Toll-like Receptors and Cytokine Modulation by Goat Milk Extracellular Vesicles in a Model of Intestinal Inflammation
Abstract
1. Introduction
2. Results
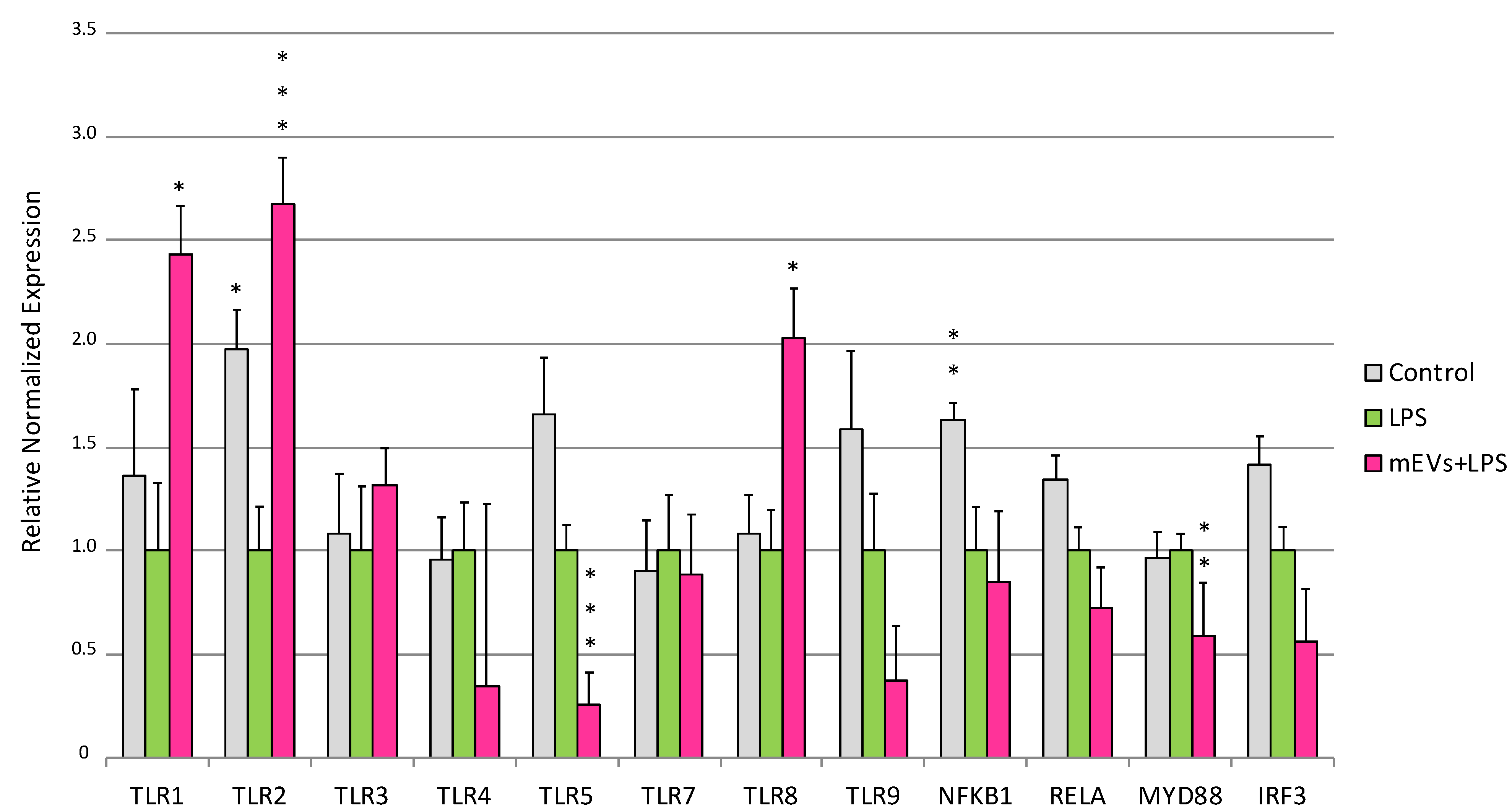
2.1. Goat mEVs Effects on IPEC-J2 Gene Expression in the LPS Model
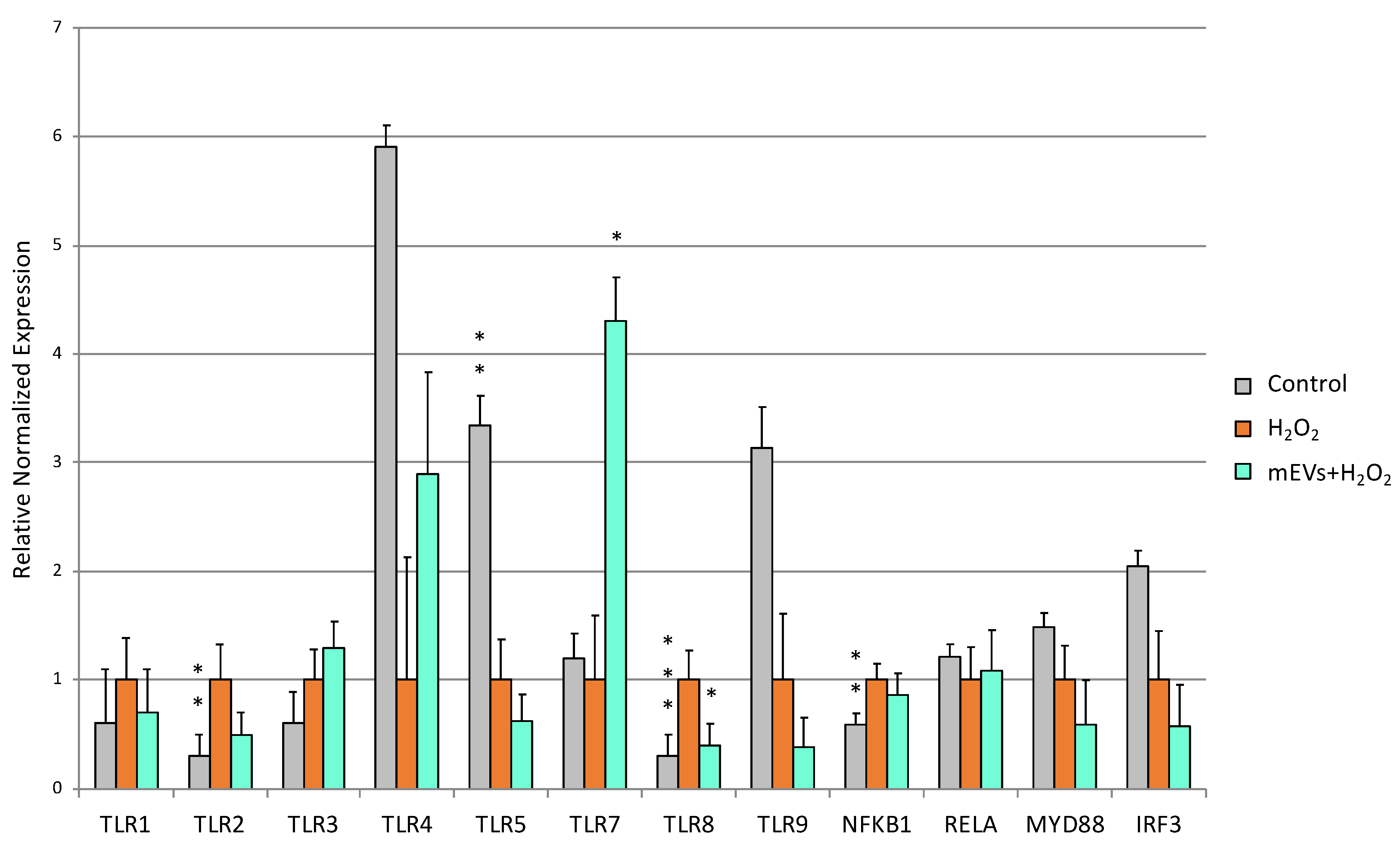
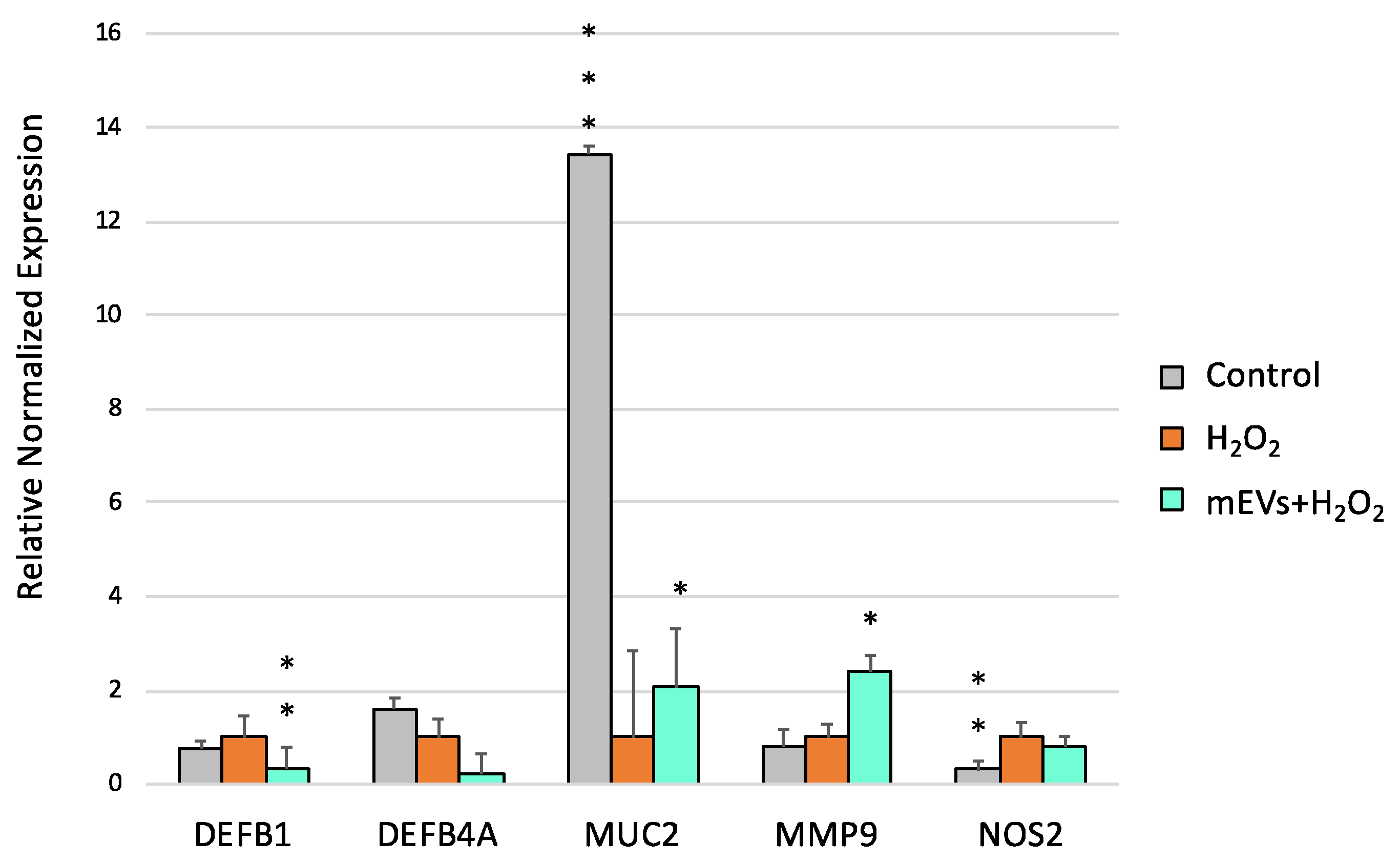
2.2. Goat mEV Effects on IPEC-J2 Gene Expression in the H2O2 Model
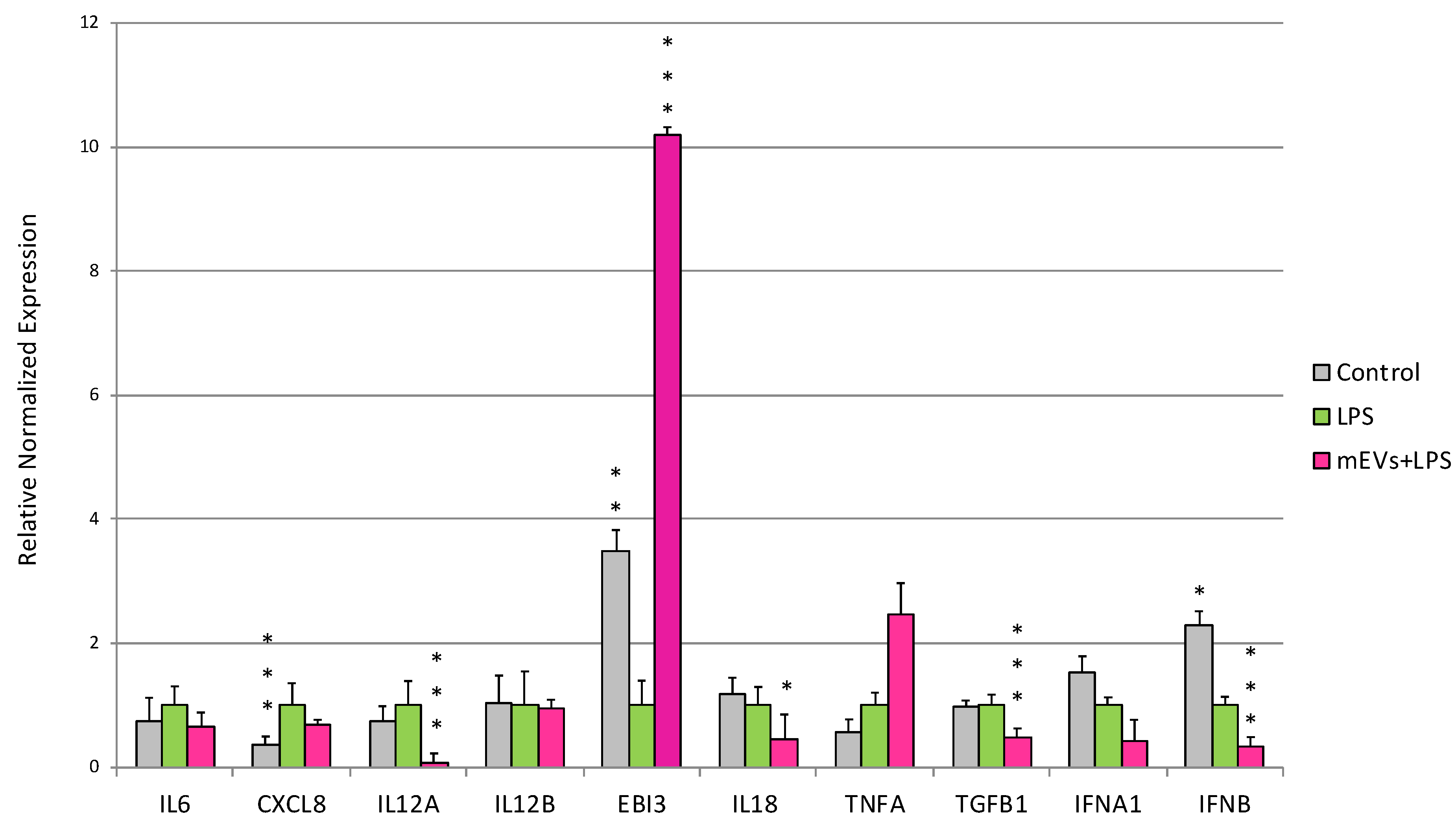
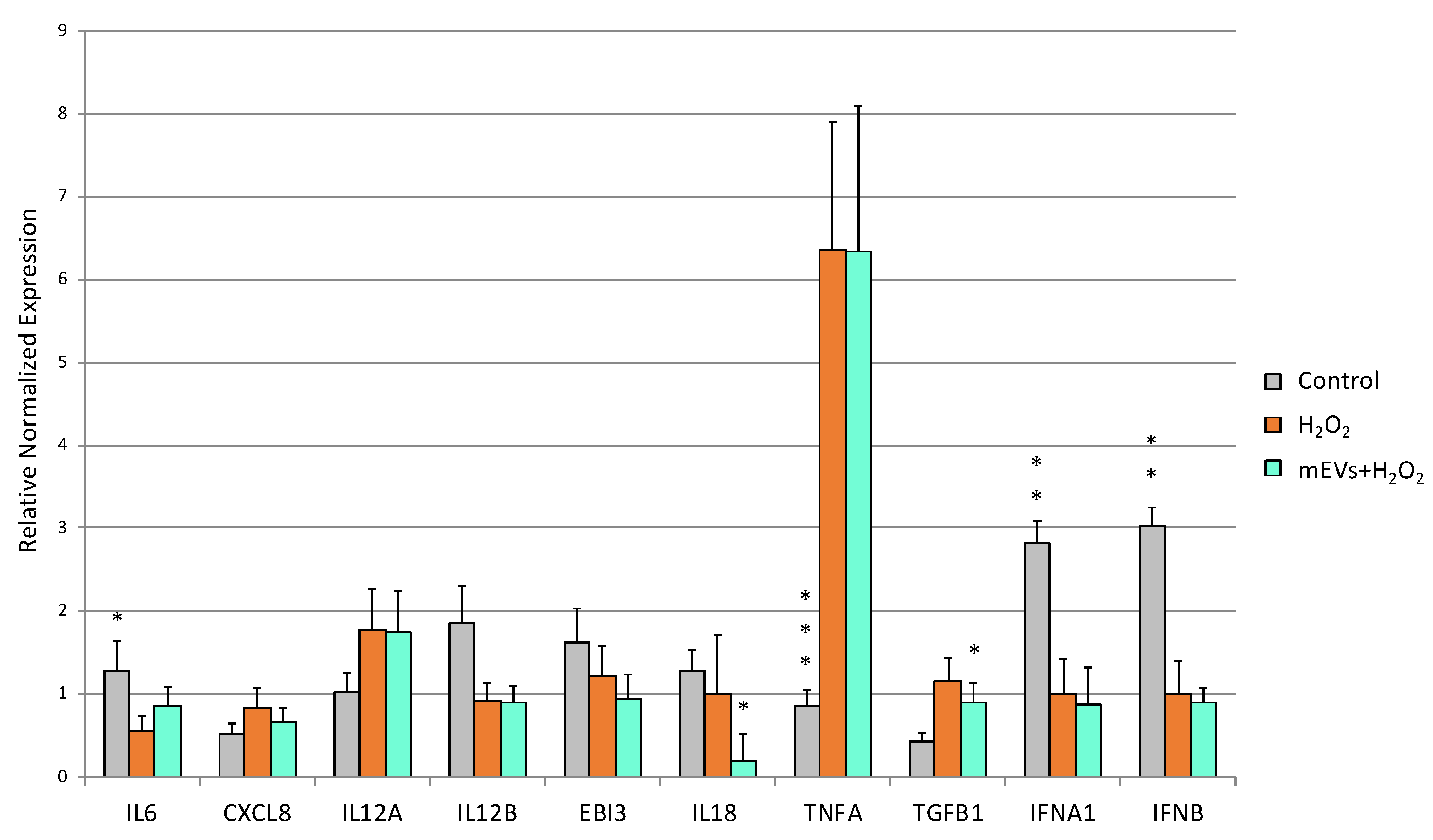
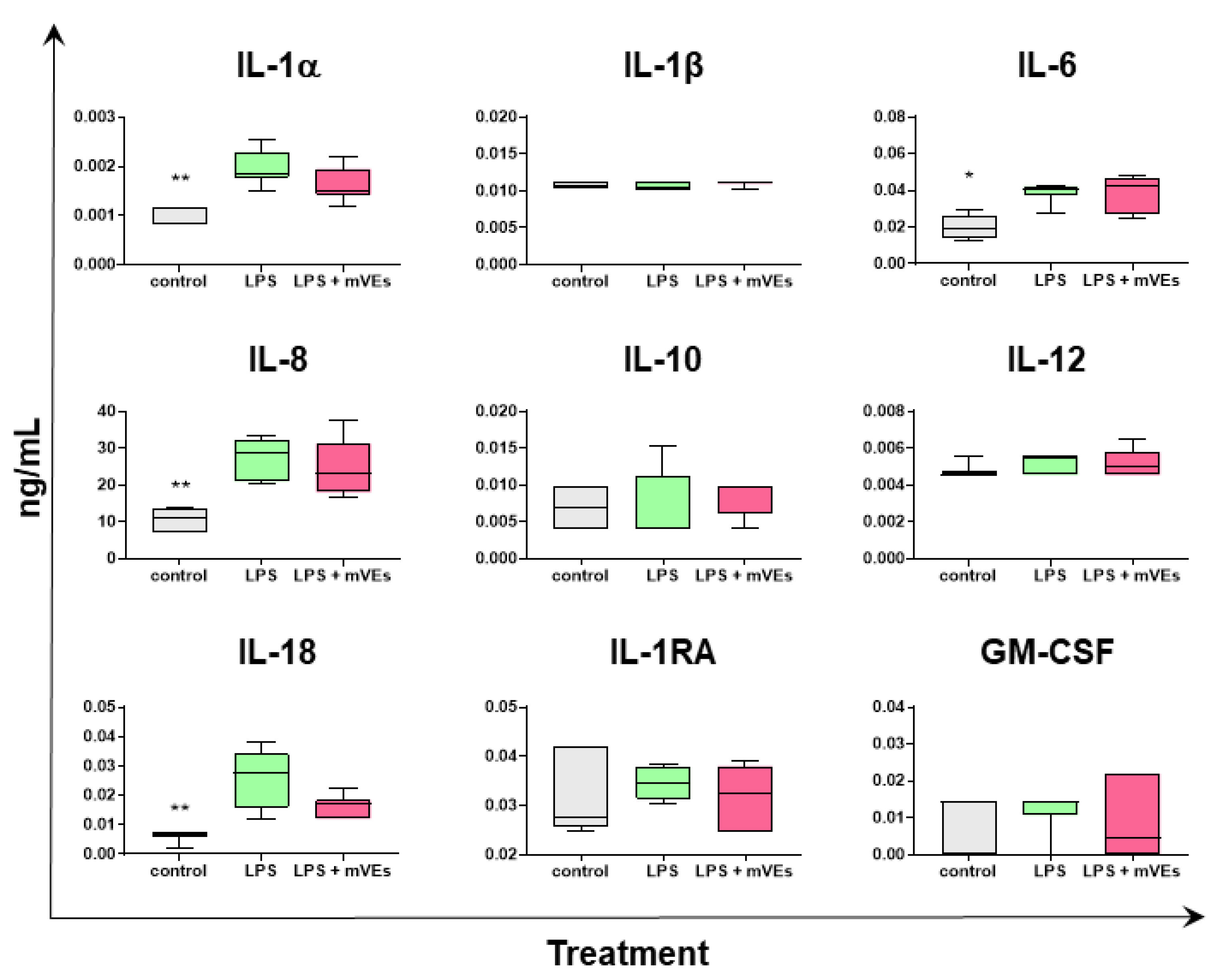
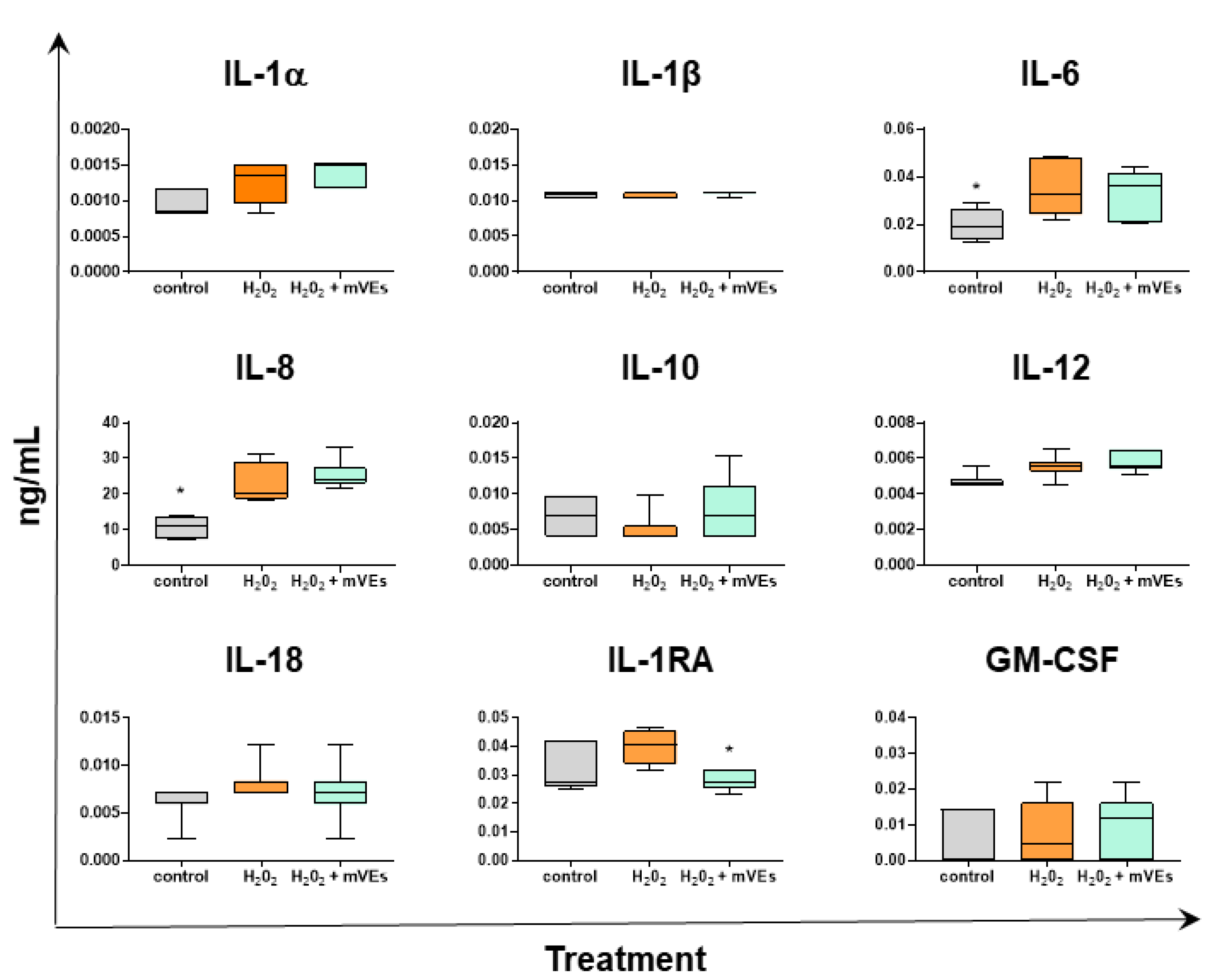
2.3. Cytokine Quantification
3. Discussion
4. Materials and Methods
4.1. Milk Collection
4.2. Extracellular Vesicle (EV) Isolation, Characterization and Size Distribution Assessment
4.3. Cell Cultures
IPEC-J2 Models of Inflammatory Bowel Disease to Mimic Crohn’s Disease and Ulcerative Colitis
4.4. XTT Assay
4.5. RNA Extraction and RT-qPCR
4.6. RT-qPCR Analyses
4.7. Cytokine Quantification
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Monguió-Tortajada, M.; Morón-Font, M.; Gámez-Valero, A.; Carreras-Planella, L.; Borràs, F.E.; Franquesa, M. Extracellular-Vesicle Isolation from Different Biological Fluids by Size-Exclusion Chromatography. Curr. Protoc. Stem Cell Biol. 2019, 49, e82. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; You, L.; Zhang, Z.; Cui, X.; Zhong, H.; Sun, X.; Ji, C.; Chi, X. Biological Properties of Milk-Derived Extracellular Vesicles and Their Physiological Functions in Infant. Front. Cell Dev. Biol. 2021, 9, 693534. [Google Scholar] [CrossRef]
- Lucchetti, D.; Ricciardi Tenore, C.; Colella, F.; Sgambato, A. Extracellular Vesicles and Cancer: A Focus on Metabolism, Cytokines, and Immunity. Cancers 2020, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Capomaccio, S.; Cappelli, K.; Bazzucchi, C.; Coletti, M.; Gialletti, R.; Moriconi, F.; Passamonti, F.; Pepe, M.; Petrini, S.; Mecocci, S.; et al. Equine Adipose-Derived Mesenchymal Stromal Cells Release Extracellular Vesicles Enclosing Different Subsets of Small RNAs. Stem Cells Int. 2019, 2019, 4957806. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, Y.; Dou, Y. The Potential of Milk-Derived Exosomes for Drug Delivery. Curr. Drug. Deliv. 2021, 18, 688–699. [Google Scholar] [CrossRef]
