An Ex Vivo Electroretinographic Apparatus for the mL-Scale Testing of Drugs to One Day and Beyond
Abstract
1. Introduction
2. Results
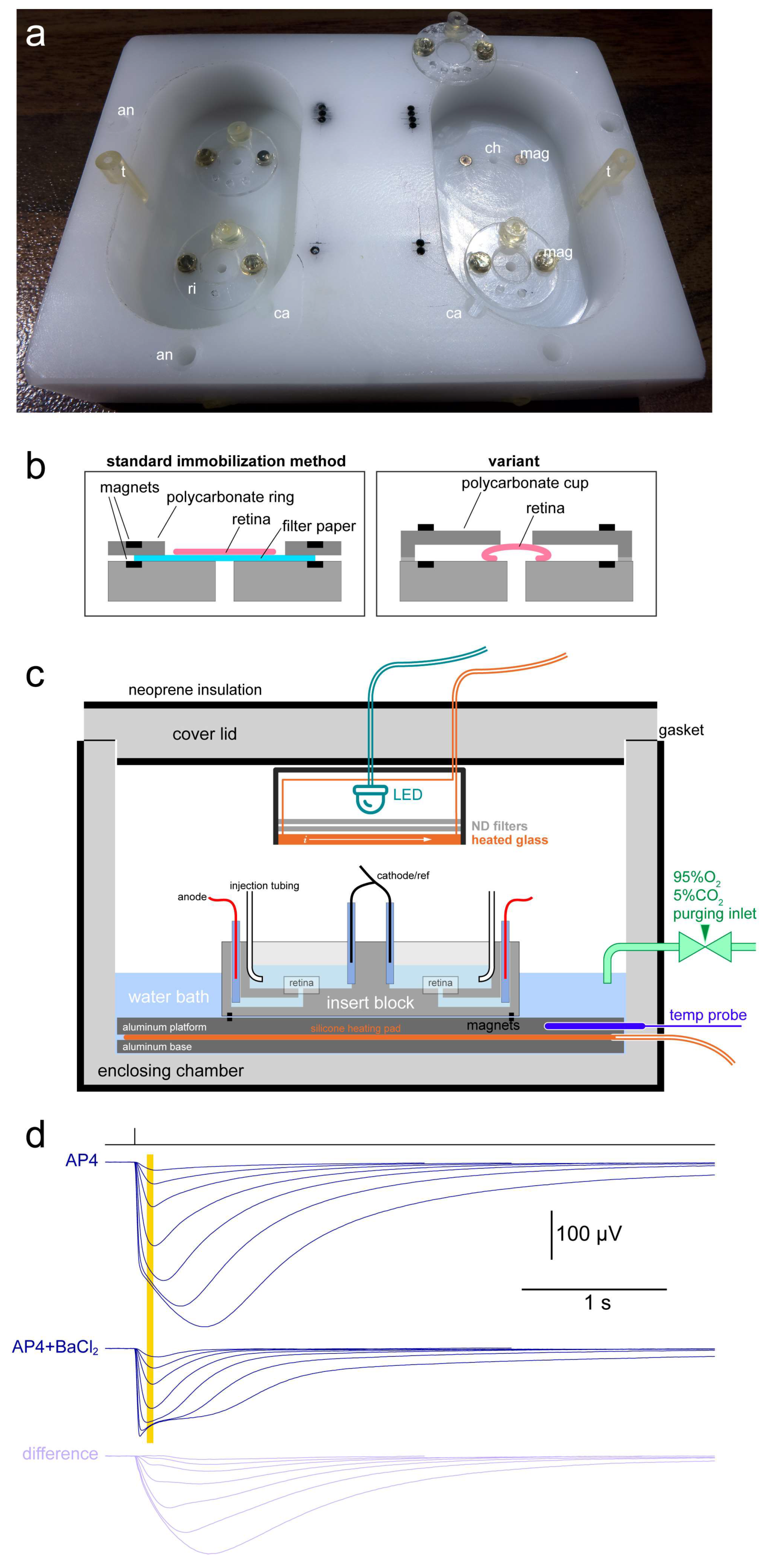
2.1. Basic Description of the Apparatus and Setting up of an Experiment
2.2. Barium Is Not Required to Obtain Good Estimates of Light Sensitivity and Response Kinetics
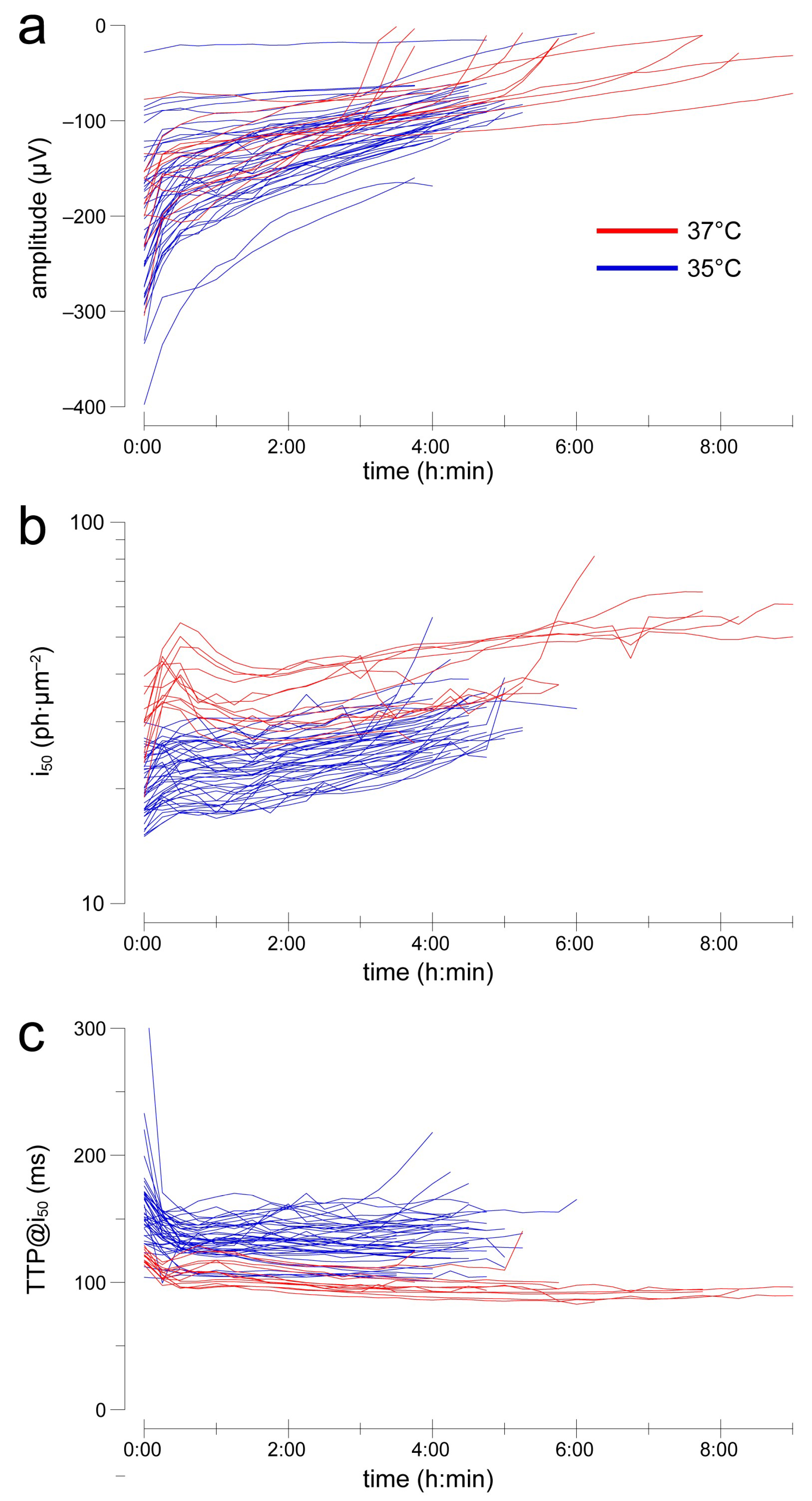
2.3. After an Initial Settling, Sensitivity and Kinetics Are Reasonably Stable over Several Hours
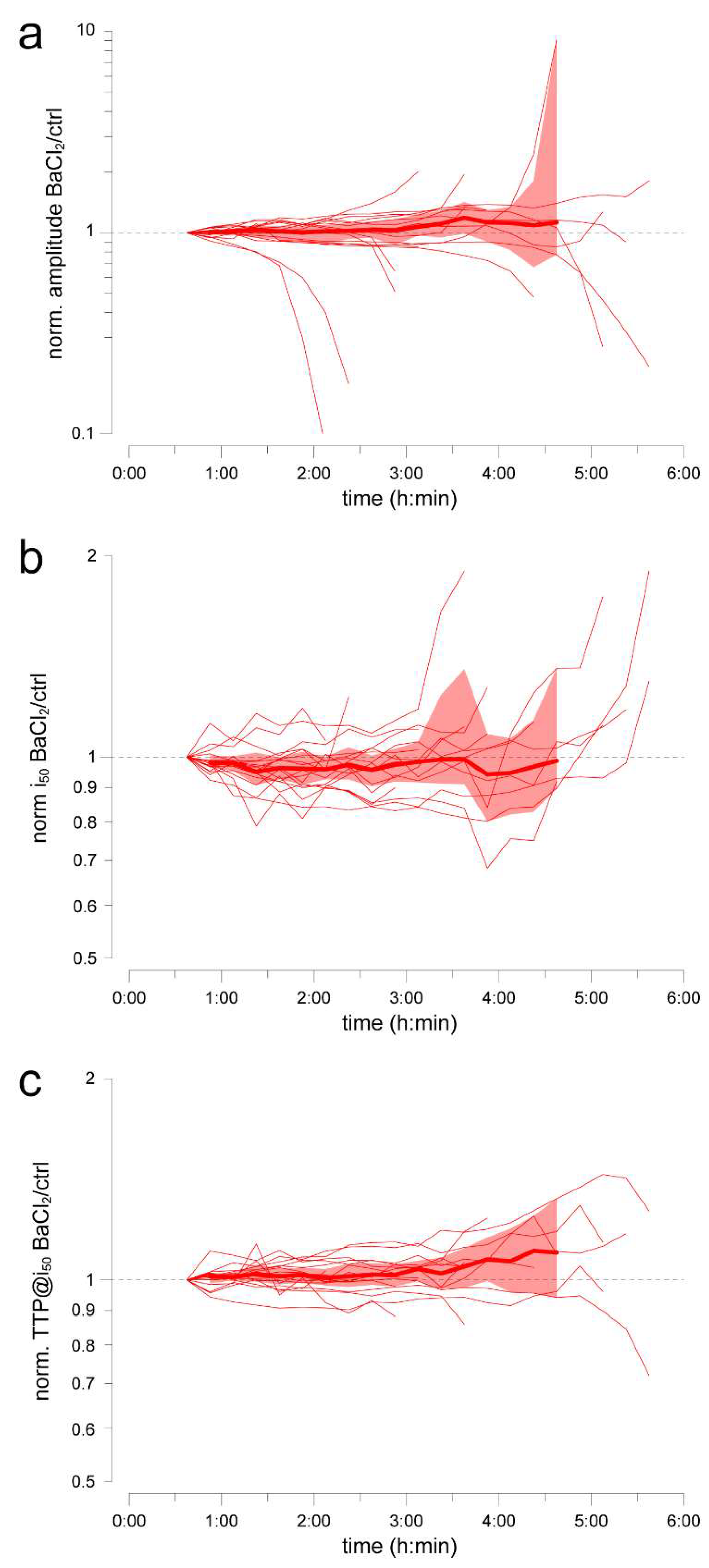
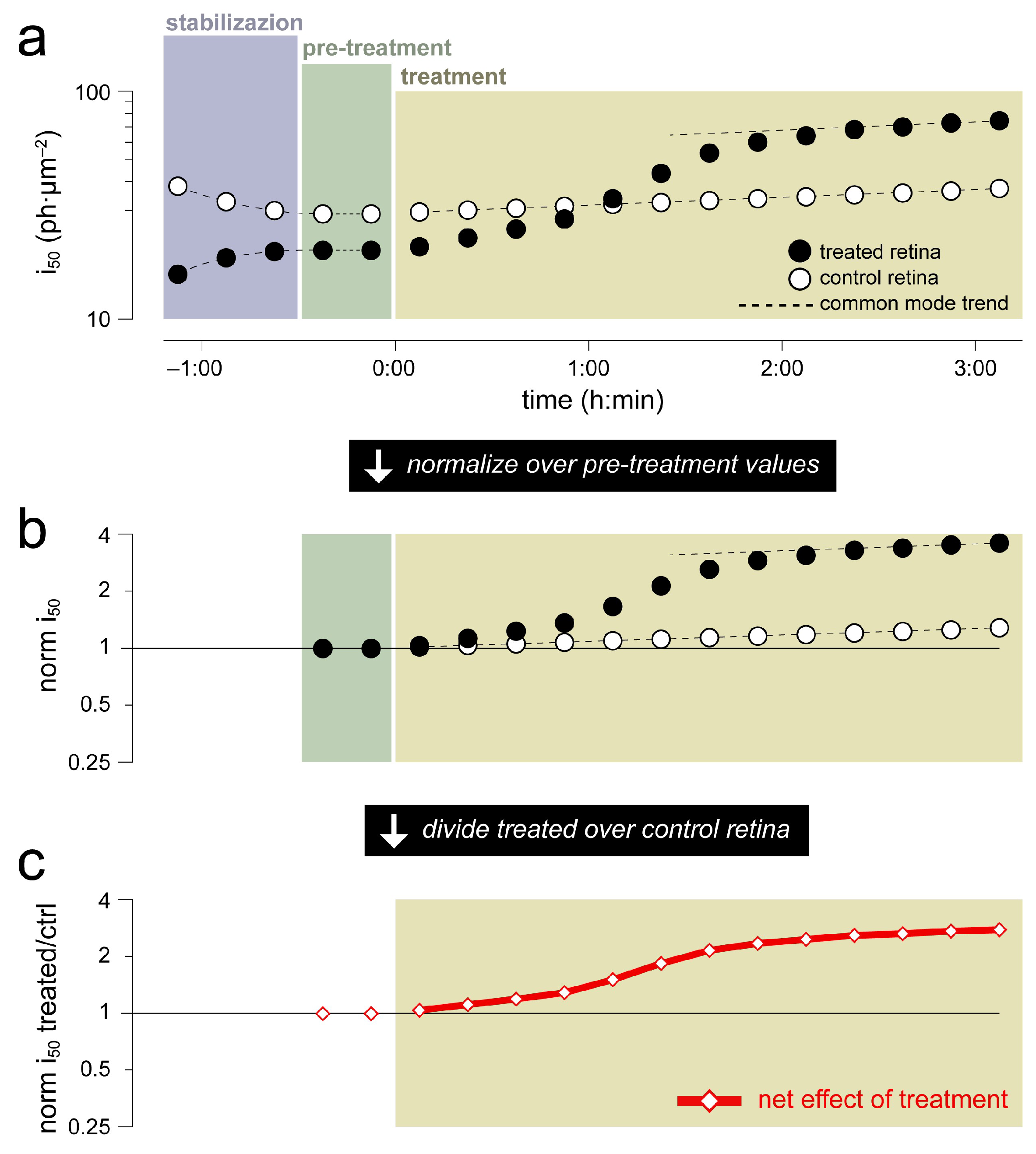
2.4. Example Application 1 (Assessing Substance Effects over the Medium Term): Barium Chloride
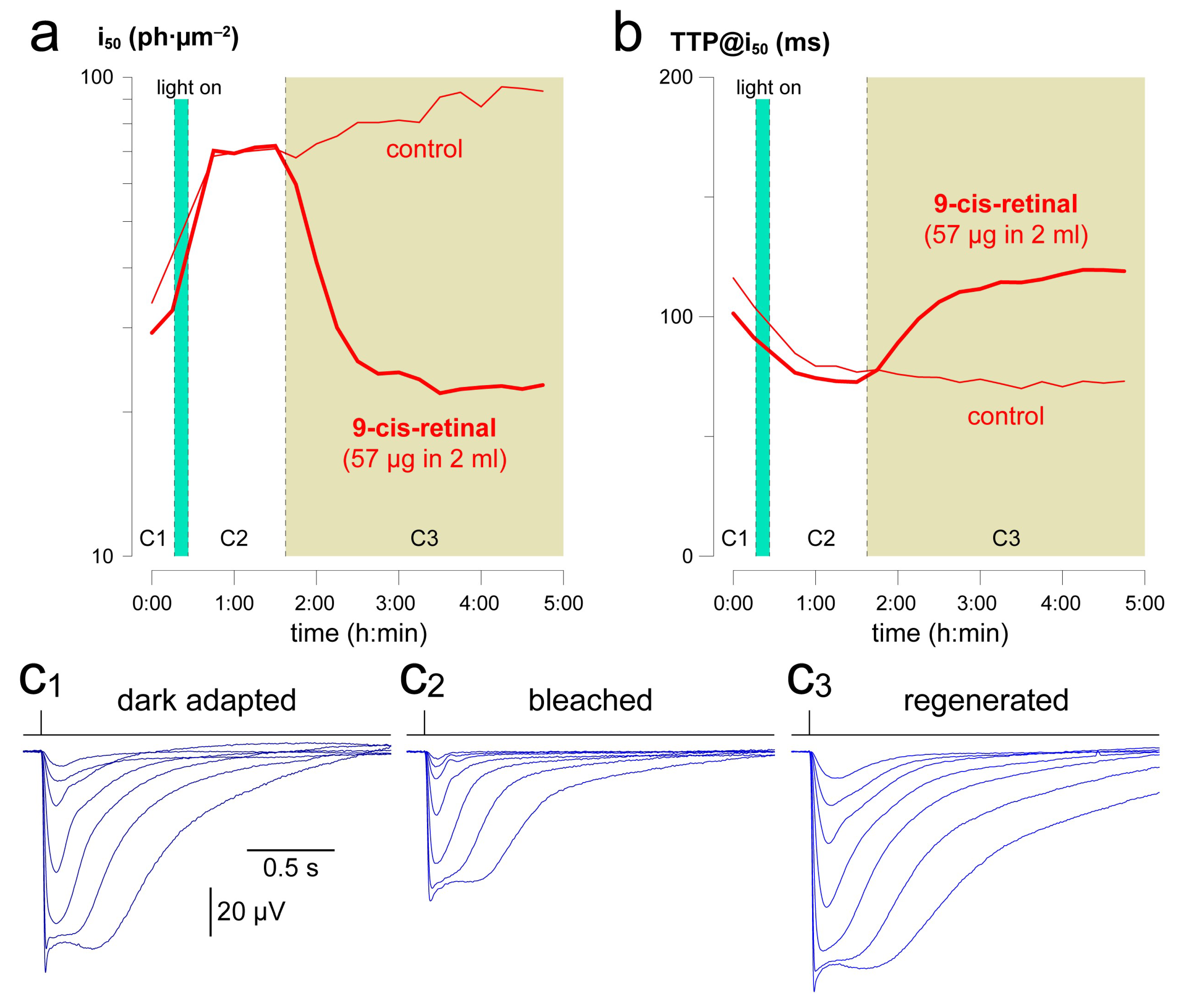
2.5. Example Application 2 (mL-Scale Drug Testing): Pigment Regeneration after a Mild Bleach
2.6. Antibiotics Extend the Recordings to One Day and Beyond
3. Discussion
4. Materials and Methods
4.1. Dissection and Handling
4.2. Insert Block
4.3. Enclosing Chamber
4.4. Solutions
4.5. Electrophysiological Recordings and Automated Parameter Extraction
4.6. Data Processing Pipeline
4.7. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Robson, J.G.; Frishman, L.J. The rod-driven a-wave of the dark-adapted mammalian electroretinogram. Prog. Retin. Eye Res. 2014, 39, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Turunen, T.T.; Koskelainen, A. Transretinal ERG in studying mouse rod phototransduction: Comparison with local ERG across the rod outer segments. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6133–6145. [Google Scholar] [CrossRef]
