Melanin’s Journey from Melanocytes to Keratinocytes: Uncovering the Molecular Mechanisms of Melanin Transfer and Processing
Abstract
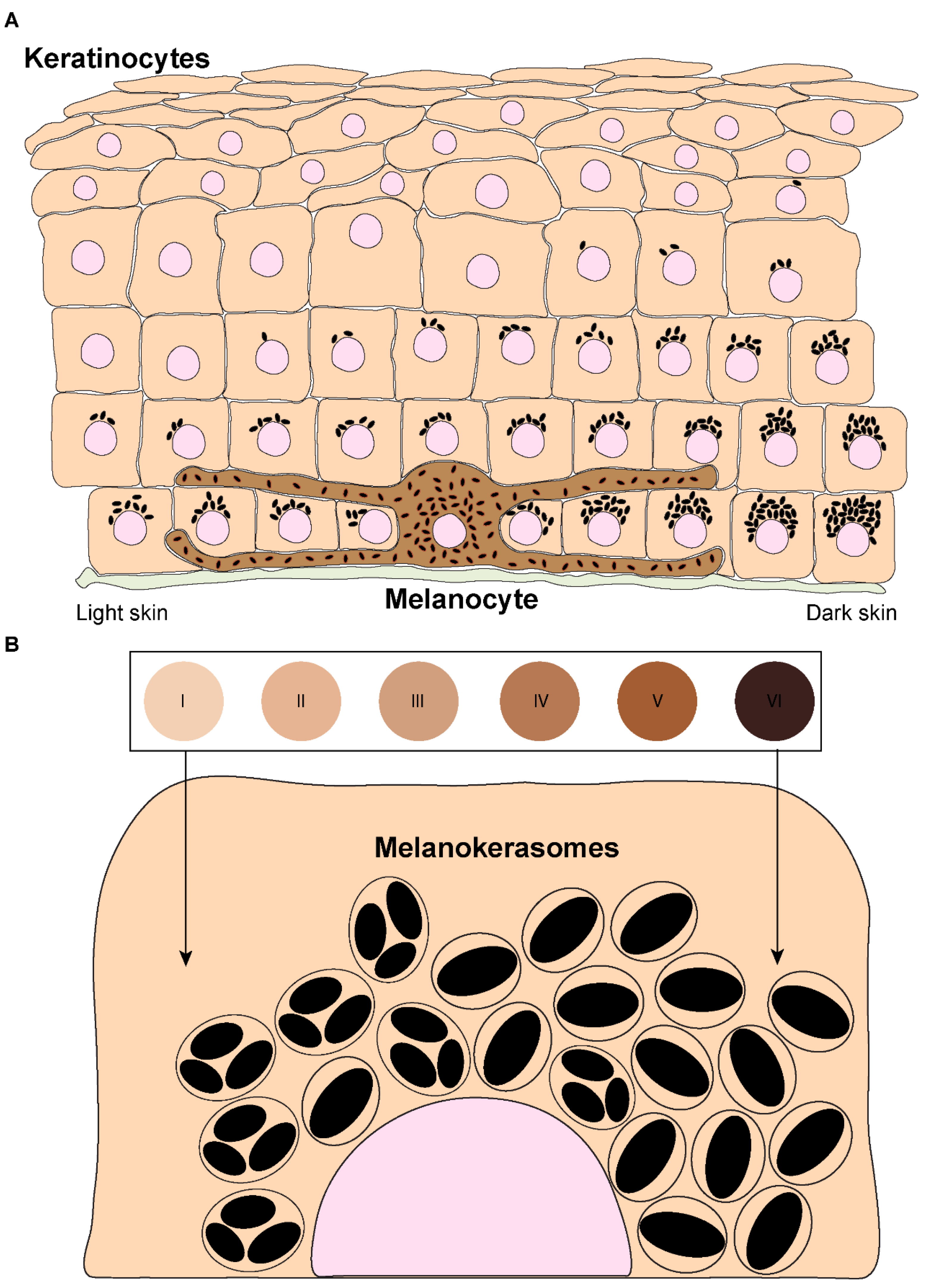
1. Introduction
2. Melanin Transfer Models
- Cytophagocytosis of melanocyte dendrites by keratinocytes.
- 2.
- Fusion of melanocyte and keratinocyte membranes.
- 3.
- Shedding of melanosome-laden globules.
- 4.
- Coupled exocytosis/phagocytosis of the melanin core.
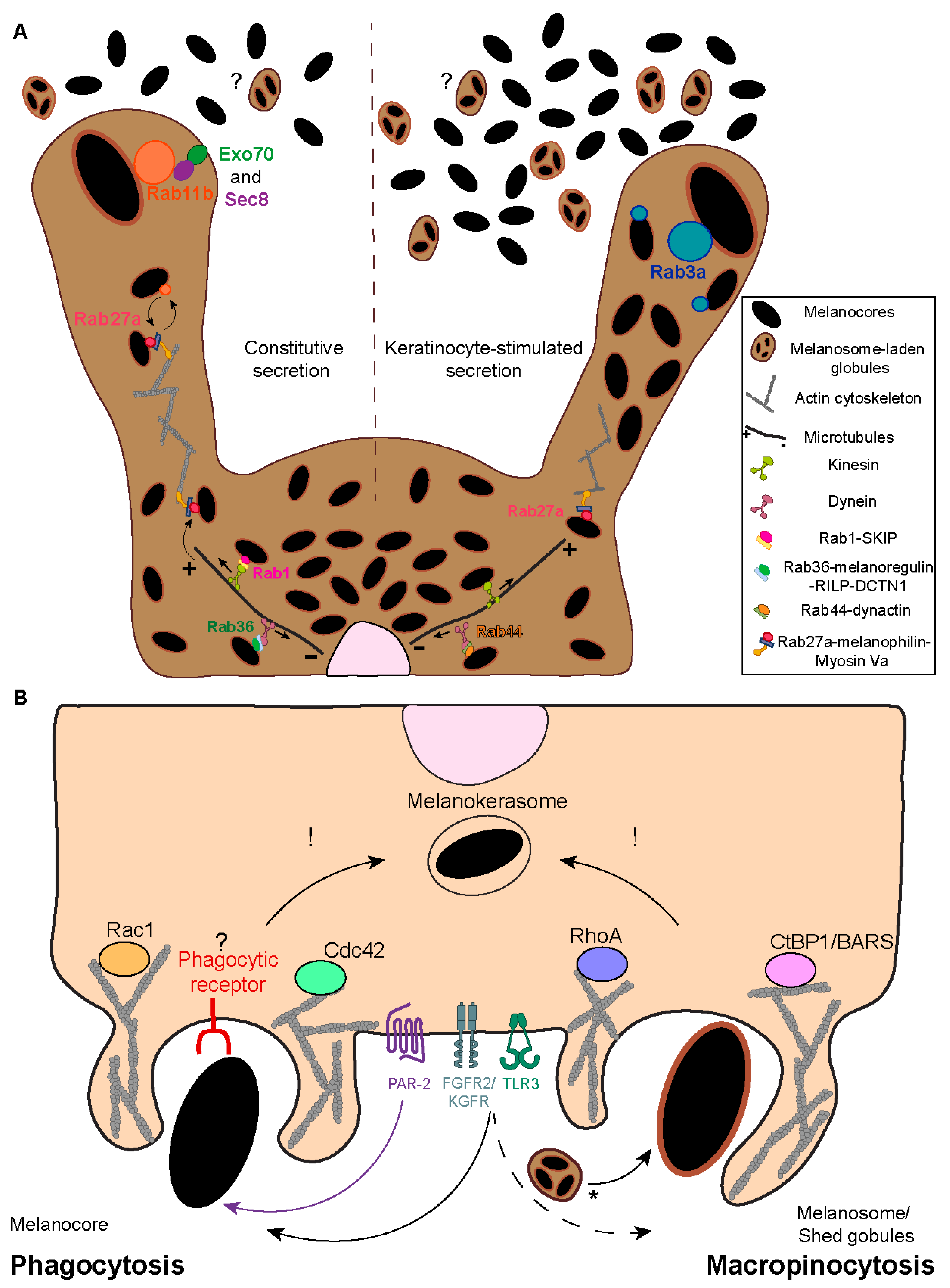
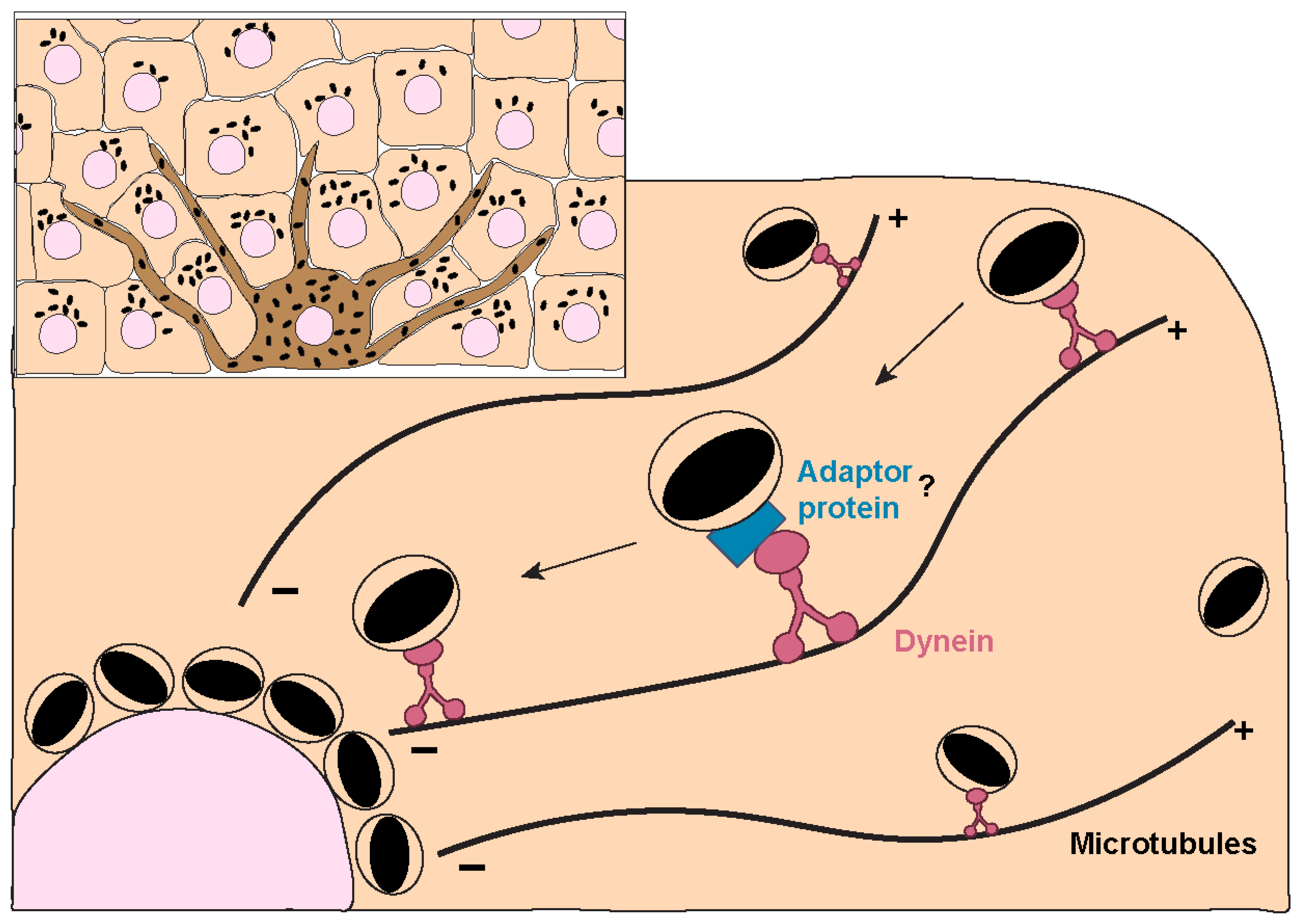
3. Melanosome Transport and Exocytosis
4. Melanin Internalization
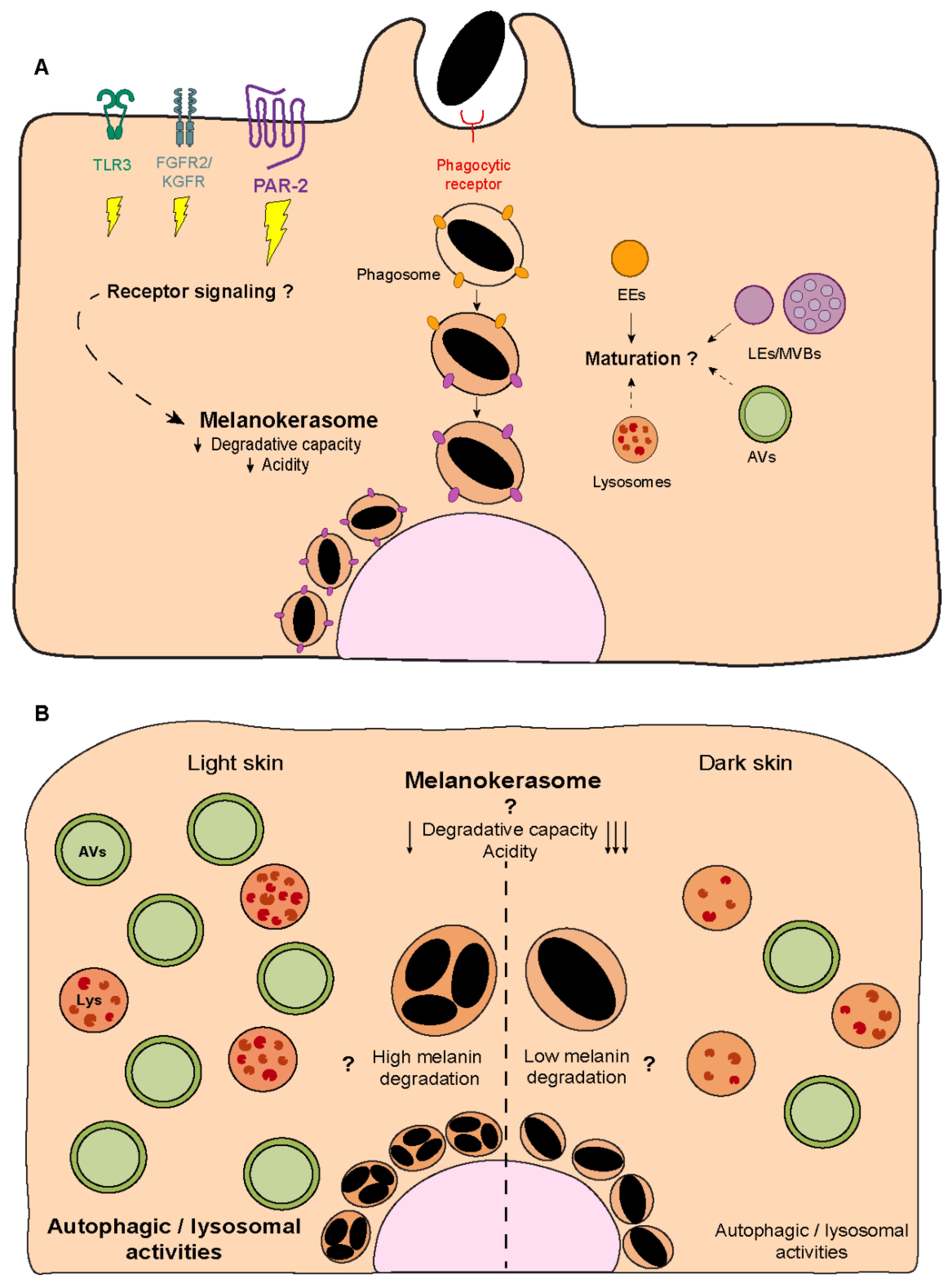
5. Melanin Processing
6. Melanin Polarization
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Solanas, G.; Benitah, S.A. Regenerating the skin: A task for the heterogeneous stem cell pool and surrounding niche. Nat. Rev. Mol. Cell Biol. 2013, 14, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and Physiology of the Skin. J. Dermatol. Nurses Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- Plonka, P.M.; Passeron, T.; Brenner, M.; Tobin, D.J.; Shibahara, S.; Thomas, A.; Slominski, A.; Kadekaro, A.L.; Hershkovitz, D.; Peters, E.; et al. What are melanocytes really doing all day long…? Exp. Dermatol. 2009, 18, 799–819. [Google Scholar] [CrossRef]
- Cox, N.H. Fitzpatrick’s Dermatology in General Medicine. Br. J. Dermatol. 2004, 150, 794. [Google Scholar] [CrossRef]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef]
- Wasmeier, C.; Hume, A.N.; Bolasco, G.; Seabra, M.C. Melanosomes at a glance. J. Cell Sci. 2008, 121, 3995–3999. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Chemistry of mixed melanogenesis–pivotal roles of dopaquinone. Photochem. Photobiol. 2008, 84, 582–592. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Human hair melanins: What we have learned and have not learned from mouse coat color pigmentation. Pigment Cell Melanoma Res. 2011, 24, 63–74. [Google Scholar] [CrossRef]
- Raposo, G.; Tenza, D.; Murphy, D.M.; Berson, J.F.; Marks, M.S. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J. Cell Biol. 2001, 152, 809–823. [Google Scholar] [CrossRef]
- Raposo, G.; Marks, M.S. The dark side of lysosome-related organelles: Specialization of the endocytic pathway for melanosome biogenesis. Traffic 2002, 3, 237–248. [Google Scholar] [CrossRef]
- Byers, H.R.; Maheshwary, S.; Amodeo, D.M.; Dykstra, S.G. Role of Cytoplasmic Dynein in Perinuclear Aggregation of Phagocytosed Melanosomes and Supranuclear Melanin Cap Formation in Human Keratinocytes. J. Investig. Dermatol. 2003, 121, 813–820. [Google Scholar] [CrossRef]
- Cruickshank, C.N.; Harcourt, S.A. Pigment donation in vitro. J. Investig. Dermatol. 1964, 42, 183–184. [Google Scholar] [CrossRef]
- Okazaki, K.; Uzuka, M.; Morikawa, F.; Toda, K.; Seiji, M. Transfer mechanism of melanosomes in epidermal cell culture. J. Investig. Dermatol. 1976, 67, 541–547. [Google Scholar] [CrossRef]
- Wolff, K. Melanocyte-keratinocyte interactions in vivo: The fate of melanosomes. Yale J. Biol. Med. 1973, 46, 384. [Google Scholar]
- Yamamoto, O.; Bhawan, J. Three modes of melanosome transfers in Caucasian facial skin: Hypothesis based on an ultrastructural study. Pigment Cell Res. 1994, 7, 158–169. [Google Scholar] [CrossRef]
- Scott, G.; Leopardi, S.; Printup, S.; Madden, B.C. Filopodia are conduits for melanosome transfer to keratinocytes. J. Cell Sci. 2002, 115, 1441–1451. [Google Scholar] [CrossRef]
- Hurbain, I.; Romao, M.; Sextius, P.; Bourreau, E.; Marchal, C.; Bernerd, F.; Duval, C.; Raposo, G. Melanosome Distribution in Keratinocytes in Different Skin Types: Melanosome Clusters Are Not Degradative Organelles. J. Investig. Dermatol. 2018, 138, 647–656. [Google Scholar] [CrossRef]
- Tarafder, A.K.; Bolasco, G.; Correia, M.S.; Pereira, F.J.; Iannone, L.; Hume, A.N.; Kirkpatrick, N.; Picardo, M.; Torrisi, M.R.; Rodrigues, I.P.; et al. Rab11b mediates melanin transfer between donor melanocytes and acceptor keratinocytes via coupled exo/endocytosis. J. Investig. Dermatol. 2014, 134, 1056–1066. [Google Scholar] [CrossRef]
