Inhibition of Ferroptosis Enables Safe Rewarming of HEK293 Cells following Cooling in University of Wisconsin Cold Storage Solution
Abstract
1. Introduction
2. Results
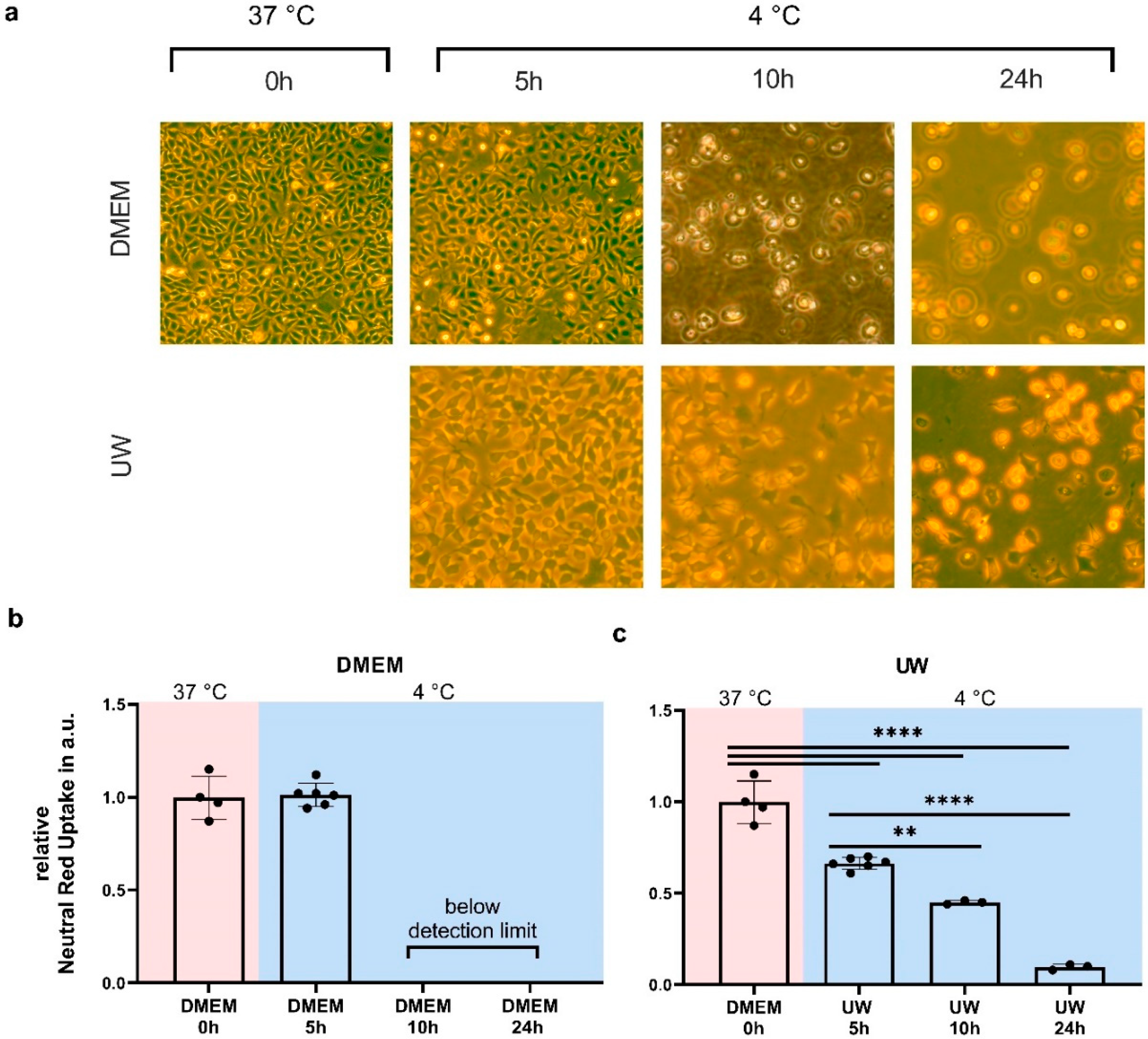
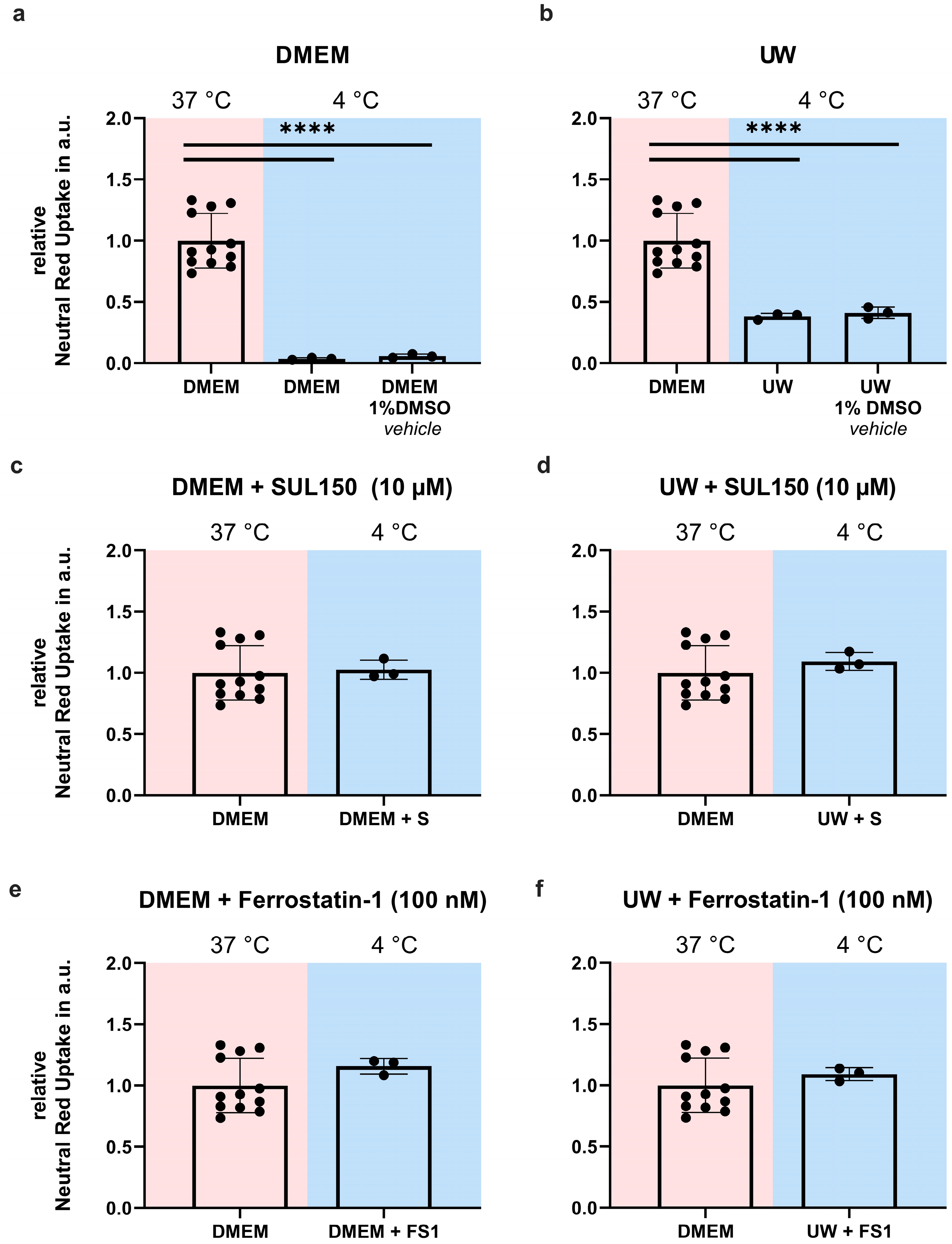
2.1. Hypothermia Causes Cell Death in HEK293 Cells That Is Partially Ameliorated by UW
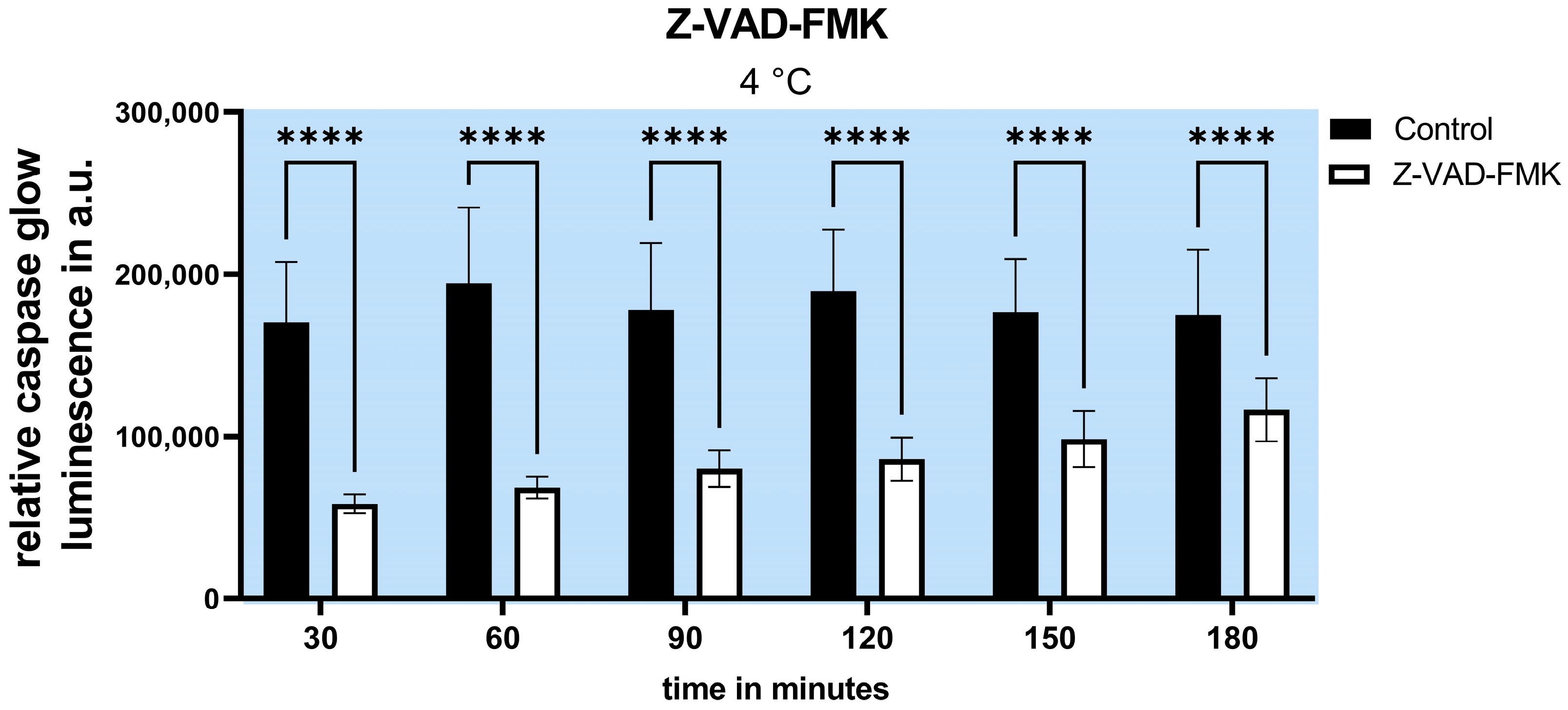
2.2. Inhibition of Apoptosis Does Not Increase Cell Survival
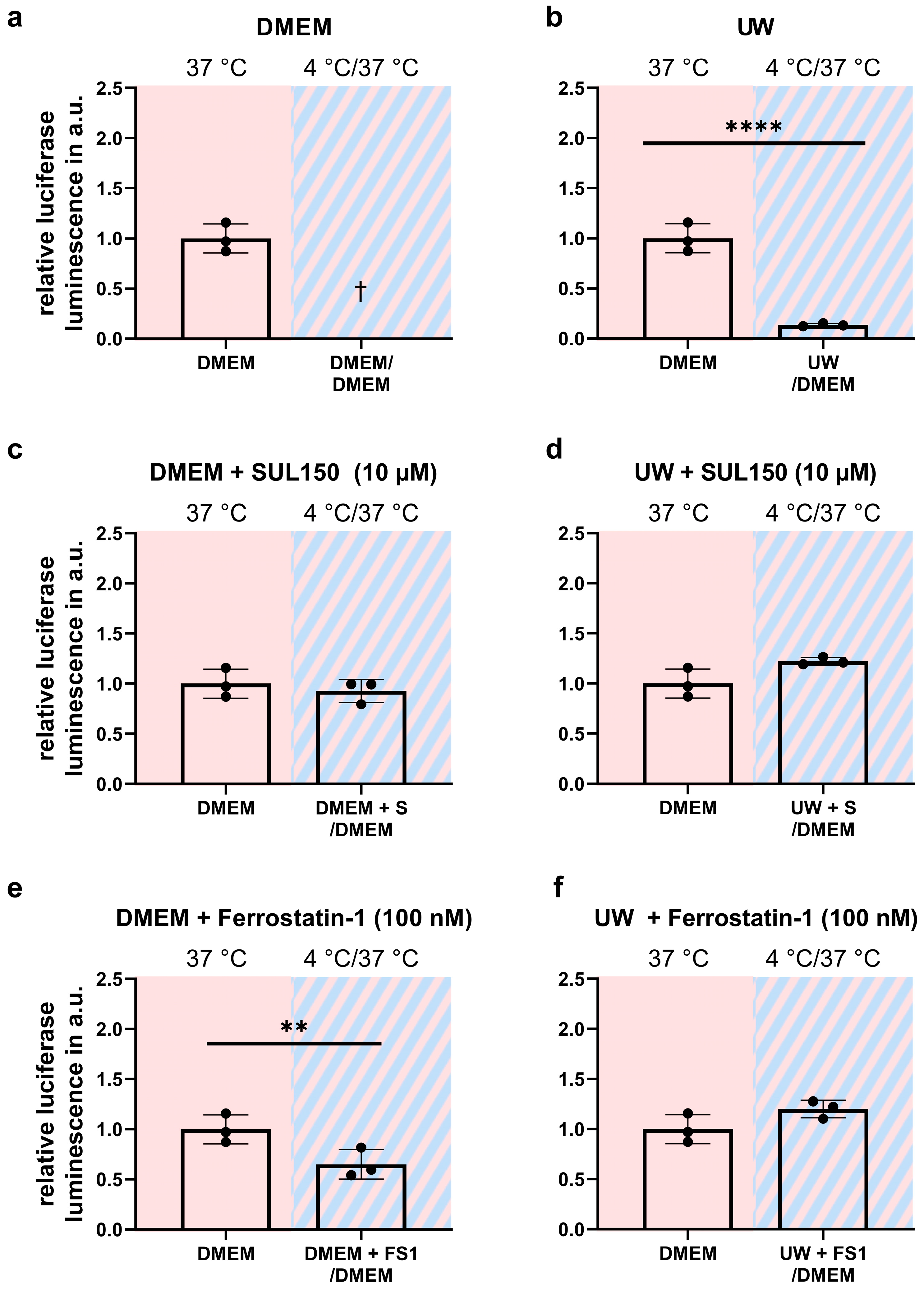
2.3. Addition of SUL150 and Ferrostatin-1 Preventing Ferroptosis
2.4. Rewarming Causes ATP Depletion and Lipid Peroxidation in UW Cold-Preserved HEK293 Cells
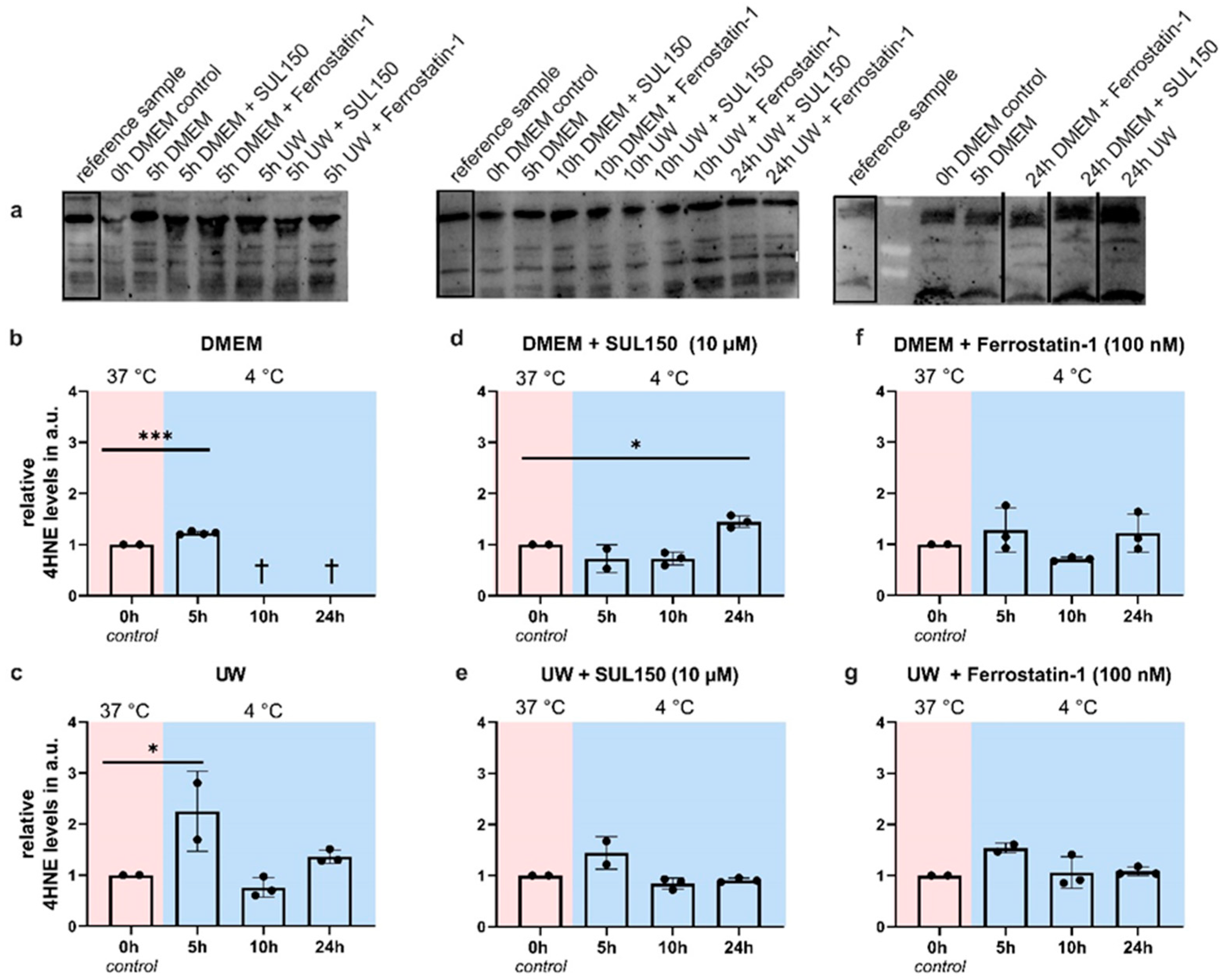
2.5. UW Prevents Oxidative Stress in the Cold
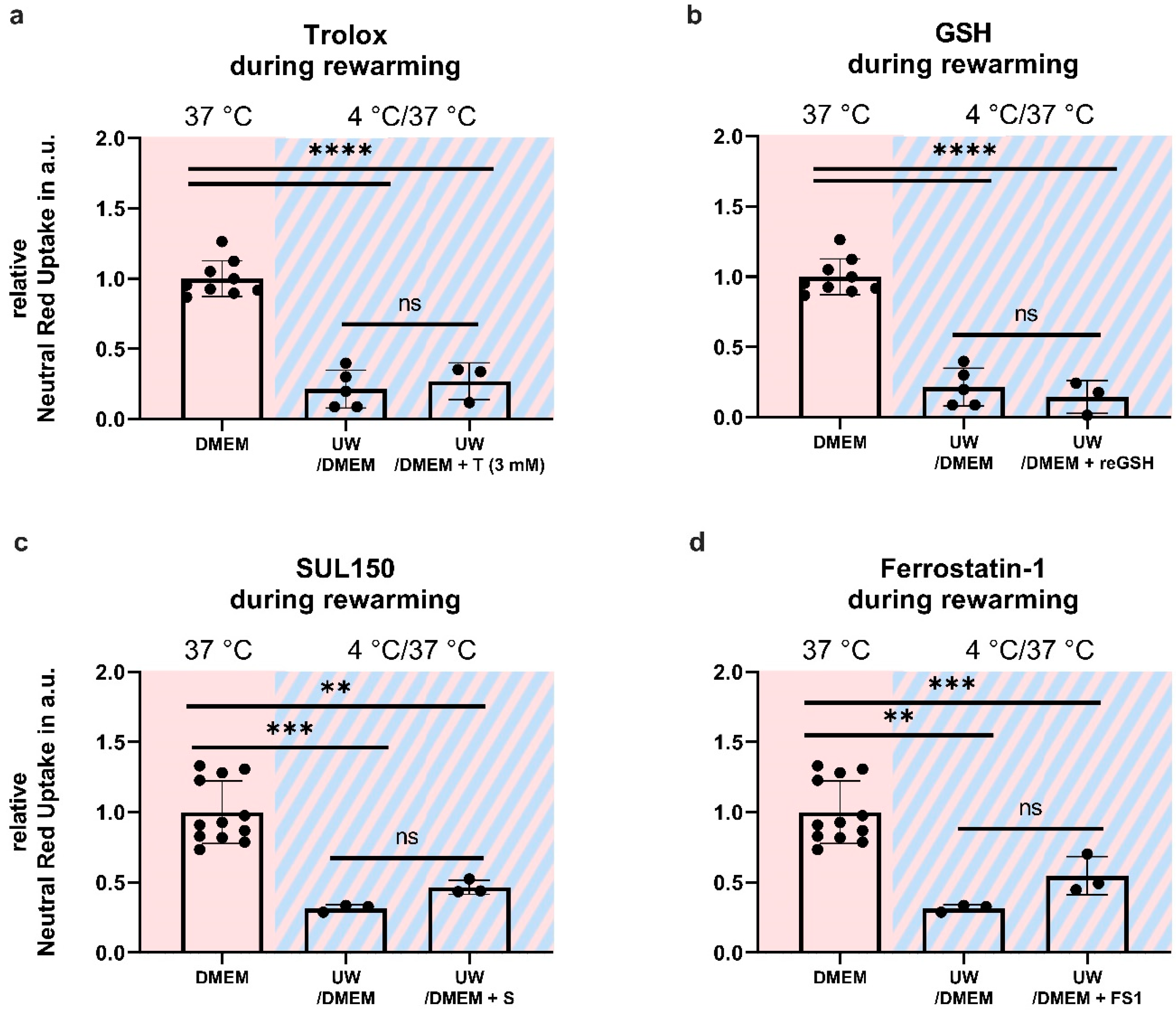
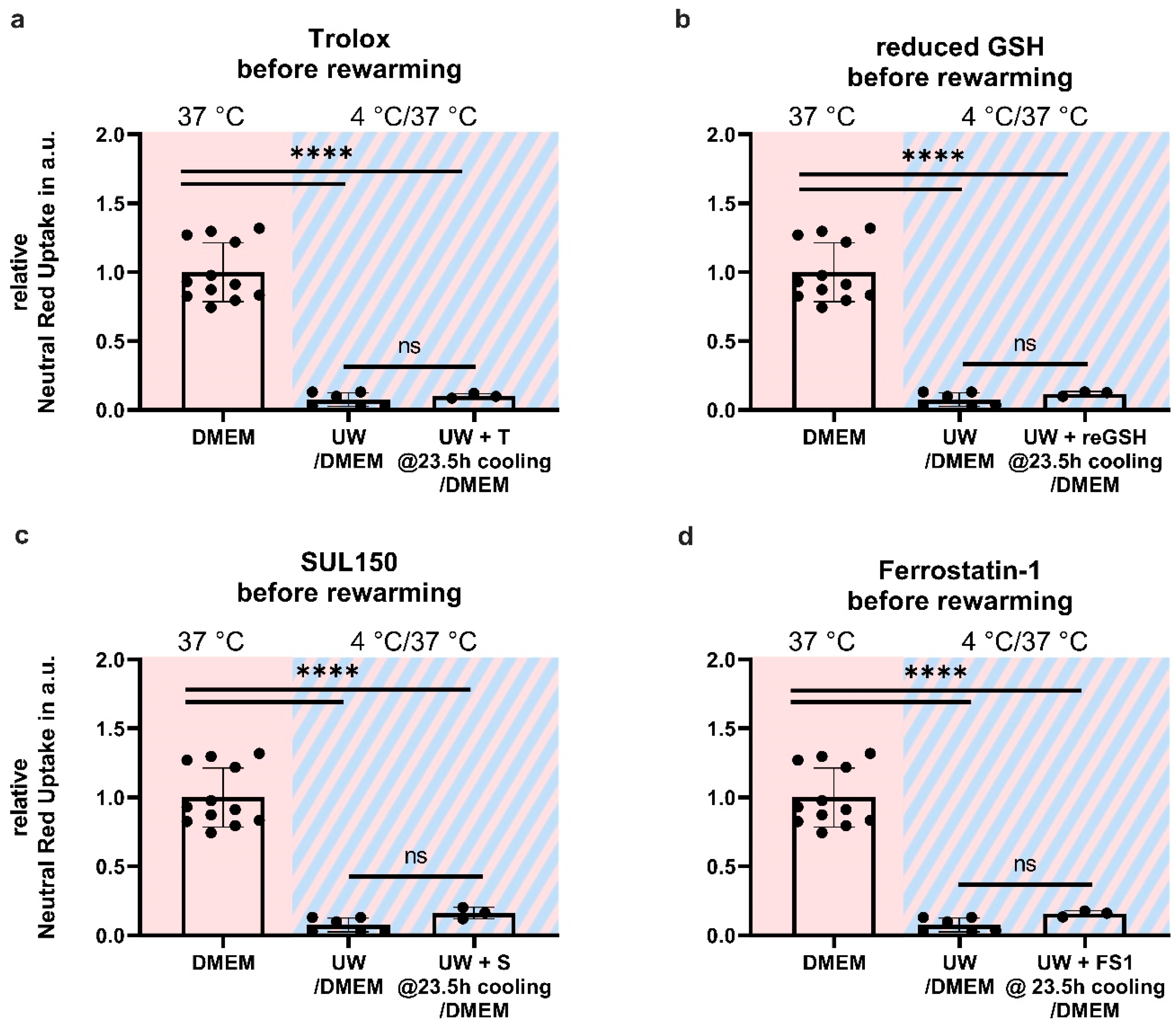
2.6. Antioxidant Supplementation Shortly before or during Rewarming Does Not Prevent Cell Death
2.7. UW Does Not Preclude Progressive Loss of GPx4
2.8. Proteasome Inhibition Does Not Prevent GPx4 Loss or Cell Death
3. Discussion