- Sanwlani, R.; Fonseka, P.; Chitti, S.V.; Mathivanan, S. Milk-Derived Extracellular Vesicles in Inter-Organism, Cross-Species Communication and Drug Delivery. Proteomes 2020, 8, 11. [Google Scholar] [CrossRef]
- Ngu, A.; Wang, S.; Wang, H.; Khanam, A.; Zempleni, J. Milk Exosomes in Nutrition and Drug Delivery. Am. J. Physiol. Cell Physiol. 2022, 322, C865–C874. [Google Scholar] [CrossRef]
- Roerig, J.; Schiller, L.; Kalwa, H.; Hause, G.; Vissiennon, C.; Hacker, M.C.; Wölk, C.; Schulz-Siegmund, M. A Focus on Critical Aspects of Uptake and Transport of Milk-Derived Extracellular Vesicles across the Caco-2 Intestinal Barrier Model. Eur. J. Pharm. Biopharm. 2021, 166, 61–74. [Google Scholar] [CrossRef]
- Mecocci, S.; Gevi, F.; Pietrucci, D.; Cavinato, L.; Luly, F.R.; Pascucci, L.; Petrini, S.; Ascenzioni, F.; Zolla, L.; Chillemi, G.; et al. Anti-Inflammatory Potential of Cow, Donkey and Goat Milk Extracellular Vesicles as Revealed by Metabolomic Profile. Nutrients 2020, 12, 2908. [Google Scholar] [CrossRef]
- Mecocci, S.; Pietrucci, D.; Milanesi, M.; Pascucci, L.; Filippi, S.; Rosato, V.; Chillemi, G.; Capomaccio, S.; Cappelli, K. Transcriptomic Characterization of Cow, Donkey and Goat Milk Extracellular Vesicles Reveals Their Anti-Inflammatory and Immunomodulatory Potential. Int. J. Mol. Sci. 2021, 22, 12759. [Google Scholar] [CrossRef]
- Mecocci, S.; De Paolis, L.; Fruscione, F.; Pietrucci, D.; De Ciucis, C.G.; Giudici, S.D.; Franzoni, G.; Chillemi, G.; Cappelli, K.; Razzuoli, E. In Vitro Evaluation of Immunomodulatory Activities of Goat Milk Extracellular Vesicles (MEVs) in a Model of Gut Inflammation. Res. Vet. Sci. 2022, 152, 546–556. [Google Scholar] [CrossRef]
- Mecocci, S.; Ottaviani, A.; Razzuoli, E.; Fiorani, P.; Pietrucci, D.; De Ciucis, C.G.; Dei Giudici, S.; Franzoni, G.; Chillemi, G.; Cappelli, K. Cow Milk Extracellular Vesicle Effects on an In Vitro Model of Intestinal Inflammation. Biomedicines 2022, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Kordjazy, N.; Haj-Mirzaian, A.; Haj-Mirzaian, A.; Rohani, M.M.; Gelfand, E.W.; Rezaei, N.; Abdolghaffari, A.H. Role of Toll-like Receptors in Inflammatory Bowel Disease. Pharmacol. Res. 2018, 129, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, X.; Liu, S.; Zhang, Y.; Zhang, D. Toll-like Receptors and Inflammatory Bowel Disease. Front. Immunol. 2018, 9, 72. [Google Scholar] [CrossRef]