- Vinberg, F.; Kolesnikov, A.V.; Kefalov, V.J. Ex vivo ERG analysis of photoreceptors using an in vivo ERG system. Vis. Res. 2014, 101, 108–117. [Google Scholar] [CrossRef]
- Asteriti, S.; Marino, V.; Avesani, A.; Biasi, A.; Dal Cortivo, G.; Cangiano, L.; Dell’Orco, D. Recombinant protein delivery enables modulation of the phototransduction cascade in mouse retina. bioRxiv 2022. [Google Scholar] [CrossRef]
- Albanna, W.; Lueke, J.N.; Sjapic, V.; Kotliar, K.; Hescheler, J.; Clusmann, H.; Sjapic, S.; Alpdogan, S.; Schneider, T.; Schubert, G.A.; et al. Electroretinographic assessment of inner retinal signaling in the isolated and superfused murine retina. Curr. Eye Res. 2017, 42, 1518–1526. [Google Scholar] [CrossRef]
- Abbas, F.; Vinberg, F.; Becker, S. Optimizing the setup and conditions for ex vivo electroretinogram to study retina function in small and large eyes. J. Vis. Exp. 2022, 27, e62763. [Google Scholar] [CrossRef]
- Bonezzi, P.J.; Tarchick, M.J.; Renna, J.M. Ex vivo electroretinograms made easy: Performing ERGs using 3D printed components. J. Physiol. 2020, 598, 4821–4842. [Google Scholar] [CrossRef]
