C.; Sweiss, N.; et al. Metabolic regulation of RA macrophages is distinct from RA fibroblasts and blockade of glycolysis alleviates inflammatory phenotype in both cell types. Cell. Mol. Life Sci. 2021, 78, 7693–7707. [Google Scholar] [CrossRef]
- Pålsson-McDermott, E.M.; O’Neill, L.A.J. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 2020, 30, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Montanaro, L.; Campoccia, D.; Rizzi, S.; Donati, M.E.; Breschi, L.; Prati, C.; Arciola, C.R. Evaluation of bacterial adhesion of Streptococcus mutans on dental restorative materials. Biomaterials 2004, 25, 4457–4463. [Google Scholar] [CrossRef]
- Belibasakis, G.N. Microbiological and immuno-pathological aspects of peri-implant diseases. Arch. Oral Biol. 2014, 59, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A. Microbiology and antimicrobial therapy of peri-implantitis. Periodontol 2000 2002, 28, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.M.; Knight, E.T.; Russell, A.A.; Tawse-Smith, A.; Leichter, J.W. Peri-implant disease: Current understanding and future direction. N. Z. Dent. J. 2013, 109, 55–62. [Google Scholar]
- Beyth, N.; Bahir, R.; Matalon, S.; Domb, A.J.; Weiss, E.I. Streptococcus mutans biofilm changes surface-topography of resin composites. Dent. Mater. 2008, 24, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.; Ponthiaux, P.; Henriques, M.; Oliveira, R.; Teughels, W.; Celis, J.P.; Rocha, L.A. Corrosion behaviour of titanium in the presence of Streptococcus mutans. J. Dent. 2013, 41, 528–534. [Google Scholar] [CrossRef]
- Prathapachandran, J.; Suresh, N. Management of peri-implantitis. Dent. Res. J. 2012, 9, 516–521. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Wisitrasameewon, W.; Humagain, M.; Thunyakitpisal, P. Peri-implantitis Update: Risk Indicators, Diagnosis, and Treatment. Eur. J. Dent. 2020, 14, 672–682. [Google Scholar] [CrossRef]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammacher, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis--a review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.; Aaboe, M.; Araujo, M.; Carrion, J.B.; Cavalcanti, R.; Cionca, N.; Cochran, D.; Darby, I.; Funakoshi, E.; Gierthmuehlen, P.C.; et al. Group 4 ITI Consensus Report: Risks and biologic complications associated with implant dentistry. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 351–358. [Google Scholar] [CrossRef]
- Lang, N.P.; Wilson, T.G.; Corbet, E.F. Biological complications with dental implants: Their prevention, diagnosis and treatment. Clin. Oral Implant. Res. 2000, 11 (Suppl. S1), 146–155. [Google Scholar] [CrossRef]
- Qin, W.; Wang, C.; Jiang, C.; Sun, J.; Yu, C.; Jiao, T. Graphene Oxide Enables the Reosteogenesis of Previously Contaminated Titanium In Vitro. J. Dent. Res. 2020, 99, 922–929. [Google Scholar] [CrossRef]
- Matthes, R.; Duske, K.; Kebede, T.G.; Pink, C.; Schluter, R.; von Woedtke, T.; Weltmann, K.D.; Kocher, T.; Jablonowski, L. Osteoblast growth, after cleaning of biofilm-covered titanium discs with air-polishing and cold plasma. J. Clin. Periodontol. 2017, 44, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Toma, S.; Lasserre, J.; Brecx, M.C.; Nyssen-Behets, C. In vitro evaluation of peri-implantitis treatment modalities on Saos-2osteoblasts. Clin. Oral Implant. Res. 2016, 27, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Hachim, D.; LoPresti, S.T.; Yates, C.C.; Brown, B.N. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials 2017, 112, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Giro, G.; Tebar, A.; Franco, L.; Racy, D.; Bastos, M.F.; Shibli, J.A. Treg and TH17 link to immune response in individuals with peri-implantitis: A preliminary report. Clin. Oral Investig. 2021, 25, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Cham, C.M.; Driessens, G.; O’Keefe, J.P.; Gajewski, T.F. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur. J. Immunol. 2008, 38, 2438–2450. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.Z.; Wang, R.; Huang, G.; Vogel, P.; Neale, G.; Green, D.R.; Chi, H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 2011, 208, 1367–1376. [Google Scholar] [CrossRef]
- Zhong, D.; Liu, X.; Schafer-Hales, K.; Marcus, A.I.; Khuri, F.R.; Sun, S.Y.; Zhou, W. 2-Deoxyglucose induces Akt phosphorylation via a mechanism independent of LKB1/AMP-activated protein kinase signaling activation or glycolysis inhibition. Mol. Cancer Ther. 2008, 7, 809–817. [Google Scholar] [CrossRef]
- Kim, H.S.; Jang, W.; Im, Y.G.; Lim, H.P. Antibacterial Effect of Zirconia Nanoparticles on Polyethyl Methacrylate Resin for Provisional Crowns. Int. J. Nanomed. 2022, 17, 6551–6560. [Google Scholar] [CrossRef]
- Rimondini, L.; Cerroni, L.; Carrassi, A.; Torricelli, P. Bacterial colonization of zirconia ceramic surfaces: An in vitro and in vivo study. Int. J. Oral Maxillofac. Implant. 2002, 17, 793–798. [Google Scholar]
- Li, P.; Hao, Z.; Wu, J.; Ma, C.; Xu, Y.; Li, J.; Lan, R.; Zhu, B.; Ren, P.; Fan, D.; et al. Comparative Proteomic Analysis of Polarized Human THP-1 and Mouse RAW264.7 Macrophages. Front Immunol. 2021, 12, 700009. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Awasthi, A.; Yosef, N.; Quintana, F.J.; Xiao, S.; Peters, A.; Wu, C.; Kleinewietfeld, M.; Kunder, S.; Hafler, D.A.; et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 2012, 13, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, T.; Yoshimura, A. In Vitro Th Differentiation Protocol. Methods Mol. Biol. 2016, 1344, 183–191. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Deng, S.; Xie, J.; Xu, C.; Huang, Z.; Huang, B.; Chen, Z.; Chen, S. 2-DG Regulates Immune Imbalance on the Titanium Surface after Debridement. Int. J. Mol. Sci. 2023, 24, 11431. https://doi.org/10.3390/ijms241411431
Liu X, Deng S, Xie J, Xu C, Huang Z, Huang B, Chen Z, Chen S. 2-DG Regulates Immune Imbalance on the Titanium Surface after Debridement. International Journal of Molecular Sciences. 2023; 24(14):11431. https://doi.org/10.3390/ijms241411431
Chicago/Turabian StyleLiu, Xingchen, Shudan Deng, Jiaxin Xie, Chunxin Xu, Zhuwei Huang, Baoxin Huang, Zhuofan Chen, and Shoucheng Chen. 2023. "2-DG Regulates Immune Imbalance on the Titanium Surface after Debridement" International Journal of Molecular Sciences 24, no. 14: 11431. https://doi.org/10.3390/ijms241411431
APA StyleLiu, X., Deng, S., Xie, J., Xu, C., Huang, Z., Huang, B., Chen, Z., & Chen, S. (2023). 2-DG Regulates Immune Imbalance on the Titanium Surface after Debridement. International Journal of Molecular Sciences, 24(14), 11431. https://doi.org/10.3390/ijms241411431




