Genome-Wide Identification and Expression Analysis of Auxin Response Factor Gene Family in Linum usitatissimum
Abstract
1. Introduction
2. Results
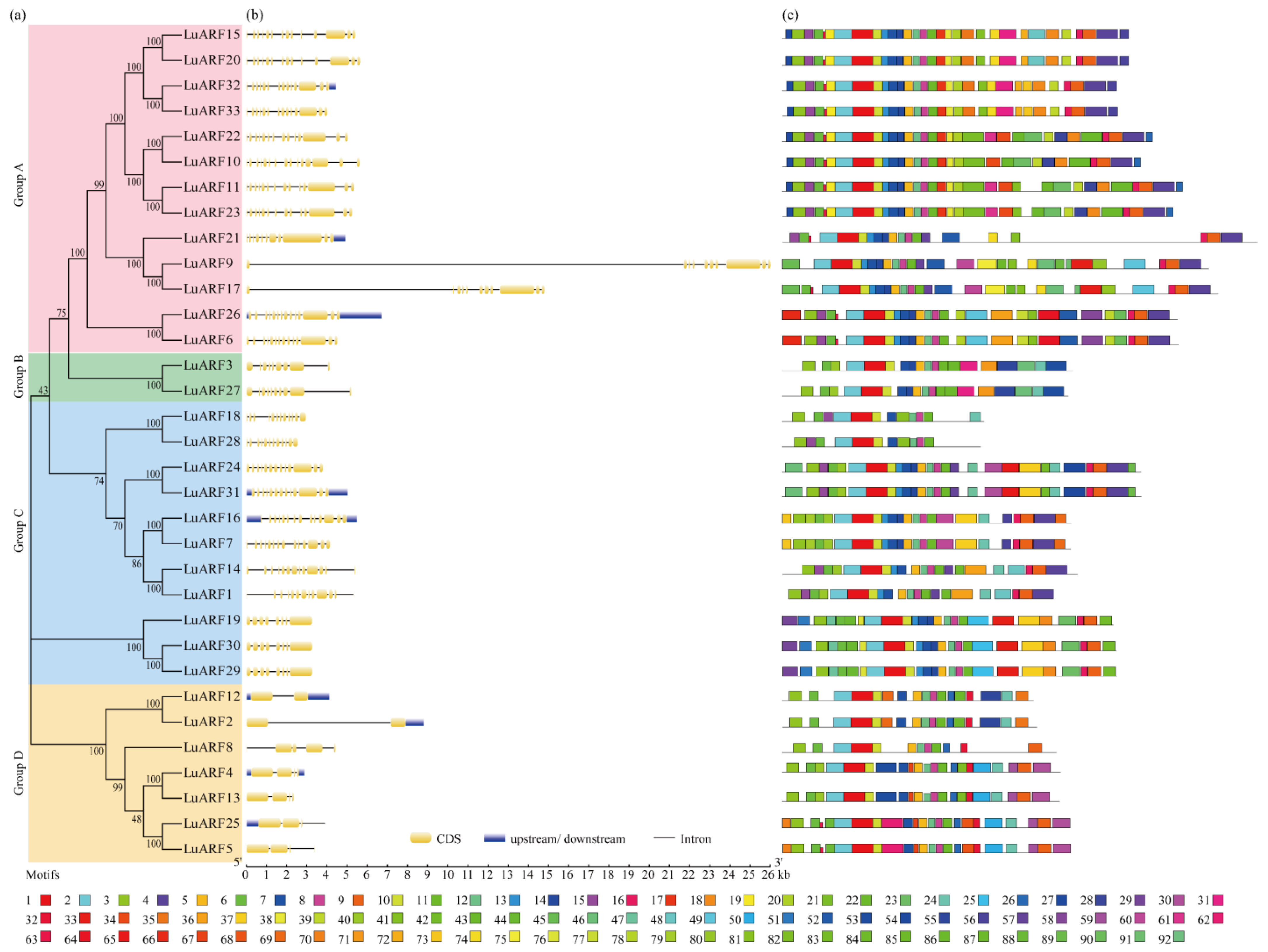
2.1. Identification of ARF Genes in Flax
2.2. Sequence Analysis of Flax ARFs
2.3. Phylogenetic Analysis of ARF Proteins
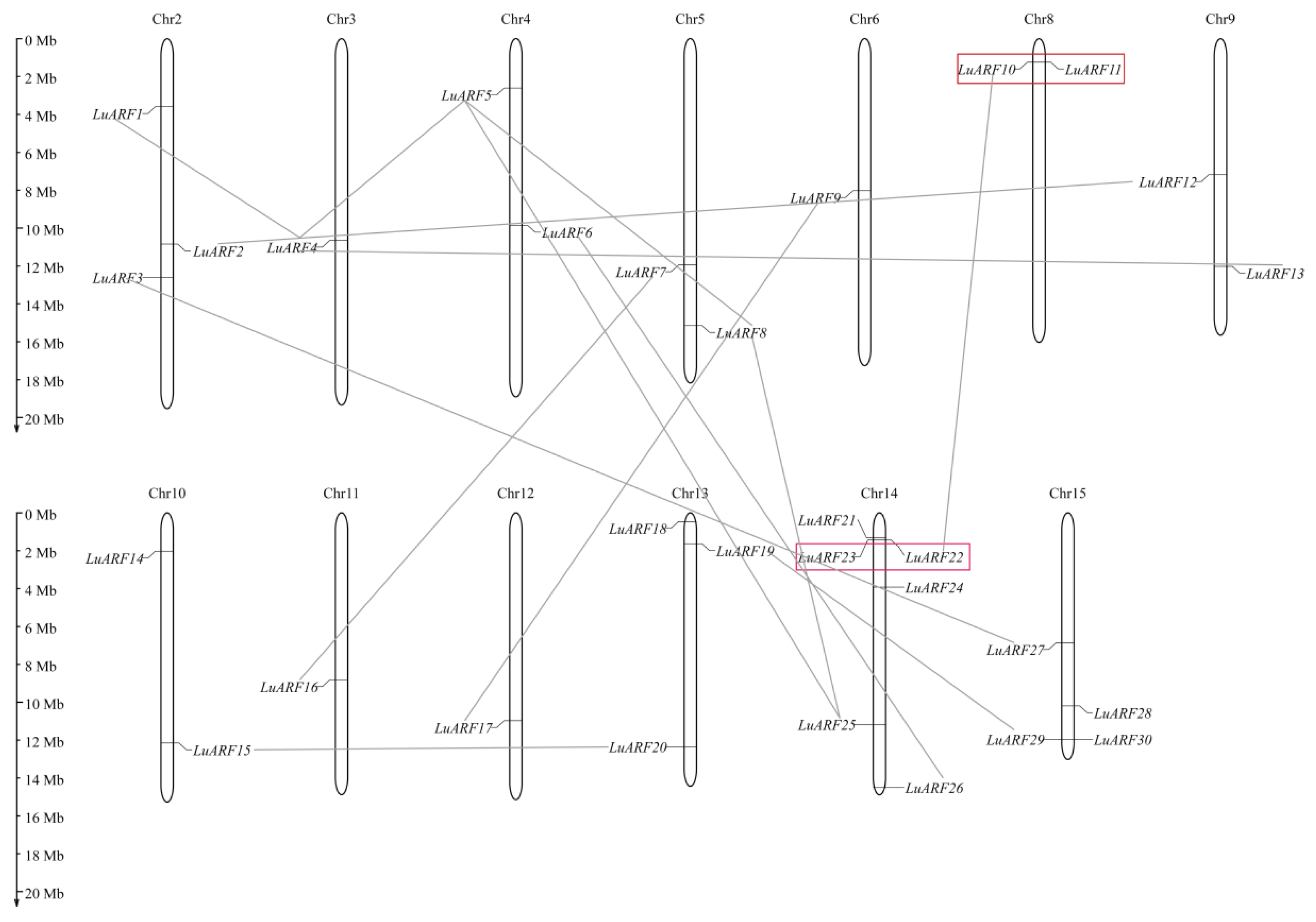
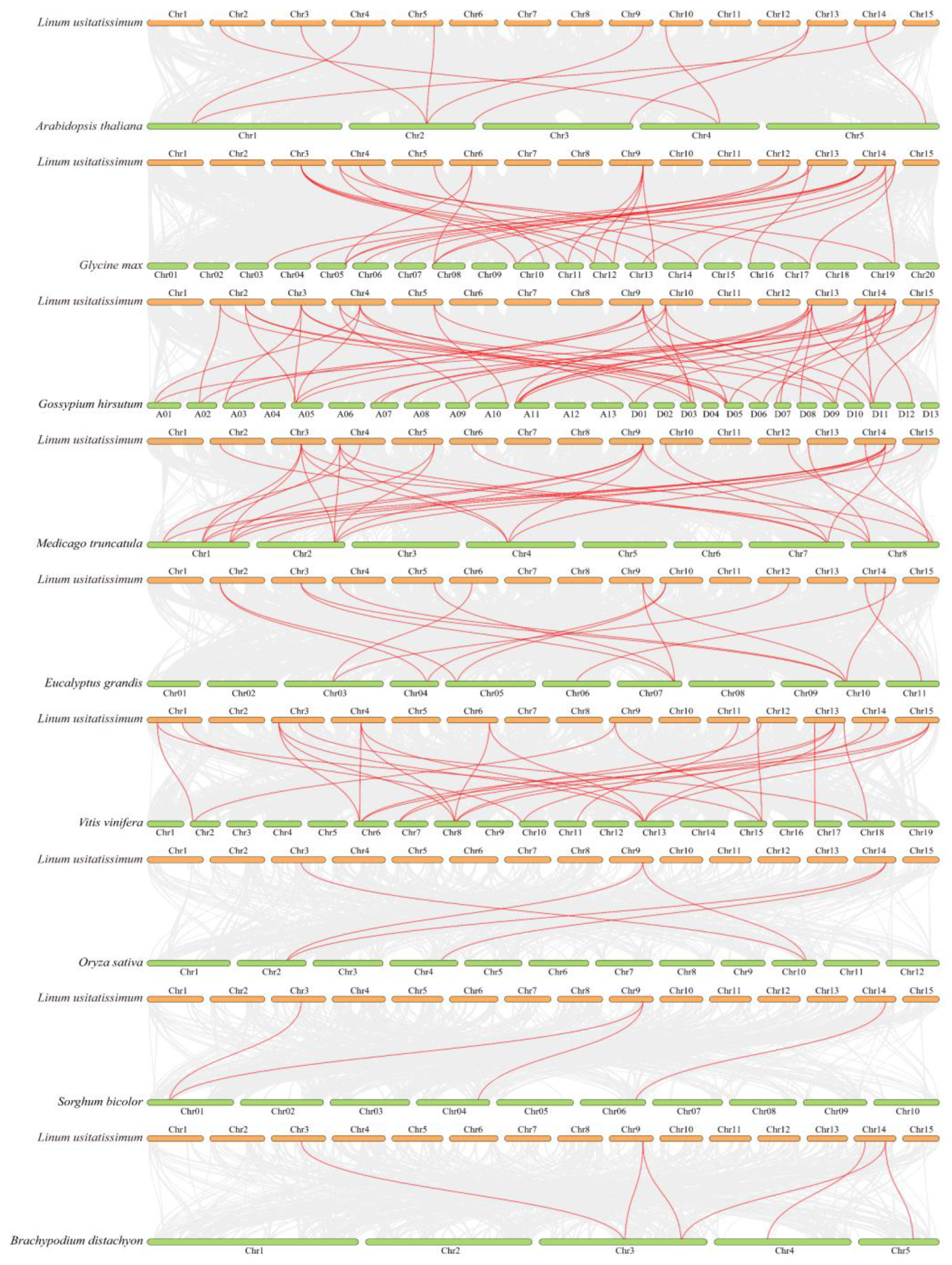
2.4. Chromosomal Distribution and Synteny Analysis
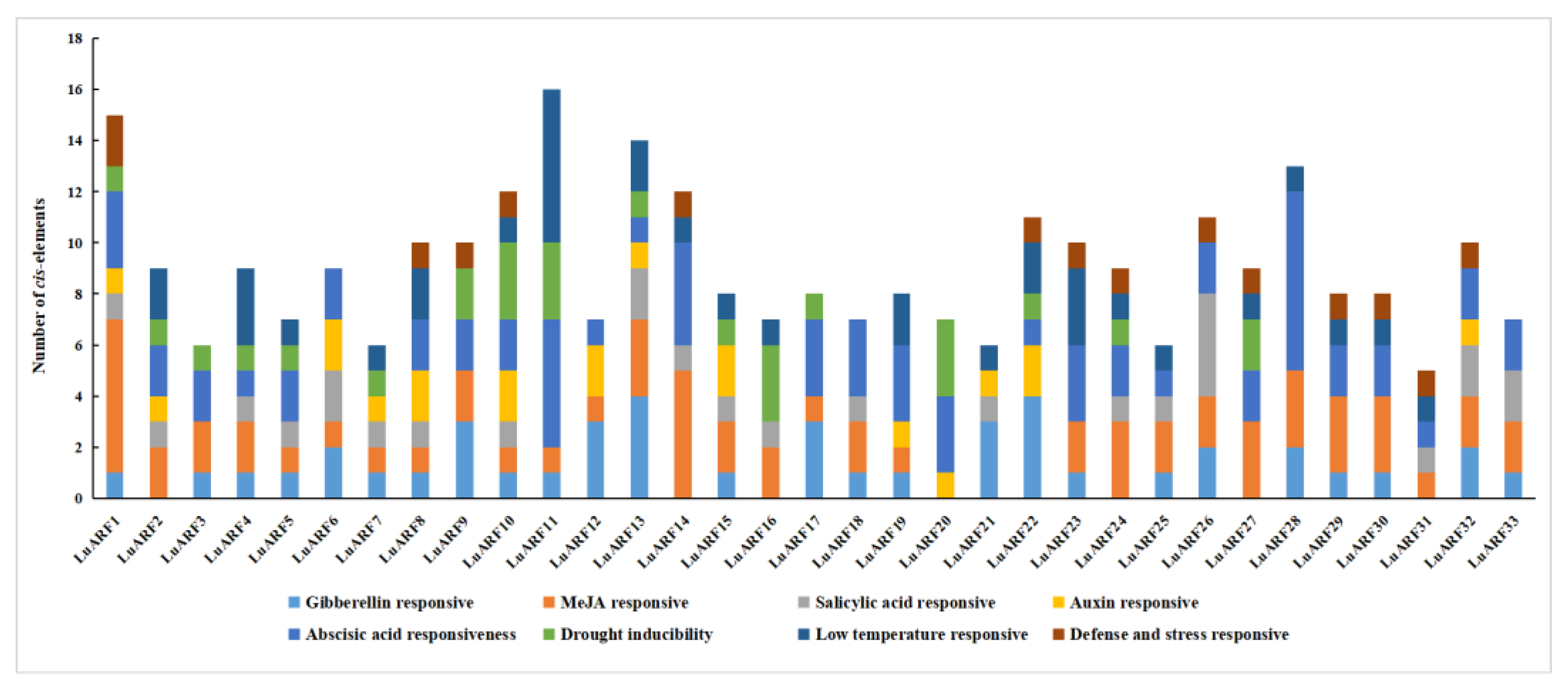
2.5. Phytohormone- and Abiotic Stress-Related Cis-Acting Elements on LuARF Promoter
2.6. Tissue-Specific Expression of LuARF Genes
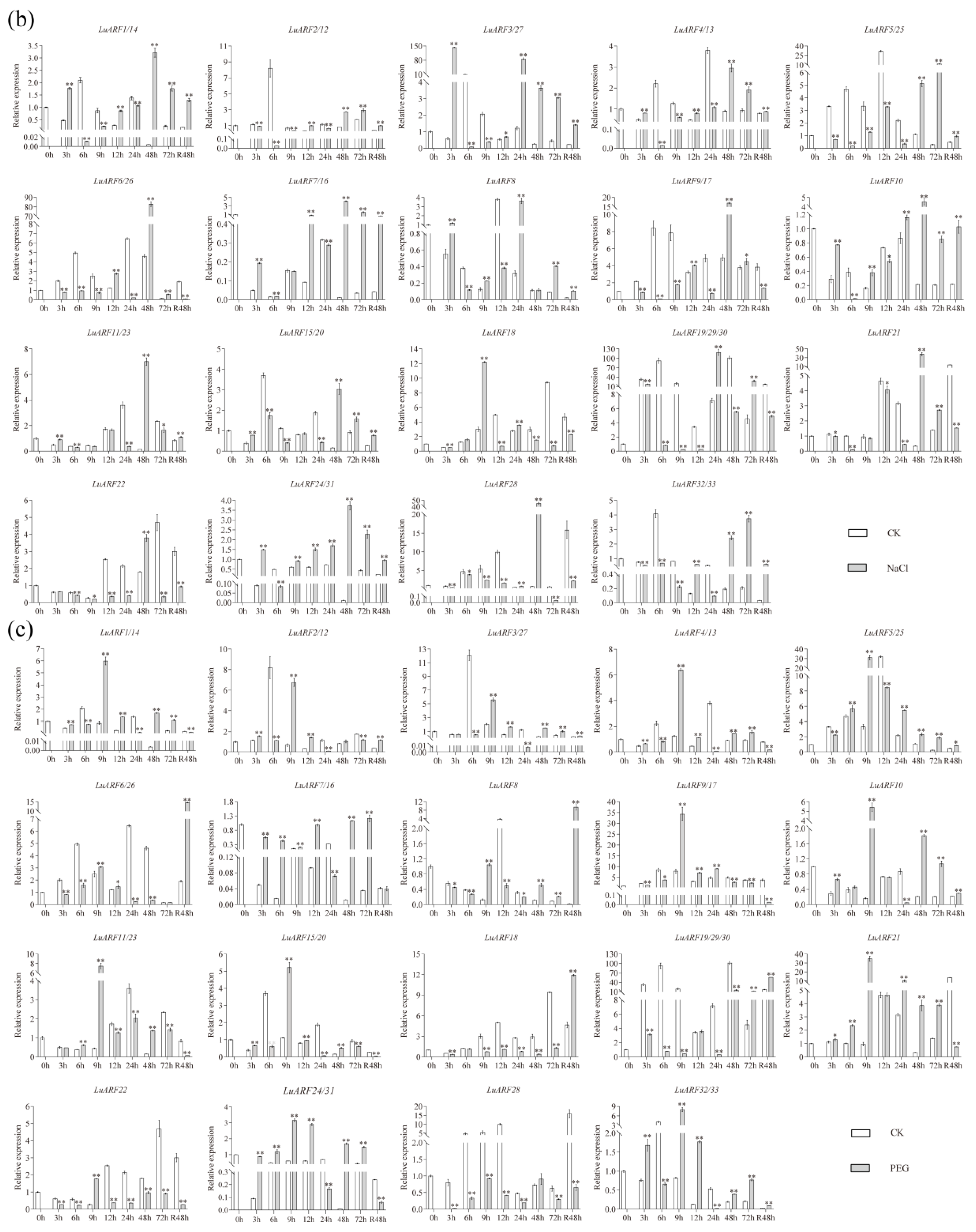
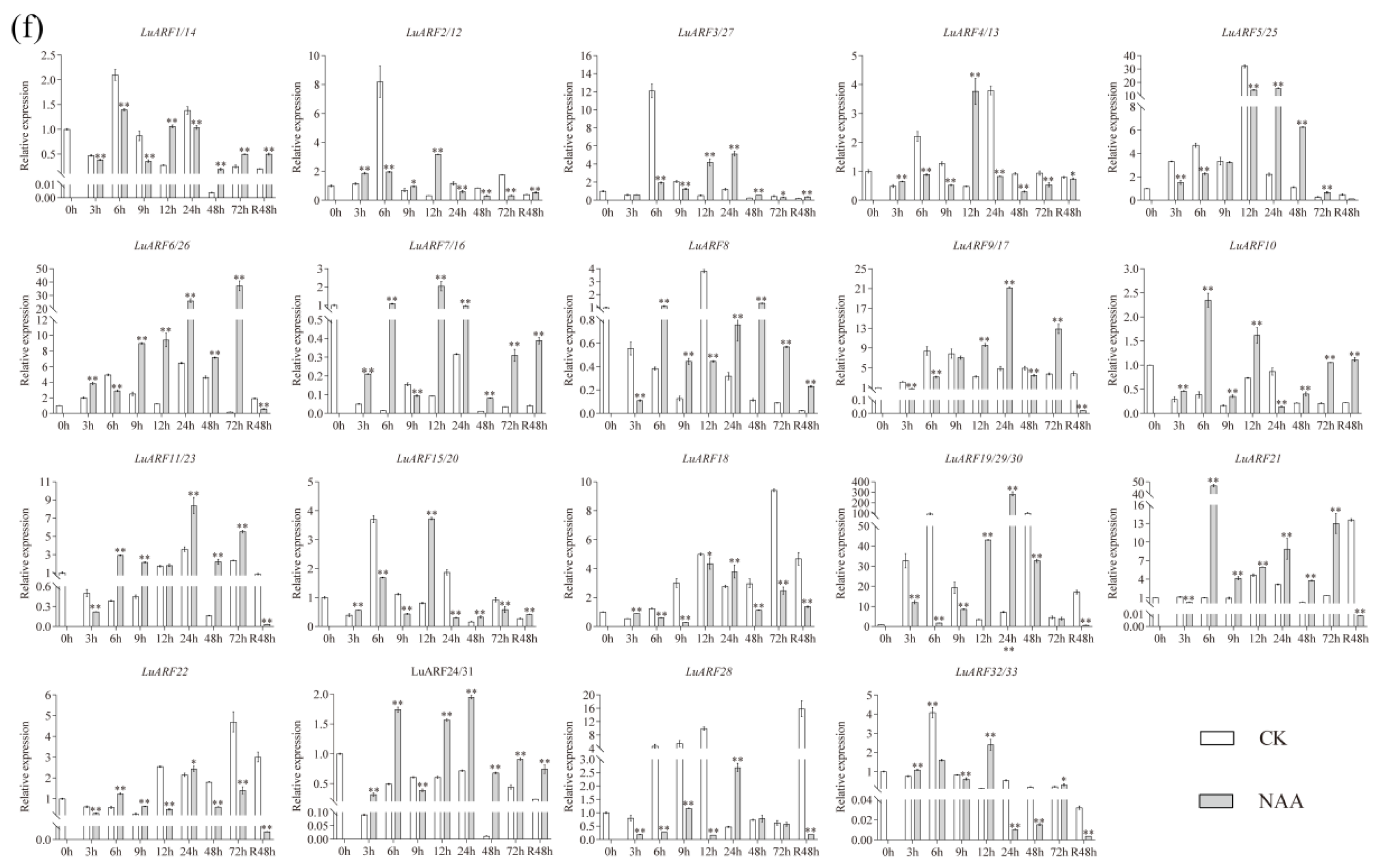
2.7. Expression Profiling of LuARFs under Various Stresses
3. Discussion
3.1. ARF Genes in Flax
3.2. LuARFs’ Possible Role in Regulating the Development of Organs and Responses to Exogenous Stimuli
4. Materials and Methods
4.1. Plant Growth and Abiotic Stress Treatments
4.2. Identification of ARF Genes
4.3. Chromosomal Mapping and Collinearity Analysis
4.4. Gene Structure and Promoter Analysis
4.5. Phylogenetic Analysis
4.6. Gene Expression Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hagen, G.; Guilfoyle, T. Auxin-responsive gene expression: Genes, promotersand regulatory factors. Plant Mol. Biol. 2002, 49, 373–385. [Google Scholar] [CrossRef]
- Li, S.B.; Xie, Z.Z.; Hu, C.G.; Zhang, J.Z. A review of auxin response factors (ARFs) in plants. Front. Plant Sci. 2016, 7, 47. [Google Scholar] [CrossRef]
- Roosjen, M.; Paque, S.; Weijers, D. Auxin Response Factors: Output control in auxin biology. J. Exp. Bot. 2018, 69, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Sáez, R.; Voß, U. Auxin Metabolism Controls Developmental Decisions in Land Plants. Trends Plant Sci. 2019, 24, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Hagen, G. Auxin Signal Transduction. Essays Biochem. 2015, 58, 1–12. [Google Scholar]
- Ulmasov, T.; Hagen, G.; Guilfoyle, T.J. Activation and repression of transcription by auxin-response factors. Proc. Natl. Acad. Sci. USA 1999, 96, 5844–5849. [Google Scholar] [CrossRef] [PubMed]
- Guilfoyle, T.J.; Hagen, G. Auxin response factor. Curr. Opin. Plant Biol. 2007, 10, 453–460. [Google Scholar] [CrossRef]
- Freire-Rios, A.; Tanaka, K.; Crespo, I.; van der Wijk, E.; Sizentsova, Y.; Levitsky, V.; Lindhoud, S.; Fontana, M.; Hohlbein, J.; Boer, D.R.; et al. Architecture of DNA elements mediating ARF transcription factor binding and auxin-responsive gene expression in Arabidopsis. Proc. Natl. Acad. Sci. USA 2020, 117, 24557–24566. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Hagen, G.; Guilfoyle, T. The roles of auxin response factor domains in auxin-responsive transcription. Plant Cell 2003, 15, 533–543. [Google Scholar] [CrossRef]
