1. Introduction
Despite the significant strides that have been made towards the development of targeted anticancer therapies, chemotherapy remains the first-line treatment for most cancers [
1,
2]. Platinum-based antitumor drugs, such as cisplatin (CDDP) (
Figure 1), are established chemotherapeutic agents in the treatment of a number of cancers and sarcomas; however, the development of cisplatin resistance is a major obstacle to curative cancer treatment [
3]. Drug resistance phenomena can be developed through several mechanisms, such as the overexpression of drug efflux pumps, activation of DNA repair efficiency, escape of drug-induced apoptosis, active cell survival signals, and enhanced drug detoxifying systems [
4,
5].
Under normal physiological conditions, the cellular redox homeostasis ensures that the cells respond appropriately to endogenous and exogenous stimuli. The redox systems are perfectly suited to the regulation of the cell’s life-or-death decisions [
6]. Both antioxidant enzymes (superoxide dismutase (SOD), glutathione reductase (GR), catalase (CAT), etc.) and low molecular weight antioxidants are, effectively, inbuilt defense strategies to maintain cellular redox homeostasis during oxidative stress [
7], which is defined as a disturbance in the balance between the generation of reactive oxygen and nitrogen species (RONS) and the antioxidant systems in the cell. The electron transport chain (ETC) in mitochondria and the NADPH oxidases (NOX) are the major endogenous sources of ROS, including the superoxide anion (O
2•–), hydrogen peroxide (H
2O
2), organic hydroperoxides (ROOH), the hydroxyl radical (
•OH), and the peroxyl radical (ROO), inside the cell. Enzymatic antioxidant defense for ROS elimination occurs as a series of the following reactions: SODs catalyze the dismutation of the O
2•– into H
2O
2. GPx catalyzes the detoxification of H
2O
2 by reduced glutathione (GSH). Further on, GR reduces oxidized glutathione (GSSG) to (GSH) using NADPH as the electron donor, while H
2O
2, which is found mainly in peroxisomes and cytosol, is neutralized by the CAT enzyme [
8].
In addition to its role in defense against oxidative stress, the thioredoxin (Trx) system also regulates DNA synthesis, the cell cycle, and the apoptosis process in mammalian cells [
9]. The over-activated Trx system, which contributes significantly to cancer progression and therapy resistance, has been recognized in several human tumors, including such highly aggressive types as lung, liver, pancreas, ovarian, and breast cancers. This antioxidant system is composed of cytosolic/nuclear and mitochondrial thioredoxin reductases (TrxR-1 and TrxR-2) and thioredoxins (Trx-1 and Trx-2), respectively. TrxR-1 and TrxR-2 are able to transfer electrons from NADPH to oxidized forms of Trx-1 and Trx-2, respectively, which reduce their substrates using a highly conserved dithiol form of the active site sequence [
8]. Oxidized signal transducers and activators of transcription 3 (STAT3) dimers, being one of the Trx target proteins, receive electrons from a reduced Trx form to regenerate reduced STAT3 monomers. Upon Tyr705 phosphorylation, the transcriptionally active phosphorylated STAT3 (pSTAT3) homodimer is formed, which in turn translocates to the nucleus and interacts with specific DNA sequences to promote STAT3-dependent gene expression [
10]. The accumulating evidence shows that the overexpression of STAT3, a transcription factor, has been observed in several cancer types and that it correlates with increased tumor cell proliferation, survival, self-renewal, tumor invasion, and angiogenesis, as well as higher cellular resistance to cisplatin [
11,
12]. Another important signaling pathway that correlates with pro-survival, the mechanistic target of rapamycin (mTOR) signaling, is also associated with CDDP resistance; it is necessary to phosphorylate STAT3 on serine
727 to ensure its maximum activation [
11].
A number of epidemiological studies have validated the fact that the consumption of dietary polyphenols, mainly quercetin (QU) (
Figure 1), is inversely correlated with cancer incidence; this has been attributed to the antioxidant properties of QU, which prevent cancer through the increasing expression of antioxidant enzymes [
13]. Moreover, QU exerts its anticancer activity by modulating cell cycle progression, reducing cell proliferation, and inhibiting angiogenesis and metastasis progression [
14], as well as by promoting apoptosis and autophagy via the modification of various pathways, such as the PI3K/Akt/mTOR, Wnt/β-catenin, and MAPK/ERK1/2 pathways [
15]. QU, a DNA intercalator, causes S-phase arrest during cell cycle progression and is consistent with the plausible induction of DNA damage following DNA intercalation within the cancer cells, which can eventually lead to the inducing of apoptosis [
16]. In addition to these mechanisms, QU can also trigger apoptosis via various mechanisms that depend on the type of cancer cells. For instance, QU inhibited the growth of human A375SM melanoma cells through inducing apoptosis via activation of the JNK/P38 MAPK signaling pathway [
17]. In vitro and in vivo mitochondrial-derived apoptosis following QU treatment in HL-60 AML cells was found to occur via reactive-oxygen-species-mediated ERK activation [
18].
However, the clinical application of QU is limited due to its poor solubility, low bioavailability, poor permeability, and instability [
19]. The bioavailability of quercetin can be significantly enhanced when it is consumed as an integral food component [
20]. The bioavailability of QU can be improved by encapsulating it in polymer micelles. The intravenous administration of QU encapsulated in biodegradable monomethoxy poly (ethylene glycol)-poly (ε-caprolactone) (MPEG-PCL) micelles significantly inhibited the growth of established xenograft A2780S ovarian tumors through the activation of apoptosis and the inhibition of angiogenesis in vivo. This confirms the effectiveness of the clinical application of QU in the treatment of ovarian cancer [
21].
