Bio-Approach for Obtaining Enantiomerically Pure Clopidogrel with the Use of Ionic Liquids
Abstract
1. Introduction
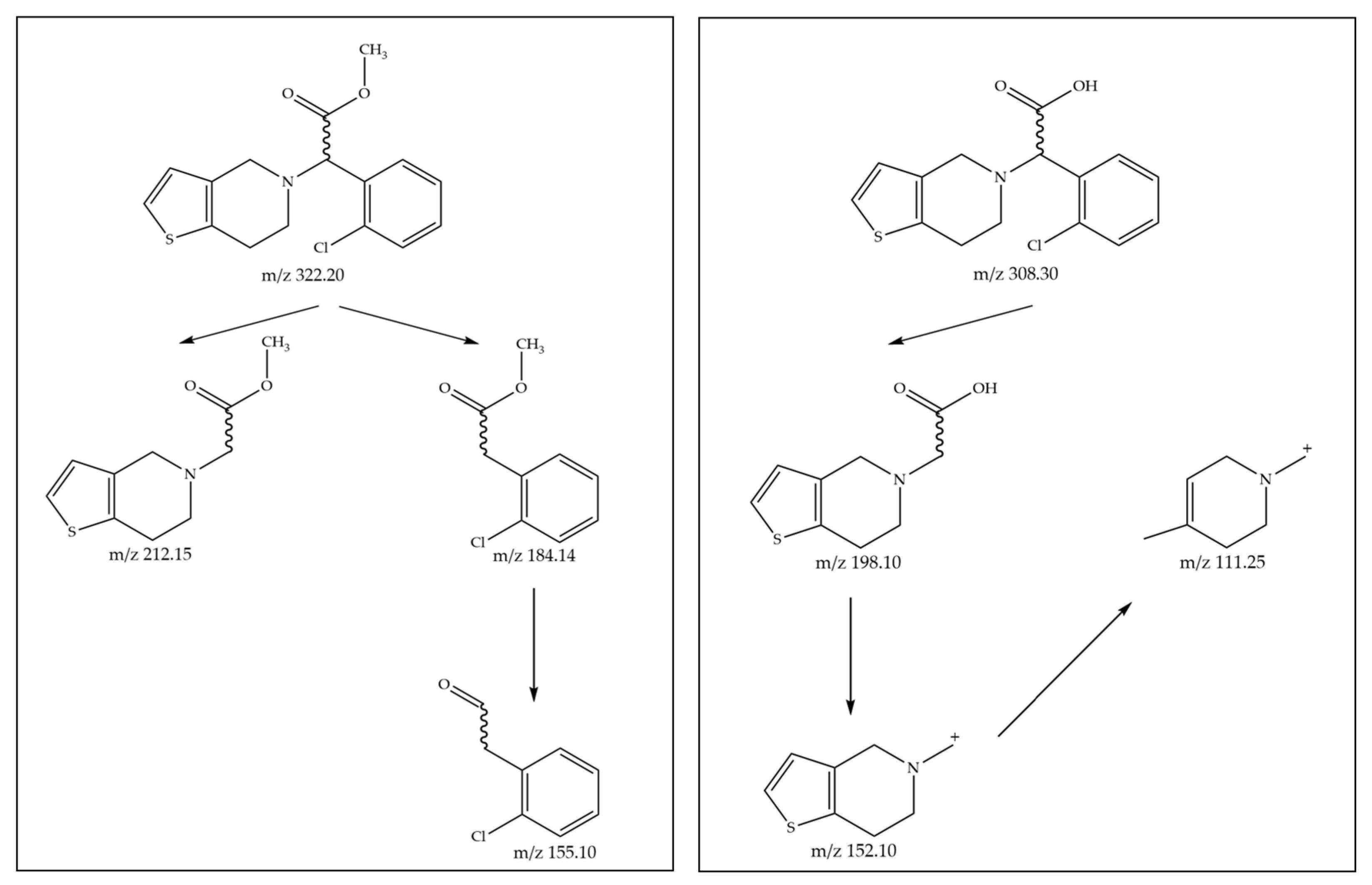
2. Results and Discussion
2.1. Enantioselective Biotransformation of Racemic Clopidogrel
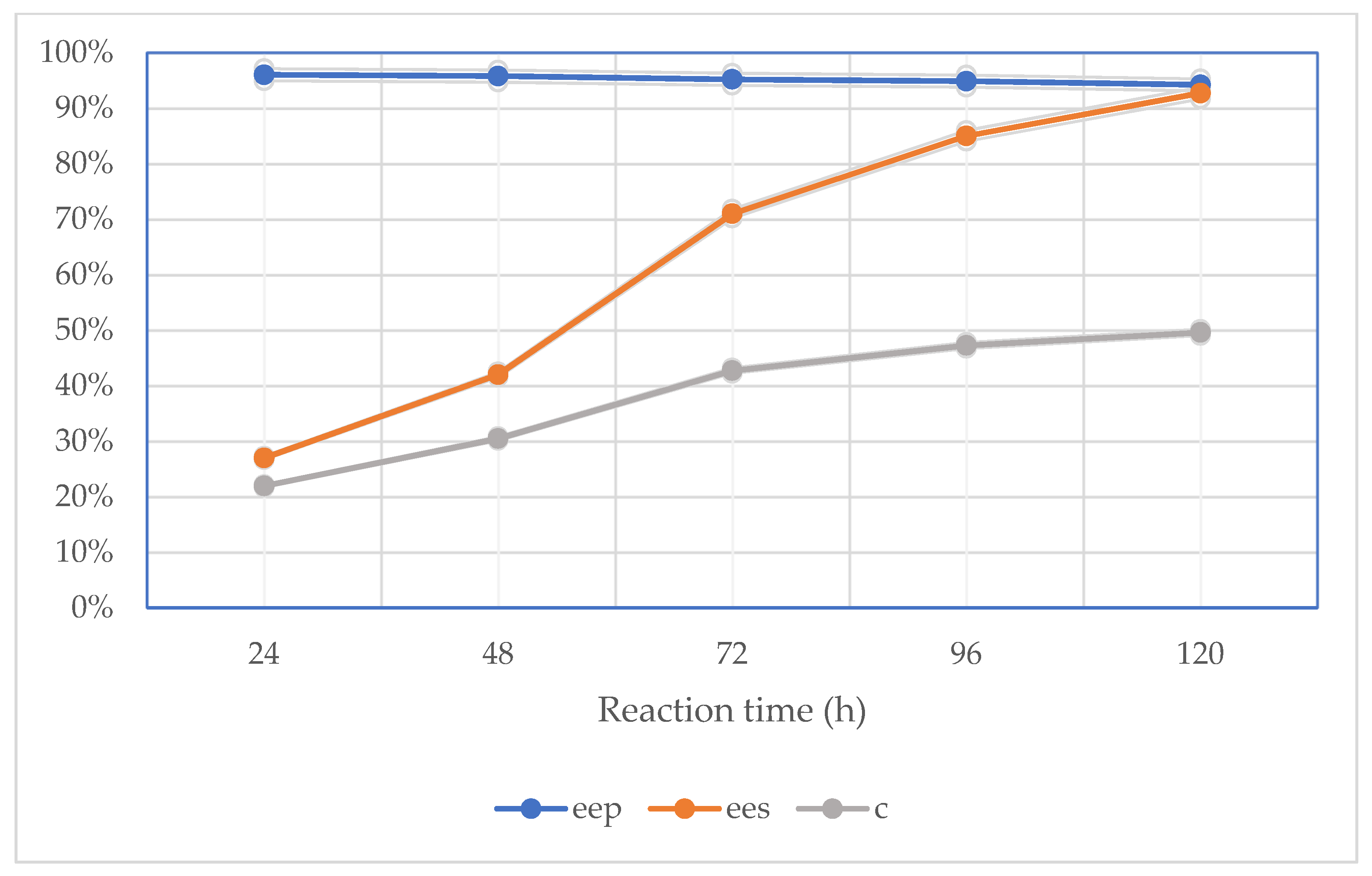
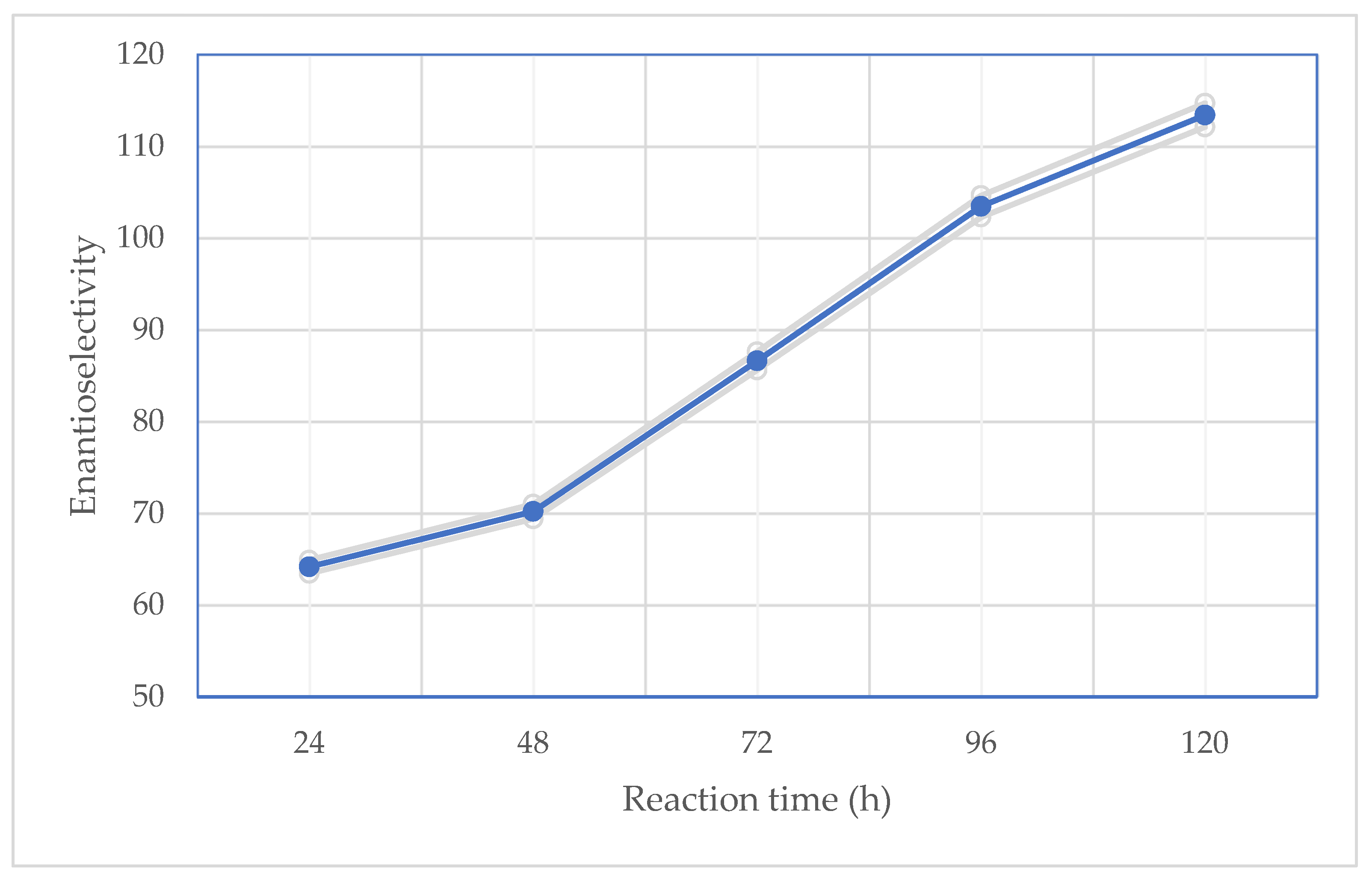
2.2. Effect of Reaction Time
2.3. Effect of Reaction Medium
3. Materials and Methods
3.1. Chemicals
3.2. Instrumentation
3.3. Chromatographic Conditions
3.4. Kinetic Resolution of (R,S)-Clopidogrel Carboxylic Acid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carlin, S.; Eikelboom, J. Are P2Y12 inhibitors superior to aspirin for long-term secondary prevention of cardiovascular disease? Expert Rev. Cardiovasc. Ther. 2023, 21, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Erlinge, D.; Amisten, S.; Braun, O.; Johansson, L.; Ridderstrale, M.; Melander, O. The P2Y12/P2Y13 haplotype is not associated with acute myocardial infarction, cardiovascular risk factors or diabetes. Eur. Heart J. 2008, 29, 826. [Google Scholar]
- Fujisaki, T.; Kuno, T.; Ando, T.; Briasoulis, A.; Takagi, H.; Bangalore, S. Potent P2Y12 inhibitors versus Clopidogrel in elderly patients with acute coronary syndrome: Systematic review and meta-analysis. Am. Heart J. 2021, 237, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Girotra, S.; Stebbins, A.; Wruck, L.M.; Riley, D.; Kripalani, S.; Munoz, D.; Effron, M.B.; Gupta, K.; Whittle, J.; Benziger, C.; et al. Effect of Aspirin Dosing in Patients Treated with P2Y12 Inhibitors for Secondary Prevention of Cardiovascular Events. Circulation 2022, 146, A14192. [Google Scholar] [CrossRef]
- Mannan, F.; Saluja, S.; Contractor, H.; Abidin, N.; Fitchet, A.; Tin, L.; Woolfson, P.; Garg, S.; Anderson, S. Next generation P2Y12 inhibitors improve survival in acs: An analysis from the british cardiovascular intervention society database. Heart 2021, 107, A30. [Google Scholar] [CrossRef]
