Molecular Characterization and Marker Development of the HMW-GS Gene from Thinopyrum elongatum for Improving Wheat Quality
Abstract
1. Introduction
2. Results
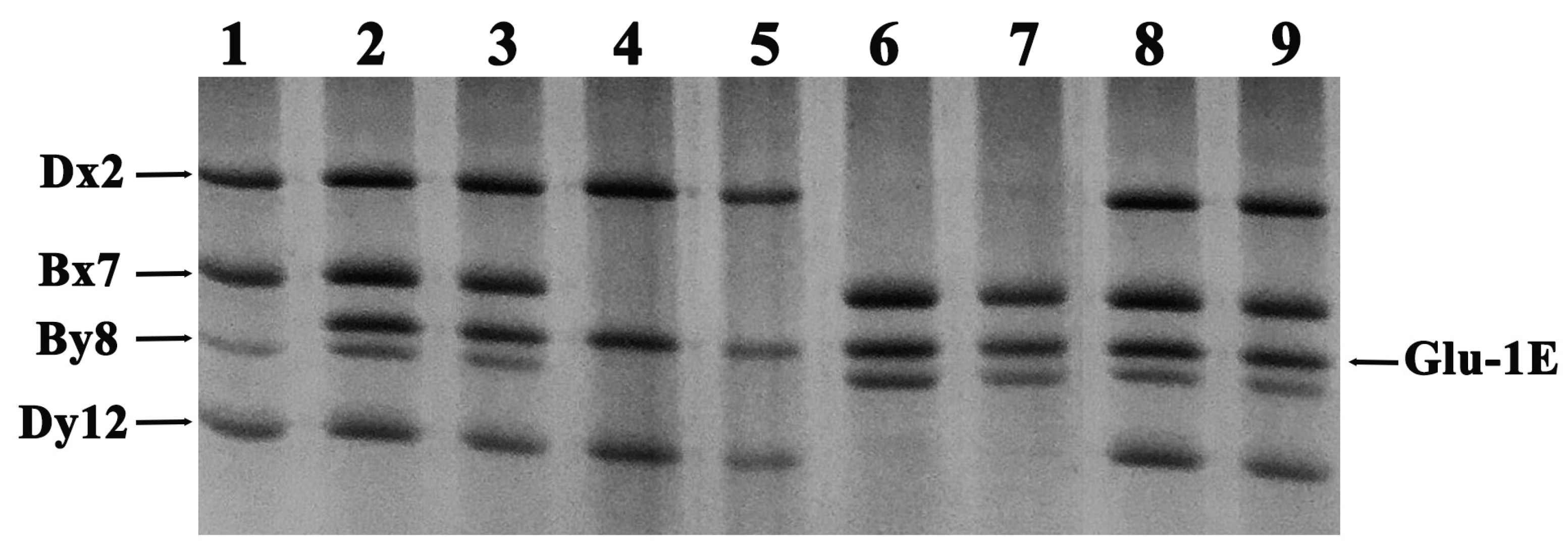
2.1. Analysis of the Relative Mobility of Th. elongatum HMW-GS
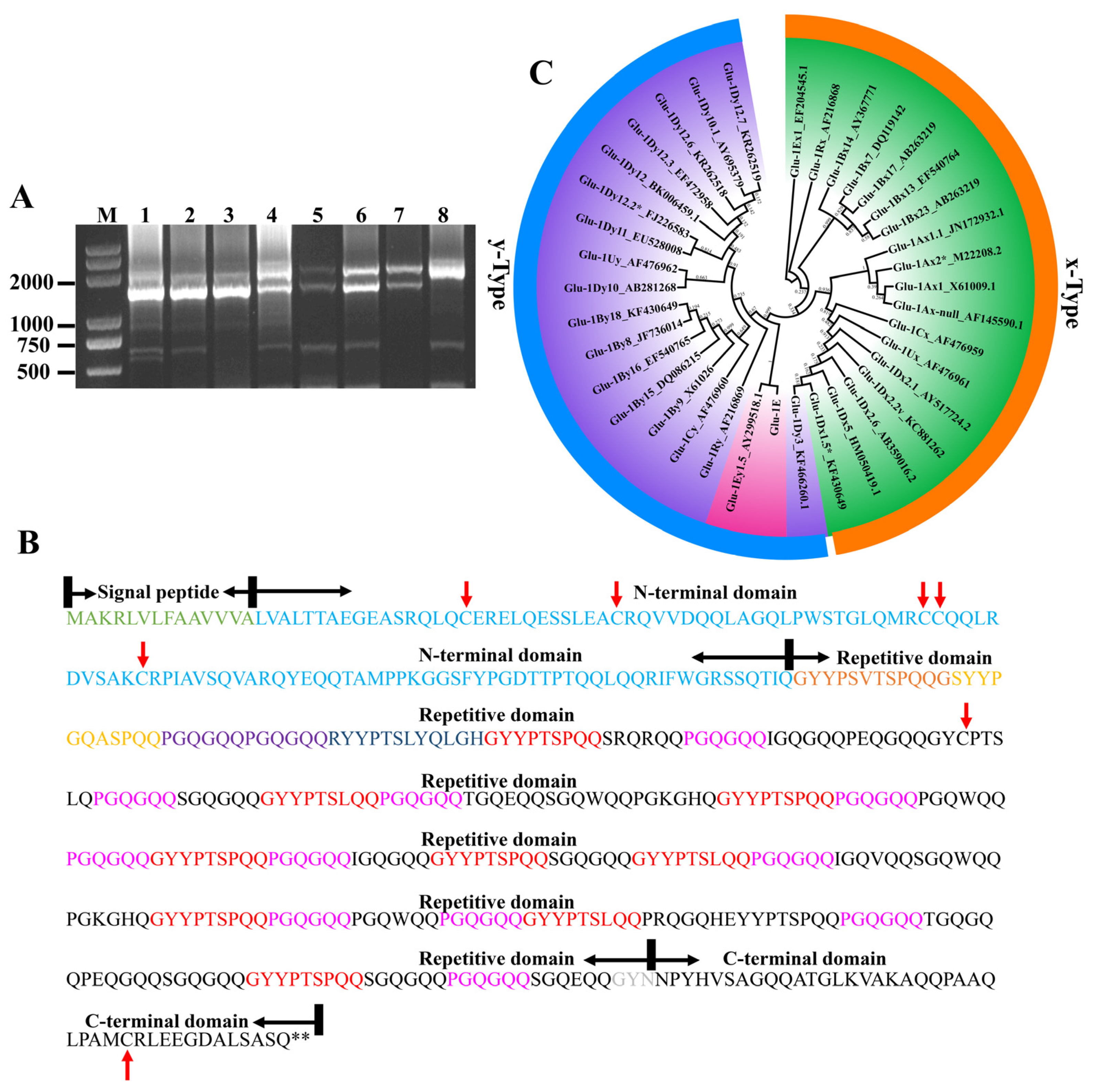
2.2. Cloning and Analysis of the Th. elongatum HMW-GS Gene
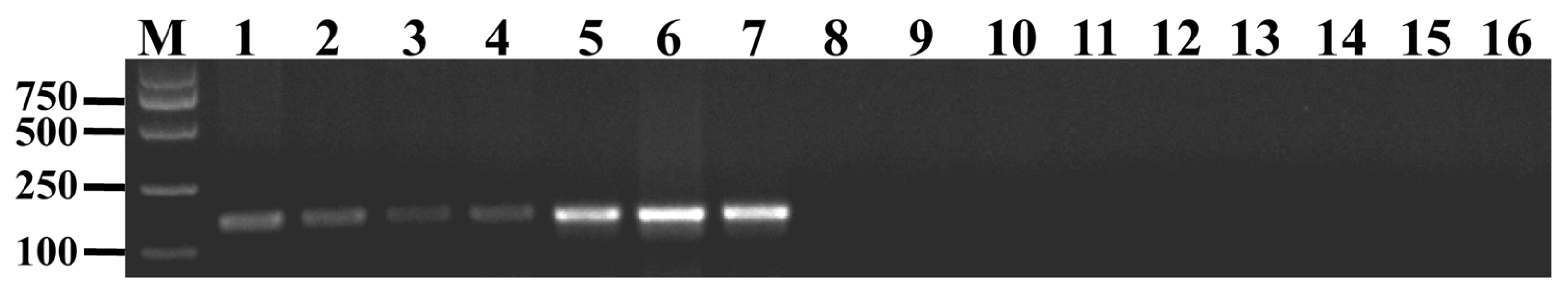
2.3. Development of a Molecular Marker for Th. elongatum HMW Glu-1Ey
2.4. Influence of Th. elongatum 1E Chromosome on Grain Quality
2.5. Effect of Th. elongatum Chromosome 1E on Flour Processing Quality
2.6. Effect of Th. elongatum Chromosome 1E on Solvent Retention Capacity (SRC)
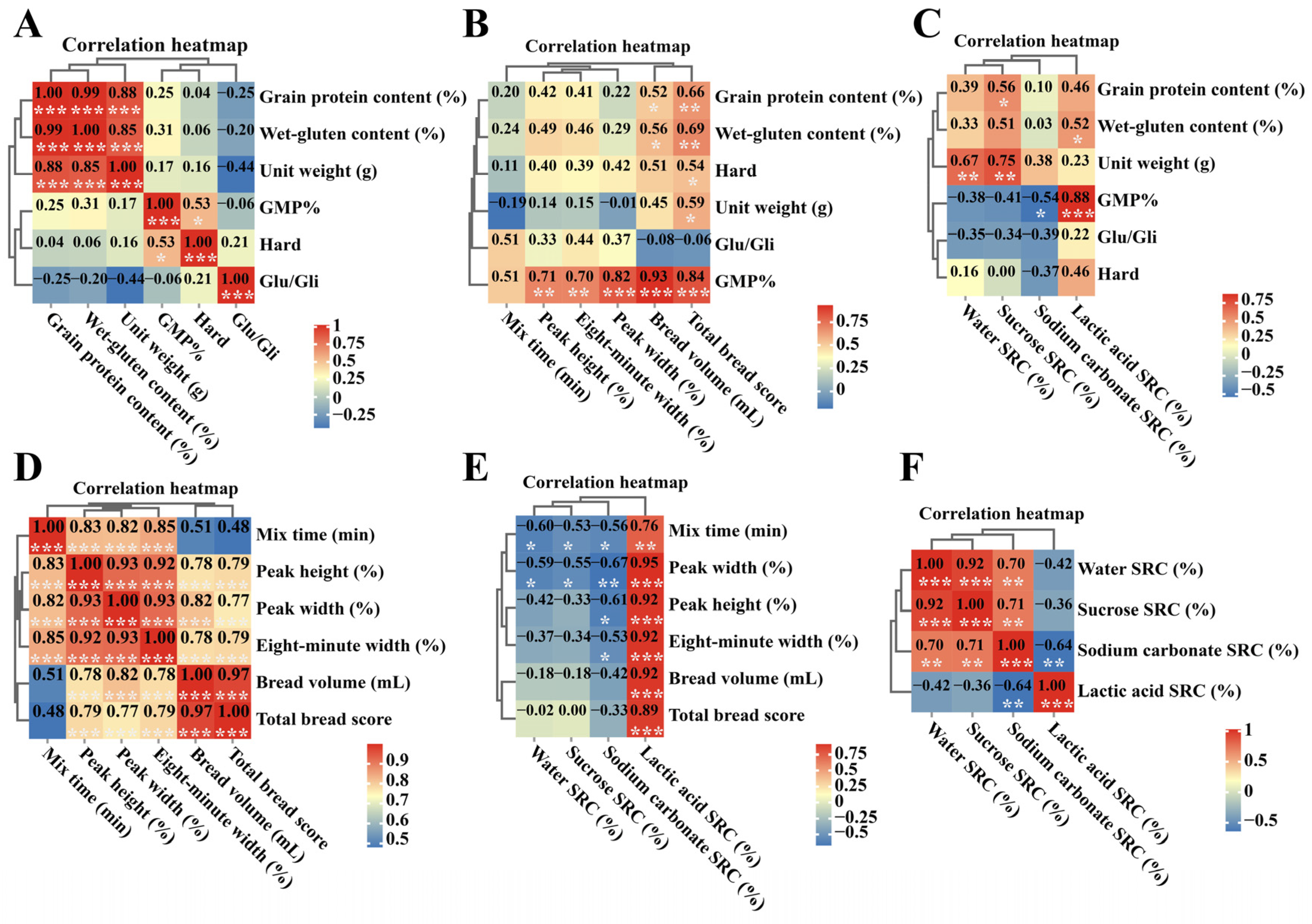
2.7. Correlational Analysis of Quality Traits of CS-Th. elongatum Chromosome 1E Substitution and Addition Lines
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Protein Extraction from Grain and SDS-PAGE
4.3. Determination of Wheat Quality
4.4. DNA Isolation and PCR Amplification
4.5. Phylogenetic Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biesiekierski, J. What is gluten? J. Gastroenterol. Hepatol. 2017, 32, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zheng, Q. Dynamic rheological properties of wheat flour dough and proteins. Trends Food Sci. Technol. 2007, 18, 132–138. [Google Scholar] [CrossRef]
- Rasheed, A.; Xia, X.; Yan, Y.; Appels, R.; Mahmood, T.; He, Z. Wheat seed storage proteins: Advances in molecular genetics, diversity and breeding applications. J. Cereal Sci. 2014, 60, 11–24. [Google Scholar] [CrossRef]
- Liu, T.; Gao, X.; Li, L.; Du, D.; Cheng, X.; Zhao, Y.; Liu, Y.; Li, X. Effects of HMW-GS at Glu-B1 locus on the polymerization of glutenin during grain development and on the secondary and micro-structures of gluten in wheat (Triticum aestivum L.). J. Cereal Sci. 2016, 72, 101–107. [Google Scholar] [CrossRef]
- Gao, X.; Liu, T.; Ding, M.; Wang, J.; Li, C.; Wang, Z.; Li, X. Effects of HMW-GS Ax1 or Dx2 absence on the glutenin polymerization and gluten micro structure of wheat (Triticum aestivum L.). Food Chem. 2018, 240, 626–633. [Google Scholar] [CrossRef]
- Lawrence, G.J.; Shepherd, K.W. Chromosomal location of genes controlling seed proteins in species related to wheat. Theor. Appl. Genet. 1981, 59, 25–31. [Google Scholar] [CrossRef]