- Masland, R.H.; Ames, A., 3rd. Dissociation of field potential from neuronal activity in the isolated retina: Failure of the b-wave with normal ganglion cell response. J. Neurobiol. 1975, 6, 305–312. [Google Scholar] [CrossRef]
- Green, D.G.; Kapousta-Bruneau, N.V. Electrophysiological properties of a new isolated rat retina preparation. Vis. Res. 1999, 39, 2165–2177. [Google Scholar] [CrossRef]
- Nymark, S.; Heikkinen, H.; Haldin, C.; Donner, K.; Koskelainen, A. Light responses and light adaptation in rat retinal rods at different temperatures. J. Physiol. 2005, 567, 923–938. [Google Scholar] [CrossRef]
- Lüke, M.; Weiergräber, M.; Brand, C.; Siapich, S.A.; Banat, M.; Hescheler, J.; Lüke, C.; Schneider, T. The isolated perfused bovine retina—A sensitive tool for pharmacological research on retinal function. Brain Res. Protoc. 2005, 16, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Vinberg, F.; Koskelainen, A. Calcium sets the physiological value of the dominant time constant of saturated mouse rod photoresponse recovery. PLoS ONE 2010, 5, e13025. [Google Scholar] [CrossRef]
- Kolesnikov, A.V.; Kefalov, V.J. Transretinal ERG recordings from mouse retina: Rod and cone photoresponses. J. Vis. Exp. 2012, 61, e3424. [Google Scholar] [CrossRef]
- Heikkinen, H.; Vinberg, F.; Pitkänen, M.; Kommonen, B.; Koskelainen, A. Flash responses of mouse rod photoreceptors in the isolated retina and corneal electroretinogram: Comparison of gain and kinetics. Investig. Ophtalmol. Vis. Sci. 2012, 53, 5653–5664. [Google Scholar] [CrossRef]