- Niezgoda, P.; Sikora, J.; Baranska, M.; Sikora, A.; Buszko, K.; Sieminska, E.; Marszall, M.P.; Siller-Matula, J.M.; Jilma, B.; Alexopoulos, D.; et al. Crushed sublingual versus oral ticagrelor administration strategies in patients with unstable angina. Thromb. Haemost. 2017, 117, 718–726. [Google Scholar] [CrossRef]
- Piao, J.X.; Yoo, C.; Kim, S.; Whang, Y.W.; Shin, S.; Choi, C.U. Assessment of therapeutic platelet inhibition in cardiac patients: Comparative study between VerifyNow-P2Y12 and Anysis-P2Y12 assay. Clin. Hemorheol. Microcirc. 2021, 78, 439–448. [Google Scholar] [CrossRef]
- Senzel, L.; Ahmed, T.; Spitzer, E.D. Laboratory Monitoring of Platelet P2Y12 Receptor Inhibitors and Reversal of Antiplatelet Agents: An ACLPS Critical Review. Am. J. Clin. Pathol. 2019, 152, 1–6. [Google Scholar] [CrossRef]
- Seung, H.; Wrobel, J.; Wadle, C.; Buehler, T.; Chiang, D.; Rettkowski, J.; Cabezas-Wallscheid, N.; Hechler, B.; Wolf, D.; Duerschmied, D.; et al. The role of P2Y12 in cardiovascular disease beyond atherothrombosis: P2Y12 signaling promotes emergency hematopoiesis after myocardial infarction. Eur. Heart J. 2022, 43, 3006. [Google Scholar]
- Wang, L.; Wang, J.X.; Xu, J.X.; Qin, W.X.; Wang, Y.M.; Luo, S.S.; Wang, G.X. The Role and Molecular Mechanism of P2Y12 Receptors in the Pathogenesis of Atherosclerotic Cardiovascular Diseases. Appl. Sci. 2021, 11, 9078. [Google Scholar] [CrossRef]
- DeRaad, R.; Jennings, L.K.; Hord, E.; Welder, J.S. Antiplatelet Effects of Ticagrelor versus Clopidogrel in Native American Patients With Stable Coronary Artery Disease. Circulation 2015, 132, A12498. [Google Scholar] [CrossRef]
- Geisler, T.; Langer, H.; Wydymus, M.; Gohring, K.; Zurn, C.; Bigalke, B.; Stellos, K.; May, A.E.; Gawaz, M. Low response to clopidogrel is associated with cardiovascular outcome after coronary stent implantation. Eur. Heart J. 2006, 27, 2420–2425. [Google Scholar] [CrossRef] [PubMed]