- Payne, P.I.; Holt, L.M.; Law, C.N. Structural and genetical studies on the high-molecular-weight subunits of wheat glutenin: Part 1: Allelic variation in subunits amongst varieties of wheat (Triticum aestivum). Theor. Appl. Genet. 1981, 60, 229–236. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G.; Tatham, A.S. High molecular weight subunits of wheat glutenin. J. Cereal Sci. 1992, 15, 105–120. [Google Scholar] [CrossRef]
- Redaelli, R.; Pogna, N.E.; Ng, P.K.W.; Wang, C.; Wang, K.; Liu, Y.; Zhou, J.; Hu, Y.; Zeller, F.J.; Yan, Y.; et al. Effects of Prolamins Encoded by Chromosomes 1B and 1D on the Rheological Properties of Dough in Near-Isogenic Lines of Bread Wheat. Cereal Chem. 1997, 74, 102–107. [Google Scholar] [CrossRef]
- Liu, L.; He, Z.; Yan, J.; Zhang, Y.; Xia, X.; Peña, R.J. Allelic variation at the Glu-1 and Glu-3 loci, presence of the 1B.1R translocation, and their effects on mixographic properties in Chinese bread wheats. Euphytica 2005, 142, 197–204. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, Y.; An, X.; Pei, Y.; Li, X.; Zhang, Y.; Wang, A.; He, Z.; Xia, X.; Bekes, F.; et al. Cloning, expression and functional analysis of HMW glutenin subunit 1By8 gene from Italy pasta wheat (Triticum turgidum L. ssp. durum). J. Cereal Sci. 2009, 50, 398–406. [Google Scholar] [CrossRef]
- Guo, H.; Wu, J.; Lu, Y.; Yan, Y. High-Molecular-Weight Glutenin 1Bx17 and 1By18 Subunits Encoded by Glu-B1i Enhance Rheological Properties and Breadmaking Quality of Wheat Dough. J. Food Qual. 2019, 2019, 1958747. [Google Scholar] [CrossRef]
- Jiang, P.; Xue, J.; Duan, L.; Gu, Y.; Mu, J.; Han, S.; Chen, L.; Li, Y.; Ma, W.; Yan, Y.; et al. Effects of high-molecular-weight glutenin subunit combination in common wheat on the quality of crumb structure. J. Sci. Food Agric. 2018, 99, 1501–1508. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, D.; Shewry, P.; Halford, N. Isolation and characterization of five novel high molecular weight subunit of glutenin genes from Triticum timopheevi and Aegilops cylindrica. Theor. Appl. Genet. 2002, 104, 828–839. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, Z.; Wan, Y.; Liu, K.; Zheng, Y.; Wang, D. Analysis of HMW glutenin subunits and their coding sequences in two diploid Aegilops species. Theor. Appl. Genet. 2003, 106, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, X.; Zhang, H.; Cao, S.; Wang, D.; Hao, S.; Li, L.; Li, H.; Wang, X. Isolation and characterization of a novel variant of HMW glutenin subunit gene from the St genome of Pseudoroegneria stipifolia. J. Cereal Sci. 2008, 47, 429–437. [Google Scholar] [CrossRef]
