Heterotrimeric G Protein-Mediated Signaling Is Involved in Stress-Mediated Growth Inhibition in Arabidopsis thaliana
Abstract
1. Introduction
2. Results
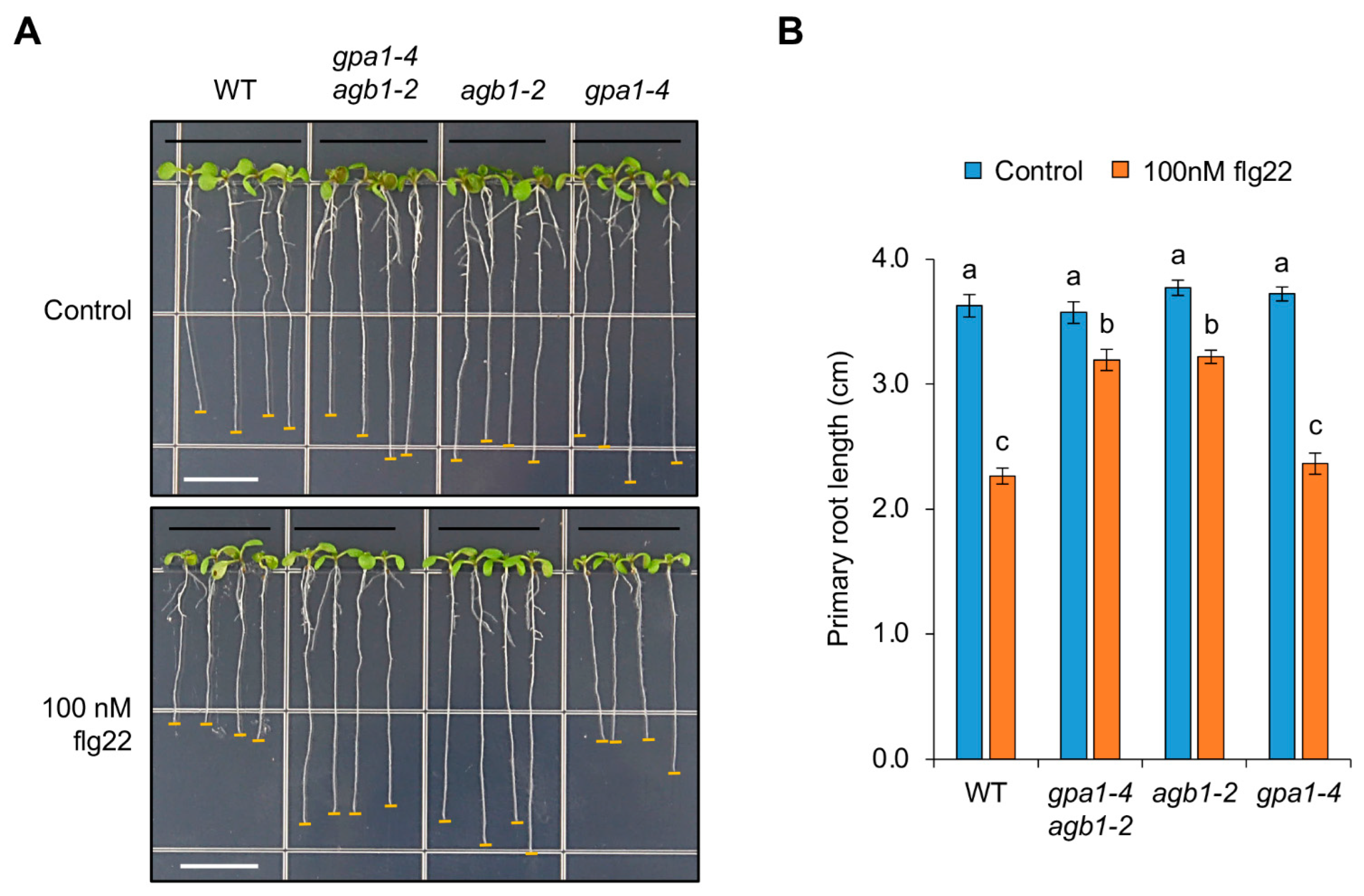
2.1. The agb1-2 Mutant Shows a More Resistant Root Growth in Response to flg22 Than WT
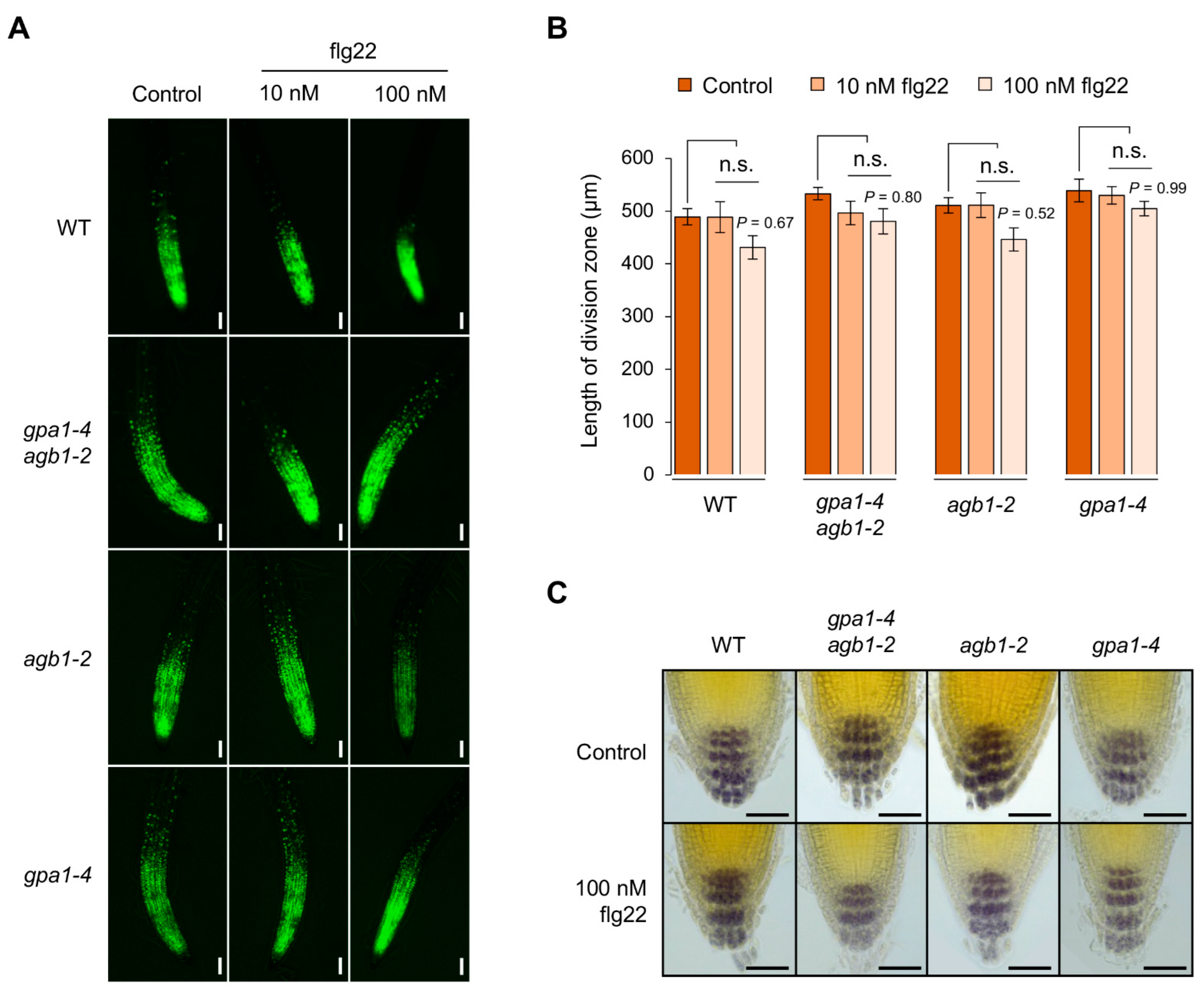
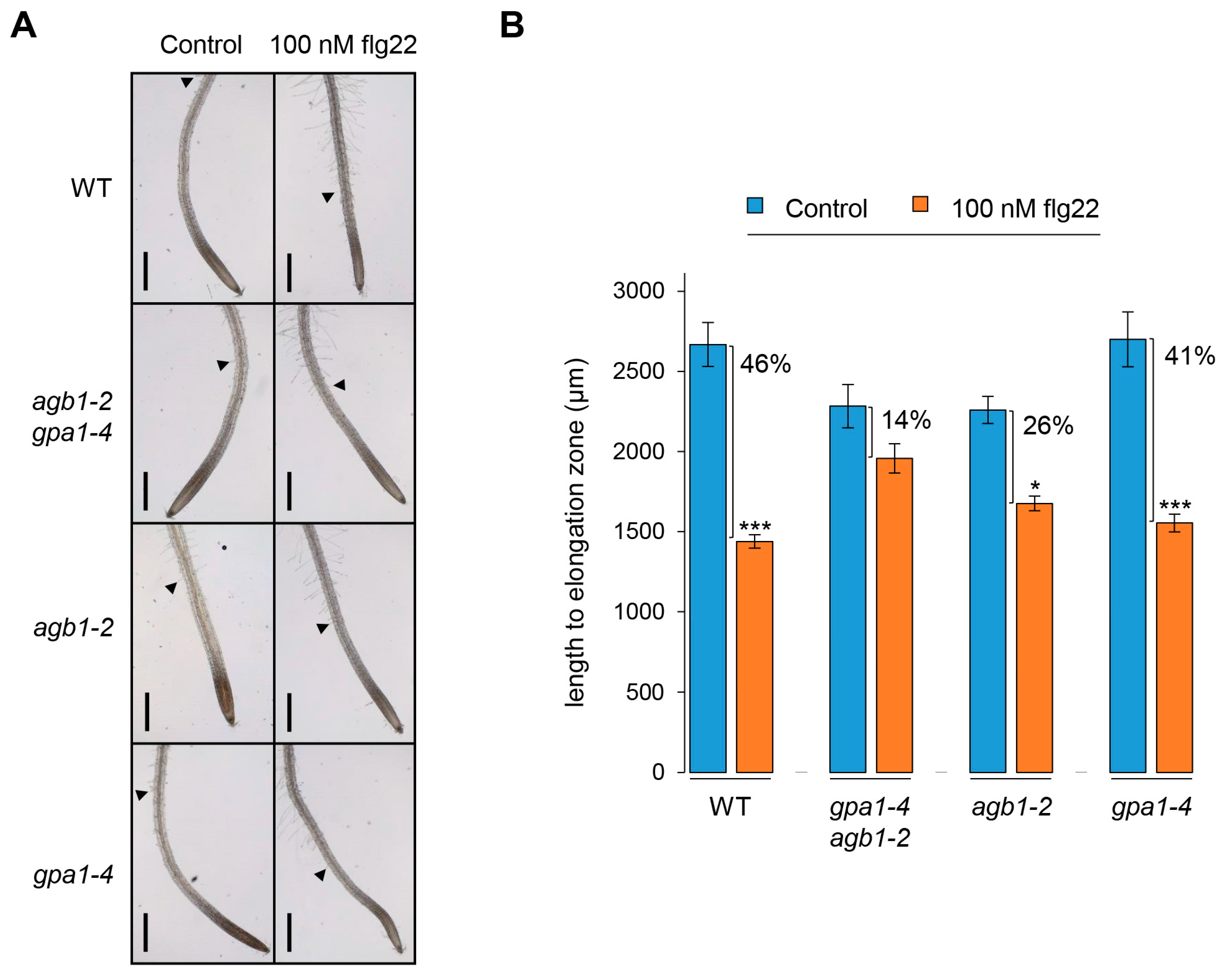
2.2. The agb1-2 Mutant Shows a Less Sensitive Defect in the Elongation Zone in Response to flg22
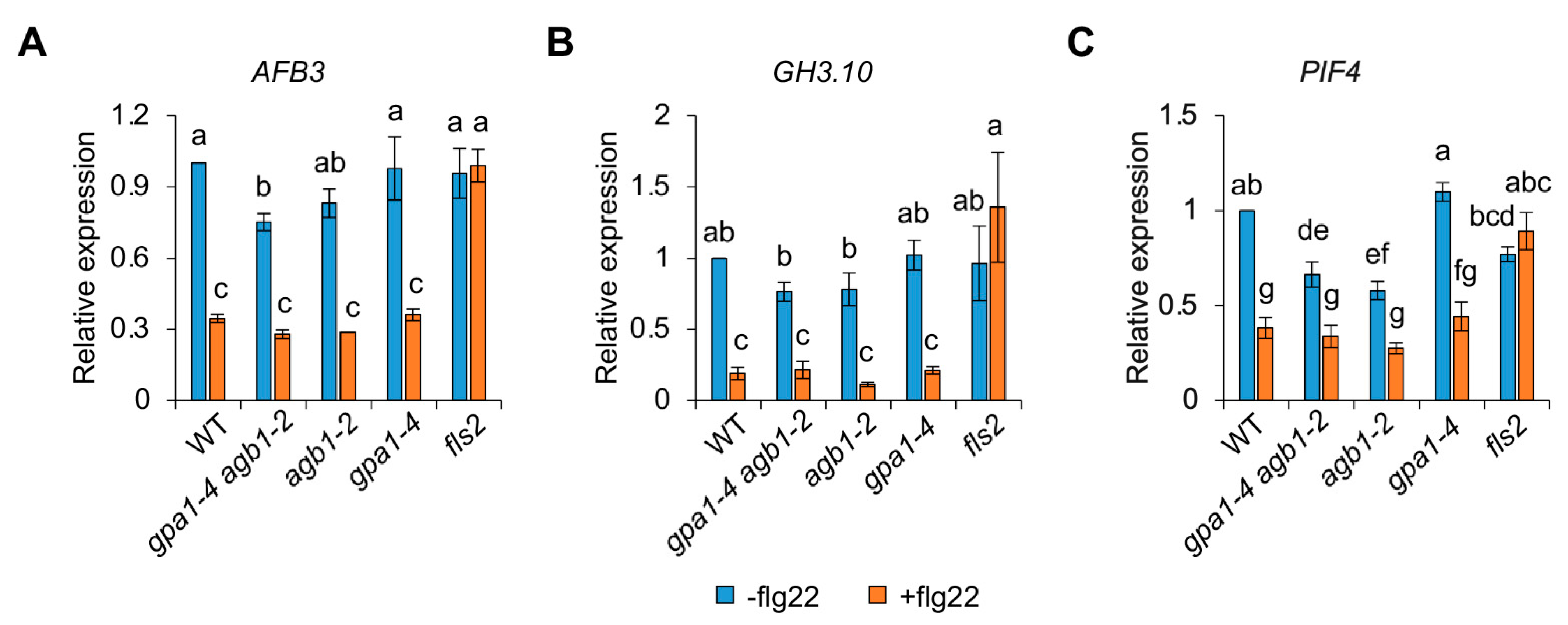
2.3. The Response of PIF4 Expression in the agb1-2 Mutant Is Less Sensitive to flg22
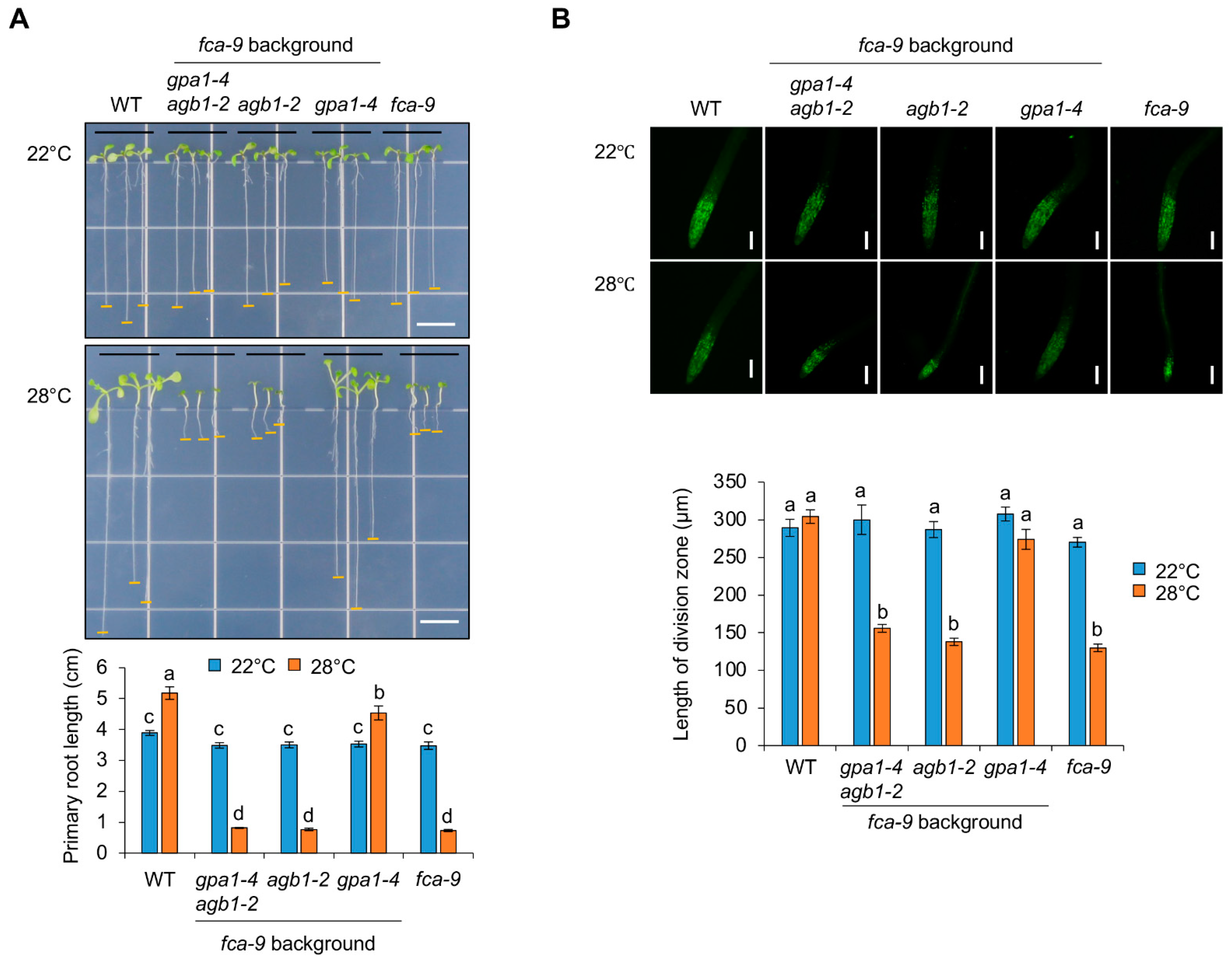
2.4. The Role of G Protein Signaling Is Involved in FCA-Mediated Thermomorphogenesis under Heat Stress Conditions
3. Discussion
3.1. The Diverse Roles of G Protein Signaling in Plant Immunity
3.2. The Diverse Roles of G Protein Signaling in Plant Growth
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Root Growth Assay
4.3. Protein Extraction and Immunoblot Assay
4.4. Gene Expression Assay
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urano, D.; Jones, A.M. Heterotrimeric G protein-coupled signaling in plants. Annu. Rev. Plant Biol. 2014, 65, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Wootten, D.; Christopoulos, A.; Marti-Solano, M.; Babu, M.M.; Sexton, P.M. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 2018, 19, 638–653. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A. G protein-coupled receptors that influence lifespan of human and animal models. Biogerontology 2022, 23, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Pandey, S.; Assmann, S.M. Arabidopsis extra-large G proteins (XLGs) regulate root morphogenesis. Plant J. 2008, 53, 248–263. [Google Scholar] [CrossRef]