- Tam, J.S.Y.; Coller, J.K.; Hughes, P.A.; Prestidge, C.A.; Bowen, J.M. Toll-like Receptor 4 (TLR4) Antagonists as Potential Therapeutics for Intestinal Inflammation. Indian J. Gastroenterol. 2021, 40, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Frosali, S.; Pagliari, D.; Gambassi, G.; Landolfi, R.; Pandolfi, F.; Cianci, R. How the Intricate Interaction among Toll-Like Receptors, Microbiota, and Intestinal Immunity Can Influence Gastrointestinal Pathology. J. Immunol. Res. 2015, 2015, 489821. [Google Scholar] [CrossRef]
- Sameer, A.S.; Nissar, S. Toll-Like Receptors (TLRs): Structure, Functions, Signaling, and Role of Their Polymorphisms in Colorectal Cancer Susceptibility. Biomed. Res. Int. 2021, 2021, 1157023. [Google Scholar] [CrossRef]
- Luchner, M.; Reinke, S.; Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13, 142. [Google Scholar] [CrossRef]
- Behzadi, P.; García-Perdomo, H.A.; Karpiński, T.M. Toll-Like Receptors: General Molecular and Structural Biology. J. Immunol. Res. 2021, 2021, 9914854. [Google Scholar] [CrossRef]
- Uenishi, H.; Shinkai, H. Porcine Toll-like Receptors: The Front Line of Pathogen Monitoring and Possible Implications for Disease Resistance. Dev. Comp. Immunol. 2009, 33, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Toll-like Receptors and Innate Immunity. Biochem. Biophys. Res. Commun. 2009, 388, 621–625. [Google Scholar] [CrossRef]
- Nishimura, M.; Kuboi, Y.; Muramoto, K.; Kawano, T.; Imai, T. Chemokines as Novel Therapeutic Targets for Inflammatory Bowel Disease. Ann. N. Y. Acad. Sci. 2009, 1173, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Poehlmann, A.; Reissig, K.; Schönfeld, P.; Walluscheck, D.; Schinlauer, A.; Hartig, R.; Lessel, W.; Guenther, T.; Silver, A.; Roessner, A. Repeated H2O2 Exposure Drives Cell Cycle Progression in an in Vitro Model of Ulcerative Colitis. J. Cell. Mol. Med. 2013, 17, 1619–1631. [Google Scholar] [CrossRef]