- Roy, N.; Islam, S.; Al-Habbar, Z.; Yu, Z.; Liu, H.; Lafiandra, D.; Masci, S.; Lu, M.; Sultana, N.; Ma, W. Contribution to Breadmaking Performance of Two Different HMW Glutenin 1Ay Alleles Expressed in Hexaploid Wheat. J. Agric. Food Chem. 2020, 69, 36–44. [Google Scholar] [CrossRef]
- Wang, K.; An, X.L.; Pan, L.P.; Dong, K.; Gao, L.Y.; Wang, S.L.; Xie, Z.Z.; Zhang, Z.; Appels, R.; Ma, W.; et al. Molecular characterization of HMW-GS 1Dx3t and 1Dx4t genes from Aegilops tauschii and their potential value for wheat quality improvement. Hereditas 2012, 149, 41–49. [Google Scholar] [CrossRef]
- Borrajo, C.I.; Sánchez-Moreiras, A.M.; Reigosa, M.J. Ecophysiological Responses of Tall Wheatgrass Germplasm to Drought and Salinity. Plants 2022, 11, 1548. [Google Scholar] [CrossRef]
- Zeng, J.; Zhou, C.; He, Z.; Wang, Y.; Xu, L.; Chen, G.; Zhu, W.; Zhou, Y.; Kang, H. Disomic Substitution of 3D Chromosome with Its Homoeologue 3E in Tetraploid Thinopyrum elongatum Enhances Wheat Seedlings Tolerance to Salt Stress. Int. J. Mol. Sci. 2023, 24, 1609. [Google Scholar] [CrossRef]
- Wang, H.; Sun, S.; Ge, W.; Zhao, L.; Hou, B.; Wang, K.; Lyu, Z.; Chen, L.; Xu, S.; Guo, J.; et al. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, eaba5435. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Conner, R.L.; Ahmad, F.; Laroche, A.; Fedak, G.; Thomas, J.B. Molecular characterization of the genome composition of partial amphiploids derived from Triticum aestivum × Thinopyrum ponticum and T. aestivum × T. intermedium as sources of resistance to wheat streak mosaic virus and its vector, Aceria tosichella. Theor. Appl. Genet. 1998, 97, 1–8. [Google Scholar] [CrossRef]
- Chen, Q.; Conner, R.; Laroche, A.; Thomas, J. Genome analysis of Thinopyrum intermedium and Thinopyrum ponticum using genomic in situ hybridization. Genome 1998, 41, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Sepsi, A.; Molnár, I.; Szalay, D.; Molnár-Láng, M. Characterization of a leaf rust-resistant wheat—Thinopyrum ponticum partial amphiploid BE-1, using sequential multicolor GISH and FISH. Theor. Appl. Genet. 2008, 116, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, S.W.; Sun, Q.; Xia, G.M. High frequency of HMW-GS sequence variation through somatic hybridization between Agropyron elongatum and common wheat. Planta 2010, 231, 245–250. [Google Scholar] [CrossRef]