- Vinberg, F.; Kefalov, V. Simultaneous ex vivo functional testing of two retinas by in vivo electroretinogram system. J. Vis. Exp. 2015, 99, e52855. [Google Scholar] [CrossRef]
- Leinonen, H.; Pham, N.C.; Boyd, T.; Santoso, J.; Palczewski, K.; Vinberg, F. Homeostatic plasticity in the retina is associated with maintenance of night vision during retinal degenerative disease. eLife 2020, 9, e59422. [Google Scholar] [CrossRef]
- Behnen, P.; Felline, A.; Comitato, A.; Di Salvo, M.T.; Raimondi, F.; Gulati, S.; Kahremany, S.; Palczewski, K.; Marigo, V.; Fanelli, F. A small chaperone improves folding and routing of rhodopsin mutants linked to inherited blindness. iScience 2018, 4, 1–19. [Google Scholar] [CrossRef]
- Abbas, F.; Becker, S.; Jones, B.W.; Mure, L.S.; Panda, S.; Hanneken, A.; Vinberg, F. Revival of light signalling in the postmortem mouse and human retina. Nature 2022, 606, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Müller, B. Organotypic culture of adult mouse retina. Methods Mol. Biol. 2019, 1940, 181–191. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, B.; Tolone, A.; Yan, J.; Christensen, G.; Arango-Gonzalez, B.; Ueffing, M.; Paquet-Durand, F. In vitro model systems for studies into retinal neuroprotection. Front. Neurosci. 2022, 16, 938089. [Google Scholar] [CrossRef]