QU also showed an anticancer effect on chemotherapy-resistant cells, such as CDDP, through the inhibition of the proliferation of ovarian carcinoma (SKOV3) and osteosarcoma (U2OS) human cell lines, as well as their cisplatin (CDDP)-resistant counterparts. Furthermore, the inhibition of the cyclin D1 level was associated with G1/S-phase alteration in QU-treated cells. CDK and cyclin inhibition by QU may be a viable therapeutic target in the ovarian CDDP-resistant cell line. Due to the ability of quercetin to overcome the resistance of cancers toward CDDP, a significant emphasis should be placed on using combinatory chemotherapy with cytotoxic drugs, particularly CDDP [
22].
Despite the several studies which have investigated the anticancer effects of QU in in vitro and in vivo models, some of these effects have not been observed in ovarian cancer. However, to the best of our knowledge, the underlying mechanisms by which QU sensitizes ovarian cancer cells to CDDP, particularly those with acquired CDDP resistance, e.g., SKOV-3/CDDP, remain elusive and still need to be elucidated to a large extent. In the previous study, after treatment with QU alone, cisplatin-resistant SKOV-3/CDDP ovarian cancer cell lines exhibited inhibition in the gene expressions of antioxidant enzymes (
SOD-1, SOD-2, Gpx-1, CAT, and
HO-1), transcription factor
NFE2L2, and the signaling pathway (
PI3K/Akt/mTOR), which contribute to cisplatin-resistance in these cells [
23].
In the present study, we attempted to explore the anticancer effects of QU pre-treatment on the CDDP-resistant ovarian adenocarcinoma human SKOV-3/CDDP cell line model. For the first time, we provide evidence that QU pre-treatment exerts pro-oxidant activity on the SKOV-3/CDDP cell line via alleviation of the protein expression of the cytosolic and mitochondrial thioredoxin antioxidant (Trx/TrxR) system that maintains STAT3 in a reduced/active state. We also identified the mechanism by which QU pre-treatment sensitizes SKOV-3/CDDP cells to cisplatin-induced apoptosis through a downregulation signaling (mTOR/STAT3) pathway. Thus, this research provides a better understanding of the mechanisms involved in QU-mediated CDDP sensitization in SKOV-3/CDDP ovarian cancer cells.
3. Discussion
Ovarian cancer is a continuing health problem that accounts for a significant portion of mortality among women around the world [
25,
26]. In current clinical practice, surgery combined with chemotherapy based on platinum compounds is considered the standard therapeutic strategy for ovarian cancer [
27]. CDDP is one of the most effective chemotherapeutic agents for ovarian cancer; however, resistance to this chemotherapeutic drug is a major obstacle to curative cancer therapy. Ovarian cancer cells develop resistance to anticancer drugs through various mechanisms, including DNA damage repair, cell metabolism, oxidative stress, cell cycle regulation, apoptotic pathways, and abnormal signaling pathways [
28]. Due to their various beneficial properties, polyphenols are potential therapeutic candidates in cancer when combined with classical antitumor agents such as cisplatin [
29]. For instance, QU could prevent ovarian cancer with its anti-inflammatory, pro-oxidative, and antiproliferation effects, and cell cycle arrest [
