LncRNA 220: A Novel Long Non-Coding RNA Regulates Autophagy and Apoptosis in Kupffer Cells via the miR-5101/PI3K/AKT/mTOR Axis in LPS-Induced Endotoxemic Liver Injury in Mice
Abstract
1. Introduction
2. Results
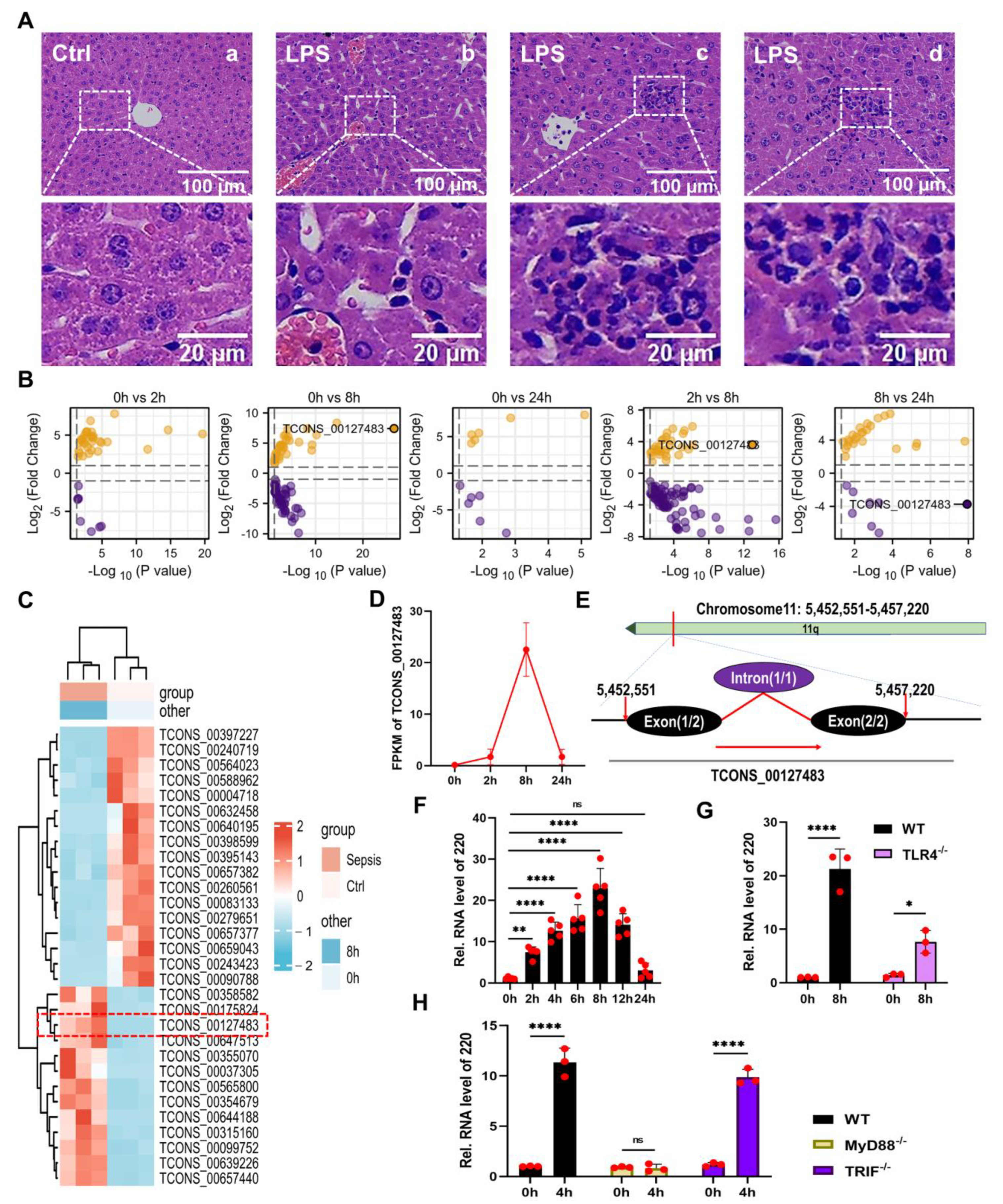
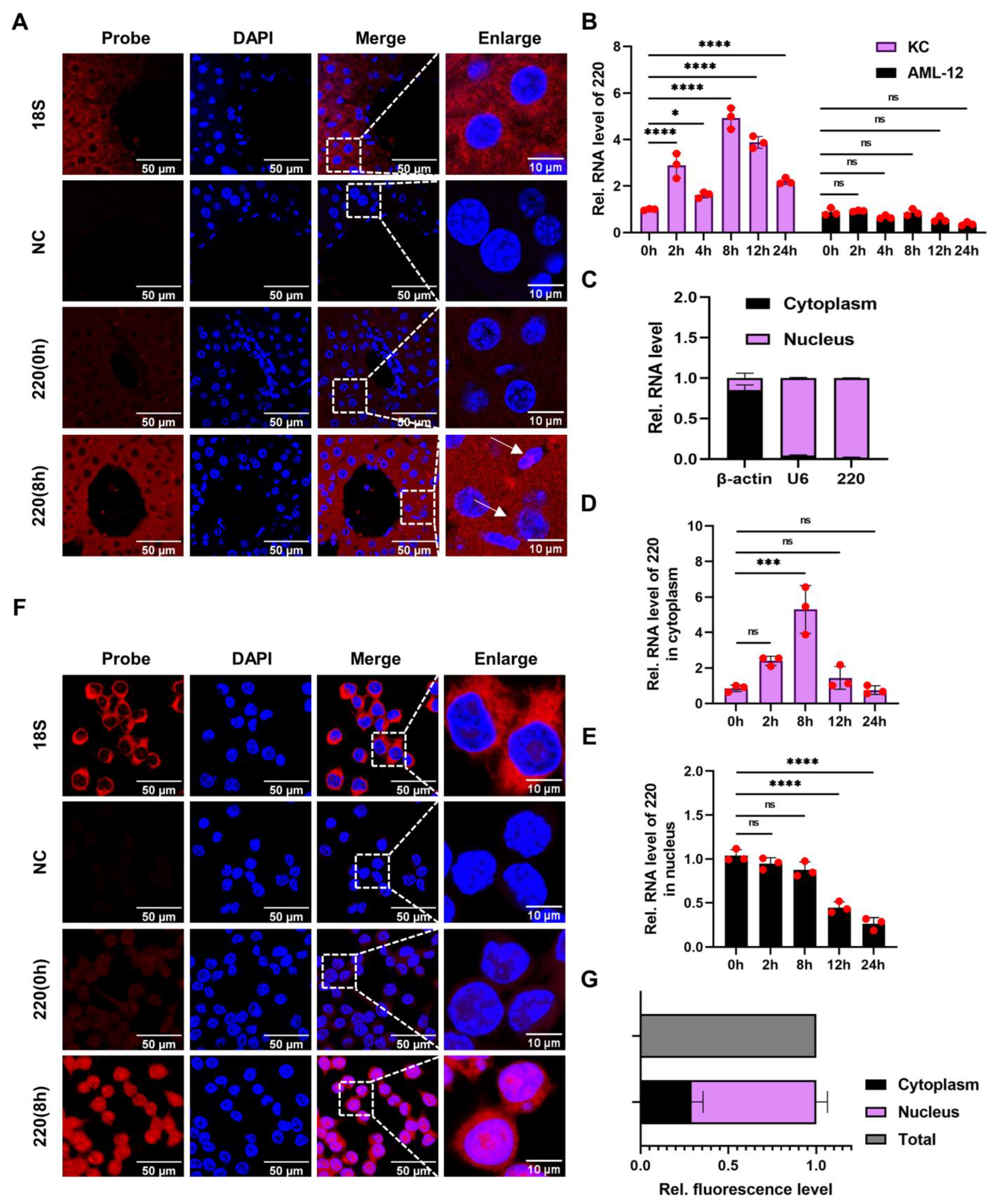
2.1. Identification and Annotation of lncRNA 220
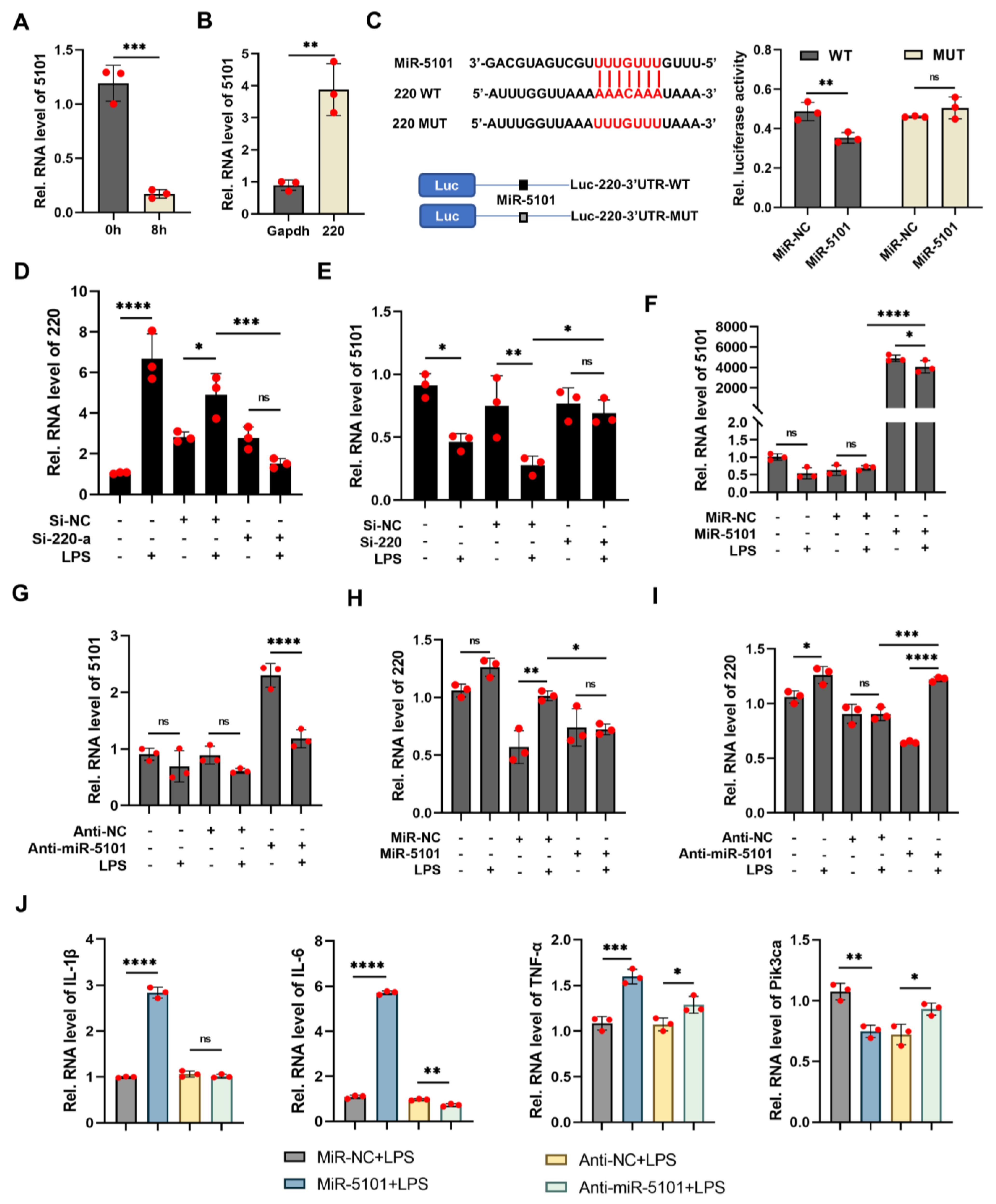
2.2. Prediction for the Interaction between lncRNA 220 and miRNA 5101 as a ceRNA Complex
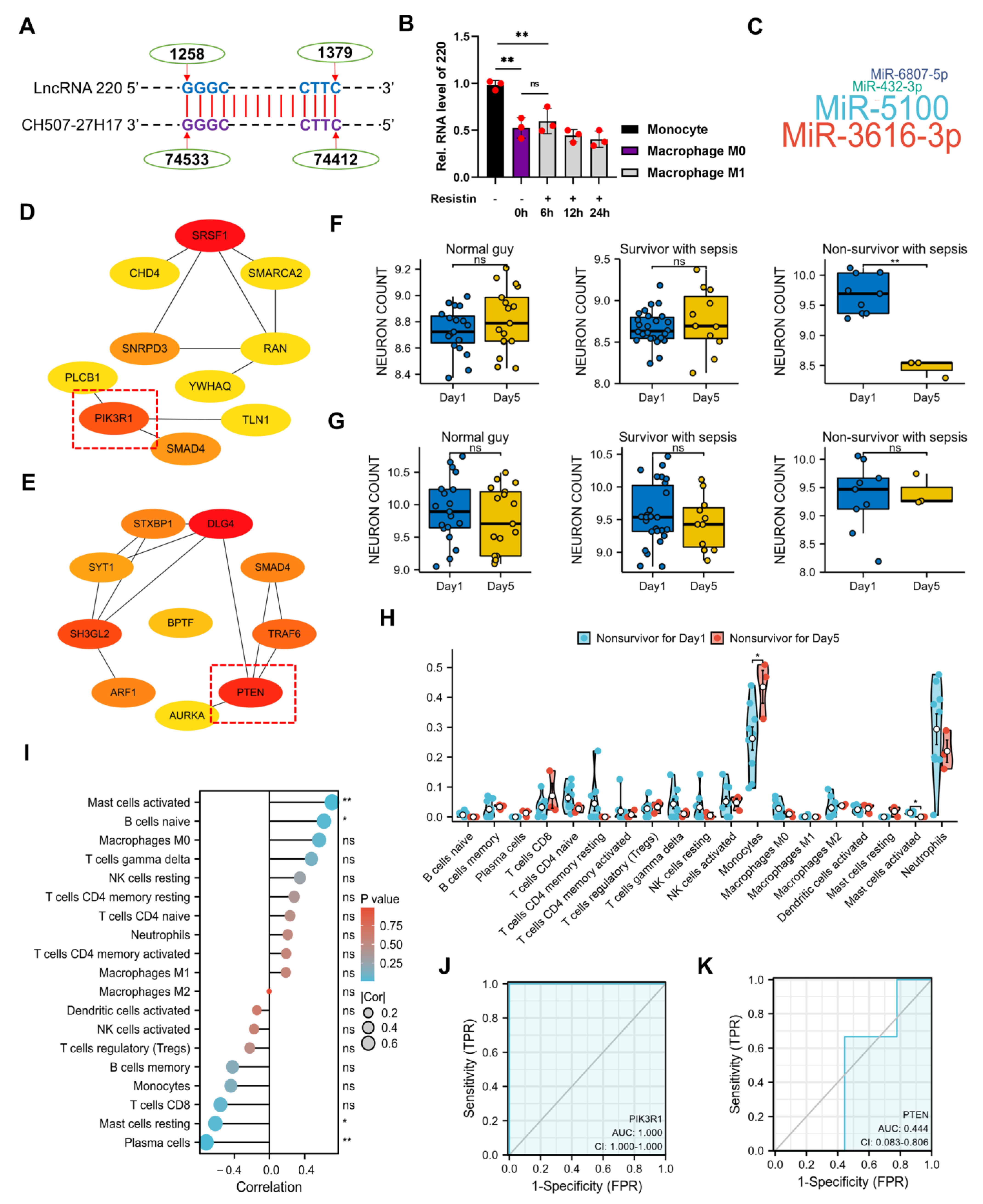
2.3. Decoy of miRNA 5101 by lncRNA 220 as a ceRNA Complex
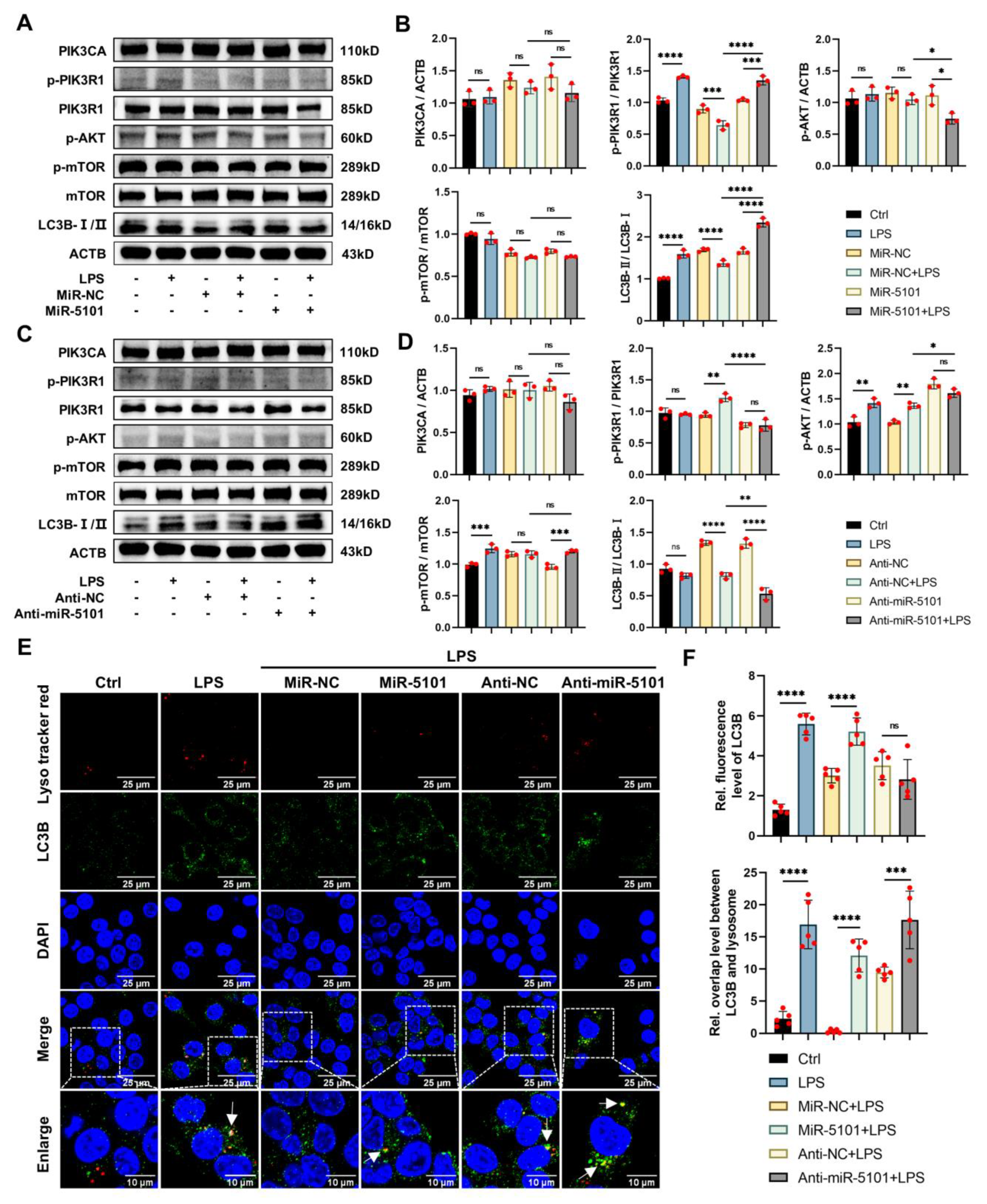
2.4. Regulation of miRNA 5101 on LPS-Induced Autophagy in Kupffer Cells via the PI3K/AKT/mTOR Axis
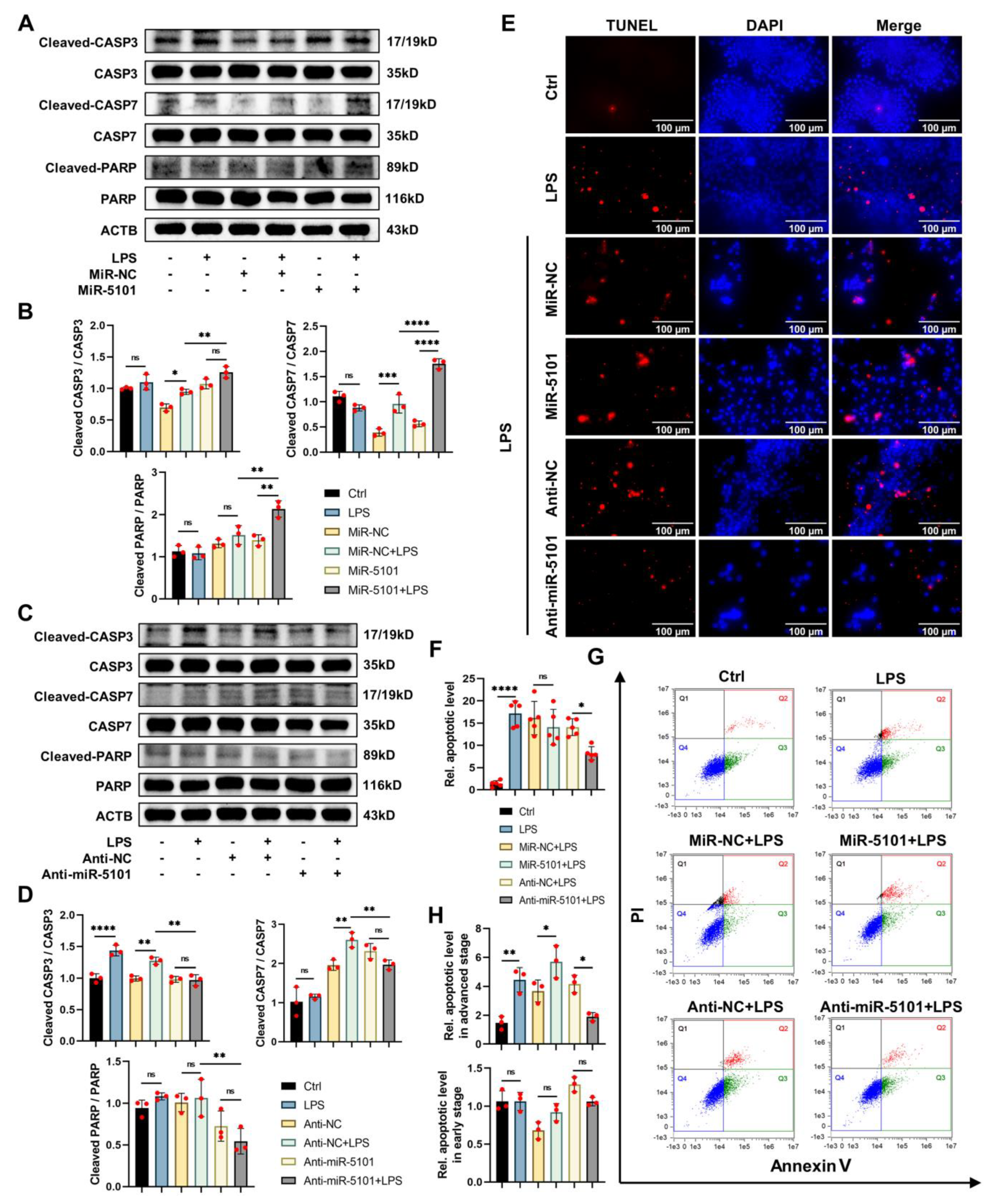
2.5. Regulation of miRNA 5101 on LPS-Induced Apoptosis in Kupffer Cells via the PI3K/AKT/mTOR Axis
2.6. The Clinical Significance of lncRNA 220
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. RNA-Seq and Bioinformatic Analysis
4.2.1. Construction of RNA Library and RNA-Seq
4.2.2. Identification of lncRNA 220 and Construction of an Interactive Network between lncRNA–miRNA–mRNA
4.2.3. Research for the Clinical Significance of lncRNA 220
4.3. Cell Culture and Treatment
4.4. Cell Transfection
4.5. Quantitative Real-Time PCR (qRT-PCR)
4.6. RNA Pull-Down Assay
4.7. RNA Fluorescence In Situ Hybridization (FISH) and Nucleocytoplasmic RNA Separation Assays
4.8. Western Blot Assay
4.9. Dual-Luciferase Reporter Assay
4.10. Flow Cytometry
4.11. Immunofluorescence Assay
4.12. TUNEL Assay
4.13. Hematoxylin and Eosin (H&E) Staining
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senousy, S.R.; Ahmed, A.F.; Abdelhafeez, D.A.; Khalifa, M.M.A.; Abourehab, M.A.S.; El-Daly, M. Alpha-Chymotrypsin Protects Against Acute Lung, Kidney, and Liver Injuries and Increases Survival in CLP-Induced Sepsis in Rats Through Inhibition of TLR4/NF-κB Pathway. Drug Des. Dev. Ther. 2022, 16, 3023–3039. [Google Scholar] [CrossRef]