- Singh, S.K.; Kurfurst, R.; Nizard, C.; Schnebert, S.; Perrier, E.; Tobin, D.J. Melanin transfer in human skin cells is mediated by filopodia—A model for homotypic and heterotypic lysosome-related organelle transfer. FASEB J. 2010, 24, 3756–3769. [Google Scholar] [CrossRef]
- Singh, S.K.; Baker, R.; Sikkink, S.K.; Nizard, C.; Schnebert, S.; Kurfurst, R.; Tobin, D.J. E-cadherin mediates ultraviolet radiation- and calcium-induced melanin transfer in human skin cells. Exp. Dermatol. 2017, 26, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Cerdan, D.; Redziniak, G.; Bourgeois, C.A.; Monsigny, M.; Kieda, C. C32 human melanoma cell endogenous lectins: Characterization and implication in vesicle-mediated melanin transfer to keratinocytes. Exp. Cell Res. 1992, 203, 164–173. [Google Scholar] [CrossRef]
- Aspengren, S.; Hedberg, D.; Wallin, M. Studies of pigment transfer between Xenopus laevis melanophores and fibroblasts in vitro and in vivo. Pigment Cell Res. 2006, 19, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Niki, Y.; Yoshida, M.; Ito, M.; Akiyama, K.; Kim, J.H.; Yoon, T.J.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. Involvement of pigment globules containing multiple melanosomes in the transfer of melanosomes from melanocytes to keratinocytes. Cell. Logist. 2011, 1, 12–20. [Google Scholar] [CrossRef]
- Ando, H.; Niki, Y.; Ito, M.; Akiyama, K.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J. Investig. Dermatol. 2012, 132, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Swift, J.A. Transfer of melanin granules from melanocytes to the cortical cells of human hair. Nature 1964, 203, 976–977. [Google Scholar] [CrossRef]
- Correia, M.S.; Moreiras, H.; Pereira, F.J.; Neto, M.V.; Festas, T.C.; Tarafder, A.K.; Ramalho, J.S.; Seabra, M.C.; Barral, D.C. Melanin Transferred to Keratinocytes Resides in Nondegradative Endocytic Compartments. J. Investig. Dermatol. 2018, 138, 637–646. [Google Scholar] [CrossRef]
- Tadokoro, R.; Murai, H.; Sakai, K.I.; Okui, T.; Yokota, Y.; Takahashi, Y. Melanosome transfer to keratinocyte in the chicken embryonic skin is mediated by vesicle release associated with Rho-regulated membrane blebbing. Sci. Rep. 2016, 6, 38277. [Google Scholar] [CrossRef]
- Wu, X.; Hammer, J.A. Melanosome transfer: It is best to give and receive. Curr. Opin. Cell Biol. 2014, 29, 1–7. [Google Scholar] [CrossRef]
- Cabaço, L.C.; Tomás, A.; Pojo, M.; Barral, D.C. The dark side of melanin secretion in cutaneous melanoma aggressiveness. Front. Oncol. 2022, 12, 887366. [Google Scholar] [CrossRef]
- Hall, M.J.; Lopes-Ventura, S.; Neto, M.V.; Charneca, J.; Zoio, P.; Seabra, M.C.; Oliva, A.; Barral, D.C. Reconstructed human pigmented skin/epidermis models achieve epidermal pigmentation through melanocore transfer. Pigment Cell Melanoma Res. 2022, 35, 425–435. [Google Scholar] [CrossRef]
- Jimenez, M.; Kameyama, K.; Maloy, W.L.; Tomita, Y.; Hearing, V.J. Mammalian tyrosinase: Biosynthesis, processing, and modulation by melanocyte-stimulating hormone. Proc. Natl. Acad. Sci. USA 1988, 85, 3830–3834. [Google Scholar] [CrossRef]
- Noguchi, S.; Kumazaki, M.; Yasui, Y.; Mori, T.; Yamada, N.; Akao, Y. MicroRNA-203 regulates melanosome transport and tyrosinase expression in melanoma cells by targeting kinesin superfamily protein 5b. J. Investig. Dermatol. 2014, 134, 461–469. [Google Scholar] [CrossRef]
- Hara, M.; Yaar, M.; Byers, H.R.; Goukassian, D.; Gonsalves, J.; Gilchrest, B.A.; Fine, R.E. Kinesin participates in melanosomal movement along melanocyte dendrites. J. Investig. Dermatol. 2000, 114, 438–443. [Google Scholar] [CrossRef]
- Ishida, M.; Ohbayashi, N.; Maruta, Y.; Ebata, Y.; Fukuda, M. Functional involvement of Rab1A in microtubule-dependent anterograde melanosome transport in melanocytes. J. Cell Sci. 2012, 125, 5177–5187. [Google Scholar] [CrossRef]
- Ishida, M.; Ohbayashi, N.; Fukuda, M. Rab1A regulates anterograde melanosome transport by recruiting kinesin-1 to melanosomes through interaction with SKIP. Sci. Rep. 2015, 5, 8238. [Google Scholar] [CrossRef]
