Expression and Activity of the Transcription Factor CCAAT/Enhancer-Binding Protein β (C/EBPβ) Is Regulated by Specific Pulse-Modulated Radio Frequencies in Oligodendroglial Cells
Abstract
1. Introduction
2. Results
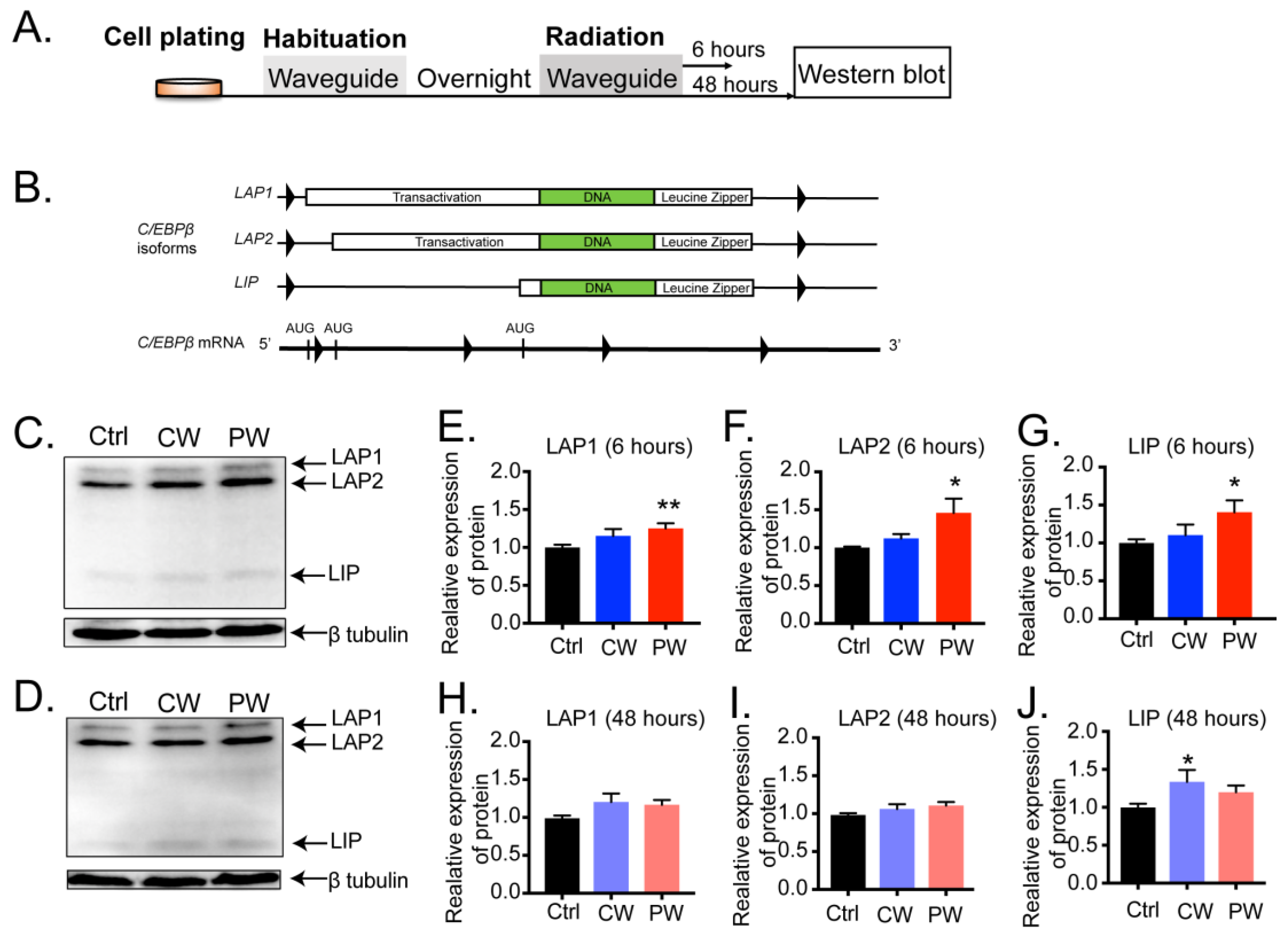
2.1. Exposure to 2.4 GHz EMR Distinctly Regulated the Transcription and Expression of C/EBPβ in Oligodendroglia, Neuronal Cells, Microglial Cells, and Astrocytes
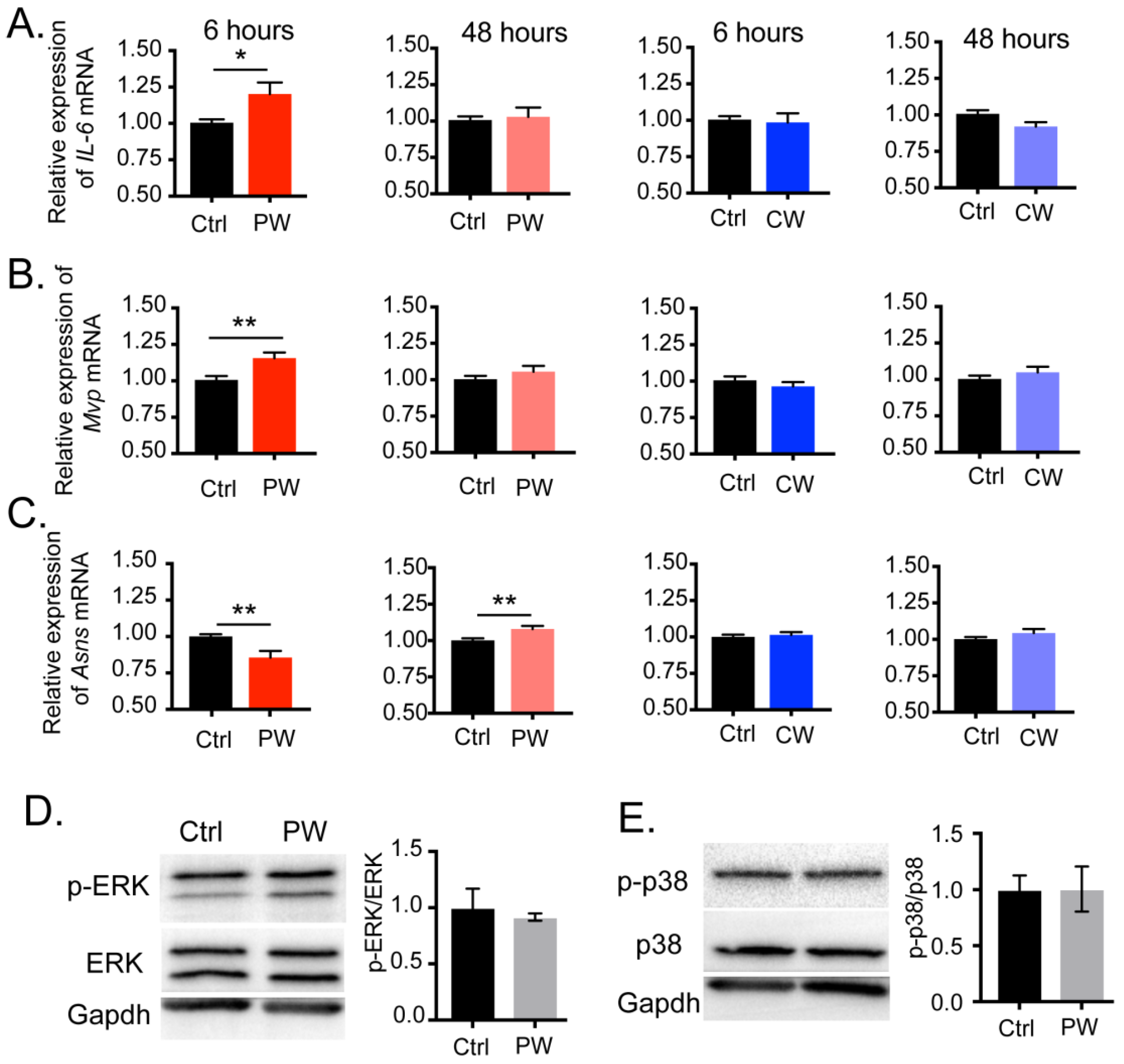
2.2. C/EBPβ-Interacting Proteins in Oligodendroglial Cells Were Altered by 2.4 GHz EMR Exposure
2.3. Transcriptional Activity of C/EBPβ Was Enhanced after Exposure to 2.4 GHz EMR in Oligodendroglial Cells
3. Discussion
4. Materials and Methods
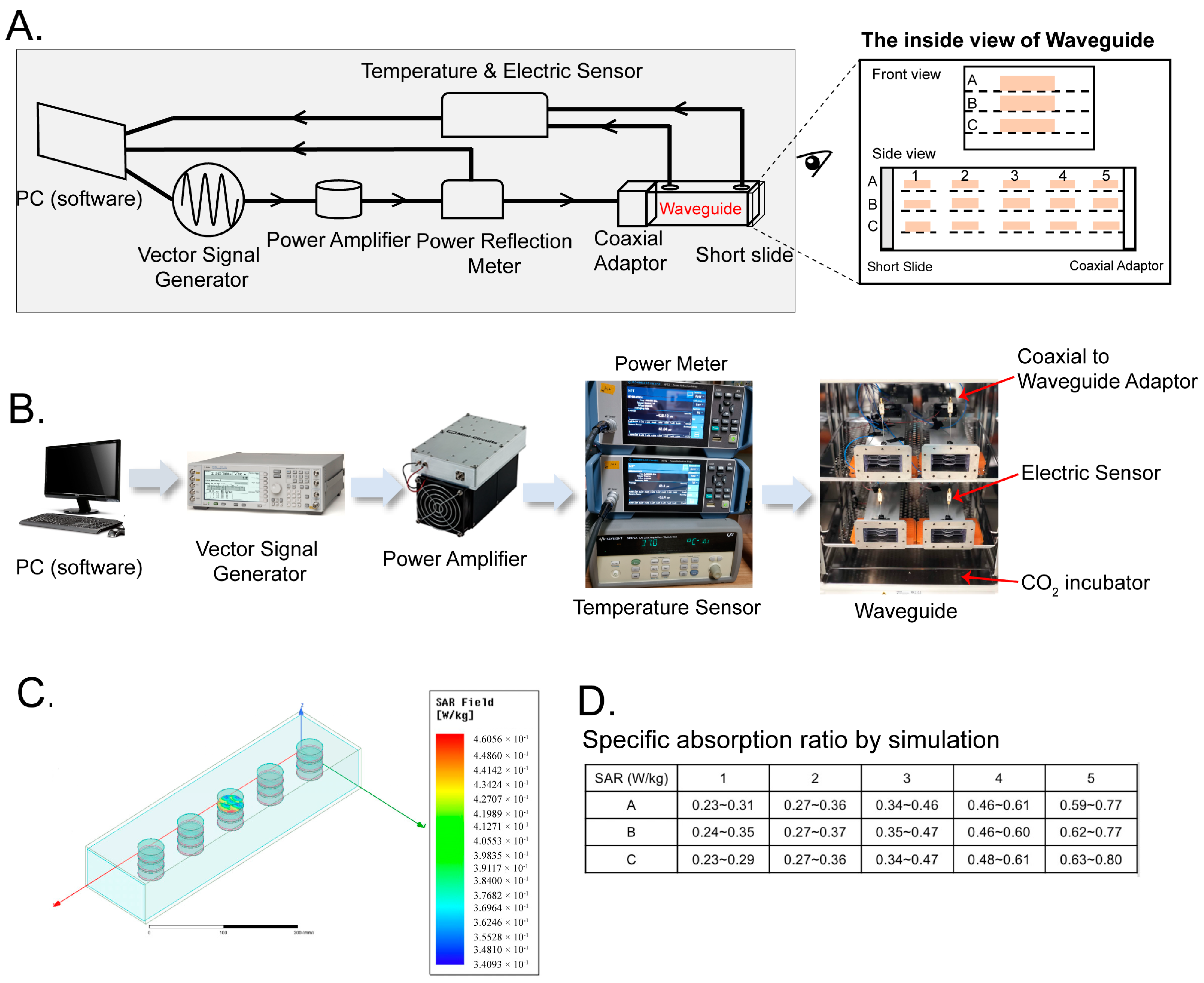
4.1. Waveguide Exposure Apparatus
4.2. Cell Culture
4.3. Isolation and Culture of Primary Astrocytes
4.4. RNA Extraction and Transcriptome Analysis
4.5. Cell Lysate Preparation and Western Blotting
4.6. Reverse Transcription and Real-Time Quantitative Polymerase Chain Reaction
4.7. Peptide Preparation
4.8. Multiple Reaction Monitoring (MRM) and Data Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zamanian, A.; Hardiman, C. Electromagnetic Radiation and Human Health—A Review of Sources and Effects. High Freq. Electron. 2005, 4, 16–26. [Google Scholar]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.-W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.R.; Meyer-Franke, A.; Lambert, S.; Bennett, V.; Duncan, I.D.; Levinson, S.R.; Barres, B.A. Induction of sodium channel clustering by oligodendrocytes. Nature 1997, 386, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Bosch, L.V.D. Amyotrophic lateral sclerosis: Mechanisms and therapeutic strategies. In Disease-Modifying Targets in Neurodegenerative Disorders; Academic Press: Cambridge, MA, USA, 2017; pp. 277–296. [Google Scholar] [CrossRef]