3.1. UW-Cooled Cell Death Is Executed through Ferroptosis Rather Than Apoptosis
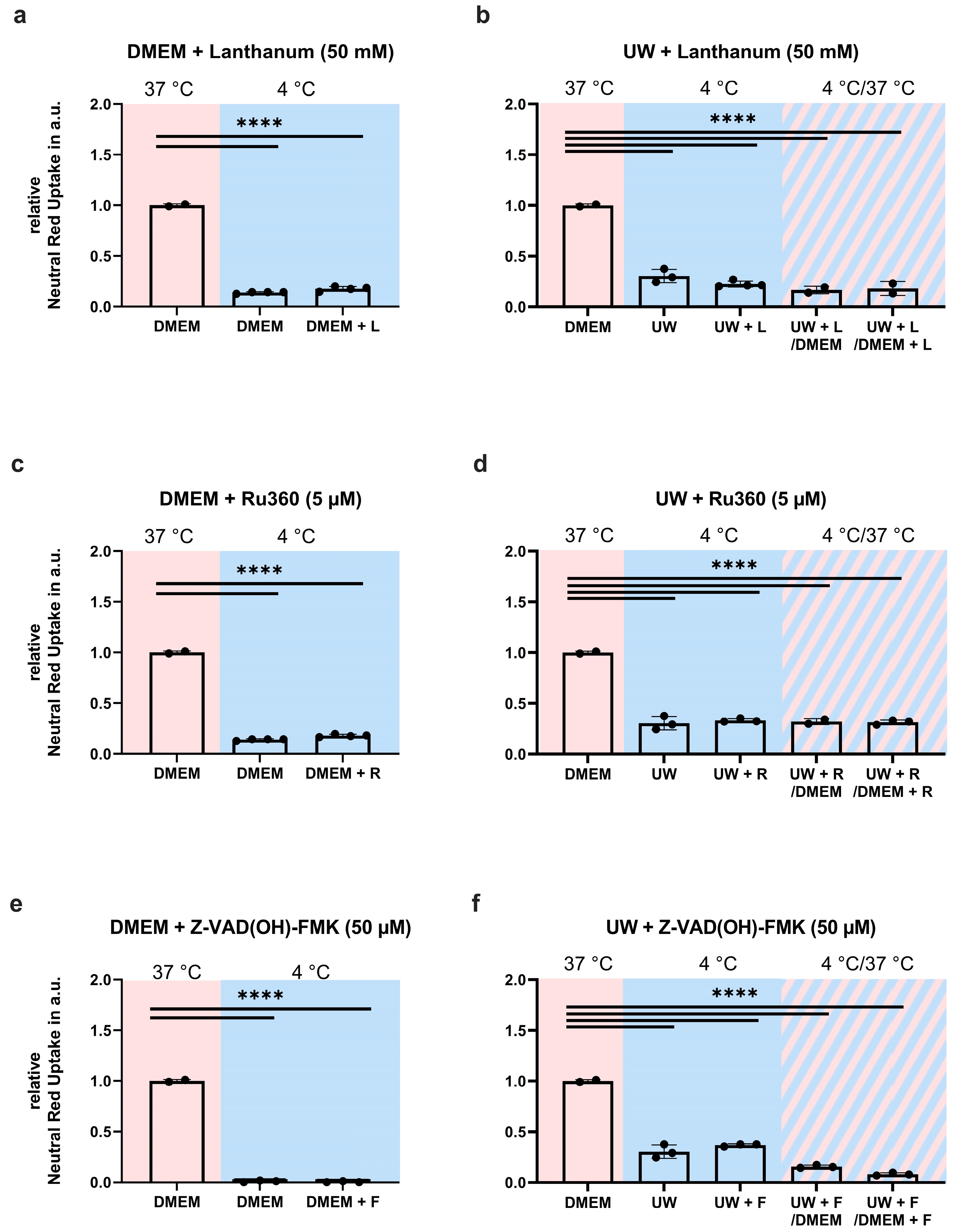
3.2. ATP Depletion, Calcium, and Hypothermic Cell Death
3.3. Early Treatment Prevents Ferroptosis
3.4. Limitations
4. Materials and Methods
4.1. Cellular Cooling and Rewarming Model
4.2. Neutral Red Cell Survival Assay
4.3. ATP Measurement
4.4. MDA Measurement
4.5. Western Blot
4.6. Caspase 3/7 Glow Assay
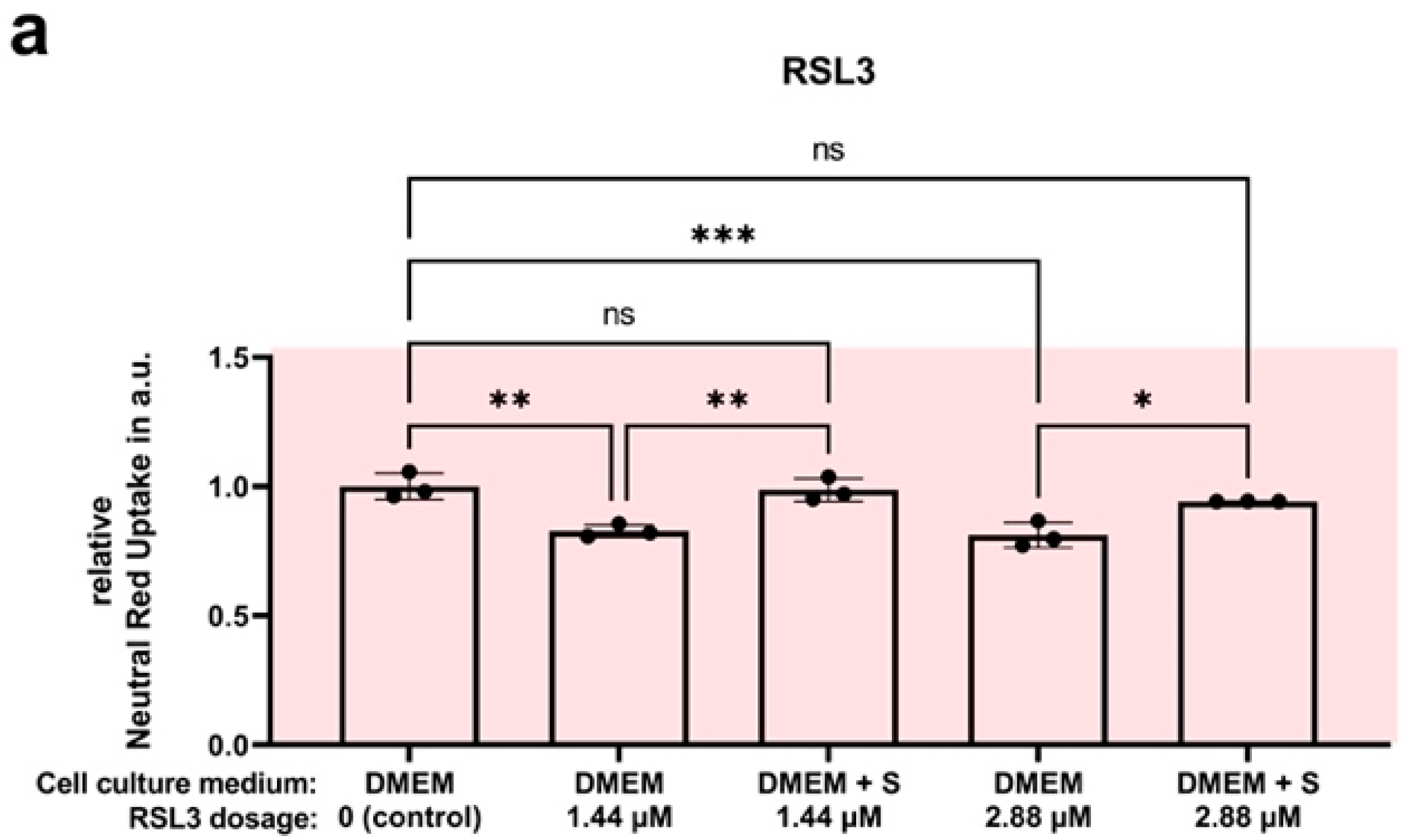
4.7. Efficacy Assay: SUL150 versus Erastin and RSL3
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- De Rosa, S.; Antonelli, M.; Ronco, C. Hypothermia and kidney: A focus on ischaemia–reperfusion injury. Nephrol. Dial. Transplant. 2016, 32, 241–247. [Google Scholar] [CrossRef]
- Jing, L.; Yao, L.; Zhao, M.; Peng, L.-P.; Liu, M. Organ preservation: From the past to the future. Acta Pharmacol. Sin. 2018, 39, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Steichen, C.; Giraud, S.; Bon, D.; Barrou, B.; Badet, L.; Salamé, E.; Kerforne, T.; Allain, G.; Roumy, J.; Jayle, C.; et al. Barriers and Advances in Kidney Preservation. BioMed Res. Int. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- van Heurn, L.W.; Talbot, D.; Nicholson, M.L.; Akhtar, M.Z.; Sanchez-Fructuoso, A.I.; Weekers, L.; Barrou, B. Recommendations for donation after circulatory death kidney transplantation in Europe. Transpl. Int. 2015, 29, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Opelz, G.; Döhler, B. Multicenter Analysis of Kidney Preservation. Transplantation 2007, 83, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Dragun, D.; Hoff, U.; Park, J.-K.; Qun, Y.; Schneider, W.; Luft, F.C.; Haller, H. Prolonged cold preservation augments vascular injury independent of renal transplant immunogenicity and function. Kidney Int. 2001, 60, 1173–1181. [Google Scholar] [CrossRef]
- Debout, A.; Foucher, Y.; Trébern-Launay, K.; Legendre, C.; Kreis, H.; Mourad, G.; Garrigue, V.; Morelon, E.; Buron, F.; Rostaing, L.; et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int. 2015, 87, 343–349. [Google Scholar] [CrossRef]
- Peters-Sengers, H.; Houtzager, J.H.; Idu, M.M.; Heemskerk, M.B.; Van Heurn, E.L.; Van Der Heide, J.J.H.; Kers, J.; Berger, S.P.; Van Gulik, T.M.; Bemelman, F.J. Impact of Cold Ischemia Time on Outcomes of Deceased Donor Kidney Transplantation: An Analysis of a National Registry. Transplant. Direct 2019, 5, e448. [Google Scholar] [CrossRef]
- Tennankore, K.K.; Kim, S.J.; Alwayn, I.P.; Kiberd, B.A. Prolonged warm ischemia time is associated with graft failure and mortality after kidney transplantation. Kidney Int. 2016, 89, 648–658. [Google Scholar] [CrossRef]
- Salvadori, M.; Rosso, G.; Bertoni, E. Update on ischemia-reperfusion injury in kidney transplantation: Pathogenesis and treatment. World J. Transplant. 2015, 5, 52–67. [Google Scholar] [CrossRef]
- Hendriks, K.D.W.; Brüggenwirth, I.M.A.; Maassen, H.; Gerding, A.; Bakker, B.; Porte, R.J.; Henning, R.H.; Leuvenink, H.G. Renal temperature reduction progressively favors mitochondrial ROS production over respiration in hypothermic kidney preservation. J. Transl. Med. 2019, 17, 265. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, K.D.W.; Lupi, E.; Hardenberg, M.C.; Hoogstra-Berends, F.; Deelman, L.E.; Henning, R.H. Differences in mitochondrial function and morphology during cooling and rewarming between hibernator and non-hibernator derived kidney epithelial cells. Sci. Rep. 2017, 7, 15482. [Google Scholar] [CrossRef] [PubMed]
- Tolouee, M.; Hendriks, K.D.W.; Lie, F.F.; Gartzke, L.P.; Goris, M.; Hoogstra-Berends, F.; Bergink, S.; Henning, R.H. Cooling of Cells and Organs Confers Extensive DNA Strand Breaks Through Oxidative Stress and ATP Depletion. Cell Transplant. 2022, 31. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, K.D.; Joschko, C.P.; Hoogstra-Berends, F.; Heegsma, J.; Faber, K.-N.; Henning, R.H. Hibernator-Derived Cells Show Superior Protection and Survival in Hypothermia Compared to Non-Hibernator Cells. Int. J. Mol. Sci. 2020, 21, 1864. [Google Scholar] [CrossRef] [PubMed]
- Bayley, J.S.; Winther, C.B.; Andersen, M.K.; Grønkjær, C.; Nielsen, O.B.; Pedersen, T.H.; Overgaard, J. Cold exposure causes cell death by depolarization-mediated Ca2+ overload in a chill-susceptible insect. Proc. Natl. Acad. Sci. USA 2018, 115, E9737–E9744. [Google Scholar] [CrossRef]