- Ohbayashi, N.; Maruta, Y.; Ishida, M.; Fukuda, M. Melanoregulin regulates retrograde melanosome transport through interaction with the RILP-p150Glued complex in melanocytes. J. Cell Sci. 2012, 125, 1508–1518. [Google Scholar] [CrossRef]
- Matsui, T.; Ohbayashi, N.; Fukuda, M. The Rab interacting lysosomal protein (RILP) homology domain functions as a novel effector domain for small GTPase Rab36: Rab36 regulates retrograde melanosome transport in melanocytes. J. Biol. Chem. 2012, 287, 28619. [Google Scholar] [CrossRef]
- Maruta, Y.; Fukuda, M. Large Rab GTPase Rab44 regulates microtubule-dependent retrograde melanosome transport in melanocytes. J. Biol. Chem. 2022, 298, 102508. [Google Scholar] [CrossRef]
- Strom, M.; Hume, A.N.; Tarafder, A.K.; Barkagianni, E.; Seabra, M.C. A family of Rab27-binding proteins: Melanophilin links Rab27a and myosin Va function in melanosome transport. J. Biol. Chem. 2002, 277, 25423–25430. [Google Scholar] [CrossRef]
- Hume, A.N.; Ushakov, D.S.; Tarafder, A.K.; Ferenczi, M.A.; Seabra, M.C. Rab27a and MyoVa are the primary Mlph interactors regulating melanosome transport in melanocytes. J. Cell Sci. 2007, 120, 3111–3122. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, K.A.; Hamilton, N.A.; Moores, M.T.; Brown, D.L.; Ohbayashi, N.; Cairncross, O.; Cook, A.L.; Smith, A.G.; Misaki, R.; Fukuda, M.; et al. The Recycling Endosome Protein Rab17 Regulates Melanocytic Filopodia Formation and Melanosome Trafficking. Traffic 2011, 12, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, N.; Gao, L.; Li, L.; Zheng, S.; Liu, Y.; Ozukum, M.; Nikiforova, A.; Zhao, G.; Song, Z. The effect of the NMDA receptor-dependent signaling pathway on cell morphology and melanosome transfer in melanocytes. J. Dermatol. Sci. 2016, 84, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Domingues, L.; Hurbain, I.; Gilles-Marsens, F.; Sirés-Campos, J.; André, N.; Dewulf, M.; Romao, M.; Viaris de Lesegno, C.; Macé, A.S.; Blouin, C.; et al. Coupling of melanocyte signaling and mechanics by caveolae is required for human skin pigmentation. Nat. Commun. 2020, 11, 2988. [Google Scholar] [CrossRef]
- Moreiras, H.; Pereira, F.J.; Neto, M.V.; Bento-Lopes, L.; Festas, T.C.; Seabra, M.C.; Barral, D.C. The exocyst is required for melanin exocytosis from melanocytes and transfer to keratinocytes. Pigment Cell Melanoma Res. 2020, 33, 366–371. [Google Scholar] [CrossRef]
- Cabaço, L.C.; Bento-Lopes, L.; Neto, M.V.; Ferreira, A.; Staubli, W.B.; Ramalho, J.S.; Seabra, M.C.; Barral, D.C. Rab3a regulates melanin exocytosis and transfer induced by keratinocyte-conditioned medium. J. Investig. Dermatol. Innov. 2022, 2, 100139. [Google Scholar] [CrossRef]
- Cicero, A.L.; Delevoye, C.; Gilles-Marsens, F.; Loew, D.; Dingli, F.; Guéré, C.; André, N.; Vié, K.; van Niel, G.; Raposo, G. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat. Commun. 2015, 6, 7506. [Google Scholar] [CrossRef]
- Koike, S.; Yamasaki, K.; Yamauchi, T.; Shimada-Omori, R.; Tsuchiyama, K.; Aiba, S. Toll-like receptor 2 utilizes RAB11A for melanosome transfer from melanocytes to keratinocytes. J. Dermatol. Sci. 2019, 94, 310–312. [Google Scholar] [CrossRef]
- Moreiras, H.; Bento-Lopes, L.; Neto, M.V.; Escrevente, C.; Cabaço, L.C.; Hall, M.J.; Ramalho, J.S.; Seabra, M.C.; Barral, D. Melanocore uptake by keratinocytes occurs through phagocytosis and involves protease-activated receptor-2 internalization. Traffic 2022, 23, 331–345. [Google Scholar] [CrossRef]
- Koike, S.; Yamasaki, K.; Yamauchi, T.; Shimada-Omori, R.; Tsuchiyama, K.; Ando, H.; Aiba, S. TLR3 stimulation induces melanosome endo/phagocytosis through RHOA and CDC42 in human epidermal keratinocyte. J. Dermatol. Sci. 2019, 96, 168–177. [Google Scholar] [CrossRef]