- Low, D.; Nguyen, D.D.; Mizoguchi, E. Animal Models of Ulcerative Colitis and Their Application in Drug Research. Drug Des. Dev. Ther. 2013, 7, 1341–1357. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef] [PubMed]
- Magro, D.O.; Kotze, P.G.; Martinez, C.A.R.; Camargo, M.G.; Guadagnini, D.; Calixto, A.R.; Vasques, A.C.J.; de Ayrizono, M.L.S.; Geloneze, B.; Pareja, J.C.; et al. Changes in Serum Levels of Lipopolysaccharides and CD26 in Patients with Crohn’s Disease. Intest. Res. 2017, 15, 352–357. [Google Scholar] [CrossRef]
- Trevisi, E.; Amadori, M.; Bakudila, A.M.; Bertoni, G. Metabolic changes in dairy cows induced by oral, low-dose interferon-alpha treatment. Anim. Sci. 2009, 87, 3020–3029. [Google Scholar] [CrossRef]
- Fioranelli, M.; Roccia, M.G. Twenty-five years of studies and trials for the therapeutic application of IL-10 immunomodulating properties. From high doses administration to low dose medicine new paradigm. J. Int. Cardiol. 2009, 1, 2–6. [Google Scholar] [CrossRef]
- Patel, P.; Asdaq, S.M.B. Immunomodulatory activity of methanolic fruit extract of Aegle marmelos in experimental animals. Saudi Pharm. J. 2010, 18, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Vergauwen, H. The IPEC-J2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; ISBN 978-3-319-15791-7. [Google Scholar]
- Wade, H.; Pan, K.; Duan, Q.; Kaluzny, S.; Pandey, E.; Fatumoju, L.; Saraswathi, V.; Wu, R.; Harris, E.N.; Su, Q. Akkermansia Muciniphila and Its Membrane Protein Ameliorates Intestinal Inflammatory Stress and Promotes Epithelial Wound Healing via CREBH and MiR-143/145. J. Biomed. Sci. 2023, 30, 38. [Google Scholar] [CrossRef] [PubMed]
- Grześkowiak, Ł.; Pieper, R.; Kröger, S.; Martínez-Vallespín, B.; Hauser, A.E.; Niesner, R.; Vahjen, W.; Zentek, J. Porcine Colostrum Protects the IPEC-J2 Cells and Piglet Colon Epithelium against Clostridioides (Syn. Clostridium) Difficile Toxin-Induced Effects. Microorganisms 2020, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Pravda, J. Evidence-Based Pathogenesis and Treatment of Ulcerative Colitis: A Causal Role for Colonic Epithelial Hydrogen Peroxide. World J. Gastroenterol. 2022, 28, 4263–4298. [Google Scholar] [CrossRef]