- Aerts-Kaya, F.S.; Visser, T.P.; Pervin, B.; Mammadova, A.; Özyüncü, Ö.; Wagemaker, G.; Uçkan-Çetinkaya, F.D. SUL-109 Protects Hematopoietic Stem Cells from Apoptosis Induced by Short-Term Hypothermic Preservation and Maintains Their Engraftment Potential. Biol. Blood Marrow Transplant. 2020, 26, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Brinkkoetter, P.-T.; Song, H.; Lösel, R.; Schnetzke, U.; Gottmann, U.; Feng, Y.; Hanusch, C.; Beck, G.; Schnuelle, P.; Wehling, M.; et al. Hypothermic Injury: The Mitochondrial Calcium, ATP and ROS Love-Hate Triangle out of Balance. Cell. Physiol. Biochem. 2008, 22, 195–204. [Google Scholar] [CrossRef]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.-S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef]
- Aslanidi, K.B.; Aslanidi, G.V.; Vachadze, D.M.; Zinchenko, V.; Yua, L.; Potapova, T.V. A possible role of cold-induced ionic stress in cold-induced cell death. Membr. Cell Biol. 1997, 11, 57–76. Available online: https://pubmed.ncbi.nlm.nih.gov/9257282/ (accessed on 2 May 2023).