- Kokkosis, A.G.; Madeira, M.M.; Mullahy, M.R.; Tsirka, S.E. Chronic stress disrupts the homeostasis and progeny progression of oligodendroglial lineage cells, associating immune oligodendrocytes with prefrontal cortex hypomyelination. Mol. Psychiatry 2022, 27, 2833–2848. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Deng, H.; Tang, X.; Lu, Y.; Zhou, J.; Wang, X.; Zhao, Y.; Huang, B.; Shi, Y. Specific electromagnetic radiation in the wireless signal range increases wakefulness in mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2105838118. [Google Scholar] [CrossRef]
- Kawashima, T.; Yashiro, M.; Kasashima, H.; Terakawa, Y.; Uda, T.; Nakajo, K.; Umaba, R.; Tanoue, Y.; Tamrakar, S.; Ohata, K. Oligodendrocytes Up-regulate the Invasive Activity of Glioblastoma Cells via the Angiopoietin-2 Signaling Pathway. Anticancer Res. 2019, 39, 577–584. [Google Scholar] [CrossRef]
- Nerlov, C. C/EBPs: Recipients of extracellular signals through proteome modulation. Curr. Opin. Cell Biol. 2008, 20, 180–185. [Google Scholar] [CrossRef]
- Spike, A.J.; Rosen, J.M. C/EBPβ Isoform Specific Gene Regulation: It’s a Lot more Complicated than you Think! J. Mammary Gland. Biol. Neoplasia 2020, 25, 1–12. [Google Scholar] [CrossRef]
- Descombes, P.; Schibler, U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the SAM mRNA. Cell 1991, 67, 569–579. [Google Scholar] [CrossRef]
- Ma, D.; Panda, S.; Lin, J.D. Temporal orchestration of circadian autophagy rhythm by C/EBPβ. EMBO J. 2011, 30, 4642–4651. [Google Scholar] [CrossRef]
- Pham, T.H.; Langmann, S.; Schwarzfischer, L.; El Chartouni, C.; Lichtinger, M.; Klug, M.; Krause, S.W.; Rehli, M. CCAAT enhancer-binding protein beta regulates constitutive gene expression during late stages of monocyte to macrophage differentiation. J. Biol. Chem. 2007, 282, 21924–21933. [Google Scholar] [CrossRef]