- Ames, A., 3rd; Nesbett, F.B. In vitro retina as an experimental model of the central nervous system. J. Neurochem. 1981, 37, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.W.; Rieke, F. Experimental protocols alter phototransduction: The implications for retinal processing at visual threshold. J. Neurosci. 2010, 31, 3670–3682. [Google Scholar] [CrossRef]
- Cangiano, L.; Asteriti, S.; Cervetto, L.; Gargini, C. The photovoltage of rods and cones in the dark-adapted mouse retina. J. Physiol. 2012, 590, 3841–3855. [Google Scholar] [CrossRef] [PubMed]
- Bolnick, D.A.; Walter, A.E.; Sillman, A.J. Barium suppresses slow PIII in perfused bullfrog retina. Vis. Res. 1979, 19, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.-G.; Yau, K.-W. Rod sensitivity of neonatal mouse and rat. J. Gen. Physiol. 2005, 126, 263–269. [Google Scholar] [CrossRef]
- Massoumi, H.; Amin, S.; Soleimani, M.; Momenaei, B.; Ashraf, M.J.; Guaiquil, V.H.; Hematti, P.; Rosenblatt, M.I.; Djalilian, A.R.; Jalilian, E. Extracellular-vesicle-based therapeutics in neuro-ophthalmic disorders. Int. J. Mol. Sci. 2023, 24, 9006. [Google Scholar] [CrossRef]
- Moritoh, S.; Tanaka, K.F.; Jouhou, H.; Ikenaka, K.; Koizumi, A. Organotypic tissue culture of adult rodent retina followed by particle-mediated acute gene transfer in vitro. PLoS ONE 2010, 5, e12917. [Google Scholar] [CrossRef]