- Weiss, C.A.; Garnaat, C.W.; Mukai, K.; Hu, Y.; Ma, H. Isolation of cDNAs encoding guanine nucleotide-binding protein beta-subunit homologues from maize (ZGB1) and Arabidopsis (AGB1). Proc. Natl. Acad. Sci. USA 1994, 91, 9554–9558. [Google Scholar] [CrossRef]
- Mason, M.G.; Botella, J.R. Completing the heterotrimer: Isolation and characterization of an Arabidopsis thaliana G protein γ-subunit cDNA. Proc. Natl. Acad. Sci. USA 2000, 97, 14784–14788. [Google Scholar] [CrossRef]
- Mason, M.G.; Botella, J.R. Isolation of a novel G-protein γ-subunit from Arabidopsis thaliana and its interaction with Gβ. Biochim. Biophys. Acta 2001, 1520, 147–153. [Google Scholar] [CrossRef]
- Chakravorty, D.; Trusov, Y.; Zhang, W.; Acharya, B.R.; Sheahan, M.B.; McCurdy, D.W.; Assmann, S.M.; Botella, J.R. An atypical heterotrimeric G-protein g-subunit is involved in guard cell K+-channel regulation and morphological development in Arabidopsis thaliana. Plant J. 2011, 67, 840–851. [Google Scholar] [CrossRef]
- Bradford, W.; Buckholz, A.; Morton, J.; Price, C.; Jones, A.M.; Urano, D. Eukaryotic G protein Signaling Evolved to Require G Protein-Coupled Receptors for Activation. Sci. Signal. 2013, 6, ra37. [Google Scholar] [CrossRef]
- Urano, D.; Chen, J.-G.; Botella, J.R.; Jones, A.M. Heterotrimeric G protein signalling in the plant kingdom. Open Biol. 2013, 3, 120186. [Google Scholar] [CrossRef]