- Ndoja, A.; Reja, R.; Lee, S.-H.; Webster, J.D.; Ngu, H.; Rose, C.M.; Kirkpatrick, D.S.; Modrusan, Z.; Chen, Y.-J.J.; Dugger, D.L.; et al. Ubiquitin Ligase COP1 Suppresses Neuroinflammation by Degrading c/EBPβ in Microglia. Cell 2020, 182, 1156–1169.e12. [Google Scholar] [CrossRef]
- Pulido-Salgado, M.; Vidal-Taboada, J.M.; Saura, J. C/EBPbeta and C/EBPdelta transcription factors: Basic biology and roles in the CNS. Prog. Neurobiol. 2015, 132, 1–33. [Google Scholar] [CrossRef]
- Carro, M.S.; Lim, W.K.; Alvarez, M.J.; Bollo, R.J.; Zhao, X.; Snyder, E.Y.; Sulman, E.P.; Anne, S.L.; Doetsch, F.; Colman, H.; et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature 2010, 463, 318–325. [Google Scholar] [CrossRef]
- Isshiki, H.; Akira, S.; Tanabe, O.; Nakajima, T.; Shimamoto, T.; Hirano, T.; Kishimoto, T. Constitutive and interleukin-1 (IL-1)-inducible factors interact with the IL-1-responsive element in the IL-6 gene. Mol. Cell. Biol. 1990, 10, 2757–2764. [Google Scholar]
- Thiaville, M.M.; Dudenhausen, E.E.; Zhong, C.; Pan, Y.X.; Kilberg, M.S. Deprivation of protein or amino acid induces C/EBPbeta synthesis and binding to amino acid response elements, but its action is not an absolute requirement for enhanced transcription. Biochem. J. 2008, 410, 473–484. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, J.; Sun, H.; Zhai, T.; Li, H.; Li, H.; Sun, Y.; Huo, R.; Shen, B.; Sheng, H.; et al. CCN1 upregulates IL-36 via AKT/NF-κB and ERK/CEBP β-mediated signaling pathways in psoriasis-like models. J. Dermatol. 2023, 50, 337–348. [Google Scholar] [CrossRef]
- Guindi, C.; Khan, F.U.; Cloutier, A.; Khongorzul, P.; Raki, A.A.; Gaudreau, S.; McDonald, P.P.; Gris, D.; Amrani, A. Inhibition of PI3K/C/EBPβ axis in tolerogenic bone marrow-derived dendritic cells of NOD mice promotes Th17 differentiation and diabetes development. Transl. Res. 2023, 255, 37–49. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Mortazavi, S.; Safari, S.; Nikseresht, M.; Mozdarani, H.; Jafari, M.; Zamani, A.; Haghani, M.; Davari, M.; Tabatabaie, A. Effects of microwave electromagnetic radiations emitted from common Wi-Fi routers on rats’ sperm count and motility. Int. J. Radiat. Res. 2015, 13, 363–368. [Google Scholar]
- Falcioni, L.; Bua, L.; Tibaldi, E.; Lauriola, M.; De Angelis, L.; Gnudi, F.; Mandrioli, D.; Manservigi, M.; Manservisi, F.; Manzoli, I.; et al. Report of final results regarding brain and heart tumors in Sprague-Dawley rats exposed from prenatal life until natural death to mobile phone radiofrequency field representative of a 1.8 GHz GSM base station environmental emission. Environ. Res. 2018, 165, 496–503. [Google Scholar] [CrossRef]
- Smith-Roe, S.L.; Wyde, M.E.; Stout, M.D.; Winters, J.W.; Hobbs, C.A.; Shepard, K.G.; Green, A.S.; Kissling, G.E.; Shockley, K.R.; Tice, R.R.; et al. Evaluation of the genotoxicity of cell phone radiofrequency radiation in male and female rats and mice following subchronic exposure. Environ. Mol. Mutagen. 2020, 61, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Inskip, P.D.; Tarone, R.E.; Hatch, E.E.; Wilcosky, T.C.; Shapiro, W.R.; Selker, R.G.; Fine, H.A.; Black, P.M.; Loeffler, J.S.; Linet, M.S. Cellular-Telephone Use and Brain Tumors. N. Engl. J. Med. 2001, 344, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Muscat, J.E.; Malkin, M.G.; Thompson, S.; Shore, R.E.; Stellman, S.D.; McRee, D.; Neugut, A.I.; Wynder, E.L. Handheld Cellular Telephone Use and Risk of Brain Cancer. JAMA 2000, 284, 3001–3007. [Google Scholar] [CrossRef]
- Wyde, M.; Cesta, M.; Blystone, C.; Elmore, S.; Foster, P.; Hooth, M.; Kissling, G.; Malarkey, D.; Sills, R.; Stout, M.; et al. Report of Partial Findings from the National Toxicology Program Carcinogenesis Studies of Cell Phone Radiofrequency Radiation in Hsd: Sprague Dawley® SD rats (Whole Body Exposures). bioRxiv 2016, 055699. [Google Scholar] [CrossRef]