- Boer, D.R.; Freire-Rios, A.; van den Berg, W.A.; Saaki, T.; Manfield, I.W.; Kepinski, S.; López-Vidrieo, I.; Franco-Zorrilla, J.M.; de Vries, S.C.; Solano, R.; et al. Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell 2014, 156, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Guilfoyle, T.J. The PB1 domain in auxin response factor and aux/IAA proteins: A versatile protein interaction module in the auxin response. Plant Cell 2015, 27, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Ulmasov, T.; Hagen, G.; Guilfoyle, T.J. ARF1, a transcription factor that binds to auxin response elements. Science 1997, 276, 1865–1868. [Google Scholar] [CrossRef]
- Weijers, D.; Wagner, D. Transcriptional Responses to the Auxin Hormone. Annu. Rev. Plant Biol. 2016, 67, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Leyser, O. Auxin signaling. Plant Physiol. 2018, 176, 465–479. [Google Scholar] [CrossRef]
- Kepinski, S.; Leyser, O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 2005, 435, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Calderon-Villalobos, L.I.A.; Sharon, M.; Zheng, C.; Robinson, C.V.; Estelle, M.; Zheng, N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 2007, 446, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.M.; Nagpal, P.; Young, J.C.; Hagen, G.; Guifoyle, T.J.; Reed, J.W. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 2005, 132, 4563–4574. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, H.; Pan, Y.; Niu, Y.; Guo, L.; Ma, Y.; Tian, S.; Wei, J.; Wang, C.; Yang, X.; et al. Cell- and noncell-autonomous AUXIN RESPONSE FACTOR3 controls meristem proliferation and phyllotactic patterns. Plant Physiol. 2022, 190, 2335–2349. [Google Scholar] [CrossRef]
- Hardtke, C.S.; Berleth, T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998, 17, 1405–1411. [Google Scholar] [CrossRef]
- Liu, Z.N.; Miao, L.M.; Huo, R.X.; Song, X.Y.; Johnson, C.; Kong, L.J.; Sundaresan, V.; Yu, X. ARF2–ARF4 and ARF5 are essential for female and male gametophyte development in Arabidopsis. Plant Cell Physiol. 2018, 59, 179–189. [Google Scholar] [CrossRef]
- Nagpal, P.; Ellis, C.M.; Weber, H.; Ploense, S.E.; Barkawi, L.S.; Guilfoyle, T.J.; Hagen, G.; Alonso, J.M.; Cohen, J.D.; Farmer, E.E.; et al. Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development 2005, 127, 3877–3888. [Google Scholar] [CrossRef] [PubMed]
- Okushima, Y.; Fukaki, H.; Onoda, M.; Theologis, A.; Tasaka, M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 2007, 19, 118–130. [Google Scholar] [CrossRef]
- Attia, K.A.; Abdelkhalik, A.F.; Ammar, M.H.; Wei, C.; Yang, J.; Lightfoot, D.A.; El-Sayed, W.M.; El-Shemy, H.A. Antisense phenotypes reveal a functional expression of OsARF1, anauxin response factor, in transgenic rice. Curr. Issues Mol. Biol. 2009, 11, i29–i34. [Google Scholar]
