How Do Microorganisms Influence the Development of Endometriosis? Participation of Genital, Intestinal and Oral Microbiota in Metabolic Regulation and Immunopathogenesis of Endometriosis
Abstract
1. Introduction
2. The Importance of Disorders of Immune Homeostasis in the Pathogenesis of Endometriosis
2.1. The Role of Immune Cells in the Development of Endometriosis
2.1.1. Macrophages
2.1.2. Neutrophils
2.1.3. Natural Killer Cells
2.1.4. T Cells
2.1.5. Tight Junctions
2.1.6. Damage-Associated Molecular Patterns (DAMPs) and Pathogen-Associated Molecular Patterns (PAMPs)
2.1.7. Toll-Like Receptors (TLRs)
2.1.8. Lipopolysaccharide
2.2. The Importance of Inflammatory Mediators in Body Fluids (Peripheral Blood, Peritoneal Fluid, Urine) in the Development of Endometriosis
2.2.1. Anti-Inflammatory Cytokines
2.2.2. Pro-Inflammatory Cytokines
2.2.3. Urinary Markers of Inflammation and Endometriosis
3. Influence of Microbiota Dysbiosis of the Reproductive Tract, Intestines and Oral Cavity on the Pathogenesis of Endometriosis
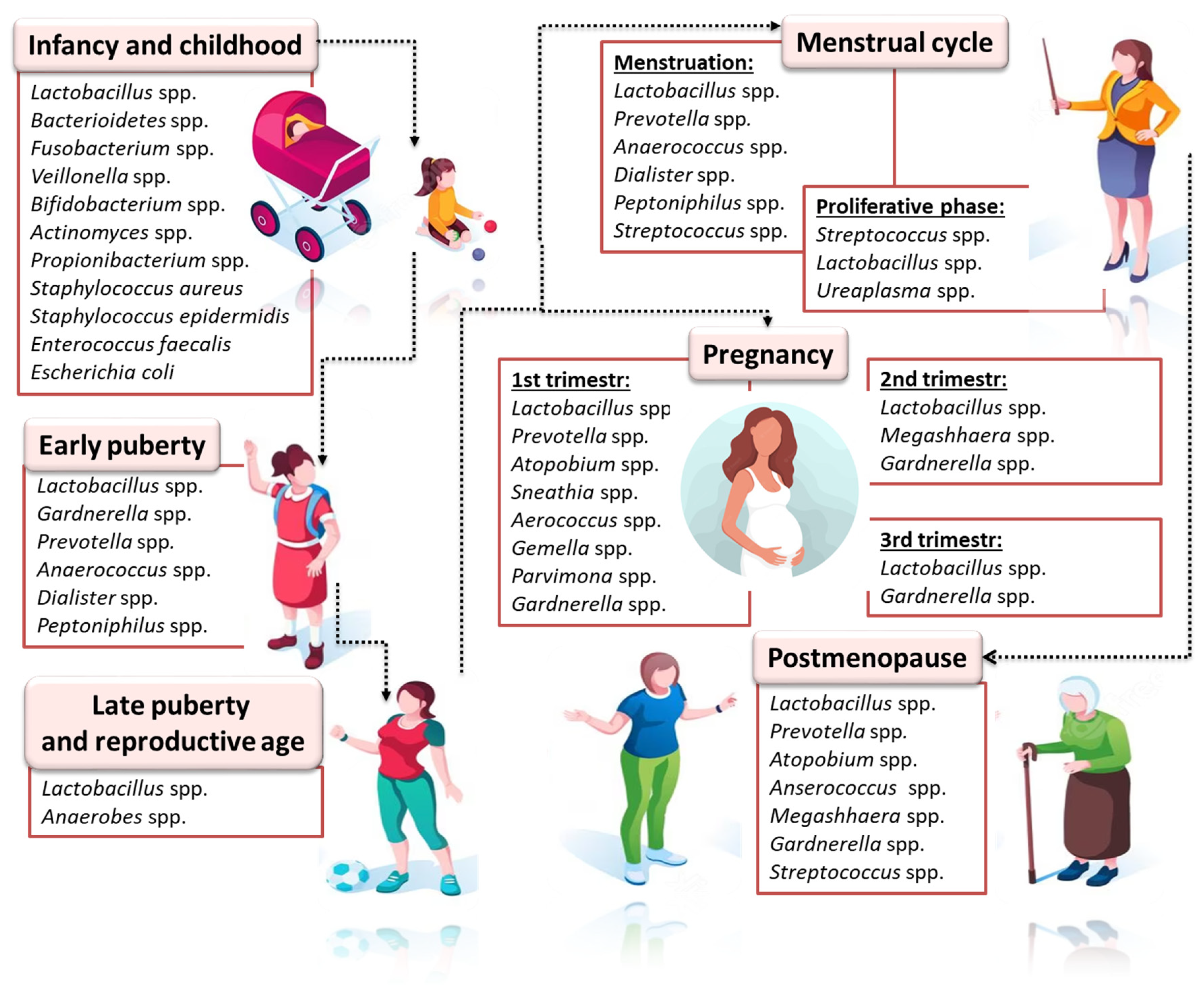
3.1. The Microbiota of the Genital Tract and Endometriosis
3.2. Gut Microbiota and Endometriosis
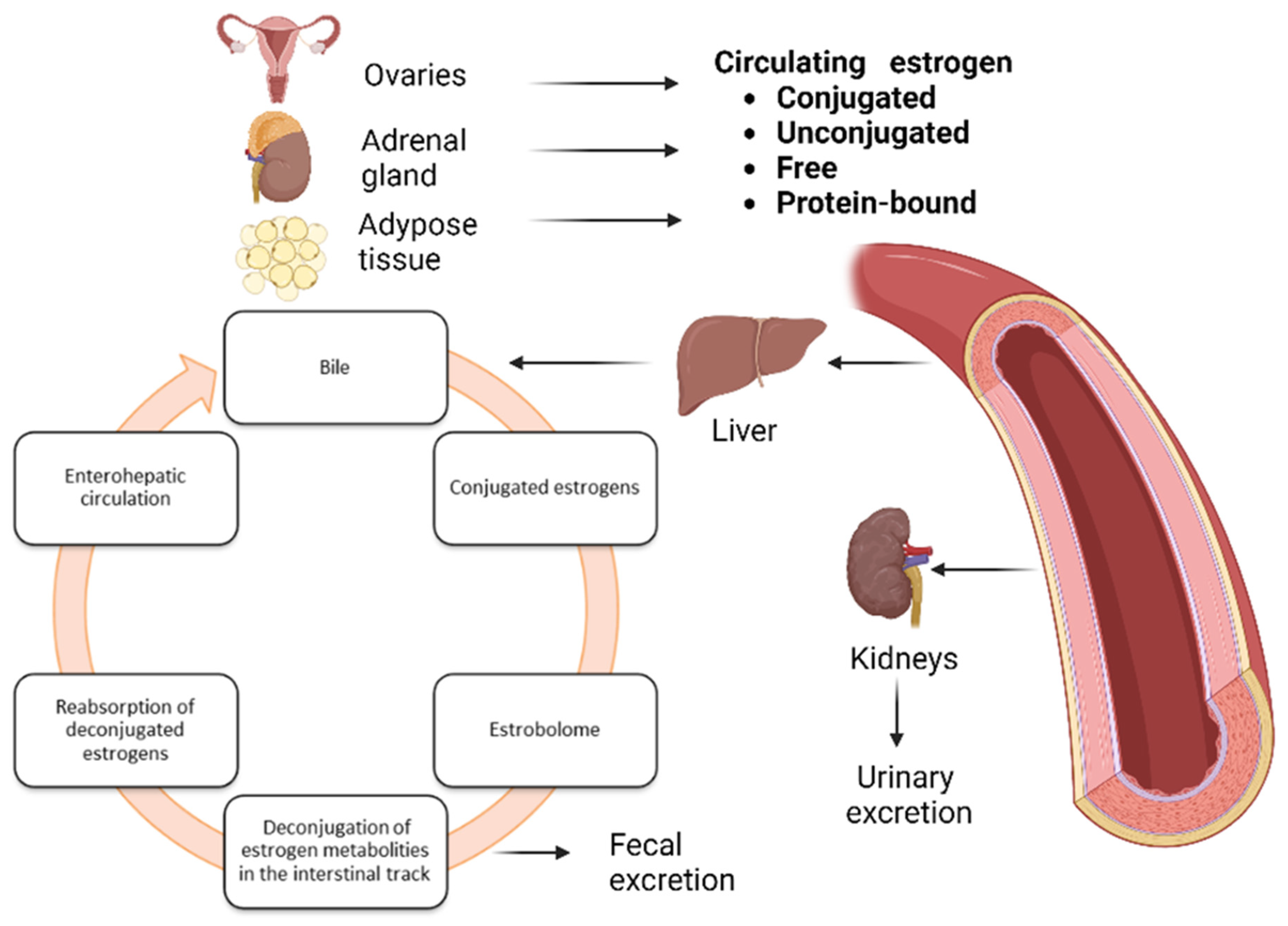
4. Metabolic Regulation of Estrogen Metabolism and Microbiota
5. Future Directions—The Role of Probiotics and Prebiotics in Endometriosis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maybin, J.A.; Critchley, H.O.D. Menstrual Physiology: Implications for Endometrial Pathology and Beyond. Hum. Reprod. Update 2015, 21, 748–761. [Google Scholar] [CrossRef]
- Karamian, A.; Paktinat, S.; Esfandyari, S.; Nazarian, H.; Ziai, S.A.; Zarnani, A.-H.; Salehpour, S.; Hosseinirad, H.; Karamian, A.; Novin, M.G. Pyrvinium Pamoate Induces In-Vitro Suppression of IL-6 and IL-8 Produced by Human Endometriotic Stromal Cells. Hum. Exp. Toxicol. 2021, 40, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T. World Endometriosis Research Foundation Global Study of Women’s Health consortium Impact of Endometriosis on Quality of Life and Work Productivity: A Multicenter Study across Ten Countries. Fertil. Steril. 2011, 96, 366–373.e8. [Google Scholar] [CrossRef]
- Rocha, A.L.L.; Reis, F.M.; Taylor, R.N. Angiogenesis and Endometriosis. Obstet. Gynecol. Int. 2013, 2013, e859619. [Google Scholar] [CrossRef] [PubMed]
- Husby, G.K.; Haugen, R.S.; Moen, M.H. Diagnostic Delay in Women with Pain and Endometriosis. Acta Obstet. Et Gynecol. Scand. 2003, 82, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Sourial, S.; Tempest, N.; Hapangama, D.K. Theories on the Pathogenesis of Endometriosis. Int. J. Reprod. Med. 2014, 2014, e179515. [Google Scholar] [CrossRef] [PubMed]