- Revathi, P.; Medoju, P.; Singh, A.K.; Sundaram, R.M.; Raju, S.; Senguttuvel, P.; Kemparaju, K.B.; Hariprasad, A.S.; Ramesha, M.S.; Neeraja, C.N.; et al. Efficiency of molecular markers in identifying fertility restoration trait of WA-CMS system in rice. Indian J. Genet. Plant Breed. 2013, 73, 89. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Huang, J.-Z.; Liu, Q.-L.; Nawaz, Z.; Lu, H.-P.; Gong, J.-Y.; Zhu, Y.-J.; Yan, W.; Shu, Q.-Y. Characterization of an RNase Z nonsense mutation identified exclusively in environment-conditioned genic male sterile rice. Mol. Breed. 2014, 34, 481–489. [Google Scholar] [CrossRef]
- Song, F.; Ni, J.; Qian, Y.; Li, L.; Ni, D.; Yang, J. Development of SNP-based dCAPS markers for identifying male sterile gene tms5 in two-line hybrid rice. Genet. Mol. Res. 2016, 15, 15038512. [Google Scholar] [CrossRef]
- Bustos, A.D.; Rubio, P.; Jouve, N. Molecular characterization of the inactive allele of the gene Glu-A1 and the development of a set of AS-PCR markers for HMW glutenins of wheat. Theor. Appl. Genet. 2000, 100, 1085–1094. [Google Scholar] [CrossRef]
- Lei, Z.S.; Gale, K.R.; He, Z.H.; Gianibelli, M.C.; Larroque, O.; Butow, B.J. Y-type gene specific markers for enhanced discrimination of high-molecular weight glutenin alleles at the Glu-B1 locus in hexaploidy wheat. J. Cereal Sci. 2006, 43, 94–101. [Google Scholar] [CrossRef]
- Zhao, X.L.; Xia, X.C.; He, Z.H.; Lei, Z.S.; Appels, R.; Yang, Y.; Sun, Q.X.; Ma, W. Novel DNA variations to characterize low molecular weight glutenin Glu-D3 genes and develop STS markers in common wheat. Theor. Appl. Genet. 2007, 114, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.P.; Struss, D.; Kazman, M.E. Rapid screening of selected European winter wheat varieties and segregating population for the Glu-D1d allele using PCR marker. Plant Breed. 1996, 115, 451–454. [Google Scholar] [CrossRef]
- Halford, N.G.; Field, J.M.; Blair, H.; Urwin, P.; Moore, K.; Robert, L.; Thompson, R.; Flavell, R.B.; Tatham, A.; Shewry, P.R. Analysis of HMW glutenin subunits encoded by chromosome 1A of bread wheat (Triticum aestivum L.) indicates quantitative effects on grain quality. Theor. Appl. Genet. 1992, 83, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Flavell, R.B.; Goldsbrough, A.P.; Robert, L.S.; Schnick, D.; Thompson, R.D. Genetic Variation in Wheat HMW Glutenin Subunits and the Molecular Basis of Bread-Making Quality. Nat. Biotechnol. 1989, 7, 1281–1285. [Google Scholar] [CrossRef]
- Li, W.; Wan, Y.; Liu, Z.; Liu, K.; Liu, X.; Li, B.; Zhang, X.; Dong, Y.; Wang, D. Molecular characterization of HMW glutenin subunit allele 1Bx14: Further insights into the evolution of Glu-B1-1 alleles in wheat and related species. Theor. Appl. Genet. 2004, 109, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, J.; Shen, Q.; Yang, D. High-Molecular-Weight Glutenin Subunits: Genetics, Structures, and Relation to End Use Qualities. Int. J. Mol. Sci. 2021, 22, 184. [Google Scholar] [CrossRef]
