Phosphoproteomics Reveal New Candidates in Abnormal Spermatogenesis of Pseudomales in Cynoglossus semilaevis
Abstract
1. Introduction
2. Results
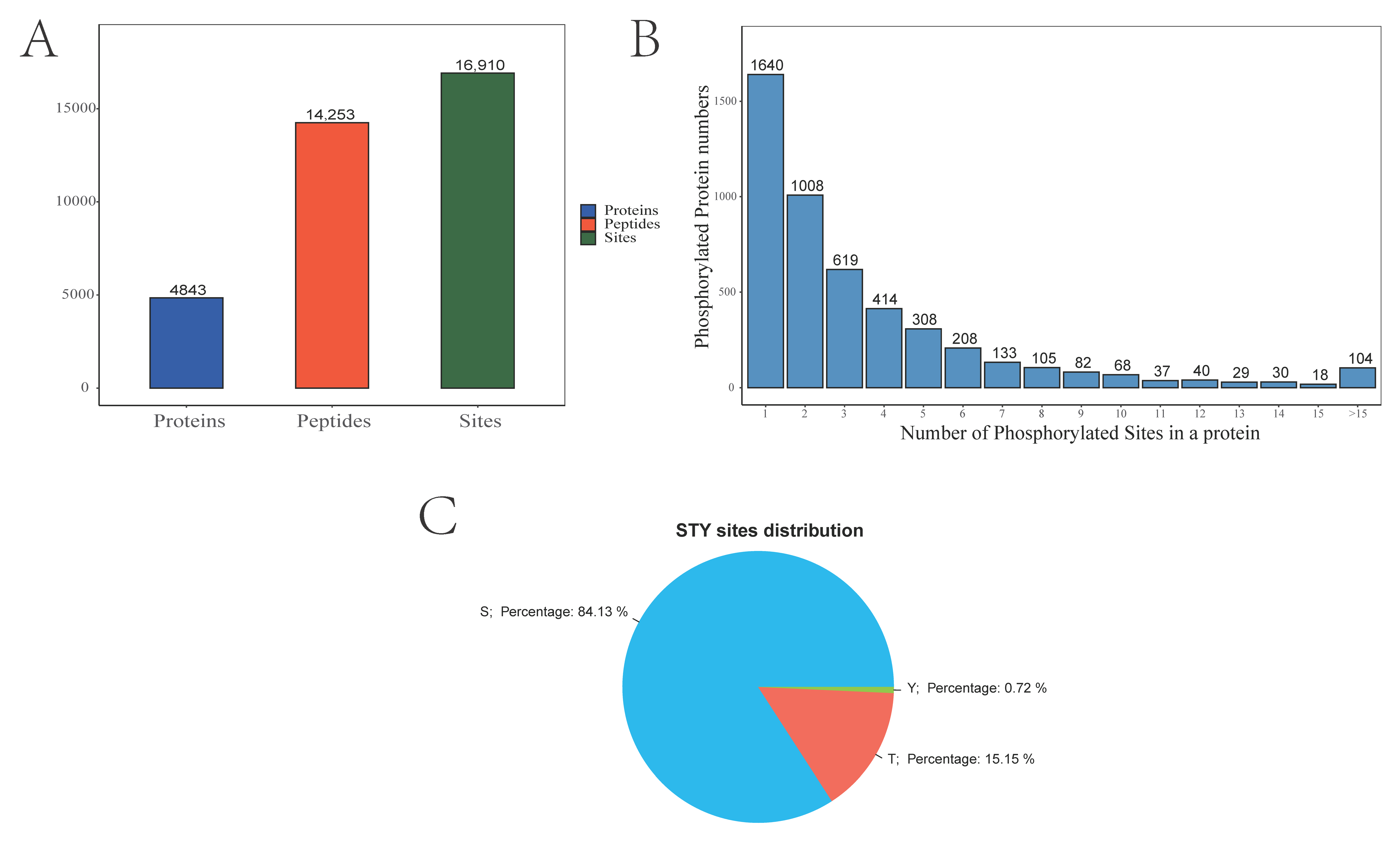
2.1. Identification and Quantification of Phosphorylation Sites in Tongue Sole Testis
2.2. Differentially Phosphorylated Proteins (DPPs) and Motif Analyses
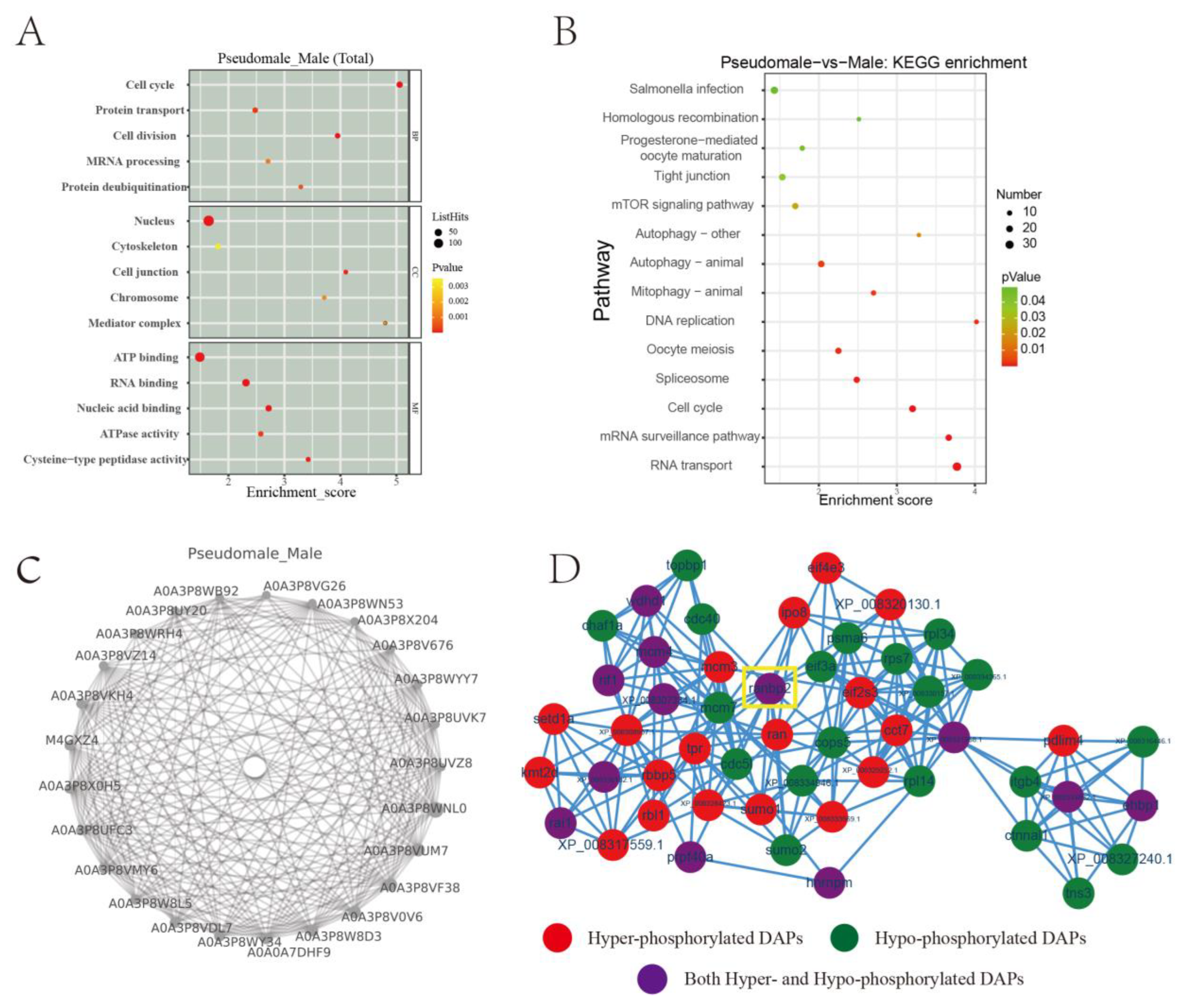
2.3. Functional Analyses of the DPPs
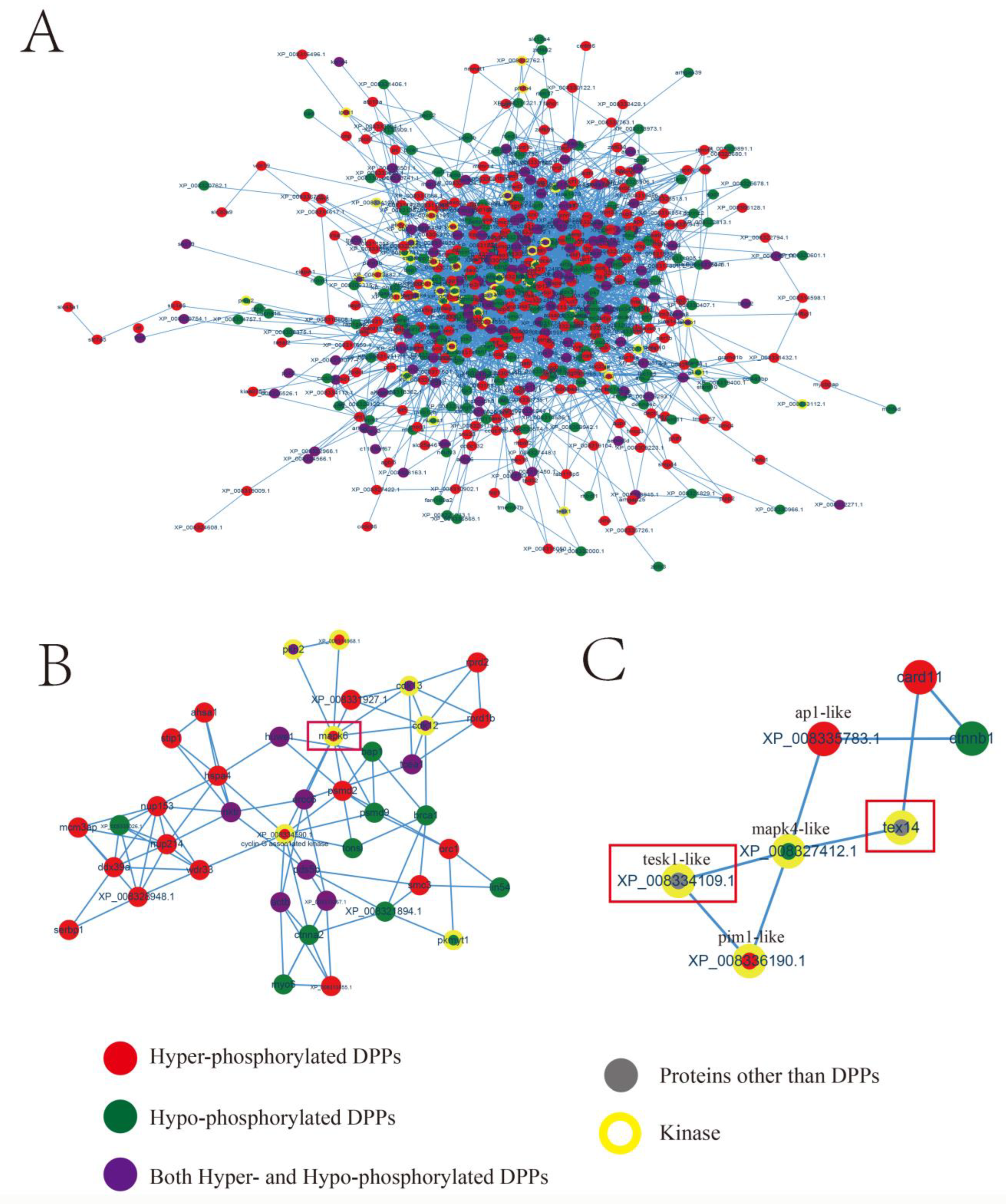
2.4. Protein-Protein Interaction (PPI) Network with Phosphorylated Proteins
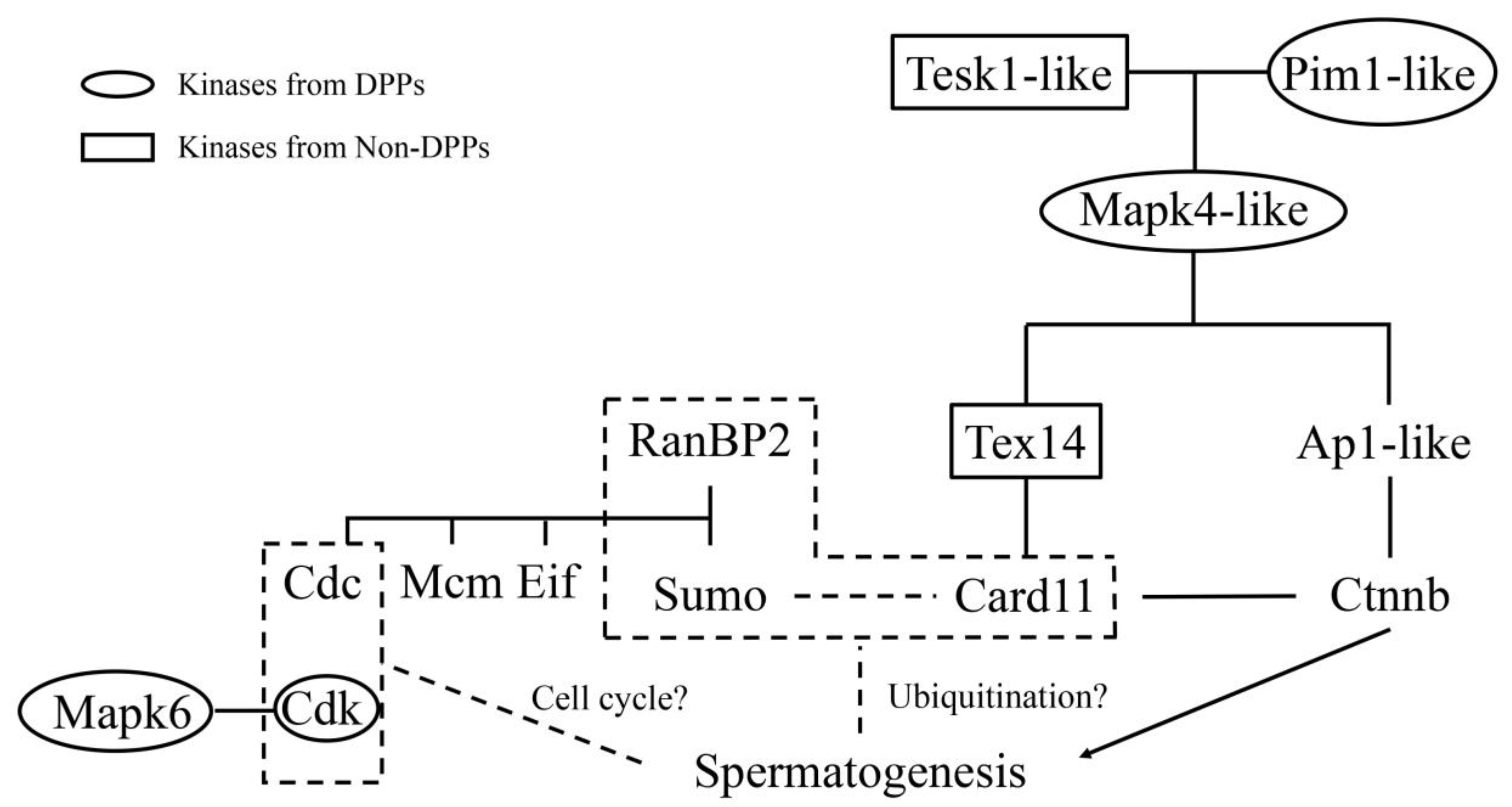
2.5. Kinase-Associated Network
3. Discussion
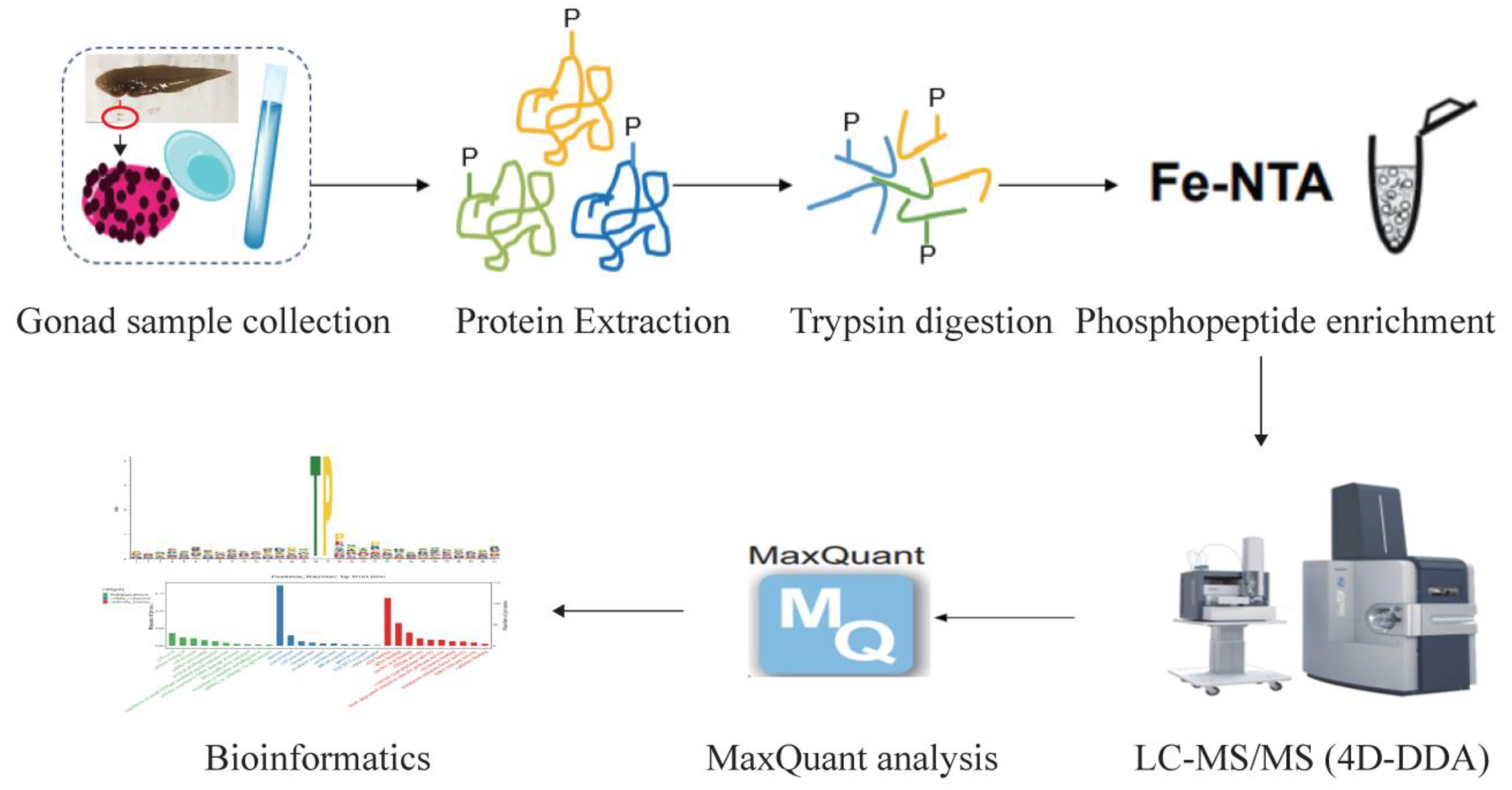
4. Materials and Methods
4.1. Experimental Fish and Sex Identification
4.2. Protein Preparation and Trypsin Digestion
4.3. Enrichment of Phosphopeptides
4.4. LC-MS/MS Analysis
4.5. Database Search
4.6. Differential Phosphorylation Site Identification and Motif Analysis
4.7. Functional Analysis and PPI Network Construction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fairbairn, D.J.; Blanckenhorn, W.U.; Székely, T. Sex, Size and Gender Roles: Evolutionary Studies of Sexual Size Dimorphism; Oxford University Press: Oxford, UK, 2007. [Google Scholar] [CrossRef]
- Horne, C.R.; Hirst, A.G.; Atkinson, D. Selection for increased male size predicts variation in sexual size dimorphism among fish species. Proc. Biol. Sci. 2020, 287, 20192640. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Gui, J.F. Genetic basis and biotechnological manipulation of sexual dimorphism and sex determination in fish. Sci. China Life Sci. 2015, 58, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Li, J.; Deng, S.P.; Tian, Y.S.; Wang, Q.Y.; Zhuang, Z.M.; Sha, Z.X.; Xu, J.Y. Isolation of female-specific AFLP markers and molecular identification of genetic sex in half-smooth tongue sole (Cynoglossus semilaevis). Mar. Biotechnol. 