- Caradonna, L.; Amati, L.; Magrone, T.; Pellegrino, N.M.; Jirillo, E.; Caccavo, D. Enteric Bacteria, Lipopolysaccharides and Related Cytokines in Inflammatory Bowel Disease: Biological and Clinical Significance. J. Endotoxin. Res. 2000, 6, 205–214. [Google Scholar]
- Laird, M.H.W.; Rhee, S.H.; Perkins, D.J.; Medvedev, A.E.; Piao, W.; Fenton, M.J.; Vogel, S.N. TLR4/MyD88/PI3K Interactions Regulate TLR4 Signaling. J. Leuk. Bio. 2009, 85, 966–977. [Google Scholar] [CrossRef]
- Surabhi, S.; Jachmann, L.H.; Shumba, P.; Burchhardt, G.; Hammerschmidt, S.; Siemens, N. Hydrogen Peroxide Is Crucial for NLRP3 Inflammasome-Mediated IL-1β Production and Cell Death in Pneumococcal Infections of Bronchial Epithelial Cells. J. Innate Immun. 2022, 14, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhang, Q.; Wang, C.; Wu, H.; Jiao, L.; Hong, Q.; Hu, C. LPS Challenge Increased Intestinal Permeability, Disrupted Mitochondrial Function and Triggered Mitophagy of Piglets. Innate Immun. 2018, 24, 221–230. [Google Scholar] [CrossRef]
- Bein, A.; Zilbershtein, A.; Golosovsky, M.; Davidov, D.; Schwartz, B. LPS Induces Hyper-Permeability of Intestinal Epithelial Cells. J. Cell. Physiol. 2017, 232, 381–390. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, Y.; Zhang, H.; Zhou, M.; Yu, Y.; Lin, S.; Jiang, B.; Zhao, X.; Miao, L.; Wei, C.-W.; et al. Integrated Cascade Nanozyme Catalyzes in Vivo ROS Scavenging for Anti-Inflammatory Therapy. Sci. Adv. 2020, 6, eabb2695. [Google Scholar] [CrossRef]
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X.; et al. Identification of Exosome-like Nanoparticle-Derived MicroRNAs from 11 Edible Fruits and Vegetables. PeerJ 2018, 6, e5186. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Kamdar, K.; Khakpour, S.; Young, G.; Karpus, W.J.; DePaolo, R.W. TLR1-Induced Chemokine Production Is Critical for Mucosal Immunity against Yersinia Enterocolitica. Mucosal Immunol. 2013, 6, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, K.; Khakpour, S.; Chen, J.; Leone, V.; Brulc, J.; Mangatu, T.; Antonopoulos, D.A.; Chang, E.B.; Kahn, S.A.; Kirschner, B.S.; et al. Genetic and Metabolic Signals during Acute Enteric Bacterial Infection Alter the Microbiota and Drive Progression to Chronic Inflammatory Disease. Cell Host Microbe 2016, 19, 21–31. [Google Scholar] [CrossRef]