- Joung, K.I.; Kim, K.H.; Hsieh, C.Y.; Shin, J.Y. Exploring pharmacogenetic difference using adverse event database: An example of clopidogrel and cardiovascular events. Pharmacogenomics 2020, 21, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.K.W.; Lam, Y.Y.; Tan, V.P.; Yan, B.P. Variability in response to clopidogrel: How important are pharmacogenetics and drug interactions? Br. J. Clin. Pharmacol. 2011, 72, 697–706. [Google Scholar] [CrossRef]
- Reist, M.; Roy-De Vos, M.; Montseny, J.P.; Mayer, J.M.; Carrupt, P.A.; Berger, Y.; Testa, B. Very slow chiral inversion of clopidogrel in rats: A pharmacokinetic and mechanistic investigation. Drug Metab. Dispos. 2000, 28, 1405–1410. [Google Scholar]
- Sashikanth, S.; Raju, V.; Somaiah, S.; Rao, P.S.; Reddy, K.V. An Asymmetric Synthesis of Clopidogrel Hydrogen Sulfate. Synth.-Stuttg. 2013, 45, 621–624. [Google Scholar] [CrossRef]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Juni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur. Heart J. 2018, 39, 213. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Hudzik, B.; Blachut, A.; Lesiak, M.; Kubica, J.; Wojakowski, W.; Gasior, M. Summary of the European Society of Cardiology guidelines on dual antiplatelet therapy in patients after percutaneous coronary interventions. Kardiol. Pol. 2022, 80, 974–989. [Google Scholar] [CrossRef]
- Adriaensen, K.; Vercammen, J.; Van Goethem, C.; Eyley, S.; Vankelecom, I.; Thielemans, W.; De Vos, D. Novel heterogeneous ruthenium racemization catalyst for dynamic kinetic resolution of chiral aliphatic amines. Green Chem. 2020, 22, 85–93. [Google Scholar] [CrossRef]
- Aguillon, A.R.; Avelar, M.N.; Gotardo, L.E.; de Souza, S.P.; Leao, R.A.C.; Itabaiana, I., Jr.; Miranda, L.S.M.; de Souza, R. Immobilized lipase screening towards continuous-flow kinetic resolution of (+/−)-1,2-propanediol. Mol. Catal. 2019, 467, 128–134. [Google Scholar] [CrossRef]