- Richter-Landsberg, C.; Heinrich, M. OLN-93: A new permanent oligodendroglia cell line derived from primary rat brain glial cultures. J. Neurosci. Res. 1996, 45, 161–173. [Google Scholar] [CrossRef]
- Adey, W.R. Tissue interactions with nonionizing electromagnetic fields. Physiol. Rev. 1981, 61, 435–514. [Google Scholar] [CrossRef]
- Mendes, M.I.; Green, L.M.; Bertini, E.; Tonduti, D.; Aiello, C.; Smith, D.; Salsano, E.; Beerepoot, S.; Hertecant, J.; Wolf, N.I.; et al. RARS1-related hypomyelinating leukodystrophy: Expanding the spectrum. Ann. Clin. Transl. Neurol. 2020, 7, 83–93. [Google Scholar] [CrossRef]
- Okuda, Y.; Sakoda, S.; Fujimura, H.; Saeki, Y.; Kishimoto, T.; Yanagihara, T. IL-6 plays a crucial role in the induction phase of myelin oligodendrocyte glucoprotein 35-55 induced experimental autoimmune encephalomyelitis. J. Neuroimmunol. 1999, 101, 188–196. [Google Scholar] [CrossRef]
- Serada, S.; Fujimoto, M.; Mihara, M.; Koike, N.; Ohsugi, Y.; Nomura, S.; Yoshida, H.; Nishikawa, T.; Terabe, F.; Ohkawara, T.; et al. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2008, 105, 9041–9046. [Google Scholar] [CrossRef]
- Alfadhel, M.; Alrifai, M.T.; Trujillano, D.; Alshaalan, H.; Al Othaim, A.; Al Rasheed, S.; Assiri, H.; Alqahtani, A.A.; Alaamery, M.; Rolfs, A.; et al. Asparagine Synthetase Deficiency: New Inborn Errors of Metabolism. JIMD Rep. 2015, 22, 11–16. [Google Scholar] [CrossRef]
- Seidahmed, M.Z.; Salih, M.A.; Abdulbasit, O.B.; Samadi, A.; Al Hussien, K.; Miqdad, A.M.; Biary, M.S.; Alazami, A.M.; Alorainy, I.A.; Kabiraj, M.M.; et al. Hyperekplexia, microcephaly and simplified gyral pattern caused by novel ASNS mutations, case report. BMC Neurol. 2016, 16, 105. [Google Scholar] [CrossRef]
