Pragmatic Considerations When Extracting DNA for Metagenomics Analyses of Clinical Samples
Abstract
1. Introduction
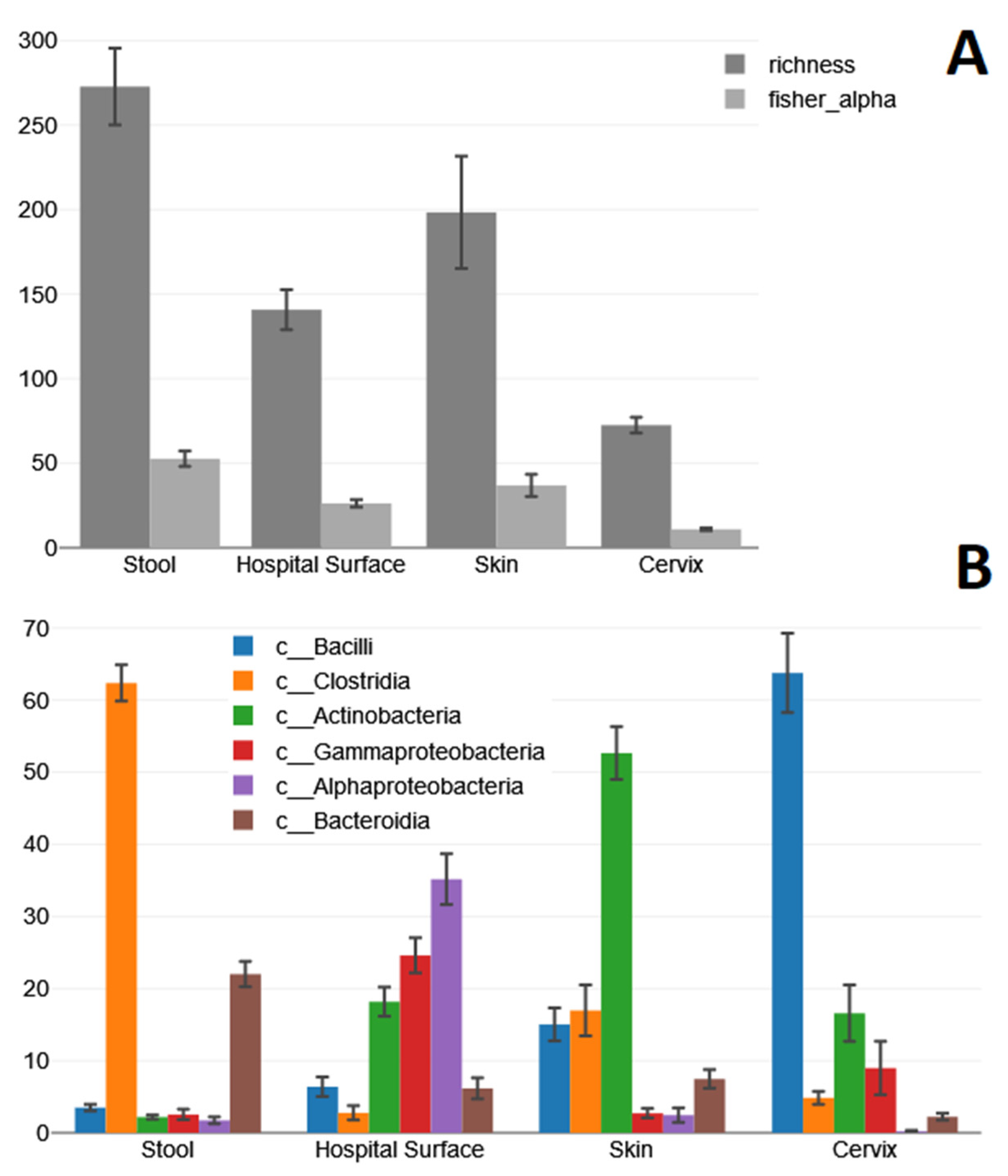
2. Results
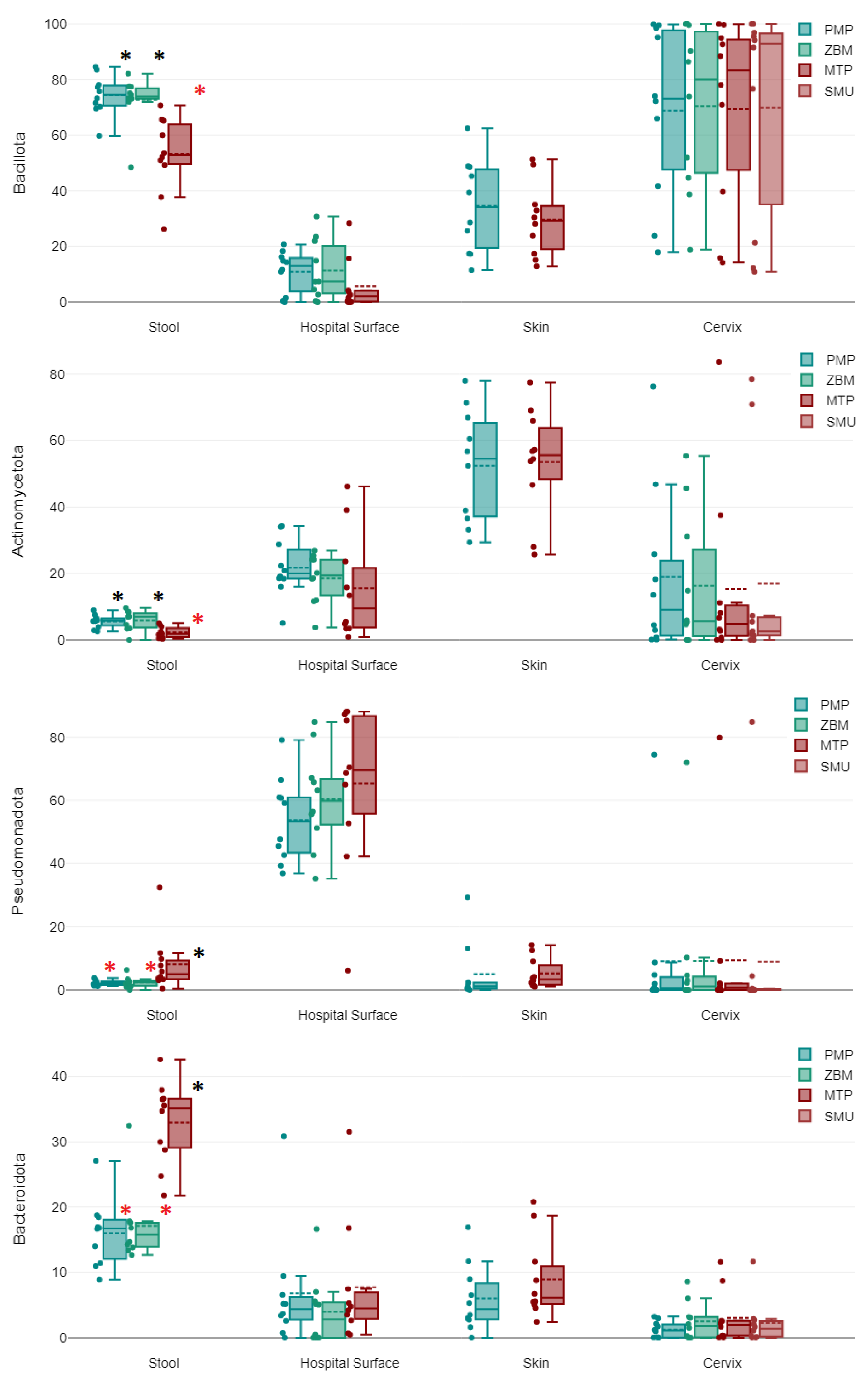
2.1. Stool Samples
2.2. Hospital Surface Swabs
2.3. Skin Swabs
2.4. Cervical Swabs
3. Discussion
4. Materials and Methods
4.1. Origin of the Samples
4.2. DNA Extraction
4.3. Library Preparation and Sequencing
4.4. Bioinformatic Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liwinski, T.; Leshem, A.; Elinav, E. Breakthroughs and bottlenecks in microbiome research. Trends Mol. Med. 2021, 27, 298–301. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Miller, R.R.; Montoya, V.; Gardy, J.L.; Patrick, D.M.; Tang, P. Metagenomics for pathogen detection in public health. Genome Med. 2013, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Bharti, R.; Grimm, D.G. Current challenges and best-practice protocols for microbiome analysis. Brief. Bioinform. 2021, 22, 178–193. [Google Scholar] [CrossRef]
- Poussin, C.; Sierro, N.; Boué, S.; Battey, J.; Scotti, E.; Belcastro, V.; Peitsch, M.C.; Ivanov, N.V.; Hoeng, J. Interrogating the microbiome: Experimental and computational considerations in support of study reproducibility. Drug Discov. Today 2018, 23, 1644–1657. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Sun, J.; Lv, A.; Wang, A. Biases from different DNA extraction methods in intestine microbiome research based on 16S rDNA sequencing: A case in the koi carp, Cyprinus carpio var. Koi. MicrobiologyOpen 2019, 8, e00626. [Google Scholar] [CrossRef]
- Videnska, P.; Smerkova, K.; Zwinsova, B.; Popovici, V.; Micenkova, L.; Sedlar, K.; Budinska, E. Stool sampling and DNA isolation kits affect DNA quality and bacterial composition following 16S rRNA gene sequencing using MiSeq Illumina platform. Sci. Rep. 2019, 9, 13837. [Google Scholar] [CrossRef]