- Benito-Martínez, S.; Salavessa, L.; Raposo, G.G.; Marks, M.S.; Delevoye, C. Melanin Transfer and Fate within Keratinocytes in Human Skin Pigmentation. Integr. Comp. Biol. 2021, 61, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Moreiras, H.; Seabra, M.C.; Barral, D.C. Melanin Transfer in the Epidermis: The Pursuit of Skin Pigmentation Control Mechanisms. Int. J. Mol. Sci. 2021, 22, 4466. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Mg, S.; Mayor, S. Endocytosis unplugged: Multiple ways to enter the cell. Cell Res. 2010, 20, 256. [Google Scholar] [CrossRef] [PubMed]
- Thottacherry, J.J.; Sathe, M.; Prabhakara, C.; Mayor, S. Spoiled for Choice: Diverse Endocytic Pathways Function at the Cell Surface. Annu. Rev. Cell Dev. Biol. 2019, 35, 55–84. [Google Scholar] [CrossRef] [PubMed]
- Wolff, K.; Konrad, K. Phagocytosis of latex beads by epidermal keratinocytes in vivo. J. Ultrastruct. Res. 1972, 39, 262–280. [Google Scholar] [CrossRef]
- Sharlow, E.R.; Paine, C.S.; Babiarz, L.; Eisinger, M.; Shapiro, S.; Seiberg, M. The protease-activated receptor-2 upregulates keratinocyte phagocytosis. J. Cell Sci. 2000, 113, 3093–3101. [Google Scholar] [CrossRef]
- Belleudi, F.; Purpura, V.; Scrofani, C.; Persechino, F.; Leone, L.; Torrisi, M.R. Expression and signaling of the tyrosine kinase FGFR2b/KGFR regulates phagocytosis and melanosome uptake in human keratinocytes. FASEB J. 2011, 25, 170–181. [Google Scholar] [CrossRef]
- Mylvaganam, S.; Freeman, S.A.; Grinstein, S. The cytoskeleton in phagocytosis and macropinocytosis. Curr. Biol. 2021, 31, R619–R632. [Google Scholar] [CrossRef]
- Seiberg, M.; Paine, C.; Sharlow, E.; Andrade-Gordon, P.; Costanzo, M.; Eisinger, M.; Shapiro, S.S. The protease-activated receptor 2 regulates pigmentation via keratinocyte-melanocyte interactions. Exp. Cell Res. 2000, 254, 25–32. [Google Scholar] [CrossRef]
- Henehan, M.; de Benedetto, A. Update on protease-activated receptor 2 in cutaneous barrier, differentiation, tumorigenesis and pigmentation, and its role in related dermatologic diseases. Exp. Dermatol. 2019, 28, 877–885. [Google Scholar] [CrossRef]
- Adams, M.N.; Ramachandran, R.; Yau, M.K.; Suen, J.Y.; Fairlie, D.P.; Hollenberg, M.D.; Hooper, J.D. Structure, function and pathophysiology of protease activated receptors. Pharmacol. Ther. 2011, 130, 248–282. [Google Scholar] [CrossRef]
- Heuberger, D.M.; Schuepbach, R.A. Protease-activated receptors (PARs): Mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb. J. 2019, 17, 4. [Google Scholar] [CrossRef]
- Scott, G.; Leopardi, S.; Parker, L.; Babiarz, L.; Seiberg, M.; Han, R. The Proteinase-Activated Receptor-2 Mediates Phagocytosis in a Rho-Dependent Manner in Human Keratinocytes. J. Investig. Dermatol. 2003, 121, 529–541. [Google Scholar] [CrossRef]
- Seiberg, M. Keratinocyte-Melanocyte Interactions During Melanosome Transfer. Pigment Cell Res. 2001, 14, 236–242. [Google Scholar] [CrossRef]
- Seiberg, M.; Paine, C.; Sharlow, E.; Eisinger, M.; Shapiro, S.S.; Andrade-Gordon, P.; Costanzo, M. Inhibition of melanosome transfer results in skin lightening. J. Investig. Dermatol. 2000, 115, 162–167. [Google Scholar] [CrossRef]
- Babiarz-Magee, L.; Chen, N.; Seiberg, M.; Lin, C.B. The expression and activation of protease-activated receptor-2 correlate with skin color. Pigment Cell Res. 2004, 17, 241–251. [Google Scholar] [CrossRef]
- Lin, C.B.; Chen, N.; Scarpa, R.; Guan, F.; Babiarz-Magee, L.; Liebel, F.; Li, W.H.; Kizoulis, M.; Shapiro, S.; Seiberg, M. LIGR, a protease-activated receptor-2-derived peptide, enhances skin pigmentation without inducing inflammatory processes. Pigment Cell Melanoma Res. 2008, 21, 172–183. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wu, W.; Liu, Y.; Xiao, Y.; Qi, D.; Zhao, G.; Zhou, M.; Wang, H.; Liu, J.; et al. TRPA1 promotes melanosome phagocytosis in keratinocytes via PAR-2/CYLD axis. J. Dermatol. Sci. 2022, 106, 181–188. [Google Scholar] [CrossRef]
- Cardinali, G.; Ceccarelli, S.; Kovacs, D.; Aspite, N.; Lotti, L.V.; Torrisi, M.R.; Picardo, M. Keratinocyte growth factor promotes melanosome transfer to keratinocytes. J. Investig. Dermatol. 2005, 125, 1190–1199. [Google Scholar] [CrossRef]