- Wang, S.; Zhang, S.; Sun, C.; Xu, Y.; Chen, Y.; Yu, C.; Qian, Q.; Jiang, D.; Qi, Y. (OsARF12), a novel regulator for phosphate homeostasis inrice (Oryza sativa). New Phytol. 2014, 201, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, K.; Chen, L.; Zou, Y.; Liu, H.; Tian, Y.; Li, D.; Wang, R.; Zhao, F.; Ferguson, B.J.; et al. Microrna167-directed regulation of the auxin response factors, GmARF8a and GmARF8b, is required for soybean (Glycine max L.) nodulation and lateral root development. Plant Physiol. 2015, 168, 984–999. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; He, P.; Zhao, P.; Liu, H.; Zhang, L.; Pang, C.; Yu, J. Genome-wide identification of the GhARF gene family reveals that GhARF2 and GhARF18 are involved in cotton fibre cell initiation. J. Exp. Bot. 2018, 69, 4323–4337. [Google Scholar] [CrossRef]
- Hu, J.; Su, H.; Cao, H.; Wei, H.; Fu, X.; Jiang, X.; Song, Q.; He, X.; Xu, C.; Luo, K. AUXIN RESPONSE FACTOR7 integrates gibberellin and auxin signaling via interactions between DELLA and AUX/IAA proteins to regulate cambial activity in poplar. Plant Cell 2022, 34, 2688–2707. [Google Scholar] [CrossRef]
- Mei, M.; Ai, W.; Liu, L.; Xu, X.; Lu, X. Genome-wide identification of the auxin response factor (ARF) gene family in Magnolia sieboldii and functional analysis of MsARF5. Front. Plant Sci. 2022, 13, 958816. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Qi, Z.; Ren, Z.; Tong, J.; Wang, B.; Wu, Z.; Hao, J.; Liu, N. Genome-Wide Analysis of Auxin Response Factors in Lettuce (Lactuca sativa L.) Reveals the Positive Roles of LsARF8a in Thermally Induced Bolting. Int. J. Mol. Sci. 2022, 23, 13509. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Dong, L.; Deng, X.; Liu, D.; Liu, Y.; Li, M.; Hu, Y.; Yan, Y. Genome-wide identification, molecular evolution, and expression analysis of auxin response factor (ARF) gene family in Brachypodium distachyon L. BMC Plant Biol. 2018, 18, 336. [Google Scholar] [CrossRef]
- Die, J.V.; Gil, J.; Millan, T. Genome-wide identification of the auxin response factor gene family in Cicer arietinum. BMC Genom. 2018, 19, 301. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Qiu, Z.; Wen, Z.; Zhang, H.; Li, Z.; Hong, Y.; Qiao, G.; Wen, X. Genome-Wide Identification of ARF Gene Family Suggests a Functional Expression Pattern during Fruitlet Abscission in Prunus avium L. Int. J. Mol. Sci. 2021, 22, 11968. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhu, X.; Liu, X.; Wu, C.; Yu, C.; Hu, G.; Chen, L.; Chen, R.; Bouzayen, M.; Zouine, M.; et al. Knockout of Auxin Response Factor SlARF4 Improves Tomato Resistance to Water Deficit. Int. J. Mol. Sci. 2021, 22, 3347. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, R.; Yu, J.; Huang, S.; Zhang, Y.; Wei, H.; Wei, Z. Genome-Wide Identification and Characterization of Auxin Response Factor (ARF) Gene Family Involved in Wood Formation and Response to Exogenous Hormone Treatment in Populus trichocarpa. Int. J. Mol. Sci. 2023, 24, 740. [Google Scholar] [CrossRef]