- Matouk, A.I.; El-Daly, M.; Habib, H.A.; Senousy, S.; Naguib Abdel Hafez, S.M.; Kasem, A.W.; Almalki, W.H.; Alzahrani, A.; Alshehri, A.; Ahmed, A.F. Protective effects of menthol against sepsis-induced hepatic injury: Role of mediators of hepatic inflammation, apoptosis, and regeneration. Front. Pharmacol. 2022, 13, 952337. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.C.; Zhuang, H.Z.; Lin, J.; Zhi, D.Y.; Duan, M.L. Epidemiology of sepsis in Beijing from 2012 to 2018: Analysis of hospital homepage databases derived from the Beijing Public Health System. BMC Public Health 2022, 22, 2237. [Google Scholar] [CrossRef] [PubMed]
- Catton, E.A.; Bonsor, D.A.; Herrera, C.; Stålhammar-Carlemalm, M.; Lyndin, M.; Turner, C.E.; Soden, J.; van Strijp, J.A.G.; Singer, B.B.; van Sorge, N.M.; et al. Human CEACAM1 is targeted by a Streptococcus pyogenes adhesin implicated in puerperal sepsis pathogenesis. Nat. Commun. 2023, 14, 2275. [Google Scholar] [CrossRef]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and clinical management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef] [PubMed]
- Purcarea, A.; Sovaila, S. Sepsis, a 2020 review for the internist. Rom. J. Intern. Med. 2020, 58, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Dellinger, R.P.; Townsend, S.R.; Linde-Zwirble, W.T.; Marshall, J.C.; Bion, J.; Schorr, C.; Artigas, A.; Ramsay, G.; Beale, R.; et al. The Surviving Sepsis Campaign: Results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010, 36, 222–231. [Google Scholar] [CrossRef]
- Wang, H.E.; Shapiro, N.I.; Angus, D.C.; Yealy, D.M. National estimates of severe sepsis in United States emergency departments. Crit. Care Med. 2007, 35, 1928–1936. [Google Scholar] [CrossRef]
- Fan, X.; Mai, C.; Zuo, L.; Huang, J.; Xie, C.; Jiang, Z.; Li, R.; Yao, X.; Fan, X.; Wu, Q.; et al. Herbal formula BaWeiBaiDuSan alleviates polymicrobial sepsis-induced liver injury via increasing the gut microbiota Lactobacillus johnsonii and regulating macrophage anti-inflammatory activity in mice. Acta Pharm. Sin. B 2023, 13, 1164–1179. [Google Scholar] [CrossRef]
- Woźnica, E.A.; Inglot, M.; Woźnica, R.K.; Łysenko, L. Liver dysfunction in sepsis. Adv. Clin. Exp. Med. 2018, 27, 547–551. [Google Scholar] [CrossRef]