- Chen, J.-G.; Willard, F.S.; Huang, J.; Liang, J.; Chasse, S.A.; Jones, A.M.; Siderovski, D.P. A seven-transmembrane RGS protein that modulates plant cell proliferation. Science 2003, 301, 1728–1731. [Google Scholar] [CrossRef]
- Johnston, C.A.; Taylor, J.P.; Gao, Y.; Kimple, A.J.; Grigston, J.C.; Chen, J.-G.; Siderovski, D.P.; Jones, A.M.; Willard, F.S. GTPase acceleration as the rate-limiting step in Arabidopsis G protein-coupled sugar signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 17317–17322. [Google Scholar] [CrossRef]
- Perfus-Barbeoch, L.; Jones, A.M.; Assmann, S.M. Plant heterotrimeric G protein function: Insights from Arabidopsis and rice mutants. Curr. Opin. Plant Biol. 2004, 7, 719–731. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, Y.; Chen, L.; Wang, W.; Liu, R.; Gao, R.; Zhou, Y.; Li, H. G protein and PLDδ are involved in JA to regulate osmotic stress responses in Arabidopsis thaliana. Biochem. Biophys. Rep. 2021, 26, 100952. [Google Scholar] [CrossRef]
- Luo, M.; Hu, L.; Li, W.; Ge, L.; Qin, Y.; Zhou, Y.; Tang, W.; Wang, C.; Xu, Z.; Chen, J.; et al. Heterotrimeric G protein α subunit (GPA1) regulates the response to low-nitrogen stress in Arabidopsis by interacting with AtNRT1.4 and AtATG8a. bioRxiv 2022. [Google Scholar] [CrossRef]
- Colaneri, A.C.; Tunc-Ozdemir, M.; Huang, J.P.; Jones, A.M. Growth attenuation under saline stress is mediated by the heterotrimeric G protein complex. BMC Plant Biol. 2014, 14, 129. [Google Scholar] [CrossRef]