26]. Discovering the main quercetin mechanisms that reduce cisplatin resistance is important for improving cancer treatment. However, the role of quercetin in SKOV-3/CDDP has not yet been completely investigated.
We initially focused on the effect of QU alone on the ovarian adenocarcinoma SKOV-3/CDDP cell lines to establish a baseline for comparison with our later study [
23]. Hence, we hypothesized that the pre-exposure to QU might impair cisplatin resistance in ovarian adenocarcinoma cell lines. In the present study, we utilized two adenocarcinoma cell lines, i.e., the SKOV-3 (wild type) and SKOV-3/CDDP cisplatin-resistant sublines. Optimum doses for treatment were decided for QU and CDDP after examining the IC
50 values from our previously published data [
23,
24]. Pre-treatment with QU at low (15 µM) and high doses (100 µM) for 24 h followed by CDDP showed an antiproliferative effect in the SKOV-3/CDDP cisplatin-resistant subline, as well as in the SKOV-3 wild cells, and it showed a sensitization of the resistant subline to the cytotoxicity of CDDP (
Figure S1), while a long incubation for 48 h showed a maximum antiproliferative effect (
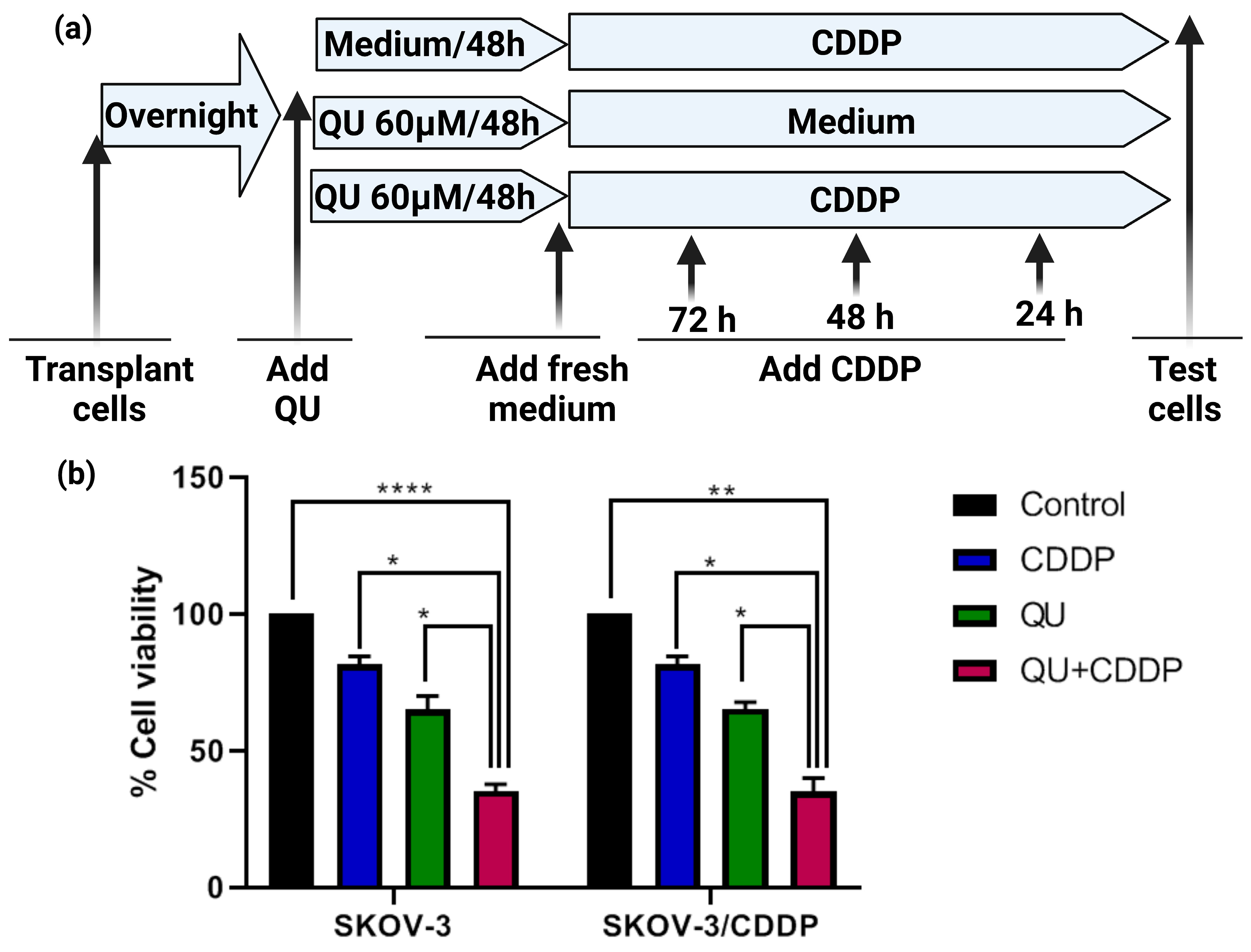
Figure 2b).
The pre-treatment of leiomyosarcoma cells with epigallocatechin-3-gallate, a polyphenol compound, for 24 h failed to alter the S-phase cell cycle arrest induced by CDDP and to modulate the CDDP effects on mitochondrial function [
30]. Furthermore, a recent study revealed that the higher efficacy of the prolonged pemetrexed pre-treatment of malignant pleural mesothelioma (MPM) for 48 h induced a cell cycle arrest, mainly in the G2/M phase, the accumulation of persistent DNA damage, and the induction of a senescence phenotype, thereby sensitizing cancer cells to subsequent CDDP treatment [
31]. In our study, the treatment of ovarian cancer cells with only QU for 24 h was shown to lead to a slightly increasing cell cycle in the S phase, whereas a long treatment for 48 h significantly induced a cell cycle arrest in the G1/S phase (
Figure S3). The conditions of a long treatment for 48 h significantly induced the cells to accumulate in sub-G1 after treatment with CDDP and increased the sensitization of the SKOV-3/CDDP subline to the subsequent CDDP treatment (
Figure 3b,d). The sensitization of the ovarian cells to CDDP was observed when the polyphenols, curcumin or QU, were added synergistically with CDDP, as well as when they were added 24 h before [
32]. In this study, two strategies (synergistic and pre-treatment) were designed. The result revealed that the SKOV-3/CDDP cells failed to enter into the sub-G1 phase even after a long incubation using the synergistic approach, while the cells effectively accumulated in sub-G1 using the QU pre-treatment strategy (
Figure S4). Our strategy allowed the progression of the QU-treated cells into the S phase, whereby re-exposure of the QU-exhausted cells to CDDP after washing out the QU from the culture medium was sufficient to increase the duration of the S phase, which in turn led to further cell damage. The evaluation of this pre-treatment therapy in cisplatin-resistant ovarian cancer cells may lead to more efficient CDDP treatment.