- Agustian, J.; Kamaruddin, A.H.; Aboul-Enein, H.Y. Enantio-conversion and -selectivity of racemic atenolol kinetic resolution using free Pseudomonas fluorescens lipase (Amano) conducted via transesterification reaction. RSC Adv. 2016, 6, 26077–26085. [Google Scholar] [CrossRef]
- Agustian, J.; Kamaruddin, A.H. The Reaction Mechanism and Kinetics Data of Racemic Atenolol Kinetic Resolution via Enzymatic Transesterification Process Using Free Pseudomonas fluorescence Lipase. Int. J. Chem. Kinet. 2016, 48, 253–265. [Google Scholar] [CrossRef]
- Bozan, A.; Songur, R.; Mehmetoglu, U. The production of enantiomerically pure 1-phenylethanol by enzymatic kinetic resolution method using response surface methodology. Turk. J. Chem. 2020, 44, 1352–1365. [Google Scholar] [CrossRef]
- de Almeida, D.K.C.; da Silva, M.R.; de Mattos, M.C.; Nunes, F.M.; Ballereau, S.; Genisson, Y.; Maraval, V.; Chauvin, R.; Oliveira, M.C.F. Lipase-catalysed enantioselective kinetic resolution of rac-lipidic alkynylcarbinols and a C-5 synthon thereof via a hydrolysis approach. Mol. Catal. 2020, 488, 809. [Google Scholar]
- Fukawa, Y.; Mizuno, Y.; Kawade, K.; Mitsukura, K.; Yoshida, T. Novel (S)-Selective Hydrolase from Arthrobacter sp. K5 for Kinetic Resolution of Cyclic Amines. Catalysts 2021, 11, 809. [Google Scholar] [CrossRef]
- Lesniarek, A.; Chojnacka, A.; Gladkowski, W. Application of Lecitase (R) Ultra-Catalyzed Hydrolysis to the Kinetic Resolution of (E)-4-phenylbut-3-en-2-yl Esters. Catalysts 2018, 8, 423. [Google Scholar] [CrossRef]
- Chalupka, J.; Sikora, A.; Kozicka, A.; Marszall, M.P. Overview: Enzyme-catalyzed Enantioselective Biotransformation of Chiral Active Compounds Used in Hypertension Treatment. Curr. Org. Chem. 2020, 24, 2782–2791. [Google Scholar] [CrossRef]