- Xu, D.-B.; Chen, M.; Ma, Y.-N.; Xu, Z.-S.; Li, L.-C.; Chen, Y.-F.; Ma, Y.-Z. A G-protein β subunit, AGB1, negatively regulates the ABA response and drought tolerance by down-regulating AtMPK6-related pathway in Arabidopsis. PLoS ONE 2015, 10, e0116385. [Google Scholar] [CrossRef]
- Gómez-Gómez, L.; Felix, G.; Boller, T. A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J. 1999, 18, 277–284. [Google Scholar] [CrossRef]
- Boller, T.; Felix, G. A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef]
- Hong, J.H.; Chu, H.; Zhang, C.; Ghosh, D.; Gong, X.; Xu, J. A quantitative analysis of stem cell homeostasis in the Arabidopsis columella root cap. Front. Plant Sci. 2015, 6, 206. [Google Scholar] [CrossRef]
- Petricka, J.J.; Winter, C.M.; Benfey, P.N. Control of Arabidopsis Root Development. Annu. Rev. Plant Biol. 2012, 63, 563–590. [Google Scholar] [CrossRef] [PubMed]
- Navarro, L.; Dunoyer, P.; Jay, F.; Arnold, B.; Dharmasiri, N.; Estelle, M.; Voinnet, O.; Jones, J.D.G. A Plant miRNA Contributes to Antibacterial Resistance by Repressing Auxin Signaling. Science 2006, 312, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Long, X.; Chern, M.; Chen, X. Understanding the molecular mechanisms of trade-offs between plant growth and immunity. Sci. China Life Sci. 2021, 64, 234–241. [Google Scholar] [CrossRef] [PubMed]