- Youseflu, S.; Jahanian Sadatmahalleh, S.; Mottaghi, A.; Kazemnejad, A. The Association of Food Consumption and Nutrient Intake with Endometriosis Risk in Iranian Women: A Case-Control Study. Int. J. Reprod. BioMed. 2019, 17, 661–670. [Google Scholar] [CrossRef]
- Christodoulakos, G.; Augoulea, A.; Lambrinoudaki, I.; Sioulas, V.; Creatsas, G. Pathogenesis of Endometriosis: The Role of Defective ‘Immunosurveillance’. Eur. J. Contracept. Reprod. Health Care 2007, 12, 194–202. [Google Scholar] [CrossRef]
- Sampson, J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Ferguson, B.R.; Bennington, J.L.; Haber, S.L. Histochemistry of mucosubstances and histology of mixed Müllerian pelvic lymph node glandular inclusions. Evidence for histogenesis by Müllerian metaplasia of coelomic epithelium. Obstet. Gynecol. 1969, 33, 617–625. [Google Scholar]
- Sampson, J.A. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93–110. [Google Scholar] [PubMed]
- Sasson, I.E.; Taylor, H.S. Stem cells and the pathogenesis of endometriosis. Ann. N. Y. Acad. Sci. 2008, 1127, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Lousse, J.-C.; Langendonckt, A.V.; Defrere, S.; Ramos, R.G.; Colette, S.; Donnez, J. Peritoneal Endometriosis Is an Inflammatory Disease. FBE 2012, 4, 23–40. [Google Scholar] [CrossRef]
- Gazvani, R.; Templeton, A. Peritoneal Environment, Cytokines and Angiogenesis in the Pathophysiology of Endometriosis. Reproduction 2002, 123, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Van Langendonckt, A.; Casanas-Roux, F.; Donnez, J. Oxidative Stress and Peritoneal Endometriosis. Fertil. Steril. 2002, 77, 861–870. [Google Scholar] [CrossRef]
- Sikora, J.; Mielczarek-Palacz, A.; Kondera-Anasz, Z. Role of oxidative stress and mitochondrial abnormalities in endometriosis development. Front. Biosci. 2018, 23, 1010–1024. [Google Scholar]
- Silva, E.J.R.; do Amara, V.F.; Mendonça, J.M.; Nakao, L.S.; Neto, O.P.; Ferriani, R.A. Serum markers of oxidative stress and endometriosis. Clin. Exp. Obstet. Gynecol. 2014, 41, 371–374. [Google Scholar] [CrossRef]
- Harlev, A.; Gupta, S.; Agarwal, A. Targeting oxidative stress to treat endometriosis. Expert Opin. Ther. Targets 2015, 19, 1447–1464. [Google Scholar] [CrossRef]