2007, 9, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Yang, Y.; Cheng, J.; Cheng, X.; Chen, S. Genetic parameter estimates for female proportion in tongue sole (Cynoglossus semilaevis). Aquaculture 2022, 548, 737652. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, G.; Shao, C.; Huang, Q.; Liu, G.; Zhang, P.; Song, W.; An, N.; Chalopin, D.; Volff, J.N.; et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat. Genet. 2014, 46, 253–260. [Google Scholar] [CrossRef]
- Shao, C.; Li, Q.; Chen, S.; Zhang, P.; Lian, J.; Hu, Q.; Sun, B.; Jin, L.; Liu, S.; Wang, Z.; et al. Epigenetic modification and inheritance in sexual reversal of fish. Genome Res. 2014, 24, 604–615. [Google Scholar] [CrossRef]
- Tang, L.; Huang, F.; You, W.; Poetsch, A.; Nobrega, R.H.; Power, D.M.; Zhu, T.; Liu, K.; Wang, H.Y.; Wang, Q.; et al. ceRNA crosstalk mediated by ncRNAs is a novel regulatory mechanism in fish sex determination and differentiation. Genome Res. 2022, 32, 1502–1515. [Google Scholar] [CrossRef]
- Mao, B.; Zhang, W.; Zheng, Y.; Li, D.; Chen, M.Y.; Wang, Y.F. Comparative phosphoproteomics reveal new candidates in the regulation of spermatogenesis of Drosophila melanogaster. Insect Sci. 2022, 29, 1703–1720. [Google Scholar] [CrossRef]
- Zhang, R.; Liang, C.; Guo, X.; Bao, P.; Pei, J.; Wu, F.; Yin, M.; Chu, M.; Yan, P. Quantitative phosphoproteomics analyses reveal the regulatory mechanisms related to frozen-thawed sperm capacitation and acrosome reaction in yak (Bos grunniens). Front. Physiol. 2022, 13, 1013082. [Google Scholar] [CrossRef]