- Scott, G.; Rodriguez-Burford, C.; Seiberg, M.; Han, R.; Babiarz, L.; Grizzle, W.; Bell, W.; Pentland, A.; Deng, A. Protease-activated receptor 2, a receptor involved in melanosome transfer, is upregulated in human skin by ultraviolet irradiation. J. Investig. Dermatol. 2001, 117, 1412–1420. [Google Scholar] [CrossRef]
- Marchese, C.; Maresca, V.; Cardinali, G.; Belleudi, F.; Ceccarelli, S.; Bellocci, M.; Frati, L.; Torrisi, M.R.; Picardo, M. UVB-induced activation and internalization of keratinocyte growth factor receptor. Oncogene 2003, 22, 2422–2431. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.S.; Wu, Q.; Dong, T.T.; Tsim, K.W.K. The UV-induced uptake of melanosome by skin keratinocyte is triggered by α7 nicotinic acetylcholine receptor-mediated phagocytosis. FEBS J. 2023, 290, 724–744. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Querol, E.; Rosales, C. Phagocytosis: Our Current Understanding of a Universal Biological Process. Front. Immunol. 2020, 11, 1066. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.A.; Grinstein, S. Phagocytosis: Receptors, signal integration, and the cytoskeleton. Immunol. Rev. 2014, 262, 193–215. [Google Scholar] [CrossRef]
- Senoo, H.; Sesaki, H.; Iijima, M. A GPCR Handles Bacterial Sensing in Chemotaxis and Phagocytosis. Dev. Cell. 2016, 36, 354. [Google Scholar] [CrossRef]
- Pan, M.; Neilson, M.P.; Grunfeld, A.M.; Cruz, P.; Wen, X.; Insall, R.H.; Jin, T. A G-protein-coupled chemoattractant receptor recognizes lipopolysaccharide for bacterial phagocytosis. PLoS Biol. 2018, 16, e2005754. [Google Scholar] [CrossRef]
- Wen, X.; Xu, X.; Sun, W.; Chen, K.; Pan, M.; Wang, J.M.; Bolland, S.M.; Jin, T. G-protein–coupled formyl peptide receptors play a dual role in neutrophil chemotaxis and bacterial phagocytosis. Mol. Biol. Cell 2019, 30, 346–356. [Google Scholar] [CrossRef]
- Doyle, S.E.; O’Connell, R.M.; Miranda, G.A.; Vaidya, S.A.; Chow, E.K.; Liu, P.T.; Suzuki, S.; Suzuki, N.; Modlin, R.L.; Yeh, W.C.; et al. Toll-like receptors induce a phagocytic gene program through p38. J. Exp. Med. 2004, 199, 81–90. [Google Scholar] [CrossRef]
- Deng, T.; Feng, X.; Liu, P.; Yan, K.; Chen, Y.; Han, D. Toll-like receptor 3 activation differentially regulates phagocytosis of bacteria and apoptotic neutrophils by mouse peritoneal macrophages. Immunol. Cell Biol. 2013, 91, 52–59. [Google Scholar] [CrossRef]
- Del Bino, S.; Duval, C.; Bernerd, F. Clinical and biological characterization of skin pigmentation diversity and its consequences on UV impact. Int. J. Mol. Sci. 2018, 19, 2668. [Google Scholar] [CrossRef]
- Nordlund, J.J. The Melanocyte and the Epidermal Melanin Unit: An Expanded Concept. Dermatol. Clin. 2007, 25, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Alaluf, S.; Atkins, D.; Barrett, K.; Blount, M.; Carter, N.; Heath, A. Ethnic variation in melanin content and composition in photoexposed and photoprotected human skin. Pigment Cell Res. 2002, 15, 112–118. [Google Scholar] [CrossRef]
- Stappers, M.H.; Clark, A.E.; Aimanianda, V.; Bidula, S.; Reid, D.M.; Asamaphan, P.; Hardison, S.E.; Dambuza, I.M.; Valsecchi, I.; Kerscher, B.; et al. Recognition of DHN-melanin by MelLec, is required for protective immunity to Aspergillus. Nature 2018, 555, 382. [Google Scholar] [CrossRef]
- Van den Berg, L.M.; Zijlstra-Willems, E.M.; Richters, C.D.; Ulrich, M.M.W.; Geijtenbeek, T.B.H. Dectin-1 activation induces proliferation and migration of human keratinocytes enhancing wound re-epithelialization. Cell Immunol. 2014, 289, 49–54. [Google Scholar] [CrossRef]
- Lee, H.M.; Shin, D.M.; Choi, D.K.; Lee, Z.W.; Kim, K.H.; Yuk, J.M.; Kim, C.D.; Lee, J.H.; Jo, E.K. Innate immune responses to Mycobacterium ulcerans via toll-like receptors and dectin-1 in human keratinocytes. Cell. Microbiol. 2009, 11, 678–692. [Google Scholar] [CrossRef]
- Livden, J.K. Fc gamma receptors on keratinocytes in psoriasis. Arch. Dermatol. Res. 1988, 280, 12–17. [Google Scholar] [CrossRef]