- Possamai, L.A.; Thursz, M.R.; Wendon, J.A.; Antoniades, C.G. Modulation of monocyte/macrophage function: A therapeutic strategy in the treatment of acute liver failure. J. Hepatol. 2014, 61, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.G.; Khamri, W.; Abeles, R.D.; Taams, L.S.; Triantafyllou, E.; Possamai, L.A.; Bernsmeier, C.; Mitry, R.R.; O’Brien, A.; Gilroy, D.; et al. Secretory leukocyte protease inhibitor: A pivotal mediator of anti-inflammatory responses in acetaminophen-induced acute liver failure. Hepatology 2014, 59, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.J.; Barnes, M.; Tang, H.; Pritchard, M.T.; Nagy, L.E. Kupffer cells in the liver. Compr. Physiol. 2013, 3, 785–797. [Google Scholar] [PubMed]
- Han, J.; Ulevitch, R.J. Limiting inflammatory responses during activation of innate immunity. Nat. Immunol. 2005, 6, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Brea, D.; Sobrino, T.; Ramos-Cabrer, P.; Castillo, J. Inflammatory and neuroimmunomodulatory changes in acute cerebral ischemia. Cerebrovasc. Dis. 2009, 27, 48–64. [Google Scholar] [CrossRef]
- Feng, R.; Li, S.; Li, F. Toll-like receptor 4 is involved in ischemic tolerance of postconditioning in hippocampus of tree shrews to thrombotic cerebral ischemia. Brain Res. 2011, 1384, 118–127. [Google Scholar] [CrossRef]
- Obrenovitch, T.P. Molecular physiology of preconditioning-induced brain tolerance to ischemia. Physiol. Rev. 2008, 88, 211–247. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Wu, L.R.; Liu, L.; Xiong, X.Y.; Zhang, Q.; Wang, F.X.; Gong, C.X.; Zhong, Q.; Yang, Y.R.; Meng, Z.Y.; Yang, Q.W. Vinpocetine alleviate cerebral ischemia/reperfusion injury by down-regulating TLR4/MyD88/NF-κB signaling. Oncotarget 2017, 8, 80315–80324. [Google Scholar] [CrossRef]
- Hritz, I.; Mandrekar, P.; Velayudham, A.; Catalano, D.; Dolganiuc, A.; Kodys, K.; Kurt-Jones, E.; Szabo, G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology 2008, 48, 1224–1231. [Google Scholar] [CrossRef]
- Zhao, X.J.; Dong, Q.; Bindas, J.; Piganelli, J.D.; Magill, A.; Reiser, J.; Kolls, J.K. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J. Immunol. 2008, 181, 3049–3056. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.G.; Albrecht, U.; Haussinger, D.; Heinrich, P.C.; Schaper, F. Hepatic acute phase proteins–regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-kappaB-dependent signaling. Eur. J. Cell Biol. 2012, 91, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Welch, K.; Siddiqui, J.; Remick, D.G. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J. Immunol. 2006, 177, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Faix, J.D. Biomarkers of sepsis. Crit. Rev. Clin. Lab. Sci. 2013, 50, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.H.; Liu, H.B.; Guo, S.X.; Zhang, J.; Li, D.X.; Chen, Z.G.; Lin, F.; Zhao, G.A. Long non-coding RNAs: Modulators of phenotypic transformation in vascular smooth muscle cells. Front. Cardiovasc. Med. 2022, 9, 959955. [Google Scholar] [CrossRef] [PubMed]
- Hombach, S.; Kretz, M. Non-coding RNAs: Classification, Biology and Functioning. Adv. Exp. Med. Biol. 2016, 937, 3–17. [Google Scholar]
- Shi, L.; Yang, Y.; Li, M.; Li, C.; Zhou, Z.; Tang, G.; Wu, L.; Yao, Y.; Shen, X.; Hou, Z.; et al. LncRNA IFITM4P promotes immune escape by up-regulating PD-L1 via dual mechanism in oral carcinogenesis. Mol. Ther. 2022, 30, 1564–1577. [Google Scholar] [CrossRef]
- Kirstein, N.; Dokaneheifard, S.; Cingaram, P.R.; Valencia, M.G.; Beckedorff, F.; Gomes Dos Santos, H.; Blumenthal, E.; Tayari, M.M.; Gaidosh, G.S.; Shiekhattar, R. The Integrator complex regulates microRNA abundance through RISC loading. Sci. Adv. 2023, 9, eadf0597. [Google Scholar] [CrossRef]
- Singh Kakan, S.; Li, X.; Edman, M.C.; Okamoto, C.T.; Hjelm, B.E.; Hamm-Alvarez, S.F. The miRNA Landscape of Lacrimal Glands in a Murine Model of Autoimmune Dacryoadenitis. Investig. Ophthalmol. Vis. Sci. 2023, 64, 1. [Google Scholar] [CrossRef]
- Yekta, S.; Shih, I.H.; Bartel, D.P. MicroRNA-directed cleavage of HOXB8 mRNA. Science 2004, 304, 594–596. [Google Scholar] [CrossRef]
- Ni, Q.; Zhang, H.; Shi, X.; Li, X. Exosomal lncRNA HCG18 contributes to cholangiocarcinoma growth and metastasis through mediating miR-424-5p/SOX9 axis through PI3K/AKT pathway. Cancer Gene Ther. 2023, 30, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Juan, L.; Wang, G.; Radovich, M.; Schneider, B.P.; Clare, S.E.; Wang, Y.; Liu, Y. Potential roles of microRNAs in regulating long intergenic noncoding RNAs. BMC Med. Genom. 2013, 6 (Suppl. 1), S7. [Google Scholar] [CrossRef]