- Maukonen, J.; Simões, C.; Saarela, M. The currently used commercial DNA-extraction methods give different results of clostridial and actinobacterial populations derived from human fecal samples. FEMS Microbiol. Ecol. 2012, 79, 697–708. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, B.W.; Waite, D.; Taylor, M.W. Evaluating variation in human gut microbiota profiles due to DNA extraction method and inter-subject differences. Front. Microbiol. 2015, 6, 130. [Google Scholar] [CrossRef]
- Carrigg, C.; Rice, O.; Kavanagh, S.; Collins, G.; O’flaherty, V. DNA extraction method affects microbial community profiles from soils and sediment. Appl. Microbiol. Biotechnol. 2007, 77, 955–964. [Google Scholar] [CrossRef]
- Kuske, C.R.; Banton, K.L.; Adorada, D.L.; Stark, P.C.; Hill, K.K.; Jackson, P.J. Small-scale DNA sample preparation method for field PCR detection of microbial cells and spores in soil. Appl. Environ. Microbiol. 1998, 64, 2463–2472. [Google Scholar] [CrossRef]
- Bjerre, R.D.; Hugerth, L.W.; Boulund, F.; Seifert, M.; Johansen, J.D.; Engstrand, L. Effects of sampling strategy and DNA extraction on human skin microbiome investigations. Sci. Rep. 2019, 9, 17287. [Google Scholar] [CrossRef]
- Wu, W.-K.; Chen, C.-C.; Panyod, S.; Chen, R.-A.; Wu, M.-S.; Sheen, L.-Y.; Chang, S.-C. Optimization of fecal sample processing for microbiome study—The journey from bathroom to bench. J. Formos. Med. Assoc. 2019, 118, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Lear, G.; Dickie, I.; Banks, J.; Boyer, S.; Buckley, H.; Buckley, T.; Cruickshank, R.; Dopheide, A.; Handley, K.; Hermans, S.; et al. Methods for the extraction, storage, amplification and sequencing of DNA from environmental samples. N. Z. J. Ecol. 2018, 42, 10. [Google Scholar] [CrossRef]
- Marotz, C.A.; Sanders, J.G.; Zuniga, C.; Zaramela, L.S.; Knight, R.; Zengler, K. Improving saliva shotgun metagenomics by chemical host DNA depletion. Microbiome 2018, 6, 42. [Google Scholar] [CrossRef]
- Kim, D.; Hofstaedter, C.E.; Zhao, C.; Mattei, L.; Tanes, C.; Clarke, E.; Lauder, A.; Sherrill-Mix, S.; Chehoud, C.; Kelsen, J.; et al. Optimizing methods and dodging pitfalls in microbiome research. Microbiome 2017, 5, 52. [Google Scholar] [CrossRef]