- Wang, D.; Pei, K.; Fu, Y.; Sun, Z.; Li, S.; Liu, H.; Tang, K.; Han, B.; Tao, Y. Genome-wide analysis of the auxin response factors (ARF) gene family in rice (Oryza sativa). Gene 2007, 394, 13–24. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Zhao, Y.; Yu, W.; Si, W. Whole-Genome Duplication and Purifying Selection Contributes to the Functional Redundancy of Auxin Response Factor (ARF) Genes in Foxtail Millet (Setaria italica L.). Int. J. Genom. 2021, 2021, 2590665. [Google Scholar] [CrossRef]
- Ali, S.; Wang, W.; Zhang, Z.; Xie, L.; Boer, D.R.; Khan, N. Genome-Wide Identification, Expression and Interaction Analysis of ARF and AUX/IAA Gene Family in Soybean. Front. Biosci. 2022, 7, 251. [Google Scholar] [CrossRef] [PubMed]
- van Zeist, W.; Bakker-Heeres, J.A.H. Evidence for linseed cultivation before 6000 BC. J. Archaeol. Sci. 1975, 2, 215–219. [Google Scholar] [CrossRef]
- Zohary, D. Monophyletic vs. polyphyletic origin of the crops on which agriculture was founded in the near east. Genet. Resour. Crop Evol. 1999, 46, 133–142. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, Y.; Wang, L.; Wang, L.; Yan, X.; Dang, Z.; Li, W.; Zhao, W.; Pei, X.; Li, X. Genomic Comparison and Population Diversity Analysis Provide Insights into the Domestication and Improvement of Flax. iScience 2020, 23, 100967. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: Anupgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Nekrutenko, A.; Makova, K.D.; Li, W.H. The KA/KS ratio test for assessing the protein-coding potential of genomic regions: An empirical and simulation study. Genome Res. 2002, 12, 198–202. [Google Scholar] [CrossRef]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Wan, S.; Li, W.; Zhu, Y.; Liu, Z.; Huang, W.; Zhan, J. Genome-wide identification, characterization and expression analysis of the auxin response factor gene family in Vitis vinifera. Plant Cell Rep. 2014, 33, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Guo, Z.; Lu, S. Genome-Wide Identification and Expression Analysis of the Aux/IAA and Auxin Response Factor Gene Family in Medicago truncatula. Int. J. Mol. Sci. 2021, 22, 10494. [Google Scholar] [CrossRef] [PubMed]
- Okushima, Y.; Overvoorde, P.J.; Arima, K.; Alonso, J.M.; Chan, A.; Chang, C.; Ecker, J.R.; Hughes, B.; Lui, A.; Nguyen, D.; et al. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: Unique and overlapping functions of ARF7 and ARF19. Plant Cell 2005, 17, 444–463. [Google Scholar] [CrossRef]
- Kubeš, M.; Napier, R. Non-canonical auxin signalling: Fast and curious. J. Exp. Bot. 2019, 70, 2609. [Google Scholar] [CrossRef]