- Andrisani, A.; Donà, G.; Brunati, A.M.; Clari, G.; Armanini, D.; Ragazzi, E.; Ambrosini, G.; Bordin, L. Increased oxidation-related glutathionylation and carbonic anhydrase activity in endometriosis. Reprod. Biomed. Online 2014, 28, 773–779. [Google Scholar] [CrossRef]
- Nagata, M. Inflammatory cells and oxygen radicals. Curr. Drug Targets Inflamm. Allergy 2005, 4, 503–504. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Binda, M.M.; Donnez, O.; Dolmans, M.-M. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil. Steril. 2016, 106, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Chen, Y.-H.; Chang, H.-Y.; Au, H.-K.; Tzeng, C.-R.; Huang, Y.-H. Chronic Niche Inflammation in Endometriosis-Associated Infertility: Current Understanding and Future Therapeutic Strategies. Int. J. Mol. Sci. 2018, 19, 2385. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.; Liu, H.; Lash, G.E. The Role of Decidual Macrophages During Normal and Pathological Pregnancy. Am. J. Reprod. Immunol. 2016, 75, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Yang, F.; Chen, X.; Li, J.; Zhong, C.; Chen, S. Patterns of Immune Infiltration in Endometriosis and Their Relationship to R-AFS Stages. Front. Genet. 2021, 12, 631715. [Google Scholar] [CrossRef]
- May, K.E.; Villar, J.; Kirtley, S.; Kennedy, S.H.; Becker, C.M. Endometrial Alterations in Endometriosis: A Systematic Review of Putative Biomarkers. Hum. Reprod. Update 2011, 17, 637–653. [Google Scholar] [CrossRef]
- Yang, H.; Zhu, L.; Wang, S.; Lang, J.; Xu, T. Noninvasive Diagnosis of Moderate to Severe Endometriosis: The Platelet-Lymphocyte Ratio Cannot Be a Neoadjuvant Biomarker for Serum Cancer Antigen 125. J. Minim. Invasive Gynecol. 2015, 22, 373–377. [Google Scholar] [CrossRef]
- Itoh, F.; Komohara, Y.; Takaishi, K.; Honda, R.; Tashiro, H.; Kyo, S.; Katabuchi, H.; Takeya, M. Possible Involvement of Signal Transducer and Activator of Transcription-3 in Cell-Cell Interactions of Peritoneal Macrophages and Endometrial Stromal Cells in Human Endometriosis. Fertil. Steril. 2013, 99, 1705–1713. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, B.; Yu, J.-J.; Wei, C.-Y.; Zhou, W.-J.; Chang, K.-K.; Yang, H.-L.; Jin, L.-P.; Zhu, X.-Y.; Li, M.-Q. Macrophages Promote the Growth and Invasion of Endometrial Stromal Cells by Downregulating IL-24 in Endometriosis. Reproduction 2016, 152, 673–682. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infec-tious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Milewski, Ł.; Dziunycz, P.; Barcz, E.; Radomski, D.; Roszkowski, P.I.; Korczak-Kowalska, G.; Kamiński, P.; Malejczyk, J. Increased Levels of Human Neutrophil Peptides 1, 2, and 3 in Peritoneal Fluid of Patients with Endometriosis: Association with Neutrophils, T Cells and IL-8. J. Reprod. Immunol. 2011, 91, 64–70. [Google Scholar] [CrossRef]