- Nearing, J.T.; Comeau, A.M.; Langille, M.G.I. Identifying biases and their potential solutions in human microbiome studies. Microbiome 2021, 9, 113. [Google Scholar] [CrossRef]
- Watts, G.S.; Hurwitz, B.L. Metagenomic next-generation sequencing in clinical microbiology. Clin. Microbiol. Newsl. 2020, 42, 53–59. [Google Scholar] [CrossRef]
- Shibata, T.; Nakagawa, M.; Coleman, H.N.; Owens, S.M.; Greenfield, W.W.; Sasagawa, T.; Ii, M.S.R. Evaluation of DNA extraction protocols from liquid-based cytology specimens for studying cervical microbiota. PLoS ONE 2021, 16, e0237556. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Kohler, C.; Alsallaq, R.; Hayden, R.; Maron, G.; Margolis, E.; Gutaker, R.M.; Reiter, E.; Furtwängler, A.; Schuenemann, V.J.; et al. Improved yield and accuracy for DNA extraction in microbiome studies with variation in microbial biomass. Biotechniques 2019, 66, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Neidhöfer, C.; Sib, E.; Benhsain, A.-H.; Mutschnik-Raab, C.; Schwabe, A.; Wollkopf, A.; Wetzig, N.; Sieber, M.A.; Thiele, R.; Döhla, M.; et al. Examining Different Analysis Protocols Targeting Hospital Sanitary Facility Microbiomes. Microorganisms 2023, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Greathouse, K.L.; Sinha, R.; Vogtmann, E. DNA extraction for human microbiome studies: The issue of standardization. Genome Biol. 2019, 20, 212. [Google Scholar] [CrossRef]
- Condic, M.; Neidhöfer, C.; Ralser, D.J.; Wetzig, N.; Thiele, R.; Sieber, M.; Otten, L.A.; Warwas, L.K.; Hoerauf, A.; Mustea, A.; et al. Analysis of the cervical microbiome in women from the German national cervical cancer screening program. J. Cancer Res. Clin. Oncol. 2023, 149, 1–12. [Google Scholar] [CrossRef]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, L.; Wang, Y.; Zhao, M.; Chen, R.; Tao, Z.; Lyu, T.; Huang, Z.; Liao, Q. An optimized 16S rRNA sequencing protocol for vaginal microbiome to avoid biased abundance estimation. Biorxiv 2019, 11, 857052. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2′s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Robeson, M.S., 2nd; O’Rourke, D.R.; Kaehler, B.D.; Ziemski, M.; Dillon, M.R.; Foster, J.T.; Bokulich, N.A. RESCRIPt: Reproducible sequence taxonomy reference database management. PLoS Comput. Biol. 2021, 17, e1009581. [Google Scholar] [CrossRef] [PubMed]
- DATAtab Team. DATAtab: Online Statistics Calculator; DATAtab e.U.: Graz, Austria, 2023; Available online: https://datatab.net (accessed on 8 July 2023).
| Sample Type | Amount | Storage Medium | PMP | MTP | ZBM | SMU | Total |
|---|---|---|---|---|---|---|---|
| Stool samples | 10 | DNA/RNA Shield | yes | yes | yes | 30 | |
| Hospital swabs | 10 | eNAT | yes | yes | yes | 30 | |
| Skin swabs | 10 | eNAT | yes | yes | 20 | ||
| Cervical swabs | 10 | eNAT | yes | yes | yes | yes | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neidhöfer, C.; Bagniceva, M.; Wetzig, N.; Sieber, M.A.; Thiele, R.; Parčina, M. Pragmatic Considerations When Extracting DNA for Metagenomics Analyses of Clinical Samples. Int. J. Mol. Sci. 2023, 24, 11262. https://doi.org/10.3390/ijms241411262
Neidhöfer C, Bagniceva M, Wetzig N, Sieber MA, Thiele R, Parčina M. Pragmatic Considerations When Extracting DNA for Metagenomics Analyses of Clinical Samples. International Journal of Molecular Sciences. 2023; 24(14):11262. https://doi.org/10.3390/ijms241411262
Chicago/Turabian StyleNeidhöfer, Claudio, Maria Bagniceva, Nina Wetzig, Martin A. Sieber, Ralf Thiele, and Marijo Parčina. 2023. "Pragmatic Considerations When Extracting DNA for Metagenomics Analyses of Clinical Samples" International Journal of Molecular Sciences 24, no. 14: 11262. https://doi.org/10.3390/ijms241411262
APA StyleNeidhöfer, C., Bagniceva, M., Wetzig, N., Sieber, M. A., Thiele, R., & Parčina, M. (2023). Pragmatic Considerations When Extracting DNA for Metagenomics Analyses of Clinical Samples. International Journal of Molecular Sciences, 24(14), 11262. https://doi.org/10.3390/ijms241411262