- Chalupka, J.; Duleba, J.; Sikora, A.; Siodmiak, T.; Marszall, M.P. The Application of Two-Phase Catalytic System in Enantioselective Separation of Racemic (R,S)-1-Phenylethanol. Catalysts 2023, 13, 292. [Google Scholar] [CrossRef]
- Sikora, A.; Siodmiak, T.; Marszall, M.P. Kinetic Resolution of Profens by Enantioselective Esterification Catalyzed by Candida antarctica and Candida rugosa Lipases. Chirality 2014, 26, 663–669. [Google Scholar] [CrossRef]
- Sikora, A.; Sroka, W.D.; Siodmiak, T.; Marszall, M.P. Kinetic Resolution of (R,S)-atenolol with the Use of Lipases in Various Organic Solvents. Curr. Org. Synth. 2017, 14, 747–754. [Google Scholar] [CrossRef]
- Di, X.H.; Zhang, Y.; Fu, J.Y.; Yu, Q.; Wang, Z.M.; Yuan, Z.H. Ionic Liquid-Strengthened Immobilized Rhizomucor miehei Lipase for Catalytic Esterification of Itaconic Acid in Aqueous Media. ACS Sustain. Chem. Eng. 2020, 8, 1805–1812. [Google Scholar] [CrossRef]
- Hussain, A.; AlAjmi, M.F.; Hussain, I.; Ali, I. Future of Ionic Liquids for Chiral Separations in High-Performance Liquid Chromatography and Capillary Electrophoresis. Crit. Rev. Anal. Chem. 2019, 49, 289–305. [Google Scholar] [CrossRef]
- Itoh, T. Biotransformation in ionic liquid. In Future Directions in Biocatalysis, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–67. [Google Scholar] [CrossRef]
- Lozano, P.; Alvarez, E.; Bernal, J.M.; Nieto, S.; Gomez, C.; Sanchez-Gomez, G. Ionic Liquids for Clean Biocatalytic Processes. Curr. Green Chem. 2017, 4, 116–129. [Google Scholar] [CrossRef]
- Teixeira, R.; Lourenco, N.M.T. Enzymatic kinetic resolution of sec-alcohols using an ionic liquid anhydride as acylating agent. Tetrahedron-Asymmetry 2014, 25, 944–948. [Google Scholar] [CrossRef]
- Wei, T.; Yang, K.P.; Bai, B.; Zang, J.; Yu, X.; Mao, D.B. Enzymatic Hydrolytic Resolution of Racemic Ibuprofen Ethyl Ester Using an Ionic Liquid as Cosolvent. Molecules 2016, 21, 905. [Google Scholar] [CrossRef]