- Belyaev, I.; Dean, A.; Eger, H.; Hubmann, G.; Jandrisovits, R.; Kern, M.; Kundi, M.; Moshammer, H.; Lercher, P.; Müller, K.; et al. EUROPAEM EMF Guideline 2016 for the prevention, diagnosis and treatment of EMF-related health problems and illnesses. Rev. Environ. Health 2016, 31, 363–397. [Google Scholar] [CrossRef]
- Rosch, P.J. Bioelectromagnetic and Subtle Energy Medicine. Ann. N. Y. Acad. Sci. 2009, 1172, 297–311. [Google Scholar] [CrossRef]
- ICNIRP. Guidelines for Limiting Exposure to Electromagnetic Fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef]
- Höytö, A.; Luukkonen, J.; Juutilainen, J.; Naarala, J. Proliferation, Oxidative Stress and Cell Death in Cells Exposed to 872 MHz Radiofrequency Radiation and Oxidants. Radiat. Res. 2008, 170, 235–243. [Google Scholar] [CrossRef]
- Luukkonen, J.; Hakulinen, P.; Mäki-Paakkanen, J.; Juutilainen, J.; Naarala, J. Enhancement of chemically induced reactive oxygen species production and DNA damage in human SH-SY5Y neuroblastoma cells by 872 MHz radiofrequency radiation. Mutat. Res. Mol. Mech. Mutagen. 2009, 662, 54–58. [Google Scholar] [CrossRef]
- Juutilainen, J.; Höytö, A.; Kumlin, T.; Naarala, J. Review of possible modulation-dependent biological effects of radiofrequency fields. Bioelectromagnetics 2011, 32, 511–534. [Google Scholar] [CrossRef]
- Park, B.-H.; Kook, S.; Lee, S.; Jeong, J.-H.; Brufsky, A.; Lee, B.-C. An Isoform of C/EBPβ, LIP, Regulates Expression of the Chemokine Receptor CXCR4 and Modulates Breast Cancer Cell Migration. J. Biol. Chem. 2013, 288, 28656–28667. [Google Scholar] [CrossRef]
- Liu, Q.; Boudot, A.; Ni, J.; Hennessey, T.; Beauparlant, S.L.; Rajabi, H.N.; Zahnow, C.; Ewen, M.E. Cyclin D1 and C/EBPβ LAP1 Operate in a Common Pathway To Promote Mammary Epithelial Cell Differentiation. Mol. Cell. Biol. 2014, 34, 3168–3179. [Google Scholar] [CrossRef]