- Cauza, K.; Grassauer, A.; Hinterhuber, G.; Wolff, K.; Foedinger, D.; Horvat, R.; Rappersberger, K. FcgammaRIII expression on cultured human keratinocytes and upregulation by interferon-gamma. J. Investig. Dermatol. 2002, 119, 1074–1079. [Google Scholar] [CrossRef]
- SzabÓ, G.; Gerald, A.B.; Pathak, M.A.; Fitzpatrick, T.B. Racial differences in the fate of melanosomes in human epidermis. Nature 1969, 222, 1081–1082. [Google Scholar] [CrossRef]
- Minwalla, L.; Zhao, Y.; Boissy, R.E.; le Poole, I.C.; Wickett, R.R. Keratinocytes Play a Role in Regulating Distribution Patterns of Recipient Melanosomes In Vitro. J. Investig. Dermatol. 2001, 117, 341–347. [Google Scholar] [CrossRef]
- Yoshida, Y.; Hachiya, A.; Sriwiriyanont, P.; Ohuchi, A.; Kitahara, T.; Takema, Y.; Visscher, M.O.; Boissy, R.E. Functional analysis of keratinocytes in skin color using a human skin substitute model composed of cells derived from different skin pigmentation types. FASEB J. 2007, 21, 2829–2839. [Google Scholar] [CrossRef]
- Thong, H.Y.; Jee, S.H.; Sun, C.C.; Boissy, R.E. The patterns of melanosome distribution in keratinocytes of human skin as one determining factor of skin colour. Br. J. Dermatol. 2003, 149, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Ebanks, J.P.; Koshoffer, A.; Wickett, R.R.; Schwemberger, S.; Babcock, G.; Hakozaki, T.; Boissy, R.E. Epidermal keratinocytes from light vs. dark skin exhibit differential degradation of melanosomes. J. Investig. Dermatol. 2011, 131, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Murase, D.; Hachiya, A.; Takano, K.; Hicks, R.; Visscher, M.O.; Kitahara, T.; Hase, T.; Takema, Y.; Yoshimori, T. Autophagy has a significant role in determining skin color by regulating melanosome degradation in keratinocytes. J. Investig. Dermatol. 2013, 133, 2416–2424. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.; Ganesan, A.K. The pleiotropic roles of autophagy regulators in melanogenesis. Pigment Cell Melanoma Res. 2011, 24, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, J.; Ahn, Y.; Lee, E.J.; Hwang, S.; Almurayshid, A.; Park, K.; Chung, H.J.; Kim, H.J.; Lee, S.H.; et al. Autophagy induction can regulate skin pigmentation by causing melanosome degradation in keratinocytes and melanocytes. Pigment Cell Melanoma Res. 2020, 33, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Seiberg, M.; Lin, C.B. Cathepsin L2 levels inversely correlate with skin color. J. Investig. Dermatol. 2006, 126, 2345–2347. [Google Scholar] [CrossRef]
- Yi, W.J.; Su, M.Y.; Shi, Y.; Jiang, S.; Xu, S.Z.; Lei, T.C. Degraded melanocores are incompetent to protect epidermal keratinocytes against UV damage. Cell Cycle 2018, 17, 844–857. [Google Scholar] [CrossRef]
- Ebanks, J.P.; Koshoffer, A.; Wickett, R.R.; Hakozaki, T.; Boissy, R.E. Hydrolytic enzymes of the interfollicular epidermis differ in expression and correlate with the phenotypic difference observed between light and dark skin. J. Dermatol. 2013, 40, 27–33. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, E.J.; Ahn, Y.; Park, S.; Bae, Y.J.; Kim, T.G.; Oh, S.H. Cathepsin L, a target of hypoxia-inducible factor-1-α, is involved in melanosome degradation in melanocytes. Int. J. Mol. Sci. 2021, 22, 8596. [Google Scholar] [CrossRef]
- Homma, T.; Kageyama, S.; Nishikawa, A.; Nagata, K. Melanosome degradation in epidermal keratinocytes related to lysosomal protease cathepsin V. Biochem. Biophys. Res. Commun. 2018, 500, 339–343. [Google Scholar] [CrossRef]
- Murase, D.; Kusaka-Kikushima, A.; Hachiya, A.; Fullenkamp, R.; Stepp, A.; Imai, A.; Ueno, M.; Kawabata, K.; Takahashi, Y.; Hase, T.; et al. Autophagy declines with premature skin aging resulting in dynamic alterations in skin pigmentation and epidermal differentiation. Int. J. Mol. Sci. 2020, 21, 5708. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zeng, B.; Pan, Y.; Huang, P.; Wang, C. Autophagy participates in isoliquiritigenin-induced melanin degradation in human epidermal keratinocytes through PI3K/AKT/mTOR signaling. Biomed. Pharmacother. 2018, 97, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.Y.; Choi, N.; Lee, J.U.; Lee, E.J.; Kim, J.Y.; Choi, W.J.; Oh, S.H.; Sung, J.H. Marliolide derivative induces melanosome degradation via nrf2/p62-mediated autophagy. Int. J. Mol. Sci. 2021, 22, 3995. [Google Scholar] [CrossRef] [PubMed]