- Chen, C.S.; Fujimoto, Y.; Girdaukas, G.; Sih, C.J. Quantitative-analyses of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 1982, 104, 7294–7299. [Google Scholar] [CrossRef]
- Sih, C.J.; Wu, S.H. Resolution of enantiomers via biocatalysis. Top. Stereochem. 1989, 19, 63–125. [Google Scholar]
- Saeed, A.; Shahzad, D.; Faisal, M.; Larik, F.A.; El-Seedi, H.R.; Channar, P.A. Developments in the synthesis of the antiplatelet and antithrombotic drug (S)-clopidogrel. Chirality 2017, 29, 684–707. [Google Scholar] [CrossRef]
- Ferraboschi, P.; De Mieri, M.; Galimberti, F. Chemo-enzymatic approach to the synthesis of the antithrombotic clopidogrel. Tetrahedron-Asymmetry 2010, 21, 2136–2141. [Google Scholar] [CrossRef]
- Montalban, M.G.; Collado-Gonzalez, M.; Lozano-Perez, A.A.; Banos, F.G.D.; Villora, G. Extraction of organic compounds involved in the kinetic resolution of rac-2-pentanol from n-hexane by imidazolium-based ionic liquids: Liquid-liquid equilibrium. J. Mol. Liq. 2018, 252, 445–453. [Google Scholar] [CrossRef]
- Ramos-Martin, J.; Khiari, O.; Alcantara, A.R.; Sanchez-Montero, J.M. Biocatalysis at Extreme Temperatures: Enantioselective Synthesis of both Enantiomers of Mandelic Acid by Transesterification Catalyzed by a Thermophilic Lipase in Ionic Liquids at 120 °C. Catalysts 2020, 10, 1055. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.; Muniruzzaman, M.; Salleh, H.M.; Alam, M.D.Z. Ionic Liquids as a Green Solvent for Lipase-Catalyzed Reactions. In Industrial Applications of Green Solvents, Vol I; Inamuddin, D., Ahamed, M.I., Asiri, A.M., Eds.; Materials Research Foundations; Materials Research Forum: Millersvile, PA, USA, 2019; Volume 50, pp. 21–60. [Google Scholar]
- Park, S.; Doan, T.T.N.; Koo, Y.M.; Oh, K.K.; Lee, S.H. Ionic liquids as cosolvents for the lipase-catalyzed kinetic resolution of ketoprofen. Mol. Catal. 2018, 459, 113–118. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, H.; He, J.R.; Yao, Q.X.; Li, L.L.; Liang, Z.H.; Li, X. Enzymes-Catalyzed Knoevenagel Condensation Promoted by Ionic Liquid and Deep Eutectic Solvent. Catal. Lett. 2022, 152, 1215–1223. [Google Scholar] [CrossRef]