- Bagge, M.; Xia, X.; Lübberstedt, T. Functional markers in wheat. Curr. Opin. Plant Biol. 2007, 10, 211–216. [Google Scholar] [CrossRef]
- Liu, C.; Jun, J.H.; Dixon, R.A. MYB5 and MYB14 Play Pivotal Roles in Seed Coat Polymer Biosynthesis in Medicago truncatula. Plant Physiol. 2014, 165, 1424–1439. [Google Scholar] [CrossRef]
- Azmach, G.; Gedil, M.; Menkir, A.; Spillane, C. Marker-trait association analysis of functional gene markers for provitamin A levels across diverse tropical yellow maize inbred lines. BMC Plant Biol. 2013, 13, 227. [Google Scholar] [CrossRef]
- Wei, J.X.; Geng, H.W.; Zhang, Y.; Liu, J.D.; Wen, W.; Zhang, Y.; Xia, X.C.; Chen, X.M.; He, Z.H. Mapping quantitative trait loci for peroxidase activity and developing gene-specific markers for TaPod-A1 on wheat chromosome 3AL. Theor. Appl. Genet. 2015, 128, 2067–2076. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, J.; Mao, X.; Li, A.; Jia, J.; Jing, R. Polymorphism of TaSAP1-A1 and its association with agronomic traits in wheat. Planta 2013, 237, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
- Uthayakumaran, S.; Lukow, O.M.; Jordan, M.C.; Cloutier, S. Development of genetically modified wheat to assess its dough functional properties. Mol. Breed. 2003, 11, 249–258. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.-Q.; Wu, H.-Y.; Lu, C.-B.; Lü, G.-F.; Liu, D.-T.; Li, M.; Jiang, W.; Song, G.-H.; Gao, D.-R. Effect of high-molecular-weight glutenin subunit deletion on soft wheat quality properties and sugar-snap cookie quality estimated through near-isogenic lines. J. Integr. Agric. 2018, 17, 1066–1073. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Q.; Su, M.; Yan, B.; Zhang, X.; Jiao, Z. High-molecular-weight glutenin subunit-deficient mutants induced by ion beam and the effects of Glu-1 loci deletion on wheat quality properties. J. Sci. Food Agric. 2015, 96, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- De Bustos, A.; Rubio, P.; Jouve, N. Characterisation of two gene subunits on the 1R chromosome of rye as orthologs of each of the Glu-1 genes of hexaploid wheat. Theor. Appl. Genet. 2001, 103, 733–742. [Google Scholar] [CrossRef]