- Joly-Tonetti, N.; Wibawa, J.I.D.; Bell, M.; Tobin, D.J. An explanation for the mysterious distribution of melanin in human skin: A rare example of asymmetric (melanin) organelle distribution during mitosis of basal layer progenitor keratinocytes. Br. J. Dermatol. 2018, 179, 1115–1126. [Google Scholar] [CrossRef]
- Mottaz, J.H.; Zelickson, A.S. Melanin transfer: A possible phagocytic process. J. Investig. Dermatol. 1967, 49, 605–610. [Google Scholar] [CrossRef]
- Marubashi, S.; Fukuda, M. Rab7B/42 is functionally involved in protein degradation on melanosomes in keratinocytes. Cell Struct. Funct. 2020, 45, 45–55. [Google Scholar] [CrossRef]
- Delevoye, C.; Marks, M.S.; Raposo, G. Lysosome-related organelles as functional adaptations of the endolysosomal system. Curr. Opin. Cell Biol. 2019, 59, 147–158. [Google Scholar] [CrossRef]
- Du, C.; Zhang, T.; Xiao, X.; Shi, Y.; Duan, H.; Ren, Y. Protease-activated receptor-2 promotes kidney tubular epithelial inflammation by inhibiting autophagy via the PI3K/Akt/mTOR signalling pathway. Biochem. J. 2017, 474, 2733–2747. [Google Scholar] [CrossRef]
- Chandrabalan, A.; Ramachandran, R. Molecular mechanisms regulating Proteinase-Activated Receptors (PARs). FEBS J. 2021, 288, 2697–2726. [Google Scholar] [CrossRef]
- Borovanský, J.; Elleder, M. Melanosome degradation: Fact or fiction. Pigment Cell Res. 2003, 16, 280–286. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Chemical degradation of melanins: Application to identification of dopamine-melanin. Pigment Cell Res. 1998, 11, 120–126. [Google Scholar] [CrossRef]
- Akoumianaki, T.; Kyrmizi, I.; Valsecchi, I.; Gresnigt, M.S.; Samonis, G.; Drakos, E.; Boumpas, D.; Muszkieta, L.; Prevost, M.C.; Kontoyiannis, D.P.; et al. Aspergillus Cell Wall Melanin Blocks LC3-Associated Phagocytosis to Promote Pathogenicity. Cell Host Microbe 2016, 19, 79–90. [Google Scholar] [CrossRef]
- Chamilos, G.; Akoumianaki, T.; Kyrmizi, I.; Brakhage, A.; Beauvais, A.; Latge, J.P. Melanin targets LC3-associated phagocytosis (LAP): A novel pathogenetic mechanism in fungal disease. Autophagy 2016, 12, 888–889. [Google Scholar] [CrossRef]
- Moreiras, H.; Lopes-da-Silva, M.; Seabra, M.C.; Barral, D.C. Melanin processing by keratinocytes: A non-microbial type of host-pathogen interaction? Traffic 2019, 20, 301–304. [Google Scholar] [CrossRef]
- Joly-Tonetti, N.; Wibawa, J.I.D.; Bell, M.; Tobin, D. Melanin fate in the human epidermis: A reassessment of how best to detect and analyse histologically. Exp. Dermatol. 2016, 25, 501–504. [Google Scholar] [CrossRef]
- D’Ischia, M.; Napolitano, A.; Michalczyk-Wetula, D.; Płonka, P.M. Melanin “dust” or “ghost”? Exp. Dermatol. 2016, 25, 505–506. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Kobayashi, N.; Nakagawa, A.; Muramatsu, T.; Yamashina, Y.; Shirai, T.; Hashimoto, M.W.; Ishigaki, Y.; Ohnishi, T.; Mori, T. Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. J. Investig. Dermatol. 1998, 110, 806–810. [Google Scholar] [CrossRef]
- Gibbs, S.; Murli, S.; de Boer, G.; Mulder, A.A.T.; Mommaas, A.M.; Ponec, M. Melanosome capping of keratinocytes in pigmented reconstructed epidermis—Effect of ultraviolet radiation and 3-isobutyl-1-methyl-xanthine on melanogenesis. Pigment Cell Res. 2000, 13, 458–466. [Google Scholar] [CrossRef]
- Lan, Y.; Zeng, W.; Wang, Y.; Dong, X.; Shen, X.; Gu, Y.; Zhang, W.; Lu, H. Opsin 3 mediates UVA-induced keratinocyte supranuclear melanin cap formation. Commun. Biol. 2023, 6, 238. [Google Scholar] [CrossRef]
- Vaidžiulyte, K.; Coppey, M.; Schauer, K. Intracellular organization in cell polarity—Placing organelles into the polarity loop. J. Cell Sci. 2019, 132, jcs230995. [Google Scholar] [CrossRef] [PubMed]