- Almero, J.M. Influence of Toll-like receptor 2 and interleukin 10 on the intestinal epithelial barrier and their roles in inflammatory bowel disease. Inmunología 2011, 30, 8–16. [Google Scholar] [CrossRef]
- Fernandes, P.; MacSharry, J.; Darby, T.; Fanning, A.; Shanahan, F.; Houston, A.; Brint, E. Differential Expression of Key Regulators of Toll-like Receptors in Ulcerative Colitis and Crohn’s Disease: A Role for Tollip and Peroxisome Proliferator-Activated Receptor Gamma? Clin. Exp. Immunol. 2016, 183, 358–368. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Jia, R.; Wang, X.-M.; Jia, L.-G.; Petito, V.; Goodman, W.A.; Meddings, J.B.; Cominelli, F.; Reuter, B.K.; Pizarro, T.T. Epithelial-Specific Toll-like Receptor (TLR)5 Activation Mediates Barrier Dysfunction in Experimental Ileitis. Inflamm. Bowel Dis. 2017, 23, 392–403. [Google Scholar] [CrossRef]
- Sainathan, S.K.; Bishnupuri, K.S.; Aden, K.; Luo, Q.; Houchen, C.W.; Anant, S.; Dieckgraefe, B.K. Toll-like Receptor-7 Ligand Imiquimod Induces Type I Interferon and Antimicrobial Peptides to Ameliorate Dextran Sodium Sulfate-Induced Acute Colitis. Inflamm. Bowel Dis. 2012, 18, 955–967. [Google Scholar] [CrossRef]
- Saruta, M.; Michelsen, K.S.; Thomas, L.S.; Yu, Q.T.; Landers, C.J.; Targan, S.R. TLR8-Mediated Activation of Human Monocytes Inhibits TL1A Expression. Eur. J. Immunol. 2009, 39, 2195–2202. [Google Scholar] [CrossRef]
- Ramasundara, M.; Leach, S.T.; Lemberg, D.A.; Day, A.S. Defensins and Inflammation: The Role of Defensins in Inflammatory Bowel Disease. J. Gastroenterol. Hepatol. 2009, 24, 202–208. [Google Scholar] [CrossRef]
- Parronchi, P.; Romagnani, P.; Annunziato, F.; Sampognaro, S.; Becchio, A.; Giannarini, L.; Maggi, E.; Pupilli, C.; Tonelli, F.; Romagnani, S. Type 1 T-Helper Cell Predominance and Interleukin-12 Expression in the Gut of Patients with Crohn’s Disease. Am. J. Pathol. 1997, 150, 823–832. [Google Scholar] [PubMed]