- Xie, J.; Wooten, M.; Tran, V.; Chen, B.C.; Pozmanter, C.; Simbolon, C.; Betzig, E.; Chen, X. Histone H3 Threonine Phosphorylation Regulates Asymmetric Histone Inheritance in the Drosophila Male Germline. Cell 2015, 163, 920–933. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Ficca, M.L.; Scherthan, H.; Burkle, A.; Meyer, R.G. Poly(ADP-ribosyl)ation during chromatin remodeling steps in rat spermiogenesis. Chromosoma 2005, 114, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Xie, Y.M.; Li, X.; Luo, J.; Shi, X.Q.; Hong, X.; Pan, Y.H.; Ma, X. Mass spectrometry analysis of dynamic post-translational modifications of TH2B during spermatogenesis. Mol. Hum. Reprod. 2009, 15, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kang, X.; Mu, S. Histone phosphorylation and spermatogenesis. Yi Chuan = Hered. 2014, 36, 220–227. [Google Scholar]
- Shen, Y.R.; Wang, H.Y.; Kuo, Y.C.; Shih, S.C.; Hsu, C.H.; Chen, Y.R.; Wu, S.R.; Wang, C.Y.; Kuo, P.L. SEPT12 phosphorylation results in loss of the septin ring/sperm annulus, defective sperm motility and poor male fertility. PLoS Genet. 2017, 13, e1006631. [Google Scholar] [CrossRef]
- Lin, C.H.; Shen, Y.R.; Wang, H.Y.; Chiang, C.W.; Wang, C.Y.; Kuo, P.L. Regulation of septin phosphorylation: SEPT12 phosphorylation in sperm septin assembly. Cytoskeleton 2019, 76, 137–142. [Google Scholar] [CrossRef]
- Dietrich, M.A.; Nynca, J.; Ciereszko, A. Proteomic and metabolomic insights into the functions of the male reproductive system in fishes. Theriogenology 2019, 132, 182–200. [Google Scholar] [CrossRef]
- Nynca, J.; Dietrich, M.A.; Adamek, M.; Steinhagen, D.; Bilinska, B.; Hejmej, A.; Ciereszko, A. Purification, characterization and expression of transferrin from rainbow trout seminal plasma. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017, 208–209, 38–46. [Google Scholar] [CrossRef]