- Takamura, M.; Koga, K.; Izumi, G.; Urata, Y.; Nagai, M.; Hasegawa, A.; Harada, M.; Hirata, T.; Hirota, Y.; Wada-Hiraike, O.; et al. Neutrophil Depletion Reduces Endometriotic Lesion Formation in Mice. Am. J. Reprod. Immunol. 2016, 76, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Lee, D.-H.; Joo, J.-K.; Jin, J.-O.; Wang, J.-W.; Hong, Y.-S.; Kwak, J.-Y.; Lee, K.-S. ORIGINAL ARTICLE: Effects of Peritoneal Fluid from Endometriosis Patients on Interferon-γ-Induced Protein-10 (CXCL10) and Interleukin-8 (CXCL8) Released by Neutrophils and CD4+ T Cells. Am. J. Reprod. Immunol. 2009, 62, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.-Y.; Park, S.-W.; Kim, K.-H.; Na, Y.-J.; Lee, K.-S. Modulation of Neutrophil Apoptosis by Plasma and Peritoneal Fluid from Patients with Advanced Endometriosis. Hum. Reprod. 2002, 17, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Viganò, P.; Gaffuri, B.; Candiani, M.; Busacca, M.; Blasio, A.M.D.; Vignali, M. Modulation of NK Cell Lytic Function by Endometrial Secretory Factors: Potential Role in Endometriosis. Am. J. Reprod. Immunol. 1996, 36, 295–300. [Google Scholar] [CrossRef]
- Matsuoka, S.; Maeda, N.; Izumiya, C.; Yamashita, C.; Nishimori, Y.; Fukaya, T. Expression of Inhibitory-Motif Killer Immunoglobulin-Like Receptor, KIR2DL1, Is Increased in Natural Killer Cells from Women with Pelvic Endometriosis. Am. J. Reprod. Immunol. 2005, 53, 249–254. [Google Scholar] [CrossRef]
- Du, Y.; Liu, X.; Guo, S.-W. Platelets Impair Natural Killer Cell Reactivity and Function in Endometriosis through Multiple Mechanisms. Hum. Reprod. 2017, 32, 794–810. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Jeung, I.C.; Park, A.; Park, Y.-J.; Jung, H.; Kim, T.-D.; Lee, H.G.; Choi, I.; Yoon, S.R. An Increased Level of IL-6 Suppresses NK Cell Activity in Peritoneal Fluid of Patients with Endometriosis via Regulation of SHP-2 Expression. Hum. Reprod. 2014, 29, 2176–2189. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 Cells: Different Patterns of Lymphokine Secretion Lead to Different Functional Properties. Annu. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.