- de Lucas, M.; Davière, J.-M.; Rodríguez-Falcón, M.; Pontin, M.; Iglesias-Pedraz, J.M.; Lorrain, S.; Fankhauser, C.; Blázquez, M.A.; Titarenko, E.; Prat, S. A molecular framework for light and gibberellin control of cell elongation. Nature 2008, 451, 480–484. [Google Scholar] [CrossRef]
- Bai, M.-Y.; Shang, J.-X.; Oh, E.; Fan, M.; Bai, Y.; Zentella, R.; Sun, T.-P.; Wang, Z.-Y. Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat. Cell Biol. 2012, 14, 810–817. [Google Scholar] [CrossRef]
- Sun, J.; Qi, L.; Li, Y.; Chu, J.; Li, C. PIF4-Medidated Activation of YUCCA8 Expression Integrates Temperature into the Auxin Pathway in Regulating Arabidopsis Hypocotyl Growth. PLoS Genet. 2012, 8, e1002594. [Google Scholar] [CrossRef]
- Sun, J.; Qi, L.; Li, Y.; Zhai, Q.; Li, C. PIF4 and PIF5 Transcription Factors Link Blue Light and Auxin to Regulate the Phototropic Response in Arabidopsis. Plant Cell 2013, 25, 2102–2114. [Google Scholar] [CrossRef]
- Oh, E.; Zhu, J.-Y.; Bai, M.-Y.; Arenhart, R.A.; Sun, Y.; Wang, Z.-Y. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. eLife 2014, 3, e03031. [Google Scholar] [CrossRef]
- Liu, F.; Marquardt, S.; Lister, C.; Swiezewski, S.; Dean, C. Targeted 3′ processing of antisense transcripts triggers Arabidopsis FLC chromatin silencing. Science 2010, 327, 94–97. [Google Scholar] [CrossRef]
- Ha, J.-H.; Lee, H.-J.; Jung, J.-H.; Park, C.-M. Thermo-Induced Maintenance of Photo-oxidoreductases Underlies Plant Autotropic Development. Dev. Cell 2017, 41, 170–179. [Google Scholar] [CrossRef]