- di Clemente, N.; Jamin, S.P.; Lugovskoy, A.; Carmillo, P.; Ehrenfels, C.; Picard, J.Y.; Whitty, A.; Josso, N.; Pepinsky, R.B.; Cate, R.L. Processing of anti-mullerian hormone regulates receptor activation by a mechanism distinct from TGF-beta. Mol. Endocrinol. 2010, 24, 2193–2206. [Google Scholar] [CrossRef]
- Duan, W.; Gao, F.X.; Chen, Z.W.; Gao, Y.; Gui, J.F.; Zhao, Z.; Shi, Y. A sex-linked SNP mutation in amhr2 is responsible for male differentiation in obscure puffer (Takifugu obscurus). Mol. Biol. Rep. 2021, 48, 6035–6046. [Google Scholar] [CrossRef]
- Gazo, I.; Dietrich, M.A.; Pruliere, G.; Shaliutina-Kolesova, A.; Shaliutina, O.; Cosson, J.; Chenevert, J. Protein phosphorylation in spermatozoa motility of Acipenser ruthenus and Cyprinus carpio. Reproduction 2017, 154, 653–673. [Google Scholar] [CrossRef] [PubMed]
- Chauvigne, F.; Ducat, C.; Ferre, A.; Hansen, T.; Carrascal, M.; Abian, J.; Finn, R.N.; Cerda, J. A multiplier peroxiporin signal transduction pathway powers piscine spermatozoa. Proc. Natl. Acad. Sci. USA 2021, 118, e2019346118. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.V.; Orchard, S. Omics Technologies, Data, and Bioinformatics Principles. Methods Mol. Biol. 2011, 719, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, S.; Cao, X.; Gao, J. Quantitative Phosphoproteomic Analysis Reveals the Regulatory Networks of Elovl6 on Lipid and Glucose Metabolism in Zebrafish. Int. J. Mol. Sci. 2020, 21, 2860. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Li, H.; Wang, L.; Zhang, N.; Liu, Y.; Meng, L.; Xu, X.; Dong, Z.; Wei, M.; et al. iTRAQ-based analysis of 17β-estradiol induced proteome in Chinese tongue sole Cynoglossus semilaevis. J. Oceanol. Limnol. 2019, 37, 1659–1668. [Google Scholar] [CrossRef]