- Alarautalahti, V.; Ragauskas, S.; Hakkarainen, J.J.; Uusitalo-Järvinen, H.; Uusitalo, H.; Hyttinen, J.; Kalesnykas, G.; Nymark, S. Viability of Mouse Retinal Explant Cultures Assessed by Preservation of Functionality and Morphology. Investig. Ophtalmol. Vis. Sci. 2019, 60, 1914–1927. [Google Scholar] [CrossRef]
- Kawasaki, K.; Ohnogi, J.; Okayama, Y.; Yonemura, D. Effects of antibiotics on the in vitro ERG of the albino rabbit. Penicillins and cephalosporins antibiotics. Doc. Ophtalmol. 1987, 66, 75–84. [Google Scholar] [CrossRef]
- Hancock, H.A.; Guidry, C.; Read, R.W.; Ready, E.L.; Kraft, T.W. Acute aminoglycoside retinal toxicity in vivo and in vitro. Investig. Ophtalmol. Vis. Sci. 2005, 46, 4804–4808. [Google Scholar] [CrossRef]
- Fernandez-Bueno, I.; Fernández-Sánchez, L.; Gayoso, M.J.; García-Gutierrez, M.T.; Pastor, J.C.; Cuenca, N. Time course modifications in organotypic culture of human neuroretina. Exp. Eye Res. 2012, 104, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Wagner, F.; Lorenz, B.; Stieger, K. Organotypic cultures of adult mouse retina: Morphologic changes and gene expression. Investig. Ophtalmol. Vis. Sci. 2017, 58, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Asteriti, S.; Cangiano, L. Slow light response kinetics in rods points towards a perturbation of the normal cellular milieu. J. Physiol. 2015, 593, 2975–2976. [Google Scholar] [CrossRef]