- Llorente, F.; Alonso-Blanco, C.; Sánchez-Rodriguez, C.; Jorda, L.; Molina, A. ERECTA receptor-like kinase and heterotrimeric G protein from Arabidopsis are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina. Plant J. 2005, 43, 165–180. [Google Scholar] [CrossRef]
- Trusov, Y.; Rookes, J.E.; Chakravorty, D.; Armour, D.; Schenk, P.M.; Botella, J.R. Heterotrimeric G Proteins Facilitate Arabidopsis Resistance to Necrotrophic Pathogens and Are Involved in Jasmonate Signaling. Plant Physiol. 2006, 140, 210–220. [Google Scholar] [CrossRef]
- Trusov, Y.; Rookes, J.E.; Tilbrook, K.; Chakravorty, D.; Mason, M.G.; Anderson, D.; Chen, J.-G.; Jones, A.M.; Botella, J.R. Heterotrimeric G Protein γ Subunits Provide Functional Selectivity in Gβγ Dimer Signaling in Arabidopsis. Plant Cell 2007, 19, 1235–1250. [Google Scholar] [CrossRef]
- Delgado-Cerezo, M.; Sánchez-Rodríguez, C.; Escudero, V.; Miedes, E.; Fernández, P.V.; Jordá, L.; Hernández-Blanco, C.; Sánchez-Vallet, A.; Bednarek, P.; Schulze-Lefert, P.; et al. Arabidopsis Heterotrimeric G-protein Regulates Cell Wall Defense and Resistance to Necrotrophic Fungi. Mol. Plant 2012, 5, 98–114. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, J.-F.; Niu, Y.; Zhang, X.-C.; Woody, O.Z.; Xiong, Y.; Djonović, S.; Millet, Y.; Bush, J.; McConkey, B.J.; et al. Pathogen-secreted proteases activate a novel plant immune pathway. Nature 2015, 521, 213–216. [Google Scholar] [CrossRef]