- Kumar, A.; Kapoor, P.; Chunduri, V.; Sharma, S.; Garg, M. Potential of Aegilops sp. for Improvement of Grain Processing and Nutritional Quality in Wheat (Triticum aestivum). Front. Plant Sci. 2019, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Delorean, E.; Gao, L.; Lopez, J.F.C.; Mehrabi, A.; Bentley, A.; Sharon, A.; Keller, B.; Wulff, B.B.H.; Steffenson, B.; Steuernagel, B.; et al. High molecular weight glutenin gene diversity in Aegilops tauschii demonstrates unique origin of superior wheat quality. Commun. Biol. 2021, 4, 1242. [Google Scholar] [CrossRef]
- Guo, L.; Yu, L.; Tong, J.; Zhao, Y.; Yang, Y.; Ma, Y.; Cui, L.; Hu, Y.; Wang, Z.; Gao, X. Addition of Aegilops geniculata 1Ug chromosome improves the dough rheological properties by changing the composition and micro-structure of gluten. Food Chem. 2021, 358, 129850. [Google Scholar] [CrossRef]
- Hou, W.; Feng, W.; Yu, G.; Du, X.; Ren, M. Cloning and functional analysis of a novel x-type high-molecular-weight glutenin subunit with altered cysteine residues from Aegilops umbellulata. Crop. Pasture Sci. 2017, 68, 409–414. [Google Scholar] [CrossRef]
- Du, X.; Jia, Z.; Yu, Y.; Wang, S.; Che, B.; Ni, F.; Bao, Y. A wheat-Aegilops umbellulata addition line improves wheat agronomic traits and processing quality. Breed. Sci. 2019, 69, 503–507. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, C.; Yang, E.; Yin, M.; Liu, C.; Ren, Z. Characterization of a new wheat-Aegilops biuncialis addition line conferring quality-associated HMW glutenin subunits. Genet. Mol. Res. 2014, 13, 660–669. [Google Scholar] [CrossRef]
- Singh, J.; Sheikh, I.; Sharma, P.; Kumar, S.; Verma, S.K.; Kumar, R.; Mathpal, P.; Kumar, S.; Vyas, P.; Dhaliwal, H.S. Transfer of HMW glutenin subunits from Aegilops kotschyi to wheat through radiation hybridization. J. Food Sci. Technol. 2016, 53, 3543–3549. [Google Scholar] [CrossRef]
- Wen, M.; Feng, Y.; Chen, J.; Bie, T.; Fang, Y.; Li, D.; Wen, X.; Chen, A.; Cai, J.; Zhang, R. Characterization of a Triticum aestivum–Dasypyrum villosum T1VS·6BL translocation line and its effect on wheat quality. Braz. J. Bot. 2016, 40, 371–377. [Google Scholar] [CrossRef]
- Garg, M.; Tanaka, H.; Ishikawa, N.; Takata, K.; Yanaka, M.; Tsujimoto, H. Agropyron elongatum HMW-glutenins have a potential to improve wheat end-product quality through targeted chromosome introgression. J. Cereal Sci. 2009, 50, 358–363. [Google Scholar] [CrossRef]
- Liu, D.T.; Zhang, X.; Jiang, W.; Li, M.; Wu, X.J.; Gao, D.R.; Bie, T.D.; Lu, C.B. Influence of high-molecular-weight glutenin subunit deletion at Glu-A1 and Glu-D1 loci on protein body development, protein components and dough properties of wheat (Triticum aestivum L.). J. Integr. Agric. 2021, 20, 2–11. [Google Scholar] [CrossRef]