The antioxidant or pro-oxidant properties of polyphenol compounds depend predominantly on the number and positions of the substituent hydroxyl groups, along with their redox metal (Cu, Fe) chelating capacity. For example, the higher number of hydroxyl groups, including the 3-OH group of the C ring of polyphenols in the presence of Cu(II) ions, act as potential anticancer agents with moderate pro-oxidant activity, causing DNA damage via interaction with DNA and ROS formation via the Fenton reaction. Otherwise, the slight pro-oxidant activity of polyphenols acts as a preventive anticancer therapeutic agent via the inducing of cellular antioxidant systems, including antioxidant enzymes, and the synthesis of low-molecular-weight antioxidants, such as glutathione [
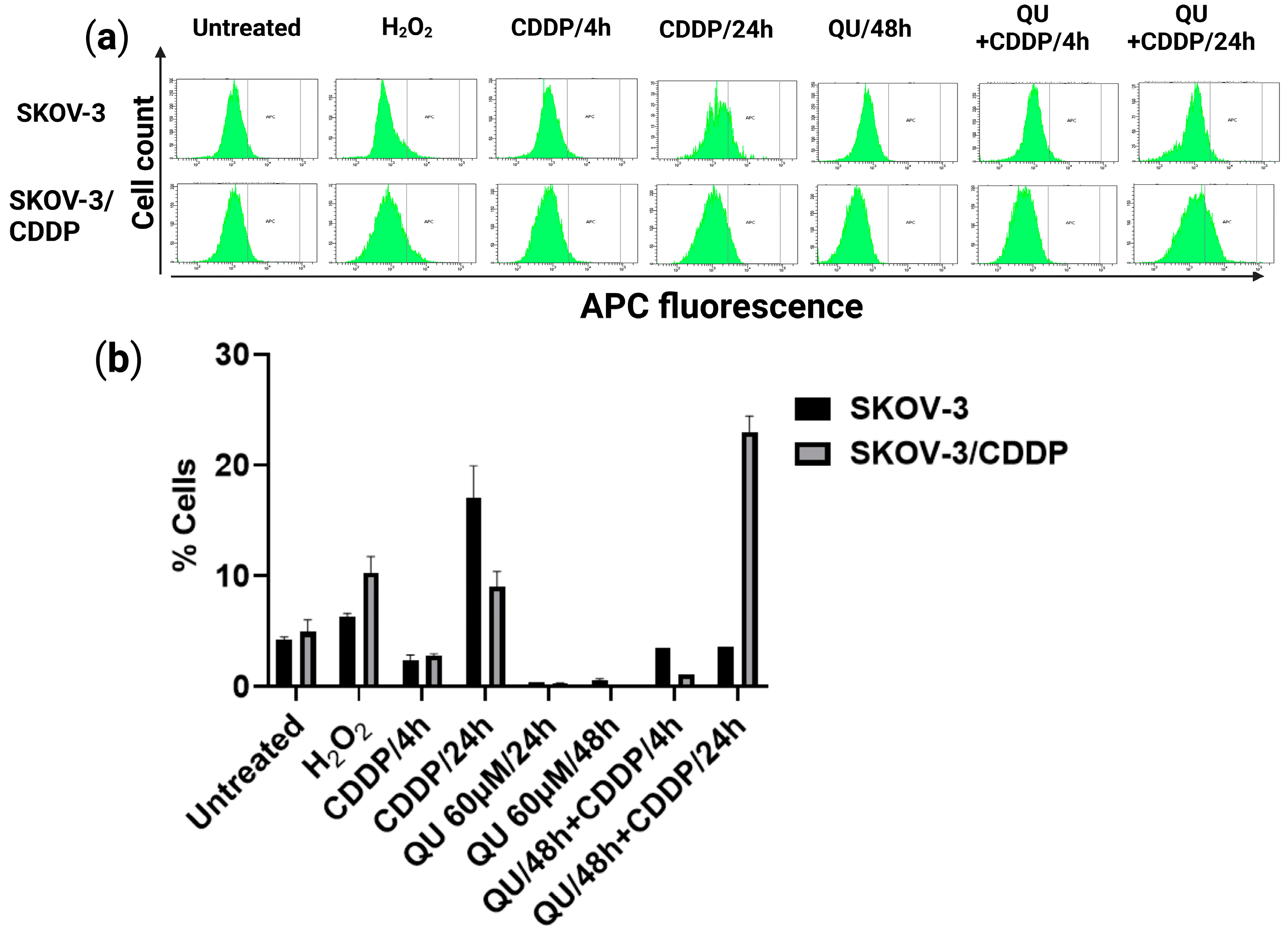
33]. In this study, QU could not effectively induce ROS generation, while CDDP slightly induced the ROS level in the SKOV/CDDP cells. However, QU pre-treatment effectively triggered the generation of ROS in the SKOV-3/CDDP cells. These data indicate that the increased oxidative stress induced by QU pre-treatment may have activated an apoptotic cascade in the SKOV-3/CDDP cells (
Figure 4) and inhibited expression of antioxidant proteins and the signaling pathway (
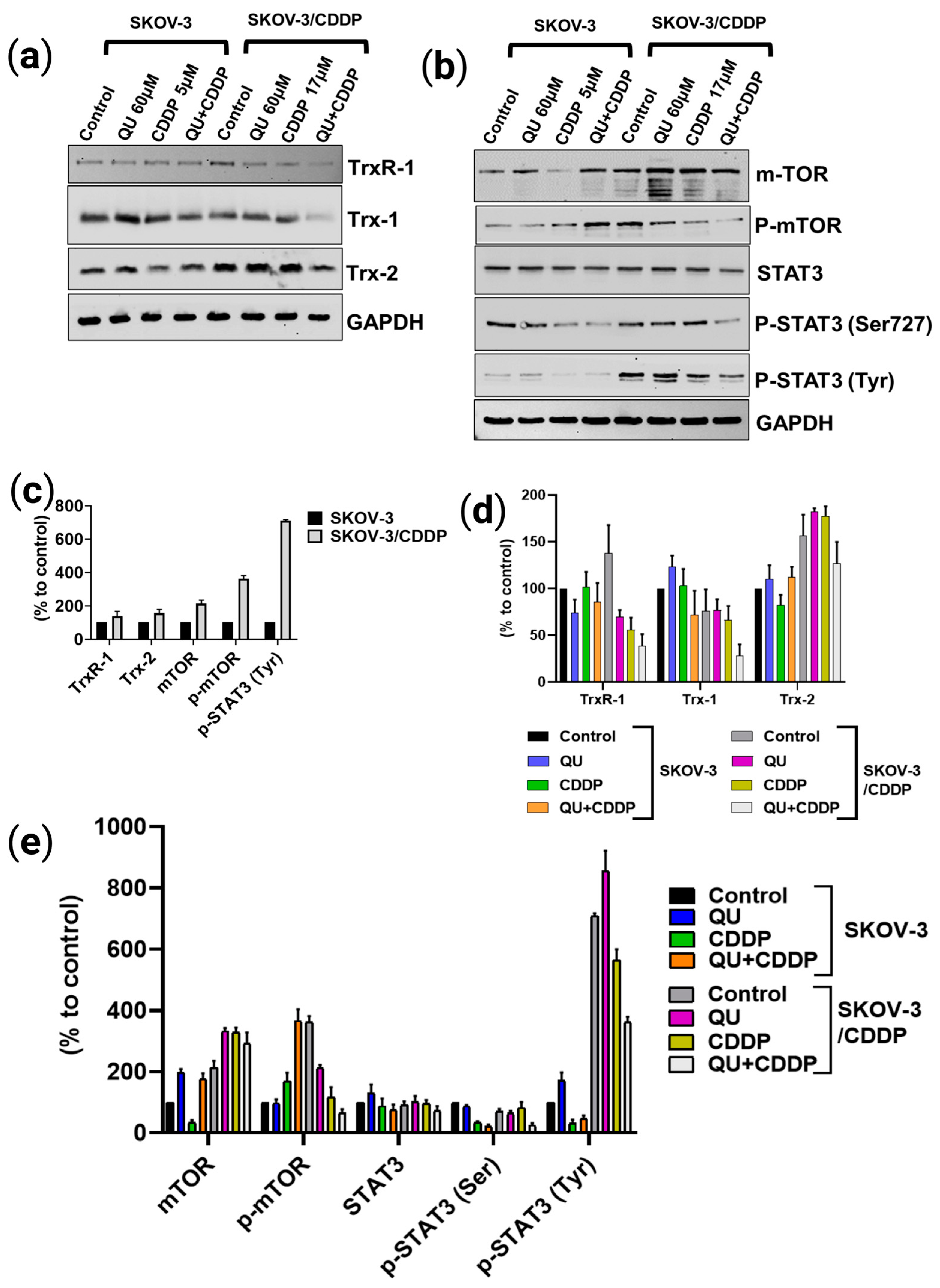
Figure 5a,b,d,e). Alternatively, QU pre-treatment in SKOV-3 acts as an antioxidant by decreasing ROS formation and inducing the expression of antioxidant proteins (