- Shen, C.; Wang, S.; Bai, Y.; Wu, Y.; Zhang, S.; Chen, M.; Guilfoyle, T.J.; Wu, P.; Qi, Y. Functional analysis of the structural domain of ARF proteins in rice (Oryza sativa L.). J. Exp. Bot. 2010, 61, 3971–3981. [Google Scholar] [CrossRef]
- Finet, C.; Berne-Dedieu, A.; Scutt, C.P.; Marletaz, F. Evolution of the ARF gene family in land plants: Old domains, new tricks. Mol. Biol. Evol. 2013, 30, 45–56. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, N.; Song, W.; Yin, G.; Qin, Y.; Yan, Y. Soybean (Glycine max) expansin gene superfamily origins: Segmental and tandem duplication events followed by divergent selection among subfamilies. BMC Plant Biol. 2014, 43, 14–93. [Google Scholar] [CrossRef]
- Ren, R.; Wang, H.; Guo, C.; Zhang, N.; Zeng, L.; Chen, Y.; Ma, H.; Qi, J. Widespread Whole Genome Duplications Contribute to Genome Complexity And Species Diversity in Angiosperms. Mol. Plant 2018, 11, 414–428. [Google Scholar] [CrossRef]
- Qiao, X.; Li, Q.; Yin, H.; Qi, K.; Li, L.; Wang, R.; Zhang, S.; Paterson, A.H. Gene duplication and evolution in recurring polyploidization-diploidization cycles in plants. Genome Biol. 2019, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Matthes, M.S.; Best, N.B.; Robil, J.M.; Malcomber, S.; Gallavotti, A.; McSteen, P. Auxin EvoDevo: Conservation and Diversification of Genes Regulating Auxin Biosynthesis, Transport, and Signaling. Mol. Plant 2019, 12, 298–320. [Google Scholar] [CrossRef]
- Gorshkova, T.A.; Sal’nikova, V.V.; Chemikosova, S.B.; Ageeva, M.V.; Pavlencheva, N.V.; van Dam, J.E.G. The snap point: A transition point in Linum usitatissimum bast fiber development. Ind. Crops Prod. 2003, 18, 213–221. [Google Scholar] [CrossRef]
- Pinzon-Latorre, D.; Deyholos, M.K. Pectinmethylesterases (PME) and Pectinmethylesterase Inhibitors (PMEI) Enriched during Phloem Fiber Development in Flax (Linum usitatissimum). PLoS ONE 2014, 9, e105386. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qiu, C.; Long, S.; Chen, P.; Hao, D.; Preisner, M.; Wang, H.; Wang, Y. Digital gene expression profiling of flax (Linum usitatissimum L.) stem peel identifies genes enriched in fiber-bearing phloem tissue. Gene 2017, 626, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Nairn, C.J.; Haselkorn, T. Three loblolly pine CesA genes expressed in developing xylem are orthologous to secondary cell wall CesA genes of angiosperms. New Phytol. 2005, 166, 907–915. [Google Scholar] [CrossRef]
- Shibasaki, K.; Uemura, M.; Tsurumi, S.; Rahman, A. Auxin response in Arabidopsis under cold stress: Underlying molecular mechanisms. Plant Cell 2009, 21, 3823–3838. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, X.; Li, T.; Jia, M.; Liu, X.; Zou, Y.; Liu, Z.; Wen, F. Identification, characterization, and expression analysis of auxin response factor (ARF) gene family in Brachypodium distachyon. Funct. Integr. Genom. 2018, 18, 709–724. [Google Scholar] [CrossRef]