- Flora, S.J.S.; Pachauri, V. Chelation in Metal Intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef]
- Knoop, S.; de Groot, H.; Rauen, U. Little evidence for a major role of Ca2+ in cold-induced injury of liver cells. Cryobiology 2008, 56, 103–113. [Google Scholar] [CrossRef]
- Rauen, U.; Kerkweg, U.; de Groot, H. Iron-dependent vs. iron-independent cold-induced injury to cultured rat hepatocytes: A comparative study in physiological media and organ preservation solutions. Cryobiology 2007, 54, 77–86. [Google Scholar] [CrossRef]
- Kerkweg, U.; Li, T.; de Groot, H.; Rauen, U. Cold-induced apoptosis of rat liver cells in University of Wisconsin solution: The central role of chelatable iron. Hepatology 2002, 35, 560–567. [Google Scholar] [CrossRef]
- Rauen, U.; Petrat, F.; Li, T.; De Groot, H. Hypothermia injury/cold-induced apoptosis—Evidence of an increase in chelatable iron causing oxidative injury in spite of low O2−/H2O2 formation. FASEB J. 2000, 14, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Y.; Dixon, S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Pratt, D.A. Ferroptosis: A flexible constellation of related biochemical mechanisms. Mol. Cell 2023, 83, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Y.; Min, J.; Wang, F. Zooming in and out of ferroptosis in human disease. Front. Med. 2023, 17, 173–206. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Kuang, F.; Liu, J.; Tang, D.; Kang, R. Oxidative Damage and Antioxidant Defense in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 586578. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef]
- Shintoku, R.; Takigawa, Y.; Yamada, K.; Kubota, C.; Yoshimoto, Y.; Takeuchi, T.; Koshiishi, I.; Torii, S. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci. 2017, 108, 2187–2194. [Google Scholar] [CrossRef]
- Hattori, K.; Ishikawa, H.; Sakauchi, C.; Takayanagi, S.; Naguro, I.; Ichijo, H. Cold stress-induced ferroptosis involves the ASK 1-p38 pathway. EMBO Rep. 2017, 18, 2067–2078. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2020, 31, 107–125. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Feng, H.; Stockwell, B.R. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? PLoS Biol. 2018, 16, e2006203. [Google Scholar] [CrossRef] [PubMed]
- Cozza, G.; Rossetto, M.; Bosello-Travain, V.; Maiorino, M.; Roveri, A.; Toppo, S.; Zaccarin, M.; Zennaro, L.; Ursini, F. Glutathione peroxidase 4-catalyzed reduction of lipid hydroperoxides in membranes: The polar head of membrane phospholipids binds the enzyme and addresses the fatty acid hydroperoxide group toward the redox center. Free. Radic. Biol. Med. 2017, 112, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef]
- Zhang, Y.; Swanda, R.V.; Nie, L.; Liu, X.; Wang, C.; Lee, H.; Lei, G.; Mao, C.; Koppula, P.; Cheng, W.; et al. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Seiler, A.; Schneider, M.; Förster, H.; Roth, S.; Wirth, E.K.; Culmsee, C.; Plesnila, N.; Kremmer, E.; Rådmark, O.; Wurst, W.; et al. Glutathione Peroxidase 4 Senses and Translates Oxidative Stress into 12/15-Lipoxygenase Dependent- and AIF-Mediated Cell Death. Cell Metab. 2008, 8, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Miotto, G.; Rossetto, M.; Di Paolo, M.L.; Orian, L.; Venerando, R.; Roveri, A.; Vučković, A.-M.; Travain, V.B.; Zaccarin, M.; Zennaro, L.; et al. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol. 2019, 28, 101328. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Wang, Y.; Lin, Y.; Mao, Q.; Feng, J.; Gao, G.; Jiang, J. Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci. Ther. 2018, 25, 465–475. [Google Scholar] [CrossRef]
- Vogelaar, P.C.; Roorda, M.; de Vrij, E.L.; Houwertjes, M.C.; Goris, M.; Bouma, H.; van der Graaf, A.C.; Krenning, G.; Henning, R.H. The 6-hydroxychromanol derivative SUL-109 ameliorates renal injury after deep hypothermia and rewarming in rats. Nephrol. Dial. Transplant. 2018, 33, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Hajmousa, G.; Vogelaar, P.; Brouwer, L.A.; van der Graaf, A.C.; Henning, R.H.; Krenning, G. The 6-chromanol derivate SUL-109 enables prolonged hypothermic storage of adipose tissue-derived stem cells. Biomaterials 2017, 119, 43–52. [Google Scholar] [CrossRef]
- Lambooy, S.P.H.; Bidadkosh, A.; Nakladal, D.; van Buiten, A.; Girgis, R.A.T.; van der Graaf, A.C.; Wiedenmann, T.J.; Koster, R.A.; Vogelaar, P.; Buikema, H.; et al. The Novel Compound Sul-121 Preserves Endothelial Function and Inhibits Progression of Kidney Damage in Type 2 Diabetes Mellitus in Mice. Sci. Rep. 2017, 7, 11165. [Google Scholar] [CrossRef]
- Petrenko, A.; Carnevale, M.; Somov, A.; Osorio, J.; Rodríguez, J.; Guibert, E.; Fuller, B.; Froghi, F. Organ Preservation into the 2020s: The Era of Dynamic Intervention. Transfus. Med. Hemotherapy 2019, 46, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Parsons, R.F.; Guarrera, J.V. Preservation solutions for static cold storage of abdominal allografts. Curr. Opin. Organ Transplant. 2014, 19, 100–107. [Google Scholar] [CrossRef]
- Compare Belzer UW® Cold Storage Solution to ViaSpan|Bridge to Life. Available online: https://bridgetolife.com/compare-belzer-uw-cold-storage-solution-to-viaspan/ (accessed on 2 May 2023).