Figure 5a,d) and the signaling of the mTOR/STAT3 pathway (
Figure 5b,e). In the SKOV-3/CDDP cells, the oxidative stress was also monitored by the measuring of the antioxidant system and the signaling pathway at the protein levels.
In addition to altered proliferation, the cell cycle, oxidative stress, cell metabolism, increased ability to repair DNA damage, and reduced susceptibility to apoptosis, ovarian cancer cells develop resistance to anticancer drugs through various other mechanisms, defined as enhancement expression and alteration of signaling pathways [
28]. In addition to the generation of ROS species, we also monitored the changes in the oxidative stress response proteins. Therefore, the marker proteins that were highly correlated with cellular sensitivity to CDDP were screened. Notably, we previously [
23] provided the molecular evidence that antioxidant enzymes (
SOD-1, SOD-2, Gpx-1, CAT, and
HO-1), transcription factor
NFE2L2, and the PI3K/Akt/mTOR signaling pathway are upregulated in SKOV-3/CDDP subline cells compared to normal line SKOV-3 cells and found that these gene expressions were associated with CDDP tolerance. The individual treatment with QU revealed marked downregulation in the expression of these genes. Our results suggest that QU pre-treatment has the potential to increase sensitivity to CDDP in SKOV-3/CDDP subline cells by regulating oxidative stress (ROS generation) and cell apoptosis via the antioxidant system and the signaling pathway.