- Xu, W.; Cui, Z.; Wang, N.; Zhang, M.; Wang, J.; Xu, X.; Liu, Y.; Chen, S. Transcriptomic analysis revealed gene expression profiles during the sex differentiation of Chinese tongue sole (Cynoglossus semilaevis). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100919. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P. The origins of protein phosphorylation. Nat. Cell Biol. 2002, 4, E127–E130. [Google Scholar] [CrossRef]
- Xu, Y.; Han, Q.; Ma, C.; Wang, Y.; Zhang, P.; Li, C.; Cheng, X.; Xu, H. Comparative Proteomics and Phosphoproteomics Analysis Reveal the Possible Breed Difference in Yorkshire and Duroc Boar Spermatozoa. Front. Cell Dev. Biol. 2021, 9, 652809. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Q.; Zhu, L.; He, W.; Yang, C.; Zhang, H.; Sun, Y.; Zhou, L.; Sun, Y.; Zhu, S.; et al. Abnormal meiosis in fertile and sterile triploid cyprinid fish. Sci. China Life Sci. 2021, 64, 1917–1928. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Liu, X.; Chen, J.-Y.; Huang, Y.; Lu, Y.; Tan, F.; Liu, Q.; Yang, M.; Li, S.; Zhang, X.; et al. Single-cell-resolution transcriptome map revealed novel genes involved in testicular germ cell progression and somatic cells specification in Chinese tongue sole with sex reversal. Sci. China Life Sci. 2023, 66, 1151–1169. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Zhou, C.-X.; Calderón-Mantilla, G.; Petsalaki, E.; He, J.-J.; Song, H.-Y.; Elsheikha, H.M.; Zhu, X.-Q. iTRAQ-Based Global Phosphoproteomics Reveals Novel Molecular Differences Between Toxoplasma gondii Strains of Different Genotypes. Front. Cell. Infect. Microbiol. 