-H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A Distinct Lineage of CD4 T Cells Regulates Tissue Inflammation by Producing Interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-Producing CD4+ Effector T Cells Develop via a Lineage Distinct from the T Helper Type 1 and 2 Lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Antsiferova, Y.S.; Sotnikova, N.Y.; Posiseeva, L.V.; Shor, A.L. Changes in the T-Helper Cytokine Profile and in Lymphocyte Activation at the Systemic and Local Levels in Women with Endometriosis. Fertil. Steril. 2005, 84, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, L.A.; Wynn, T.A.; Fisher, A.J. Cytokine Mediated Tissue Fibrosis. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2013, 1832, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Gogacz, M.; Winkler, I.; Bojarska-Junak, A.; Tabarkiewicz, J.; Semczuk, A.; Rechberger, T.; Adamiak, A. Increased Percentage of Th17 Cells in Peritoneal Fluid Is Associated with Severity of Endometriosis. J. Reprod. Immunol. 2016, 117, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Osuga, Y.; Takamura, M.; Kodama, A.; Hirota, Y.; Koga, K.; Yoshino, O.; Harada, M.; Takemura, Y.; Yano, T.; et al. Recruitment of CCR6-Expressing Th17 Cells by CCL 20 Secreted from IL-1β-, TNF-α-, and IL-17A-Stimulated Endometriotic Stromal Cells. Endocrinology 2010, 151, 5468–5476. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Z.; Chen, Q.; Ren, F.; Li, T.; Zhang, C.; Wang, D. Expression of Th1 and Th2 Cytokine-Associated Transcription Factors, T-Bet and GATA-3, in the Eutopic Endometrium of Women with Endometriosis. Acta Histochem. 2012, 114, 779–784. [Google Scholar] [CrossRef]
- Olkowska-Truchanowicz, J.; Bocian, K.; Maksym, R.B.; Białoszewska, A.; Włodarczyk, D.; Baranowski, W.; Ząbek, J.; Korczak-Kowalska, G.; Malejczyk, J. CD4+ CD25+ FOXP3+ Regulatory T Cells in Peripheral Blood and Peritoneal Fluid of Patients with Endometriosis. Hum. Reprod. 2013, 28, 119–124. [Google Scholar] [CrossRef]
- Li, M.-Q.; Wang, Y.; Chang, K.-K.; Meng, Y.-H.; Liu, L.-B.; Mei, J.; Wang, Y.; Wang, X.-Q.; Jin, L.-P.; Li, D.-J. CD4+Foxp3+ Regulatory T Cell Differentiation Mediated by Endometrial Stromal Cell-Derived TECK Promotes the Growth and Invasion of Endometriotic Lesions. Cell Death Dis. 2014, 5, e1436. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Günzel, D.; Yu, A.S.L. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Loeffelmann, A.C.; Hoerscher, A.; Riaz, M.A.; Zeppernick, F.; Meinhold-Heerlein, I.; Konrad, L. Claudin-10 Expression Is Increased in Endometriosis and Adenomyosis and Mislocalized in Ectopic Endometriosis. Diagnostics 2022, 12, 2848. [Google Scholar] [CrossRef]