- Bouzroud, S.; Gouiaa, S.; Hu, N.; Bernadac, A.; Mila, I.; Bendaou, N.; Smouni, A.; Bouzayen, M.; Zouine, M. Auxin response factors (ARFs) are potential mediators of auxin action in tomato response to biotic and abiotic stress (Solanum lycopersicum). PLoS ONE 2018, 13, e0193517. [Google Scholar] [CrossRef]
- Kang, C.; He, S.; Zhai, H.; Li, R.; Zhao, N.; Liu, Q. A sweet potato auxin response factor gene (IbARF5) is involved in carotenoid biosynthesis and salt and drought tolerance in transgenic Arabidopsis. Front. Plant Sci. 2018, 9, 1307. [Google Scholar] [CrossRef]
- Talanova, V.V.; Titov, A.F. Endogenous abscisic acid content in cucumber leaves under the influence of unfavourable temperatures and salinity. J. Exp. Bot. 1994, 45, 1031–1033. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools on the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [PubMed]
- Chou, K.C.; Shen, H.B. Plant-mPLoc: A top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Paterson, A.H. MCScanX-transposed: Detecting transposed gene duplications based on multiple colinearity scans. Bioinformatics 2013, 29, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Lynch, M.; Conery, J.S. The evolutionary fate and consequences of duplicate genes. Science 2000, 290, 1151–1155. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Huis, R.; Hawkins, S.; Neutelings, G. Selection of reference genes for Quantitative gene expression normalization in flax (Linum usitatissimum L.). BMC Plant Biol. 2010, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Antonov, J.; Goldstein, D.R.; Oberli, A.; Baltzer, A.; Pirotta, M.; Fleischmann, A.; Altermatt, H.J.; Jaggi, R. Reliable gene expression measurements from degraded RNA by quantitative real-time PCR depend on short amplicons and a proper normalization. Lab. Investig. 2005, 85, 1040–1050. [Google Scholar] [CrossRef] [PubMed]




| Gene Name | Locus ID | Chromosome Location | Length (aa) | MW (KDa) | pI | Exons | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| LuARF1 | L.us.o.m.scaffold38.111 | Chr2: 4056063-4061396(−) | 654 | 73.74 | 6.43 | 13 | Nucleus |
| LuARF2 | L.us.o.m.scaffold177.42 | Chr2:12271477-12280264(+) | 602 | 65.95 | 6.05 | 2 | Nucleus |
| LuARF3 | L.us.o.m.scaffold33.48 | Chr2:14271938-14276080(+) | 688 | 75.21 | 6.98 | 9 | Nucleus |
| LuARF4 | L.us.o.m.scaffold20.385 | Chr3:12023683-12026552(+) | 659 | 72.31 | 7.85 | 4 | Nucleus |
| LuARF5 | L.us.o.m.scaffold183.107 | Chr4:2988619-2992012(+) | 682 | 75.76 | 6.98 | 4 | Nucleus |
| LuARF6 | L.us.o.m.scaffold336.4 | Chr4:11172052-11176570(−) | 937 | 102.77 | 5.60 | 14 | Nucleus |
| LuARF7 | L.us.o.m.scaffold2.53 | Chr5:13559053-13563212(−) | 683 | 75.98 | 5.81 | 14 | Nucleus |