- Wu, P.; Yu, X.; Peng, Y.; Wang, Q.L.; Deng, L.T.; Xing, W. Ginsenoside Rg3 alleviates septic liver injury by regulating the lncRNA TUG1/miR-200c-3p/SIRT1 axis. J. Inflamm. 2021, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, J.; Xie, Z.; Liu, M.; Sun, K. Long noncoding RNA colorectal neoplasia differentially expressed alleviates sepsis-induced liver injury via regulating miR-126-5p. IUBMB Life 2020, 72, 440–451. [Google Scholar] [CrossRef]
- Yu, J.S.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Wu, D.M.; He, M.; Zhang, F.; Zhang, T.; Liu, T.; Li, J.; Li, L.; Xu, Y. Samotolisib Attenuates Acute Liver Injury Through Inhibiting Caspase-11-Mediated Pyroptosis Via Regulating E3 Ubiquitin Ligase Nedd4. Front. Pharmacol. 2021, 12, 726198. [Google Scholar] [CrossRef]
- Jiang, Z.; Meng, Y.; Bo, L.; Wang, C.; Bian, J.; Deng, X. Sophocarpine Attenuates LPS-Induced Liver Injury and Improves Survival of Mice through Suppressing Oxidative Stress, Inflammation, and Apoptosis. Mediat. Inflamm. 2018, 2018, 5871431. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. A brief history of autophagy from cell biology to physiology and disease. Nat. Cell Biol. 2018, 20, 521–527. [Google Scholar] [CrossRef]
- Jiang, P.; Mizushima, N. Autophagy and human diseases. Cell Res. 2014, 24, 69–79. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef]
- Jiang, P.; Mizushima, N. LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 2015, 75, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Kma, L.; Baruah, T.J. The interplay of ROS and the PI3K/Akt pathway in autophagy regulation. Biotechnol. Appl. Biochem. 2022, 69, 248–264. [Google Scholar] [CrossRef]
- Torii, S.; Yoshida, T.; Arakawa, S.; Honda, S.; Nakanishi, A.; Shimizu, S. Identification of PPM1D as an essential Ulk1 phosphatase for genotoxic stress-induced autophagy. EMBO Rep. 2016, 17, 1552–1564. [Google Scholar] [CrossRef]
- Ikeda, S.; Zablocki, D.; Sadoshima, J. The role of autophagy in death of cardiomyocytes. J. Mol. Cell. Cardiol. 2022, 165, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, Y.; Steller, H. Programmed cell death in animal development and disease. Cell 2011, 147, 742–758. [Google Scholar] [CrossRef]