- Gaetje, R.; Holtrich, U.; Engels, K.; Kissler, S.; Rody, A.; Karn, T.; Kaufmann, M. Differential expression of claudins in human endometrium and endometriosis. Gynecol. Endocrinol. 2008, 24, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Horné, F.; Dietze, R.; Berkes, E.; Oehmke, F.; Tinneberg, H.-R.; Meinhold-Heerlein, I.; Konrad, L. Impaired Localization of Claudin-11 in Endometriotic Epithelial Cells Compared to Endometrial Cells. Reprod. Sci. 2019, 26, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-Y.; Li, X.; Weng, Z.-P.; Wang, B. Altered expression of claudin-3 and claudin-4 in ectopic endometrium of women with endometriosis. Fertil. Steril. 2009, 91, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Hoerscher, A.; Horné, F.; Dietze, R.; Berkes, E.; Oehmke, F.; Tinneberg, H.-R.; Meinhold-Heerlein, I.; Konrad, L. Localization of claudin-2 and claudin-3 in eutopic and ectopic endometrium is highly similar. Arch. Gynecol. Obstet. 2020, 301, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, H.; Yamada, Y.; Kanayama, S.; Furukawa, N.; Noguchi, T.; Haruta, S.; Yoshida, S.; Sado, T.; Oi, H.; Kobayashi, H. New insights into the pathophysiology of endometriosis: From chronic inflammation to danger signal. Gynecol. Endocrinol. 2011, 27, 73–79. [Google Scholar] [CrossRef]
- Yu, Y.; Tang, D.; Kang, R. Oxidative stress-mediated HMGB1 biology. Front. Physiol. 2015, 6, 93. [Google Scholar] [CrossRef]
- Yun, B.H.; Chon, S.J.; Choi, Y.S.; Cho, S.; Lee, B.S.; Seo, S.K. Pathophysiology of Endometriosis: Role of High Mobility Group Box-1 and Toll-Like Receptor 4 Developing Inflammation in Endometrium. PLoS ONE 2016, 11, e0148165. [Google Scholar] [CrossRef]
- Khan, K.N.; Kitajima, M.; Imamura, T.; Hiraki, K.; Fujishita, A.; Sekine, I.; Ishimaru, T.; Masuzaki, H. Toll-like receptor 4-mediated growth of endometriosis by human heat-shock protein 70. Hum. Reprod. 2008, 23, 2210–2219. [Google Scholar] [CrossRef]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate Immune Pattern Recognition: A Cell Biological Perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Mnich, M.E.; van Dalen, R.; Van Sorge, N.M. C-Type Lectin Receptors in Host Defense Against Bacterial Pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 309. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Shin, J.-S.; Nahm, M.H. NOD-Like Receptors in Infection, Immunity, and Diseases. Yonsei Med. J. 2016, 57, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Iurescia, S.; Fioretti, D.; Rinaldi, M. Targeting cytosolic nucleic acid-sensing pathways for cancer immunotherapies. Front. Immunol. 2018, 9, 711. [Google Scholar] [CrossRef] [PubMed]