| LuARF8 | L.us.o.m.scaffold255.60 | Chr5:17150878-17155311(−) | 649 | 72.37 | 8.09 | 5 | Nucleus |
| LuARF9 | L.us.o.m.scaffold53.226 | Chr6:9052662-9078679(+) | 1010 | 112.57 | 6.67 | 10 | Nucleus |
| LuARF10 | L.us.o.m.scaffold82.237 | Chr8:1405577-1411201(+) | 847 | 93.84 | 6.04 | 15 | Nucleus |
| LuARF11 | L.us.o.m.scaffold82.236 | Chr8:1413136-1418471(+) | 948 | 105.43 | 6.39 | 14 | Nucleus |
| LuARF12 | L.us.o.m.scaffold33.141 | Chr9:8160867-8164989(−) | 595 | 65.11 | 6.76 | 2 | Nucleus |
| LuARF13 | L.us.o.m.scaffold131.61 | Chr9:13620512-13622865(−) | 657 | 72.43 | 6.81 | 4 | Nucleus |
| LuARF14 | L.us.o.m.scaffold9.422 | Chr10:2282901-2288310(+) | 698 | 78.45 | 6.78 | 13 | Nucleus |
| LuARF15 | L.us.o.m.scaffold199.64 | Chr10:13744807-13750216(+) | 820 | 91.32 | 6.16 | 14 | Nucleus |
| LuARF16 | L.us.o.m.scaffold5.303 | Chr11:9965217-9970709(−) | 684 | 76.11 | 5.72 | 14 | Nucleus |
| LuARF17 | L.us.o.m.scaffold3.144 | Chr12:12422846-12437639(+) | 1032 | 114.79 | 6.28 | 11 | Nucleus |
| LuARF18 | L.us.o.m.scaffold75.41 | Chr13:524613-527571(−) | 478 | 52.82 | 8.49 | 12 | Nucleus |
| LuARF19 | L.us.o.m.scaffold71.2 | Chr13:1846189-1849447(−) | 784 | 87.26 | 6.33 | 8 | Nucleus |
| LuARF20 | L.us.o.m.scaffold83.44 | Chr13:14011405-14017053(−) | 820 | 91.39 | 6.12 | 14 | Nucleus |
| LuARF21 | L.us.o.m.scaffold48.39 | Chr14:1479346-1484254(−) | 1123 | 125.34 | 6.31 | 11 | Nucleus |
| LuARF22 | L.us.o.m.scaffold48.78 | Chr14:1639106-1644136(+) | 876 | 97.13 | 5.87 | 14 | Nucleus |
| LuARF23 | L.us.o.m.scaffold48.79 | Chr14:1646009-1651254(+) | 924 | 102.67 | 6.23 | 14 | Nucleus |
| LuARF24 | L.us.o.m.scaffold56.318 | Chr14:4404281-4408082(−) | 849 | 94.30 | 6.31 | 13 | Nucleus |
| LuARF25 | L.us.o.m.scaffold168.120 | Chr14:12632364-12636289(+) | 682 | 75.57 | 6.55 | 4 | Nucleus |
| LuARF26 | L.us.o.m.scaffold122.81 | Chr14:16412628-16419332(+) | 936 | 102.87 | 5.45 | 14 | Nucleus |
| LuARF27 | L.us.o.m.scaffold107.81 | Chr15:7797623-7802834−) | 677 | 74.04 | 6.88 | 10 | Nucleus |
| LuARF28 | L.us.o.m.scaffold0.555 | Chr15:11542664-11545214(−) | 470 | 52.81 | 8.59 | 12 | Nucleus |
| LuARF29 | L.us.o.m.scaffold239.1 | Chr15: 13529432-13532699(−) | 792 | 88.22 | 6.45 | 8 | Nucleus |
| LuARF30 | L.us.o.m.scaffold156.62 | Chr15: 13548791-13552058(−) | 792 | 88.22 | 6.38 | 8 | Nucleus |
| LuARF31 | L.us.o.m.scaffold81.17 | 850 | 94.28 | 6.45 | 13 | Nucleus | |
| LuARF32 | L.us.o.m.scaffold165.136 | 791 | 87.68 | 5.73 | 14 | Nucleus | |
| LuARF33 | L.us.o.m.scaffold434.2 | 792 | 87.79 | 5.86 | 14 | Nucleus |
| Gene Pairs | Duplication Event | Ka | Ks | Ka/Ks | Selection Type | Divergence Time (Mya) |
|---|---|---|---|---|---|---|
| LuARF10/LuARF11 | TD | 0.0495 | 0.3311 | 0.1495 | Purifying selection | 27.1393 |
| LuARF22/LuARF23 | TD | 0.0566 | 0.3028 | 0.1869 | Purifying selection | 24.8197 |