- Thomberry, N.A.; Laxebnik, Y. Caspases: Enemies within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.W.; Su, C.H.; Ho, Y.C.; Huang-Liu, R.; Tseng, C.C.; Chiang, Y.W.; Yeh, K.L.; Lee, S.S.; Chen, W.Y.; Chen, C.J.; et al. Genotoxic effects of 1-nitropyrene in macrophages are mediated through a p53-dependent pathway involving cytochrome c release, caspase activation, and PARP-1 cleavage. Ecotoxicol. Environ. Saf. 2021, 213, 112062. [Google Scholar] [CrossRef]
- Degterev, A.; Boyce, M.; Yuan, J.Y. A decade of caspases. Oncogene 2003, 22, 8543–8567. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Lien, E.C.; Dibble, C.C.; Toker, A. PI3K signaling in cancer: Beyond AKT. Curr. Opin. Cell Biol. 2017, 45, 62–71. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, H.; Gao, J.; Liu, Y.; Li, J.; Wang, J. Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J. Mol. Cell. Cardiol. 2019, 136, 27–41. [Google Scholar] [CrossRef]
- Ulevitch, R.J.; Tobias, P.S. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr. Opin. Immunol. 1999, 11, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- Bae, H.B.; Li, M.; Kim, J.P.; Kim, S.J.; Jeong, C.W.; Lee, H.G.; Kim, W.M.; Kim, H.S.; Kwak, S.H. The effect of epigallocatechin gallate on lipopolysaccharide-induced acute lung injury in a murine model. Inflammation 2010, 33, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I. Oxidative stress, transcription factors and chromatin remodelling in lung inflammation. Biochem. Pharmacol. 2002, 64, 935–942. [Google Scholar] [CrossRef]
- Lalazar, G.; Ilyas, G.; Malik, S.A.; Liu, K.; Zhao, E.; Amir, M.; Lin, Y.; Tanaka, K.E.; Czaja, M.J. Autophagy confers resistance to lipopolysaccharide-induced mouse hepatocyte injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G377–G386. [Google Scholar] [CrossRef]
- Chen, S.N.; Tan, Y.; Xiao, X.C.; Li, Q.; Wu, Q.; Peng, Y.Y.; Ren, J.; Dong, M.L. Deletion of TLR4 attenuates lipopolysaccharide-induced acute liver injury by inhibiting inflammation and apoptosis. Acta Pharmacol. Sin. 2021, 42, 1610–1619. [Google Scholar] [CrossRef]