- Caneparo, V.; Landolfo, S.; Gariglio, M.; De Andrea, M. The Absent in Melanoma 2-Like Receptor IFN-Inducible Protein 16 as an Inflammasome Regulator in Systemic Lupus Erythematosus: The Dark Side of Sensing Microbes. Front. Immunol. 2018, 9, 1180. [Google Scholar] [CrossRef]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 493–518. [Google Scholar] [CrossRef]
- Saijo, Y.; EPi, L.; Yasuda, S. Pattern recognition receptors and signaling in plant–microbe interactions. Plant J. 2018, 93, 592–613. [Google Scholar] [CrossRef]
- He, S.; He, J.; Chen, J.; Chen, F.; Ou, S. Research advancement of innate immunity and pattern recognition receptors. Chin. J. Anim. Nutr. 2017, 29, 3844–3851. [Google Scholar]
- Mortaz, E.; Adcock, I.M.; Tabarsi, P.; Darazam, I.A.; Movassaghi, M.; Garssen, J.; Jamaati, H.; Velayati, A. Pattern recognitions receptors in immunodeficiency disorders. Eur. J. Pharmacol. 2017, 808, 49–56. [Google Scholar] [CrossRef]
- Azuma, Y.; Taniguchi, F.; Nakamura, K.; Nagira, K.; Khine, Y.M.; Kiyama, T.; Uegaki, T.; Izawa, M.; Harada, T. Lipopolysaccharide promotes the development of murine endometriosis-like lesions via the nuclear factor-kappa B pathway. Am. J. Reprod. Immunol. 2017, 77, e12631. [Google Scholar] [CrossRef]
- Herington, J.L.; Bruner-Tran, K.L.; A Lucas, J.; Osteen, K.G. Immune interactions in endometriosis. Expert Rev. Clin. Immunol. 2011, 7, 611–626. [Google Scholar] [CrossRef]
- Sobstyl, M.; Niedźwiedzka-Rystwej, P.; Grywalska, E.; Korona-Głowniak, I.; Sobstyl, A.; Bednarek, W.; Roliński, J. Toll-Like Receptor 2 Expression as a New Hallmark of Advanced Endometriosis. Cells 2020, 9, 1813. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Kitajima, M.; Hiraki, K.; Yamaguchi, N.; Katamine, S.; Matsuyama, T.; Nakashima, M.; Fujishita, A.; Ishimaru, T.; Masuzaki, H. Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil. Steril. 2010, 94, 2860–2863.e3. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Masuzaki, H.; Fujishita, A.; Kitajima, M.; Kohno, T.; Sekine, I.; Matsuyama, T.; Ishimaru, T. Regulation of hepatocyte growth factor by basal and stimulated macrophages in women with endometriosis. Hum. Reprod. 2005, 20, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Iba, Y.; Harada, T.; Horie, S.; Deura, I.; Iwabe, T.; Terakawa, N. Lipopolysaccharide-promoted proliferation of endometriotic stromal cells via induction of tumor necrosis factor α and interleukin-8 expression. Fertil. Steril. 2004, 82 (Suppl. S3), 1036–1042. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Hiraki, K.; Kitajima, M.; Nakashima, M.; Fushiki, S.; Kitawaki, J. Bacterial contamination hypothesis: A new concept in endometriosis. Reprod. Med. Biol. 2018, 17, 125–133. [Google Scholar] [CrossRef]