- Heikkinen, H.; Nymark, S.; Koskelainen, A. Mouse cone photoresponses obtained with electroretinogram from the isolated retina. Vis. Res. 2008, 48, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, A.; Stolzenburg, J.U.; Eberhardt, W.; Chao, T.I.; Dettmer, D.; Hertz, L. What do retinal Müller (glial) cells do for their neuronal ‘small siblings’? J. Chem. Neuroanat. 1993, 6, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, S.; Tolone, A.; Christensen, G.; Das, S.; Chen, Y.; Paquet-Durand, F. Long-Term, Serum-Free cultivation of organotypic mouse retina explants with intact retinal pigment epithelium. J. Vis. Exp. 2020, 165, e61868. [Google Scholar] [CrossRef]
- Marcos, L.F.; Wilson, S.L.; Roach, P. Tissue engineering of the retina: From organoids to microfluidic chips. J. Tissue Eng. 2021, 12, 20417314211059876. [Google Scholar] [CrossRef]
- Beelen, C.J.; Asteriti, S.; Cangiano, L.; Koch, K.W.; Dell’Orco, D. A hybrid stochastic/deterministic model of single photon response and light adaptation in mouse rods. Comput. Struct. Biotechnol. J. 2021, 19, 3720–3734. [Google Scholar] [CrossRef]
- Asteriti, S.; Cangiano, L. Versatile bipolar temperature controller for custom in vitro applications. HardwareX 2020, 8, e00155. [Google Scholar] [CrossRef]