Hence, we assessed the pro-oxidative therapeutic potential effect of QU pre-treatment on the antioxidative system. The thioredoxin system (Trx/TrxR) is essential to maintaining cellular redox homeostasis in living organisms through detoxification of harmful metabolites, i.e., ROS; however, the overexpression of the Trx-dependent system can also contribute to the intensifying of the process of oncogenesis, such as by the enhancement of tumor growth, angiogenesis, and resistance to therapy via the modulation of both the gene expression and the cell signaling pathways that lead to the regulation of apoptotic pathways in cancer cells [
34]. Compared to SKOV-3, which exhibits normal Trx/TrxR protein expressions, SKOV-3/CDDP subline cells display unique overexpression in both the cytosolic (TrxR-1) and mitochondrial (Trx-2) protein levels, which are directly associated with resistance to cisplatin (
Figure 5c). Accumulating evidence suggests that the tumoral activity of the Trx-dependent system can be modulated by natural polyphenolic compounds, which act as Trx/TrxR system inhibitors [
34]. The inhibitory effect of QU depends on many factors, including concentration, NADPH, and the time of exposition and involvement in an attack on the reduced COOH-terminal active site sequence -Gly-Cys-Sec-Gly of TrxR. The inhibition of TrxR activity is associated with the oxidization of Trx in the cells [
35]. As expected, QU pre-treatment effectively reduced the levels of thioredoxin system (Trx-1, TrxR-1, and Trx-2) proteins in the SKOV-3/CDDP cells (
Figure 5a,d).
Furthermore, STAT3 and its two phosphorylated forms (on Ser
727 and Tyr
705) were also found to be inhibited at the protein (
Figure 5b,e) level by the QU pre-treatment in the SKOV-3/CDDP subline cells. Several TrxR-1 inhibitors also block the signal transducers and activators of transcription 3 (STAT3) activity via accumulation of oxidized STAT3, which blocks STAT3-dependent transcription [
10,
36] and induces cancer cell death [
10]. Due to the thiol groups in the Cys residues in the active sites, reduced Trx catalyzes the reduction in disulfide bonds within oxidized target proteins [
36,
37], such as STAT3 [
36].
Previously, SKOV-3/CDDP subline cells were shown to overexpress the
mTOR gene, which was downregulated after treatment with QU [
23]. A recent study demonstrated a significant correlation between STAT3 and mTOR overexpression, which underlies cancer-mediated drug resistance and cancer progression. Furthermore, the phosphorylation of STAT3 on Ser
727 is regulated by mTOR [
11]. Our data support the findings of the study; in particular, both STAT3 and mTOR were overexpressed in SKOV-3/CDDP compared with SKOV-3, and after QU pre-treatment, SKOV-3/CDDP showed inhibition of two phosphorylated STAT3 isoforms at Ser
727 and Tyr
705, as well as the inhibition of phospho-mTOR Ser
2448. This was followed by mTOR/STAT3 pathway inactivation, which ultimately inhibits proliferation and induces caspase-dependent apoptosis in ovarian SKOV-3/CDDP cancer cells.
Apoptotic cell death can be triggered by inhibition of the mTOR/STAT3 signaling pathways that are closely linked with tumor progression. Moreover, inducing cell cycle arrest by enhancing the p53 phosphorylation and apoptosis pathway after treatment with QU in HPV-positive human cervical-cancer-derived cells may be one of the key mechanisms underlying the inhibition of cell proliferation in cancer cells [
38]. Annexin V/PI staining showing early and late apoptotic cells suggests the activation of apoptosis following pre-exposure to QU (
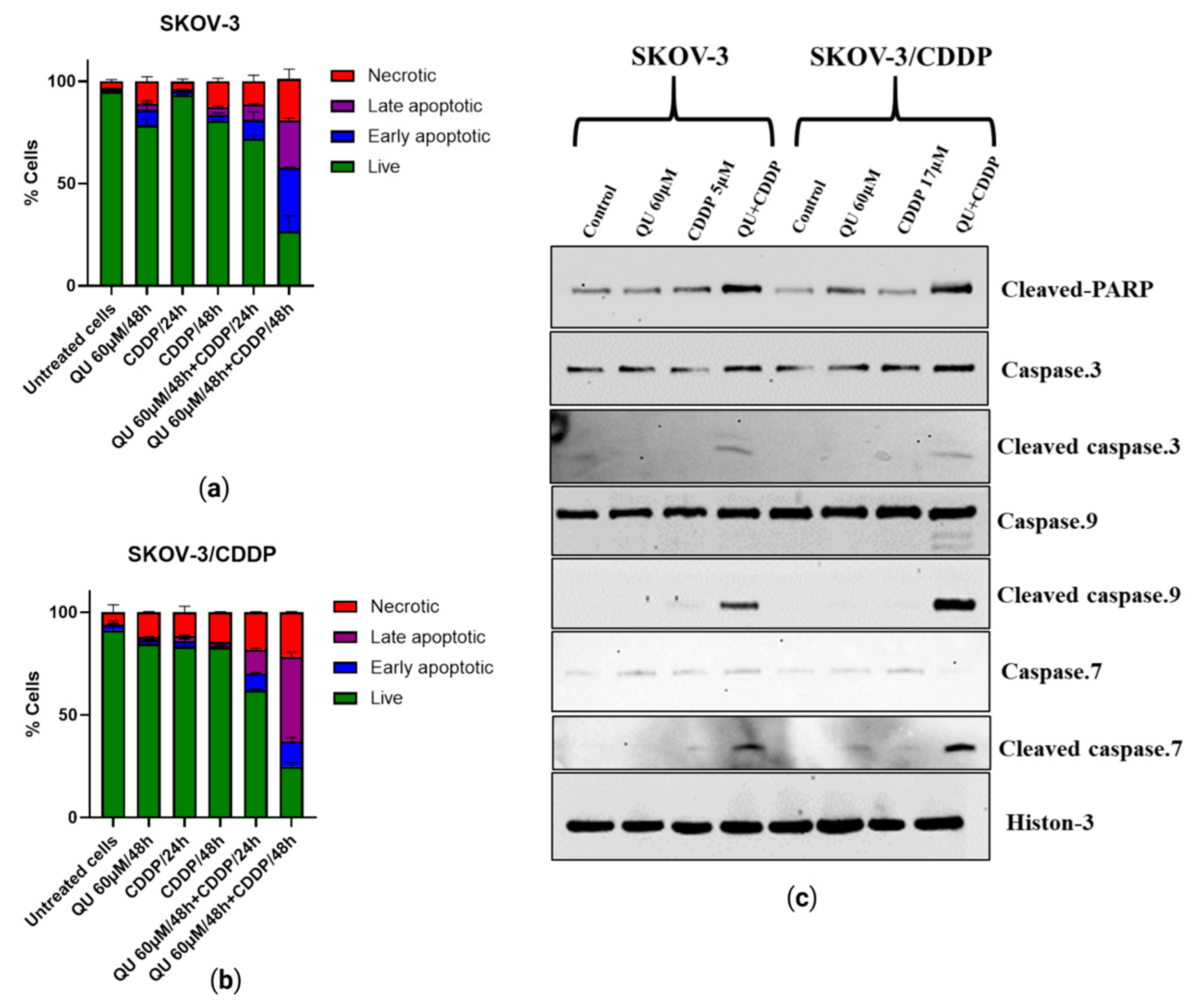
Figure 6a,b). The results of this study also indicated that QU pre-treatment induces SKOV-3 and SKOV-3/CDDP cell death through the mitochondria-dependent apoptosis pathway, where mitochondrial dysfunction, increased PARP cleavage, and activation of caspase-9,-7,-3 are critical events for apoptosis (