| LuARF1/LuARF14 | SD | 0.0224 | 0.1009 | 0.2220 | Purifying selection | 8.2705 |
| LuARF2/LuARF12 | SD | 0.0375 | 0.1097 | 0.3418 | Purifying selection | 8.9918 |
| LuARF3/LuARF27 | SD | 0.0319 | 0.1731 | 0.1843 | Purifying selection | 14.1885 |
| LuARF4/LuARF5 | SD | 0.3173 | 2.7505 | 0.1154 | Purifying selection | 225.4508 |
| LuARF4/LuARF13 | SD | 0.0404 | 0.1177 | 0.3432 | Purifying selection | 9.6475 |
| LuARF5/LuARF25 | SD | 0.0196 | 0.1073 | 0.1827 | Purifying selection | 8.7951 |
| LuARF5/LuARF8 | SD | 0.2794 | 1.9360 | 0.1443 | Purifying selection | 158.6885 |
| LuARF8/LuARF25 | SD | 0.2789 | 2.4493 | 0.1139 | Purifying selection | 200.7623 |
| LuARF6/LuARF26 | SD | 0.0090 | 0.0735 | 0.1224 | Purifying selection | 6.0246 |
| LuARF7/LuARF16 | SD | 0.0159 | 0.0556 | 0.2860 | Purifying selection | 4.5574 |
| LuARF9/LuARF17 | SD | 0.0158 | 0.1057 | 0.1495 | Purifying selection | 8.6639 |
| LuARF10/LuARF22 | SD | 0.0192 | 0.1612 | 0.1191 | Purifying selection | 13.2131 |
| LuARF15/LuARF20 | SD | 0.0372 | 0.1141 | 0.3260 | Purifying selection | 9.3525 |
| LuARF15/LuARF32 | SD | 0.1029 | 0.6960 | 0.1478 | Purifying selection | 57.0492 |
| LuARF20/LuARF32 | SD | 0.1048 | 0.7268 | 0.1442 | Purifying selection | 59.5738 |
| LuARF32/LuARF33 | SD | 0.0062 | 0.2226 | 0.0279 | Purifying selection | 18.2459 |
| LuARF19/LuARF29 | SD | 0.0250 | 0.1660 | 0.1506 | Purifying selection | 13.6066 |
| LuARF24/LuARF31 | SD | 0.0067 | 0.0681 | 0.0984 | Purifying selection | 5.5820 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Wang, L.; Li, W.; Dang, Z.; Xie, Y.; Zhao, W.; Zhao, L.; Li, W.; Yang, C.; Xu, C.; et al. Genome-Wide Identification and Expression Analysis of Auxin Response Factor Gene Family in Linum usitatissimum. Int. J. Mol. Sci. 2023, 24, 11006. https://doi.org/10.3390/ijms241311006
Qi Y, Wang L, Li W, Dang Z, Xie Y, Zhao W, Zhao L, Li W, Yang C, Xu C, et al. Genome-Wide Identification and Expression Analysis of Auxin Response Factor Gene Family in Linum usitatissimum. International Journal of Molecular Sciences. 2023; 24(13):11006. https://doi.org/10.3390/ijms241311006
Chicago/Turabian StyleQi, Yanni, Limin Wang, Wenjuan Li, Zhao Dang, Yaping Xie, Wei Zhao, Lirong Zhao, Wen Li, Chenxi Yang, Chenmeng Xu, and et al. 2023. "Genome-Wide Identification and Expression Analysis of Auxin Response Factor Gene Family in Linum usitatissimum" International Journal of Molecular Sciences 24, no. 13: 11006. https://doi.org/10.3390/ijms241311006
APA StyleQi, Y., Wang, L., Li, W., Dang, Z., Xie, Y., Zhao, W., Zhao, L., Li, W., Yang, C., Xu, C., & Zhang, J. (2023). Genome-Wide Identification and Expression Analysis of Auxin Response Factor Gene Family in Linum usitatissimum. International Journal of Molecular Sciences, 24(13), 11006. https://doi.org/10.3390/ijms241311006