- Vignali, D.A.A.; Kuchroo, V.K. IL-12 Family Cytokines: Immunological Playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Langrish, C.L.; McKenzie, B.S.; Wilson, N.J.; de Waal Malefyt, R.; Kastelein, R.A.; Cua, D.J. IL-12 and IL-23: Master Regulators of Innate and Adaptive Immunity. Immunol. Rev. 2004, 202, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A. New IL-12-Family Members: IL-23 and IL-27, Cytokines with Divergent Functions. Nat. Rev. Immunol. 2005, 5, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Yoshimoto, T.; Tsutsui, H.; Okamura, H. Interleukin-18 Is a Unique Cytokine That Stimulates Both Th1 and Th2 Responses Depending on Its Cytokine Milieu. Cytokine Growth Factor Rev. 2001, 12, 53–72. [Google Scholar] [CrossRef]
- Keyel, P.A. How Is Inflammation Initiated? Individual Influences of IL-1, IL-18 and HMGB1. Cytokine 2014, 69, 136–145. [Google Scholar] [CrossRef]
- Kaplanski, G. Interleukin-18: Biological Properties and Role in Disease Pathogenesis. Immunol. Rev. 2018, 281, 138–153. [Google Scholar] [CrossRef]
- Peluso, I.; Pallone, F.; Monteleone, G. Interleukin-12 and Th1 Immune Response in Crohn’s Disease: Pathogenetic Relevance and Therapeutic Implication. World J. Gastroenterol. 2006, 12, 5606–5610. [Google Scholar] [CrossRef]
- Monteleone, G.; Trapasso, F.; Parrello, T.; Biancone, L.; Stella, A.; Iuliano, R.; Luzza, F.; Fusco, A.; Pallone, F. Bioactive IL-18 Expression Is up-Regulated in Crohn’s Disease. J. Immunol. 1999, 163, 143–147. [Google Scholar] [CrossRef]
- Okazawa, A.; Kanai, T.; Watanabe, M.; Yamazaki, M.; Inoue, N.; Ikeda, M.; Kurimoto, M.; Ishii, H.; Hibi, T. Th1-Mediated Intestinal Inflammation in Crohn’s Disease May Be Induced by Activation of Lamina Propria Lymphocytes through Synergistic Stimulation of Interleukin-12 and Interleukin-18 without T Cell Receptor Engagement. Am. J. Gastroenterol. 2002, 97, 3108–3117. [Google Scholar] [CrossRef]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The Interleukin-1 Family: Back to the Future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef]
- Muro, M.; Mrowiec, A. Interleukin (IL)-1 Gene Cluster in Inflammatory Bowel Disease: Is IL-1RA Implicated in the Disease Onset and Outcome? Dig. Dis. Sci. 2015, 60, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Wan, J.; Zhang, L.; Luo, B.; Liu, P.; Di, A.; Gao, H.; Sun, X.; Zhao, G. Astragaloside II Alleviates the Symptoms of Experimental Ulcerative Colitis in Vitro and in Vivo. Am. J. Transl. Res. 2019, 11, 7074–7083. [Google Scholar]