Figure 6c). QU showed induction in both necrosis and apoptosis cell death in various cancer cells, including SCC-9 oral cancer cells [
39] and prostate cancer over a period of time [
40]. Apoptosis was induced by QU pre-treatment, and necrotic cells were induced in a time-dependent manner (
Figure 6a,b). The proposed anti-cancer and sensitizing mechanisms of QU pre-treatment are summarized in
Figure 7.
Taken together, the anticancer and chemosensitizing effects of QU pre-treatment are associated with the inducing of ROS-mediated apoptosis and the alleviation of the protein expressions that are highly correlated with cellular sensitivity to cisplatin, including antioxidant enzymes and the signaling pathway.
4. Materials and Methods
4.1. Reagents
The chemicals used in the experiments were purchased from the following suppliers: cisplatin (cis-platinum (ii)-diamine dichloride (CDDP; Teva, Tel Aviv-Yafo, Israel)); 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (PanEco); and quercetin (QU) (3,3′,4′,5,7-pentahydroxyflavone) from (Acros Organics, Geel, Belgium). The chemical structures of CDDP and QU are illustrated in (
Figure 1). The purity of QU was [97% (HPLC)]. The stock quercetin solution was immediately prepared before use by dissolving these test agents in dimethyl sulfoxide (DMSO) at 100 mM concentration.
4.2. Cell Culture
The SKOV-3 non-resistant human ovarian adenocarcinoma and SKOV-3/CDDP cisplatin-resistant subline cells were obtained from the All-Russian Scientific Center for Molecular Diagnostics and Treatment. The SKOV-3 and SKOV-3/CDDP cells were cultured in DMEM cell culture media supplemented with 10% heat-inactivated fetal bovine serum (HealthCare, Chicago, IL, USA), 2 mM L-glutamine, 100 U/mL penicillin, and 50 μg/mL streptomycin. All the cells were incubated at 37 °C under 5% CO2 in a humidified incubator.
4.3. Cell Viability (MTT Assay)
The inhibitory effect of the pre-treatment of QU followed by CDDP on ovarian cancer cells was assessed using the MTT assay. The SKOV-3 and SKOV-3/CDDP cells were seeded into 96-well plates (Nunc, ThermoFisher Scientific, Waltham, MA, USA) at a density of 5 × 103 cells per well overnight in triplicates. After the incubation period with QU, the culture medium was replaced with a fresh medium, and the cells were then treated with (5 µM, 1/2 IC50, for SKOV-3) or (17 µM, 1/2 IC50, for SKOV-3/CDDP) of CDDP for 72 h. The culture medium was again replaced with a fresh medium and 20 μL; 5 mg/mL MTT was added to each well; and the plates were further incubated at 37 °C for 4 h, allowing the viable cells to reduce the yellow MTT to dark blue formazan crystals. The supernatant was removed, and the formazan crystals were dissolved in 100 μL DMSO and mixed thoroughly prior to determination of the absorbance at a wavelength of 570 nm using a Multiscan FC plate reader (Thermo Scientific, Waltham, MA, USA). Cell viability was calculated according to the following equation: cell viability (%) = (absorbance of experiment group/absorbance of control group) × 100. The IC50 was developed by an inhibition curve and recorded as the mean ± standard deviation of three independent experiments.
4.4. Cell Cycle Assay
The distribution of the cell cycle was analyzed by flow cytometry using the FACS Canto II (BD Biosciences, Franklin Lakes, NJ, USA). The SKOV-3 and SKOV-3/CDDP cells in the logarithmic growth phase were seeded at a density of 1.5 × 105 cells/well in a 6-well plate, incubated at 37 °C, 5% CO2 until adherent, and treated with CDDP alone and QU alone or followed by CDDP, as described above. After incubation, the cells were harvested and washed once with phosphate-buffered saline (PBS). The pellets were then lysed in the solution containing 0.1% sodium citrate, 0.3% NP-40, 50 μg/mL RNAse A, and 10 μg/mL PI. The cells were analyzed using a BD FACS Canto II flow cytometer in a PE channel. Ten thousand ‘events’ were acquired per each sample. The data were analyzed using the FACSDiva program (BD Biosci., Franklin Lakes, NJ, USA).