2019, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zhu, Y.; Zhang, N.; Liu, W.; Liu, Y.; Shao, C.; Wang, N.; Chen, S. Cloning and characterization of tesk1, a novel spermatogenesis-related gene, in the tongue sole (Cynoglossus semilaevis). PLoS ONE 2014, 9, e107922. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hu, Q.; Xu, W.; Li, H.; Guo, H.; Meng, L.; Wei, M.; Lu, S.; Shao, C.; Wang, N.; et al. Identification and analysis of the β-catenin1 gene in half-smooth tongue sole (Cynoglossus semilaevis). PLoS ONE 2017, 12, e0176122. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhu, Y.; Cheng, P.; Zhang, M.; Wang, N.; Cui, Z.; Wei, M.; Xu, W. A Z-Linked E3 Ubiquitin Ligase Cs-rchy1 Is Involved in Gametogenesis in Chinese Tongue Sole, Cynoglossus semilaevis. Animals 2021, 11, 3265. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, H.; Dong, Z.; Cui, Z.; Zhang, N.; Meng, L.; Zhu, Y.; Liu, Y.; Li, Y.; Guo, H.; et al. Ubiquitin ligase gene neurl3 plays a role in spermatogenesis of half-smooth tongue sole (Cynoglossus semilaevis) by regulating testis protein ubiquitination. Gene 2016, 592, 215–220. [Google Scholar] [CrossRef]
- Chen, S.L.; Ji, X.S.; Shao, C.W.; Li, W.L.; Yang, J.F.; Liang, Z.; Liao, X.L.; Xu, G.B.; Xu, Y.; Song, W.T. Induction of mitogynogenetic diploids and identification of WW super-female using sex-specific SSR markers in half-smooth tongue sole (Cynoglossus semilaevis). Mar. Biotechnol. 2012, 14, 120–128. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, B.; Zhu, D.; Dufresne, D.; Jiang, T.; Chen, S. Proteomics of Homeobox7 Enhanced Salt Tolerance in Mesembryanthemum crystallinum. Int. J. Mol. Sci. 2021, 22, 6390. [Google Scholar] [CrossRef]