- Byers, H.R.; Dykstra, S.G.; Boissel, S.J.S. Requirement of dynactin p150(Glued) subunit for the functional integrity of the keratinocyte microparasol. J. Investig. Dermatol. 2007, 127, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Pellicena, I.; Morrison, C.G.; Bell, M.; O’Connor, C.; Tobin, D.J. Melanin Distribution in Human Skin: Influence of Cytoskeletal, Polarity, and Centrosome-Related Machinery of Stratum basale Keratinocytes. Int. J. Mol. Sci. 2021, 22, 3143. [Google Scholar] [CrossRef] [PubMed]
- Jordens, I.; Fernandez-Borja, M.; Marsman, M.; Dusseljee, S.; Janssen, L.; Calafat, J.; Janssen, H.; Wubbolts, R.; Neefjes, J. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 2001, 11, 1680–1685. [Google Scholar] [CrossRef]
- Risueño, I.; Valencia, L.; Jorcano, J.L.; Velasco, D. Skin-on-a-chip models: General overview and future perspectives. APL Bioeng. 2021, 5, 030901. [Google Scholar] [CrossRef]
- Dustin, M.L. The immunological synapse. Cancer Immunol. Res. 2014, 2, 1023–1033. [Google Scholar] [CrossRef]
- Südhof, T.C. The cell biology of synapse formation. J. Cell Biol. 2021, 220, e202103052. [Google Scholar] [CrossRef]
- Mizutani, Y.; Yamashita, M.; Hashimoto, R.; Atsugi, T.; Ryu, A.; Hayashi, A.; Rikimaru-Nishi, Y.; Ohta, K. Three-dimensional structure analysis of melanocytes and keratinocytes in senile lentigo. Microscopy 2021, 70, 224–231. [Google Scholar] [CrossRef]

| Autophagy Modulator | Mechanism Proposed | Model | References |
|---|---|---|---|
| Isoliquiritigenin | Decrease in melanin content through the suppression of the PI3K/AKT/mTOR pathway | Human epidermal keratinocytes | [102] |
| ATG7 silencing | Increase in melanin content | HaCaT human keratinocytes | [102] |
| 3-Methyladenine (3-MA) | Increase in melanin content | HaCaT human keratinocytes | [102] |
| Pentasodium tetracarboxymethyl palmitoyl dipeptide-12 (PTPD-12) | Decrease in melanin content | Human epidermal keratinocytes | [95] |
| ATG7 silencing | Increase in melanin content | Human epidermal keratinocytes | [93] |
| ATG13 or UVRAG silencing | Increase in melanin content | Human epidermal keratinocytes | [93] |
| Lysosome inhibitors (E-64-D and pepstatin A) | Increase in melanin content | RHPE and skin explants | [93] |
| Verapamil | Decrease in melanin content | RHPE and skin explants | [93] |
| Rapamycin | Decrease in melanin content | RHPE and skin explants | [93] |
| Hydroxychloroquine | Increase in melanin content | RHPE and skin explants | [93] |
| Marliolide derivative (5-methyl-3-tetradecylidene-dihydro-furan-2-one; DMF02) | Decrease in melanin content through Nrf2-p62 activation | HaCaT and human epidermal keratinocytes | [103] |
| Torin 1 | Decrease in melanin content through mTOR inhibition | Skin explants | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bento-Lopes, L.; Cabaço, L.C.; Charneca, J.; Neto, M.V.; Seabra, M.C.; Barral, D.C. Melanin’s Journey from Melanocytes to Keratinocytes: Uncovering the Molecular Mechanisms of Melanin Transfer and Processing. Int. J. Mol. Sci. 2023, 24, 11289. https://doi.org/10.3390/ijms241411289
Bento-Lopes L, Cabaço LC, Charneca J, Neto MV, Seabra MC, Barral DC. Melanin’s Journey from Melanocytes to Keratinocytes: Uncovering the Molecular Mechanisms of Melanin Transfer and Processing. International Journal of Molecular Sciences. 2023; 24(14):11289. https://doi.org/10.3390/ijms241411289
Chicago/Turabian StyleBento-Lopes, Liliana, Luís C. Cabaço, João Charneca, Matilde V. Neto, Miguel C. Seabra, and Duarte C. Barral. 2023. "Melanin’s Journey from Melanocytes to Keratinocytes: Uncovering the Molecular Mechanisms of Melanin Transfer and Processing" International Journal of Molecular Sciences 24, no. 14: 11289. https://doi.org/10.3390/ijms241411289
APA StyleBento-Lopes, L., Cabaço, L. C., Charneca, J., Neto, M. V., Seabra, M. C., & Barral, D. C. (2023). Melanin’s Journey from Melanocytes to Keratinocytes: Uncovering the Molecular Mechanisms of Melanin Transfer and Processing. International Journal of Molecular Sciences, 24(14), 11289. https://doi.org/10.3390/ijms241411289