- Li, Z.; Zhuang, J.; Corson, D.W. Delivery of 9-cis retinal to photoreceptors from bovine serum albumin. Photochem. Photobiol. 1999, 69, 500–504. [Google Scholar] [CrossRef]
- Hershberger, S.L. Hodges-Lehmann estimators. In International Encyclopedia of Statistical Science; Miodrag, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 635–636. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cangiano, L.; Asteriti, S. An Ex Vivo Electroretinographic Apparatus for the mL-Scale Testing of Drugs to One Day and Beyond. Int. J. Mol. Sci. 2023, 24, 11346. https://doi.org/10.3390/ijms241411346
Cangiano L, Asteriti S. An Ex Vivo Electroretinographic Apparatus for the mL-Scale Testing of Drugs to One Day and Beyond. International Journal of Molecular Sciences. 2023; 24(14):11346. https://doi.org/10.3390/ijms241411346
Chicago/Turabian StyleCangiano, Lorenzo, and Sabrina Asteriti. 2023. "An Ex Vivo Electroretinographic Apparatus for the mL-Scale Testing of Drugs to One Day and Beyond" International Journal of Molecular Sciences 24, no. 14: 11346. https://doi.org/10.3390/ijms241411346
APA StyleCangiano, L., & Asteriti, S. (2023). An Ex Vivo Electroretinographic Apparatus for the mL-Scale Testing of Drugs to One Day and Beyond. International Journal of Molecular Sciences, 24(14), 11346. https://doi.org/10.3390/ijms241411346