- Huang, L.; Gan, L.; Pan, J.; Zhong, L.; Wang, Q.; Luo, S.; Tian, J.; Liang, H. Transcriptomics combined with metabolomics analysis of the mechanism of agmatine in the treatment of septic liver injury. Ann. Transl. Med. 2022, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhu, G.; Cai, Z.; Wang, Y.; Lian, R.; Tang, X.; Ma, C.; Fu, S. miRNA, lncRNA and circRNA: Targeted Molecules Full of Therapeutic Prospects in the Development of Diabetic Retinopathy. Front. Endocrinol. 2021, 12, 771552. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef]
- Liang, H.; Ding, X.; Yu, Y.; Zhang, H.; Wang, L.; Kan, Q.; Ma, S.; Guan, F.; Sun, T. Adipose-derived mesenchymal stem cells ameliorate acute liver injury in rat model of CLP induced-sepsis via sTNFR1. Exp. Cell Res. 2019, 383, 111465. [Google Scholar] [CrossRef]
- Su, J.; Chen, D.; Ruan, Y.; Tian, Y.; Lv, K.; Zhou, X.; Ying, D.; Lu, Y. LncRNA MBNL1-AS1 represses gastric cancer progression via the TGF-β pathway by modulating miR-424-5p/Smad7 axis. Bioengineered 2022, 13, 6978–6995. [Google Scholar] [CrossRef] [PubMed]
- He, X.L.; Chen, J.Y.; Feng, Y.L.; Song, P.; Wong, Y.K.; Xie, L.L.; Wang, C.; Zhang, Q.; Bai, Y.M.; Gao, P.; et al. Single-cell RNA sequencing deciphers the mechanism of sepsis-induced liver injury and the therapeutic effects of artesunate. Acta Pharmacol. Sin. 2023. [Google Scholar] [CrossRef]
- Nishiguchi, T.; Cho, K.; Isaka, S.; Ueno, M.; Jin, J.O.; Yamaguchi, K.; Kim, D.; Oda, T. Protective effect of porphyran isolated from discolored nori (Porphyra yezoensis) on lipopolysaccharide-induced endotoxin shock in mice. Int. J. Biol. Macromol. 2016, 93 Pt A, 1273–1278. [Google Scholar] [CrossRef]
- Chu, M.; Gao, Y.; Zhou, B.; Wu, B.; Wang, J.; Xu, D. Circumferential Strain Can Be Used to Detect Lipopolysaccharide-Induced Myocardial Dysfunction and Predict the Mortality of Severe Sepsis in Mice. PLoS ONE 2016, 11, e0155346. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Wu, S.; Tong, X.; Peng, C.; Xiong, G.; Lu, K.; Hu, H.; Tan, D.; Li, C.; Han, M.; Lu, C.; et al. Comparative analysis of the integument transcriptomes of the black dilute mutant and the wild-type silkworm Bombyx mori. Sci. Rep. 2016, 6, 26114. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Twisselmann, N.; Pagel, J.; Künstner, A.; Weckmann, M.; Hartz, A.; Glaser, K.; Hilgendorff, A.; Göpel, W.; Busch, H.; Herting, E.; et al. Hyperoxia/Hypoxia Exposure Primes a Sustained Pro-Inflammatory Profile of Preterm Infant Macrophages Upon LPS Stimulation. Front. Immunol. 2021, 12, 762789. [Google Scholar] [CrossRef]