- Jadav, D.; Pandey, D.K.; Patil, T.; Singh, D.K.; Dharaskar, S.; Bandyopadhyay, R.; Tsunoji, N.; Kumar, R.; Bandyopadhyay, M. Ordered silica matrices supported ionic liquids as highly efficient catalysts for fine chemical synthesis. J. Porous Mater. 2022, 29, 2003–2017. [Google Scholar] [CrossRef]
- Chalupka, J.; Sikora, A.; Marszall, M.P. The Utilization of Two-Phase Catalytic System in Enantioselective Biotransformation of Racemic Atenolol. Catalysts 2022, 12, 1068. [Google Scholar] [CrossRef]
- Heba, M.; Wolny, A.; Kastelik-Hryniewiecka, A.; Stradomska, D.; Jurczyk, S.; Chrobok, A.; Kuznik, N. Green Dynamic Kinetic Resolution-Stereoselective Acylation of Secondary Alcohols by Enzyme-Assisted Ruthenium Complexes. Catalysts 2022, 12, 1395. [Google Scholar] [CrossRef]

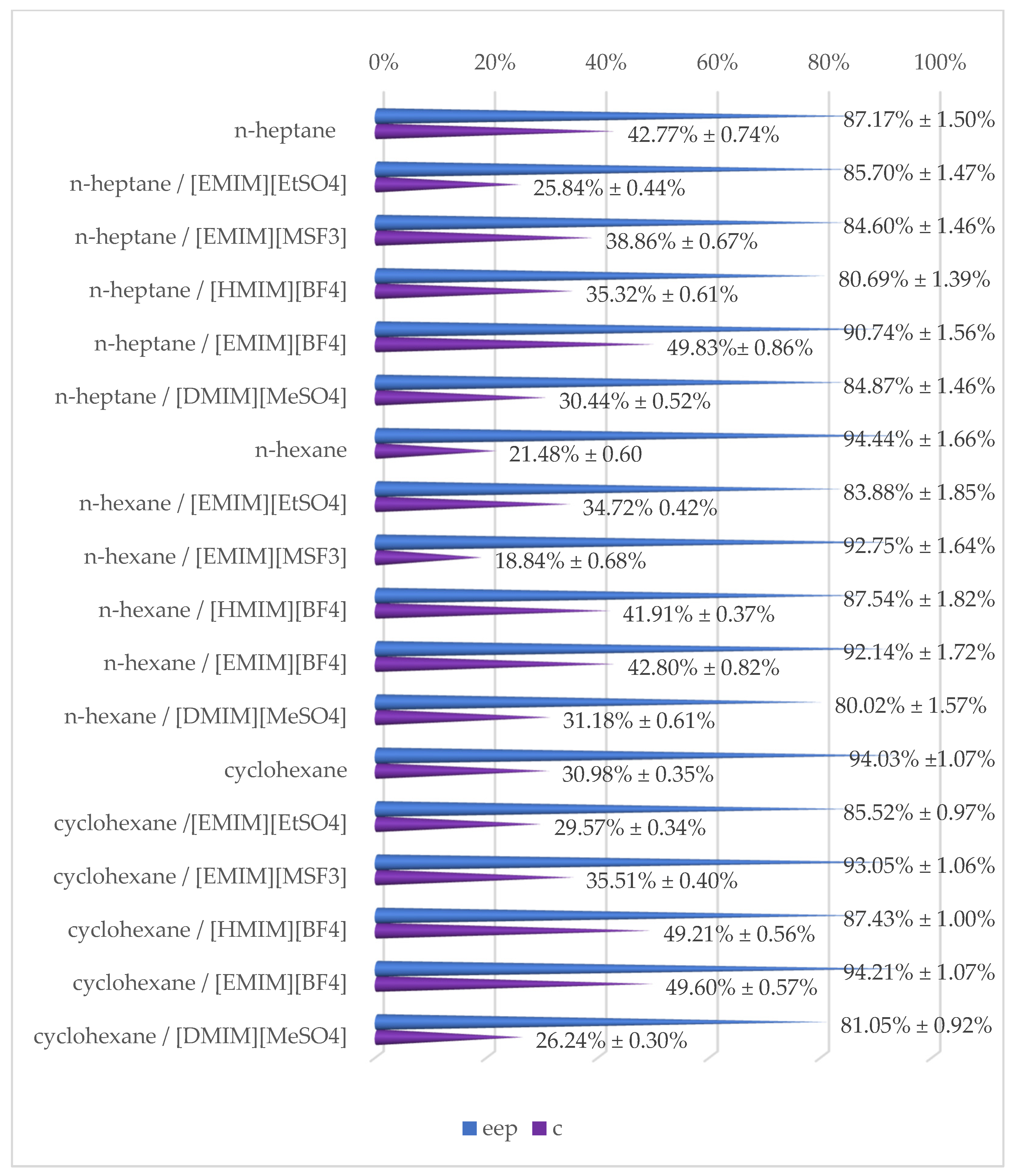
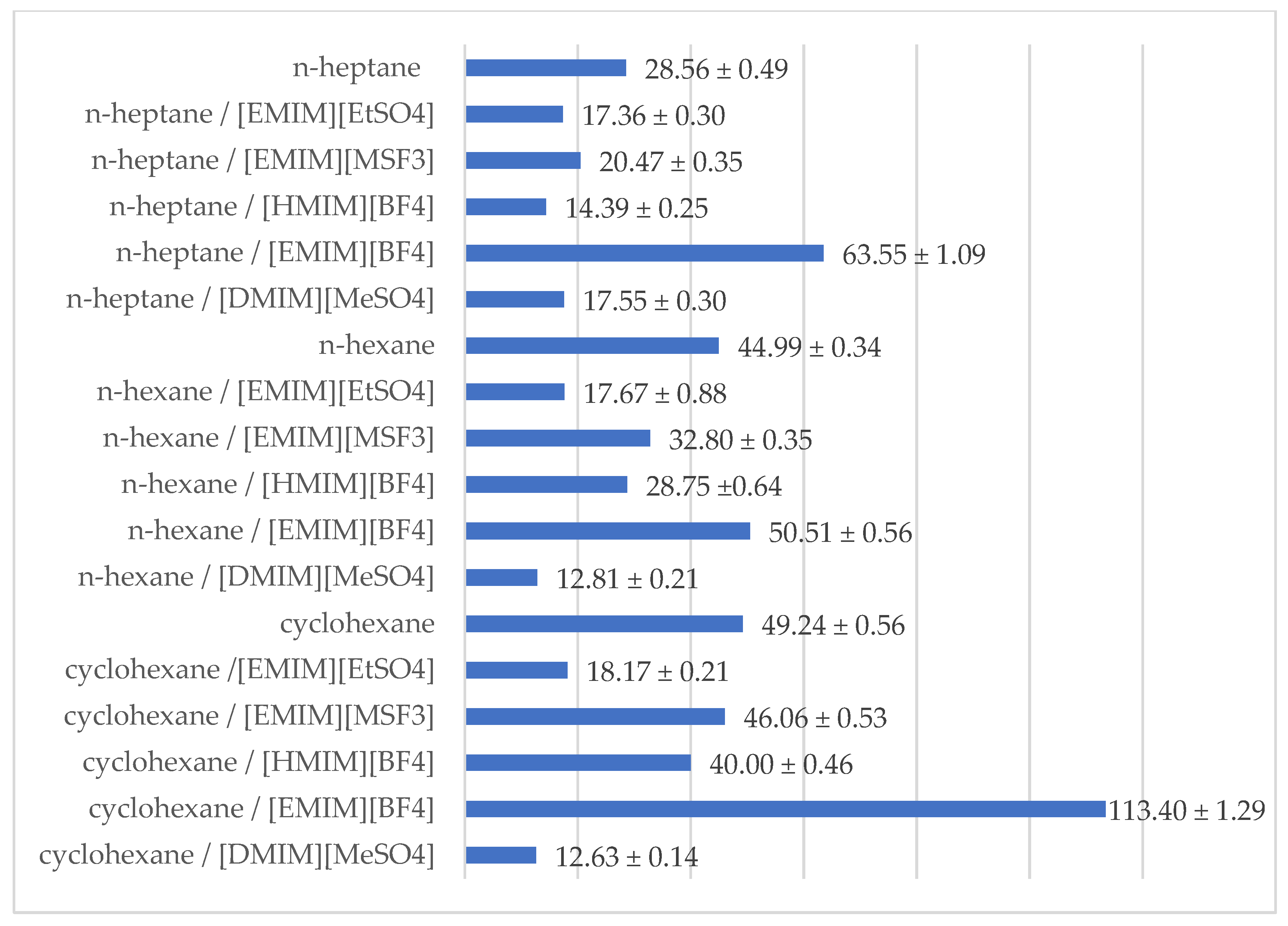

| Reaction Medium | eep | ees | c | E | |
|---|---|---|---|---|---|
| Organic Solvent | Ionic Liquid | ||||
| n-heptane | - | 87.17% ±1.50% | 65.15% ±1.12% | 42.77% ±0.74% | 28.56 ±0.49 |
| n-heptane | [EMIM][EtSO4] | 85.70% ±1.47% | 29.85% ±0.51% | 25.84% ±0.44% | 17.36 ±0.30 |
| n-heptane | [EMIM][MSF3] | 84.60% ±1.46% | 53.77% ±0.92% | 38.86% ±0.67% | 20.47 ±0.35 |
| n-heptane | [HMIM][BF4] | 80.69% ±1.39% | 44.06% ±0.76% | 35.32% ±0.61% | 14.39 ±0.25 |
| n-heptane | [EMIM][BF4] | 90.74% ±1.56% | 90.11% ±1.55% | 49.83% ±0.86% | 63.55 ±1.09 |
| n-heptane | [DMIM][MeSO4] | 84.87% ±1.46% | 37.15% ±0.64% | 30.44% ±0.52% | 17.55 ±0.30 |
| n-hexane | - | 94.44% ±1.66% | 25.83% ±0.73% | 21.48% ±0.60% | 44.99 ±0.34 |
| n-hexane | [EMIM][EtSO4] | 83.88% ±1.85% | 44.61% ±0.51% | 34.72% ±0.42% | 17.67 ±0.88 |
| n-hexane | [EMIM][MSF3] | 92.75% ±1.64% | 21.53% ±0.87% | 18.84% ±0.68% | 32.80 ±0.35 |