- Akkurt Arslan, M.; Kolman, I.; Pionneau, C.; Chardonnet, S.; Magny, R.; Baudouin, C.; Brignole-Baudouin, F.; Kessal, K. Proteomic Analysis of Tears and Conjunctival Cells Collected with Schirmer Strips Using timsTOF Pro: Preanalytical Considerations. Metabolites 2022, 12, 2. [Google Scholar] [CrossRef]

| Accession | Gene ID | Abbreviation | Protein Name | Chromosome |
|---|---|---|---|---|
| A0A3P8WNL0 | 103387747 | Cdc5l | Cell division cycle 5-like | 12 |
| A0A3P8VUM7 | 103393521 | Smg1 | Nonspecific serine/threonine protein kinase | 17 |
| A0A3P8VF38 | 103397928 | Cyclin-G-associated kinase-like | Z | |
| A0A3P8V0V6 | 103377805 | Ywhae1 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein epsilon | 4 |
| A0A3P8W8D3 | 103392282 | RanBP2 | E3 SUMO-protein ligase RanBP2 | 16 |
| A0A0A7DHF9 | 103393304 | ActB2 | Actin, beta | 17 |
| A0A3P8WY34 | 103378585 | Trip12 | Thyroid hormone receptor interactor 12 | 4 |
| A0A3P8VDL7 | 103397936 | Ttf1 | Transcription termination factor 1 | Z |
| A0A3P8W8L5 | 103388903 | Ywhaz | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta | 13 |
| A0A3P8VMY6 | 103379263 | Shank2 | SH3 and multiple ankyrin repeat domains 2a | 5 |
| A0A3P8UFC3 | 103382302 | ActA2 | Actin, alpha 2 | 8 |
| A0A3P8X0H5 | 103380891 | Hsp90ab1 | Heat shock protein HSP 90-beta | 7 |
| M4GXZ4 | 103393291 | Ubc9 | SUMO-conjugating enzyme UBC9-like | 17 |
| A0A3P8VKH4 | 103381595 | / | Serine/threonine-protein phosphatase alpha-2 isoform-like | 8 |
| A0A3P8VZ14 | 103379050 | Mapk6 | Mitogen-activated protein kinase 6 | 5 |
| A0A3P8WRH4 | 103393241 | Usp7 | Ubiquitin carboxyl-terminal hydrolase 7 | 17 |
| A0A3P8UY20 | 103380065 | Chd2 | Chromodomain-helicase-DNA-binding protein 2 | 6 |