- Maeß, M.B.; Wittig, B.; Cignarella, A.; Lorkowski, S. Reduced PMA enhances the responsiveness of transfected THP-1 macrophages to polarizing stimuli. J. Immunol. Methods 2014, 402, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.L.; Liang, K.H.; Ku, C.L.; Lo, C.C.; Cheng, Y.T.; Hsu, C.M.; Yeh, C.T.; Chiu, C.T. Resistin reinforces interferon λ-3 to eliminate hepatitis C virus with fine-tuning from RETN single-nucleotide polymorphisms. Sci. Rep. 2016, 6, 30799. [Google Scholar] [CrossRef] [PubMed]
- Heun, Y.; Gräff, P.; Lagara, A.; Schelhorn, R.; Mettler, R.; Pohl, U.; Mannell, H. The GEF Cytohesin-2/ARNO Mediates Resistin induced Phenotypic Switching in Vascular Smooth Muscle Cells. Sci. Rep. 2020, 10, 3672. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Tian, T.; Li, S.; Li, N.; Luo, H.; Jiang, Y. LncRNA 220: A Novel Long Non-Coding RNA Regulates Autophagy and Apoptosis in Kupffer Cells via the miR-5101/PI3K/AKT/mTOR Axis in LPS-Induced Endotoxemic Liver Injury in Mice. Int. J. Mol. Sci. 2023, 24, 11210. https://doi.org/10.3390/ijms241311210
Yang Y, Tian T, Li S, Li N, Luo H, Jiang Y. LncRNA 220: A Novel Long Non-Coding RNA Regulates Autophagy and Apoptosis in Kupffer Cells via the miR-5101/PI3K/AKT/mTOR Axis in LPS-Induced Endotoxemic Liver Injury in Mice. International Journal of Molecular Sciences. 2023; 24(13):11210. https://doi.org/10.3390/ijms241311210
Chicago/Turabian StyleYang, Ying, Tian Tian, Shan Li, Nanhong Li, Haihua Luo, and Yong Jiang. 2023. "LncRNA 220: A Novel Long Non-Coding RNA Regulates Autophagy and Apoptosis in Kupffer Cells via the miR-5101/PI3K/AKT/mTOR Axis in LPS-Induced Endotoxemic Liver Injury in Mice" International Journal of Molecular Sciences 24, no. 13: 11210. https://doi.org/10.3390/ijms241311210
APA StyleYang, Y., Tian, T., Li, S., Li, N., Luo, H., & Jiang, Y. (2023). LncRNA 220: A Novel Long Non-Coding RNA Regulates Autophagy and Apoptosis in Kupffer Cells via the miR-5101/PI3K/AKT/mTOR Axis in LPS-Induced Endotoxemic Liver Injury in Mice. International Journal of Molecular Sciences, 24(13), 11210. https://doi.org/10.3390/ijms241311210