- Klopffleisch, K.; Phan, N.; Augustin, K.; Bayne, R.S.; Booker, K.S.; Botella, J.R.; Carpita, N.C.; Carr, T.; Chen, J.-G.; Cooke, T.R.; et al. Arabidopsis G-protein interactome reveals connections to cell wall carbohydrates and morphogenesis. Mol. Syst. Biol. 2011, 7, 532. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Ogawa-Ohnishi, M.; Yamashita, T.; Kakita, M.; Nakayama, T.; Ohkubo, Y.; Hayashi, Y.; Yamashita, Y.; Nomura, T.; Noda, S.; Shinohara, H.; et al. Peptide ligand-mediated trade-off between plant growth and stress response. Science 2022, 378, 175–180. [Google Scholar] [CrossRef]
- McDougall, B.M.; Rovira, A.D. Sites of exudation of 14C-labelled compounds from wheat roots. New Phytol. 1970, 69, 999–1003. [Google Scholar] [CrossRef]
- Millet, Y.A.; Danna, C.H.; Clay, N.K.; Songnuan, W.; Simon, M.D.; Werck-Reichhart, D.; Ausubel, F.M. Innate Immune Responses Activated in Arabidopsis Roots by Microbe-Associated Molecular Patterns. Plant Cell 2010, 22, 973–990. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Wang, S.; Chen, J.-G.; Jones, A.M.; Fedoroff, N.V. Different Signaling and Cell Death Roles of Heterotrimeric G Protein α and β Subunits in the Arabidopsis Oxidative Stress Response to Ozone. Plant Cell 2005, 17, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Botella, J.R. Heterotrimeric G Protein Signaling in Abiotic Stress. Plants 2022, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Lease, K.A.; Wen, J.; Li, J.; Doke, J.T.; Liscum, E.; Walker, J.C. A mutant Arabidopsis heterotrimeric G-protein β-subunit affects leaf, flower, and fruit development. Plant Cell 2001, 13, 2631–2641. [Google Scholar]
- Ullah, H.; Chen, J.-G.; Young, J.C.; Im, K.H.; Sussman, M.R.; Jones, A.M. Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 2001, 292, 2066–2069. [Google Scholar] [CrossRef]