- Belzer, F.O.; Southard, J.H. Principles of Solid-Organ Preservation by Cold Storage. Transplantation 1988, 45, 673–676. [Google Scholar] [CrossRef]
- Ben Abdennebi, H.; Steghens, J.-P.; Margonari, J.; Ramella-Virieux, S.; Barbieux, A.; Boillot, O. High-Na + low-K + UW cold storage solution reduces reperfusion injuries of the rat liver graft. Transpl. Int. 1998, 11, 223–230. [Google Scholar] [CrossRef]
- Southard, J.H.; van Gulik, T.M.; Ametani, M.S.; Vreugdenhil, P.K.; Lindell, S.L.; Pienaar, B.L.; Belzer, F.O. Important components of the uw solution. Transplantation 1990, 49, 251–257. [Google Scholar] [CrossRef]
- Hernández-Gallardo, A.K.; Missirlis, F. Loss of ferritin in developing wing cells: Apoptosis and ferroptosis coincide. PLoS Genet. 2020, 16, e1008503. [Google Scholar] [CrossRef] [PubMed]
- Venema, L.H.; Brat, A.; Moers, C.; Hart, N.A.; Ploeg, R.J.; Hannaert, P.; Minor, T.; Leuvenink, H. Effects of Oxygen During Long-term Hypothermic Machine Perfusion in a Porcine Model of Kidney Donation After Circulatory Death. Transplantation 2019, 103, 2057–2064. [Google Scholar] [CrossRef]
- Jochmans, I.; Brat, A.; Davies, L.; Hofker, H.S.; van de Leemkolk, F.E.M.; Leuvenink, H.G.D.; Knight, S.R.; Pirenne, J.; Ploeg, R.J.; Abramowicz, D.; et al. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet 2020, 396, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.I.; Yiu, J.; Nozdrin, M.; Papalois, V. The Effect of Preservation Temperature on Liver, Kidney, and Pancreas Tissue ATP in Animal and Preclinical Human Models. J. Clin. Med. 2019, 8, 1421. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Eljack, N.D.; Sani, M.-A.; Separovic, F.; Rasmussen, H.H.; Kopec, W.; Khandelia, H.; Cornelius, F.; Clarke, R.J. Membrane accessibility of glutathione. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 2430–2436. [Google Scholar] [CrossRef]
- Cacciatore, I.; Cornacchia, C.; Pinnen, F.; Mollica, A.; Di Stefano, A. Prodrug Approach for Increasing Cellular Glutathione Levels. Molecules 2010, 15, 1242–1264. [Google Scholar] [CrossRef]
- Kelly-Aubert, M.; Trudel, S.; Fritsch, J.; Nguyen-Khoa, T.; Baudouin-Legros, M.; Moriceau, S.; Jeanson, L.; Djouadi, F.; Matar, C.; Conti, M.; et al. GSH monoethyl ester rescues mitochondrial defects in cystic fibrosis models. Hum. Mol. Genet. 2011, 20, 2745–2759. [Google Scholar] [CrossRef]
- Bilzer, M.; Paumgartner, G.; Gerbes, A.L. Glutathione protects the rat liver against reperfusion injury after hypothermic preservation. Gastroenterology 1999, 117, 200–210. [Google Scholar] [CrossRef]
- Czubak, K.; Antosik, A.; Cichon, N.; Zbikowska, H.M. Vitamin C and Trolox decrease oxidative stress and hemolysis in cold-stored human red blood cells. Redox Rep. 2017, 22, 445–450. [Google Scholar] [CrossRef]
- Turell, L.; Zeida, A.; Trujillo, M. Mechanisms and consequences of protein cysteine oxidation: The role of the initial short-lived intermediates. Essays Biochem. 2020, 64, 55–66. [Google Scholar] [CrossRef]
- Schulte, L.; Mao, J.; Reitz, J.; Sreeramulu, S.; Kudlinzki, D.; Hodirnau, V.-V.; Meier-Credo, J.; Saxena, K.; Buhr, F.; Langer, J.D.; et al. Cysteine oxidation and disulfide formation in the ribosomal exit tunnel. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yangab, N.C.; Hob, W.M.; Chenc, Y.H.; Hu, M.-L. A Convenient One-Step Extraction of Cellular ATP Using Boiling Water for the Luciferin–Luciferase Assay of ATP. Anal. Biochem. 2002, 306, 323–327. [Google Scholar] [CrossRef] [PubMed]




| Compound | Concentration | Manufacturer |
|---|---|---|
| SUL150 | 10 µM | Sulfateq B.V. |
| Ferrostatin-1 | 100 nM | |
| Lanthanum Chloride | 50 µM | |
| Ru360 * | 5 µM | |
| Trolox® | 100 µM to 3 mM | Sigma-Aldrich |
| Reduced GSH | 3 mM | |
| Oxidized GSH | 3 mM | |
| DMSO | n.a. | |
| Z-VAD(OH)-FMK | 50 µM | Selleck Chemicals |
| MG132 | 10 µM | |
| RSL3 | 1.44 and 2.88 µM | TargetMol |
| Erastin | 5 and 10 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gartzke, L.P.; Hendriks, K.D.W.; Hoogstra-Berends, F.; Joschko, C.P.; Strandmoe, A.-L.; Vogelaar, P.C.; Krenning, G.; Henning, R.H. Inhibition of Ferroptosis Enables Safe Rewarming of HEK293 Cells following Cooling in University of Wisconsin Cold Storage Solution. Int. J. Mol. Sci. 2023, 24, 10939. https://doi.org/10.3390/ijms241310939
Gartzke LP, Hendriks KDW, Hoogstra-Berends F, Joschko CP, Strandmoe A-L, Vogelaar PC, Krenning G, Henning RH. Inhibition of Ferroptosis Enables Safe Rewarming of HEK293 Cells following Cooling in University of Wisconsin Cold Storage Solution. International Journal of Molecular Sciences. 2023; 24(13):10939. https://doi.org/10.3390/ijms241310939
Chicago/Turabian StyleGartzke, Lucas P., Koen D. W. Hendriks, Femke Hoogstra-Berends, Christian P. Joschko, Anne-Lise Strandmoe, Pieter C. Vogelaar, Guido Krenning, and Robert H. Henning. 2023. "Inhibition of Ferroptosis Enables Safe Rewarming of HEK293 Cells following Cooling in University of Wisconsin Cold Storage Solution" International Journal of Molecular Sciences 24, no. 13: 10939. https://doi.org/10.3390/ijms241310939
APA StyleGartzke, L. P., Hendriks, K. D. W., Hoogstra-Berends, F., Joschko, C. P., Strandmoe, A.-L., Vogelaar, P. C., Krenning, G., & Henning, R. H. (2023). Inhibition of Ferroptosis Enables Safe Rewarming of HEK293 Cells following Cooling in University of Wisconsin Cold Storage Solution. International Journal of Molecular Sciences, 24(13), 10939. https://doi.org/10.3390/ijms241310939