- Echeverry-Solarte, M.; Kumar, A.; Kianian, S.; Simsek, S.; Alamri, M.S.; Mantovani, E.E.; McClean, P.E.; Deckard, E.L.; Elias, E.; Schatz, B.; et al. New QTL alleles for quality-related traits in spring wheat revealed by RIL population derived from supernumerary × non-supernumerary spikelet genotypes. Theor. Appl. Genet. 2015, 128, 893–912. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
| Wheat Variety | Abbreviation | Subunit Composition |
|---|---|---|
| Neimai 1505 | NM 1505 | Ax1, Dx5 + Dy10, Bx13 + By16, |
| Taimai 1918 | TM 1918 | N, Dx5 + Dy10, Bx17 + By18 |
| Yumai 9 | YM 9 | Ax2*, Dx5 + Dy10, Bx7 + By8 |
| Yumai 13 | YM 13 | Ax2*, Dx2 + Dy12, Bx7 + By8 |
| Yumai 18 | YM 18 | Ax2*, Dx5 + Dy10, Bx7 + By9 |
| Zhenmai 12 | ZM 12 | Dx5 + Dy10, Bx7 + By9 |
| Shannong 23 | SN 23 | Ax1, Dx5 + Dy10, Bx6 + By8 |
| Fielder | Fielder | Dx2 + Dy12, Bx14 + By15 |
| Chinese Spring | CS | Dx2 + Dy12, Bx7 + By8 |
| Materials | Grain Protein Content (%) | Wet-Gluten Content (%) | GMP (%) | Glu/Gli | Hard | Unit Weight (g) |
|---|---|---|---|---|---|---|
| DS1E/1A | 17.92 ± 0.36 b | 34.51 ± 0.51 a | 49.23 ± 1.88 a | 1.23 ± 0.23 a | 47.83 ± 0.15 b | 925.54 ± 1.32 b |
| DS1E/1B | 17.16 ± 0.17 c | 32.96 ± 0.85 b | 45.94 ± 0.96 b | 0.90 ± 0.02 c | 44.37 ± 0.12 e | 928.84 ± 2.64 b |
| DS1E/1D | 18.37 ± 0.20 a | 34.56 ± 0.41 a | 32.41 ± 0.73 d | 0.99 ± 0.11 b | 45.33 ± 0.27 d | 940.41 ± 4.76 a |
| DA1E | 17.02 ± 0.13 c | 32.38 ± 0.70 b | 48.73 ± 0.54 a | 0.91 ± 0.06 c | 49.03 ± 0.39 a | 939.11 ± 1.73 a |
| CS | 13.26 ± 0.22 d | 25.83 ± 0.25 c | 36.70 ± 2.31 c | 1.15 ± 0.02 ab | 46.15 ± 0.21 c | 899.66 ± 4.78 c |
| Materials | Mix Time (min) | Peak Height (%) | Peak Width (%) | 8-min Width (%) | Bread Volume (mL) | Total Bread Score |
|---|---|---|---|---|---|---|
| DS1E/1A | 3.71 ± 0.05 a | 59.55 ± 1.39 a | 34.14 ± 0.64 a | 10.85 ± 1.70 a | 840.21 ± 5.17 a | 81.50 ± 0.72 a |
| DS1E/1B | 2.92 ± 0.66 b | 51.64 ± 1.96 b | 25.58 ± 1.61 b | 7.15 ± 1.29 b | 783.18 ± 3.12 c | 72.11 ± 1.26 b |
| DS1E/1D | 2.30 ± 0.13 c | 47.46 ± 0.09 c | 15.48 ± 1.52 d | 4.75 ± 0.77 cd | 627.48 ± 7.94 d | 62.36 ± 1.84 c |
| DA1E | 2.35 ± 0.16 bc | 50.88 ± 2.48 b | 24.03 ± 0.53 b | 6.65 ± 1.07 bc | 823.21 ± 7.57 b | 79.01 ± 1.41 a |
| CS | 2.55 ± 0.23 b | 47.01 ± 2.09 c | 19.89 ± 1.98 c | 4.50 ± 0.30 d | 593.71 ± 8.69 e | 52.44 ± 2.22 d |
| Materials | Water SRC (%) | Sodium Carbonate SRC (%) | Lactic Acid SRC (%) | Sucrose SRC (%) |
|---|---|---|---|---|