- Ullah, H.; Chen, J.-G.; Temple, B.; Boyes, D.C.; Alonso, J.M.; Davis, K.R.; Ecker, J.R.; Jones, A.M. The β-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell 2003, 15, 393–409. [Google Scholar] [CrossRef]
- Chen, J.-G.; Gao, Y.; Jones, A.M. Differential roles of Arabidopsis heterotrimeric G-protein subunits in modulating cell division in roots. Plant Physiol. 2006, 141, 887–897. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Jung, S.; Lee, H. Heterotrimeric G Protein-Mediated Signaling Is Involved in Stress-Mediated Growth Inhibition in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 11027. https://doi.org/10.3390/ijms241311027
Yang S, Jung S, Lee H. Heterotrimeric G Protein-Mediated Signaling Is Involved in Stress-Mediated Growth Inhibition in Arabidopsis thaliana. International Journal of Molecular Sciences. 2023; 24(13):11027. https://doi.org/10.3390/ijms241311027
Chicago/Turabian StyleYang, Soeun, Seohee Jung, and Horim Lee. 2023. "Heterotrimeric G Protein-Mediated Signaling Is Involved in Stress-Mediated Growth Inhibition in Arabidopsis thaliana" International Journal of Molecular Sciences 24, no. 13: 11027. https://doi.org/10.3390/ijms241311027
APA StyleYang, S., Jung, S., & Lee, H. (2023). Heterotrimeric G Protein-Mediated Signaling Is Involved in Stress-Mediated Growth Inhibition in Arabidopsis thaliana. International Journal of Molecular Sciences, 24(13), 11027. https://doi.org/10.3390/ijms241311027