| n-hexane | [HMIM][BF4] | 87.54% ±1.82% | 63.16% ±0.42% | 41.91% ±0.37% | 28.75 ±0.64 |
| n-hexane | [EMIM][BF4] | 92.14% ±1.72% | 68.95% ±1.24% | 42.80% ±0.82% | 50.51 ±0.56 |
| n-hexane | [DMIM][MeSO4] | 80.02% ±1.57% | 36.25% ±0.71% | 31.18% ±0.61% | 12.81 ±0.25 |
| cyclohexane | - | 94.03% ±1.07% | 42.20% ±0.48% | 30.98% ±0.35% | 49.24 ±0.56 |
| cyclohexane | [EMIM][EtSO4] | 85.52% ±0.97% | 35.90% ±0.41% | 29.57% ±0.34% | 18.17 ±0.21 |
| cyclohexane | [EMIM][MSF3] | 93.05% ±1.06% | 50.78% ±0.58% | 35.31% ±0.40% | 46.06 ±0.53 |
| cyclohexane | [HMIM][BF4] | 87.43% ±1.00% | 84.70% ±0.97% | 49.21% ±0.56% | 40.00 ±0.46 |
| cyclohexane | [EMIM][BF4] | 94.21% ±1.07% | 92.71% ±1.06% | 49.60% ±0.57% | 113.40 ±1.29 |
| cyclohexane | [DMIM][MeSO4] | 81.05% ±0.92% | 28.83% ±0.33% | 26.24% ±0.30% | 12.63 ±0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chałupka, J.; Sikora, A.; Ziegler-Borowska, M.; Marszałł, M.P. Bio-Approach for Obtaining Enantiomerically Pure Clopidogrel with the Use of Ionic Liquids. Int. J. Mol. Sci. 2023, 24, 11124. https://doi.org/10.3390/ijms241311124
Chałupka J, Sikora A, Ziegler-Borowska M, Marszałł MP. Bio-Approach for Obtaining Enantiomerically Pure Clopidogrel with the Use of Ionic Liquids. International Journal of Molecular Sciences. 2023; 24(13):11124. https://doi.org/10.3390/ijms241311124
Chicago/Turabian StyleChałupka, Joanna, Adam Sikora, Marta Ziegler-Borowska, and Michał Piotr Marszałł. 2023. "Bio-Approach for Obtaining Enantiomerically Pure Clopidogrel with the Use of Ionic Liquids" International Journal of Molecular Sciences 24, no. 13: 11124. https://doi.org/10.3390/ijms241311124
APA StyleChałupka, J., Sikora, A., Ziegler-Borowska, M., & Marszałł, M. P. (2023). Bio-Approach for Obtaining Enantiomerically Pure Clopidogrel with the Use of Ionic Liquids. International Journal of Molecular Sciences, 24(13), 11124. https://doi.org/10.3390/ijms241311124