| DS1E/1A | 58.20 ± 0.63 b | 69.72 ± 0.15 c | 107.37 ± 1.37 a | 107.82 ± 2.10 c |
| DS1E/1B | 58.76 ± 0.50 b | 72.62 ± 0.95 ab | 88.48 ± 1.23 b | 109.63 ± 1.92 c |
| DS1E/1D | 63.24 ± 0.70 a | 73.94 ± 2.01 a | 70.29 ± 0.34 c | 131.74 ± 7.49 a |
| DA1E | 62.24 ± 0.87 a | 72.38 ± 0.21 ab | 88.74 ± 0.17 b | 120.04 ± 1.74 b |
| CS | 58.72 ± 0.32 b | 71.85 ± 0.52 b | 69.43 ± 1.73 d | 105.42 ± 1.67 c |
| Appearance (Out of 10) | Internal Structure (Out of 20) | Elasticity (Out of 20) | Taste (Out of 10) | Volume/mL (Out of 40) |
|---|---|---|---|---|
| Rounded top and upright rising, no scorched skin (8–10) | Bright white, very uniform, rectangular, very thin cell walls (17–20) | Very soft, high elasticity, recovers very quickly after pressing (17–20) | Fine taste, slightly sweet, yeasty and salty flavor, very delicate and resilient (8–10) | ≥850 (34–40) |
| Medium top and rising, slightly scorched skin (7) | Slightly creamy, uniform cells, thin cell walls (15–16) | Soft, medium elasticity, recovers after pressing (15–16) | No obvious aroma and odor, delicate and resilient (7) | 800–849 (30–33) |
| Flat top, no rising, dark skin (6) | Dark, non-uniform cells, thick cell walls (12–14) | Not soft, poor viscoelasticity, no recovery (12–14) | Rough and coarse taste, crumbs present (6) | 750–799 (24–29) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Li, J.; Shi, J.; Gao, Y.; Ma, H.; Wang, Y.; Ma, H. Molecular Characterization and Marker Development of the HMW-GS Gene from Thinopyrum elongatum for Improving Wheat Quality. Int. J. Mol. Sci. 2023, 24, 11072. https://doi.org/10.3390/ijms241311072
Dai Y, Li J, Shi J, Gao Y, Ma H, Wang Y, Ma H. Molecular Characterization and Marker Development of the HMW-GS Gene from Thinopyrum elongatum for Improving Wheat Quality. International Journal of Molecular Sciences. 2023; 24(13):11072. https://doi.org/10.3390/ijms241311072
Chicago/Turabian StyleDai, Yi, Jinfeng Li, Juntao Shi, Yujiao Gao, Haigang Ma, Yonggang Wang, and Hongxiang Ma. 2023. "Molecular Characterization and Marker Development of the HMW-GS Gene from Thinopyrum elongatum for Improving Wheat Quality" International Journal of Molecular Sciences 24, no. 13: 11072. https://doi.org/10.3390/ijms241311072
APA StyleDai, Y., Li, J., Shi, J., Gao, Y., Ma, H., Wang, Y., & Ma, H. (2023). Molecular Characterization and Marker Development of the HMW-GS Gene from Thinopyrum elongatum for Improving Wheat Quality. International Journal of Molecular Sciences, 24(13), 11072. https://doi.org/10.3390/ijms241311072