4.5. Intracellular Reactive Oxygen Species (ROS) Assay
The intracellular ROS level was measured to detect the anti- or pro-oxidant effect of QU pre-treatment on the SKOV-3/CDDP subline cells. ROS Deep Red dye (RDR; Cellular Reactive Oxygen Species Detection Assay Kit; Abcam, Cambridge, UK) was used to assess the production of ROS. CellROX Deep Red dye freely penetrates into cells, which in turn are oxidized by ROS into a highly fluorescent color. Briefly, the SKOV-3 and SKOV-3/CDDP cells were seeded at a density of 1.5 × 105 cells/well in a 6-well plate, treated with CDDP alone for 4 h or 24 h, QU alone for 24 h or 48 h or followed by CDDP for 4 h or 24 h, and 5 mM H2O2, as a positive control, (Ecotex, Moscow, Russia; a reference ROS inducer) for 30 min separately. After incubation, the cells were finally stained with 5 µg/mL CellROX Deep Red dye for 40 min at 37 °C in the dark and analyzed on a BD FACSCanto II (BD Biosciences, Franklin Lakes, NJ, USA) flow cytometer in the APC channel (filter 660/20). The data were analyzed using the FACSDiva program (BD Biosciences). The ROS production for each compound was represented as the percentage based on the shift of the RDR fluorescence in the drug-treated vs. untreated control cells. Ten thousand fluorescent ‘events’ were acquired per each sample.
4.6. Western Blotting Analysis
Proteins from the treated cells were extracted using RIPA lysis buffer (50 mM Tris-HCl pH 7.4, 1% NP-40, 0.1% sodium dodecylsulfate, 150 mM NaCl, 1 mM EDTA) containing a protein inhibitor cocktail and 2 mM phenylmethylsulfonyl fluoride, then kept on ice for 30 min and centrifuged at 10,000×
g for 15 min. The total protein in the lysates was quantitated by the Bradford method [
41]. The proteins were resolved by 10–15% SDS-PAGE (40 mg total protein/lane) and transferred onto a 0.2 mm nitrocellulose membrane (GE Healthcare Bio-Sci., Chicago, IL, USA). The membranes were blocked with 5% skimmed milk in 1x TBST (Tris-buffered saline with 0.1% Tween-20), incubated for 2 h, and then probed overnight at 4 °C with the indicated primary rabbit antibodies against catalase; mTOR; phospho-mTOR (S
2448); Trx1; TrxR-1(Abcam (Cambridge, MA, USA)); Trx-2; STAT3; phospho-STAT3 (Tyr
705 and ser
727) (Sigma-Aldrich (St. Louis, MO, USA)); caspase 3; caspase 7; caspase 9, PARP; the cleaved caspase 3 (Asp
175); cleaved caspase 7; cleaved caspase 9 (Asp
330); cleaved PARP (Asp
214); and Histon-3/GAPDH (as reference proteins) (Cell Signaling Technology (Danvers, MA, USA)). Next, the membranes were washed twice with 1x TBST and incubated with secondary antirabbit antibodies conjugated with horseradish peroxidase (HRP) (Cell Signaling Tech., Danvers, MA, USA) for 1 h at room temperature. The immunoreactive bands were visualized by enhanced chemiluminescence using the Image Quant LAS 4000 system (GE Healthcare, Chicago, IL, USA).
4.7. Apoptotic Programmed Cell Death Analysis
Apoptosis was measured using the Annexin V-FITC apoptosis detection kits (Thermo Fisher Scientific Inc., Waltham, MA, USA). The SKOV-3 and SKOV-3/CDDP cells in the logarithmic growth phase were seeded at a density of 1.5 × 105 cells/well in a 6-well plate, incubated overnight at 37 °C, 5% CO2, and treated with CDDP alone or QU alone and followed by cisplatin. The cells were harvested and washed once with PBS and once with 1× binding buffer. The pellets were then resuspended in 100 μL 1× binding buffer, stained with 5 μL of fluorochrome-conjugated Annexin V-APC or FITC, and incubated for 10–15 min at room temperature in the dark. After adding 2 mL of 1× binding buffer, the samples were centrifuged at 400–600 g for 5 min at room temperature. The supernatants were removed; the cells were re-suspended in 200 μL of 1× binding buffer; 5 μL of propidium iodide (PI) staining solution was added and incubated for 5–15 min on ice and analyzed by flow cytometry on a BD FACSCanto II (BD Biosciences, Franklin Lakes, NJ, USA) in the APC channel (filter 660/20 for detection of Annexin V-positive cells) and in the PerCP-Cy™5.5 channel (filter 695/40 for PI-positive cells). The data were analyzed using the FACSDiva program (BD Biosci.).
4.8. Statistical Analysis
All the in vitro data presented herein are presented as the mean ± standard deviation (SD). Each of the experiments was repeated at least three times (n = 3). Statistical analysis and graphs were generated using GraphPad Prism 8.0 software (San Diego, CA, USA).