- Mohammad, N.S.; Nazli, R.; Zafar, H.; Fatima, S. Effects of Lipid Based Multiple Micronutrients Supplement on the Birth Outcome of Underweight Pre-Eclamptic Women: A Randomized Clinical Trial. Pak. J. Med. Sci. 2022, 38, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Shi, H.; Wang, Y.; Yang, F.; Zhang, Y.; Feng, H.; Chen, J.; Wang, X. Pre-Protection and Mechanism of Crude Extracts from Dioscorea Alata L. on H2O2-Induced IPEC-J2 Cells Oxidative Damage. Animals 2023, 13, 1401. [Google Scholar] [CrossRef] [PubMed]
- Razzuoli, E.; Amadori, M.; Lazzara, F.; Bilato, D.; Ferraris, M.; Vito, G.; Ferrari, A. Salmonella Serovar-Specific Interaction with Jejun. Vet. Microbiol. 2017, 207, 219–225. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-[Delta][Delta]CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Ciucis, C.G.; Fruscione, F.; De Paolis, L.; Mecocci, S.; Zinellu, S.; Guardone, L.; Franzoni, G.; Cappelli, K.; Razzuoli, E. Toll-like Receptors and Cytokine Modulation by Goat Milk Extracellular Vesicles in a Model of Intestinal Inflammation. Int. J. Mol. Sci. 2023, 24, 11096. https://doi.org/10.3390/ijms241311096
De Ciucis CG, Fruscione F, De Paolis L, Mecocci S, Zinellu S, Guardone L, Franzoni G, Cappelli K, Razzuoli E. Toll-like Receptors and Cytokine Modulation by Goat Milk Extracellular Vesicles in a Model of Intestinal Inflammation. International Journal of Molecular Sciences. 2023; 24(13):11096. https://doi.org/10.3390/ijms241311096
Chicago/Turabian StyleDe Ciucis, Chiara Grazia, Floriana Fruscione, Livia De Paolis, Samanta Mecocci, Susanna Zinellu, Lisa Guardone, Giulia Franzoni, Katia Cappelli, and Elisabetta Razzuoli. 2023. "Toll-like Receptors and Cytokine Modulation by Goat Milk Extracellular Vesicles in a Model of Intestinal Inflammation" International Journal of Molecular Sciences 24, no. 13: 11096. https://doi.org/10.3390/ijms241311096
APA StyleDe Ciucis, C. G., Fruscione, F., De Paolis, L., Mecocci, S., Zinellu, S., Guardone, L., Franzoni, G., Cappelli, K., & Razzuoli, E. (2023). Toll-like Receptors and Cytokine Modulation by Goat Milk Extracellular Vesicles in a Model of Intestinal Inflammation. International Journal of Molecular Sciences, 24(13), 11096. https://doi.org/10.3390/ijms241311096