- Kim, H.J.; Magrané, J. Isolation and Culture of Neurons and Astrocytes from the Mouse Brain Cortex. Methods Mol. Biol. 2011, 793, 63–75. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, T. High-Throughput Proteomic Analysis of Fresh-Frozen Biopsy Tissue Samples Using Pressure Cycling Technology Coupled with SWATH Mass Spectrometry. Methods Mol. Biol. 2018, 1788, 279–287. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Zhao, W.; Cai, X.; Zhu, Y.; Lu, Y.; Zhao, J.; Xiang, N.; Wang, X.; Deng, H.; Tang, X.; et al. Expression and Activity of the Transcription Factor CCAAT/Enhancer-Binding Protein β (C/EBPβ) Is Regulated by Specific Pulse-Modulated Radio Frequencies in Oligodendroglial Cells. Int. J. Mol. Sci. 2023, 24, 11131. https://doi.org/10.3390/ijms241311131
Huang B, Zhao W, Cai X, Zhu Y, Lu Y, Zhao J, Xiang N, Wang X, Deng H, Tang X, et al. Expression and Activity of the Transcription Factor CCAAT/Enhancer-Binding Protein β (C/EBPβ) Is Regulated by Specific Pulse-Modulated Radio Frequencies in Oligodendroglial Cells. International Journal of Molecular Sciences. 2023; 24(13):11131. https://doi.org/10.3390/ijms241311131
Chicago/Turabian StyleHuang, Bing, Weihao Zhao, Xue Cai, Yumin Zhu, Yingxian Lu, Junli Zhao, Nan Xiang, Xiaofei Wang, Hu Deng, Xiaping Tang, and et al. 2023. "Expression and Activity of the Transcription Factor CCAAT/Enhancer-Binding Protein β (C/EBPβ) Is Regulated by Specific Pulse-Modulated Radio Frequencies in Oligodendroglial Cells" International Journal of Molecular Sciences 24, no. 13: 11131. https://doi.org/10.3390/ijms241311131
APA StyleHuang, B., Zhao, W., Cai, X., Zhu, Y., Lu, Y., Zhao, J., Xiang, N., Wang, X., Deng, H., Tang, X., Liu, L., Zhao, Y., & Shi, Y. (2023). Expression and Activity of the Transcription Factor CCAAT/Enhancer-Binding Protein β (C/EBPβ) Is Regulated by Specific Pulse-Modulated Radio Frequencies in Oligodendroglial Cells. International Journal of Molecular Sciences, 24(13), 11131. https://doi.org/10.3390/ijms241311131