| A0A3P8WB92 | 103391196 | Hsp4b | Heat shock protein 4b | 15 |
| A0A3P8VG26 | 103381460 | Brca1 | BRCA1 DNA repair associated | 8 |
| A0A3P8V676 | 103377844 | Ppp5c | Serine/threonine-protein phosphatase 5 | 4 |
| A0A3P8WN53 | 103378493 | Rabgef1l | RAB guanine nucleotide exchange factor (GEF) 1-like | 4 |
| A0A3P8X204 | 103392668 | Setd1a | Histone-lysine N-methyltransferase SETD1A | 17 |
| A0A3P8WYY7 | 103395053 | Hsp70 | Heat shock cognate 70 kDa protein | 19 |
| A0A3P8UVK7 | 103377749 | Usp32 | Ubiquitin specific peptidase 32 | 4 |
| A0A3P8UVZ8 | 103378868 | Pp1cb | Serine/threonine-protein phosphatase PP1-beta catalytic subunit | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, L.; Cui, Z.; Li, M.; Xu, W. Phosphoproteomics Reveal New Candidates in Abnormal Spermatogenesis of Pseudomales in Cynoglossus semilaevis. Int. J. Mol. Sci. 2023, 24, 11430. https://doi.org/10.3390/ijms241411430
Li X, Li L, Cui Z, Li M, Xu W. Phosphoproteomics Reveal New Candidates in Abnormal Spermatogenesis of Pseudomales in Cynoglossus semilaevis. International Journal of Molecular Sciences. 2023; 24(14):11430. https://doi.org/10.3390/ijms241411430
Chicago/Turabian StyleLi, Xihong, Lu Li, Zhongkai Cui, Ming Li, and Wenteng Xu. 2023. "Phosphoproteomics Reveal New Candidates in Abnormal Spermatogenesis of Pseudomales in Cynoglossus semilaevis" International Journal of Molecular Sciences 24, no. 14: 11430. https://doi.org/10.3390/ijms241411430
APA StyleLi, X., Li, L., Cui, Z., Li, M., & Xu, W. (2023). Phosphoproteomics Reveal New Candidates in Abnormal Spermatogenesis of Pseudomales in Cynoglossus semilaevis. International Journal of Molecular Sciences, 24(14), 11430. https://doi.org/10.3390/ijms241411430