- García-Gómez, E.; Vázquez-Martínez, E.R.; Reyes-Mayoral, C.; Cruz-Orozco, O.P.; Camacho-Arroyo, I.; Cerbón, M. Regulation of Inflammation Pathways and Inflammasome by Sex Steroid Hormones in Endometriosis. Front. Endocrinol. 2020, 10, 935. [Google Scholar] [CrossRef]
- Suen, J.-L.; Chang, Y.; Chiu, P.-R.; Hsieh, T.-H.; Hsi, E.; Chen, Y.-C.; Chen, Y.-F.; Tsai, E.-M. Serum Level of IL-10 Is Increased in Patients with Endometriosis, and IL-10 Promotes the Growth of Lesions in a Murine Model. Am. J. Pathol. 2014, 184, 464–471. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Chen, H.-Y.; Chen, W.; Liu, Y.-N.; Fu, Y.; Wang, L.-N. Expression of Inflammatory Cytokines in Serum and Peritoneal Fluid from Patients with Different Stages of Endometriosis. Gynecol. Endocrinol. 2018, 34, 507–512. [Google Scholar] [CrossRef]
- Drosdzol-Cop, A.; Skrzypulec-Plinta, V.; Stojko, R. Serum and Peritoneal Fluid Immunological Markers in Adolescent Girls with Chronic Pelvic Pain. Obstet. Gynecol. Surv. 2012, 67, 374. [Google Scholar] [CrossRef]
- Chang, K.-K.; Liu, L.-B.; Jin, L.-P.; Zhang, B.; Mei, J.; Li, H.; Wei, C.-Y.; Zhou, W.-J.; Zhu, X.-Y.; Shao, J.; et al. IL-27 Triggers IL-10 Production in Th17 Cells via a c-Maf/RORγt/Blimp-1 Signal to Promote the Progression of Endometriosis. Cell Death Dis. 2017, 8, e2666. [Google Scholar] [CrossRef]
- Zhang, X.; Hei, P.; Deng, L.; Lin, J. Interleukin-10 Gene Promoter Polymorphisms and Their Protein Production in Peritoneal Fluid in Patients with Endometriosis. Mol. Hum. Reprod. 2007, 13, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Malutan, A.M.; Drugan, C.; Walch, K.; Drugan, T.; Ciortea, R.; Mihu, D. The Association between Interleukin-10 (IL-10) −592C/A, −819T/C, −1082G/A Promoter Polymorphisms and Endometriosis. Arch. Gynecol. Obstet. 2017, 295, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, S.; He, B.; Pan, Y.; Li, Y.; Zeng, Q.; Jiang, H.; Chen, J. Association of Estrogen Receptor Alpha and Interleukin-10 Gene Polymorphisms with Endometriosis in a Chinese Population. Fertil. Steril. 2009, 92, 54–60. [Google Scholar] [CrossRef]
- Kaabachi, W.; Kacem, O.; Belhaj, R.; Hamzaoui, A.; Hamzaoui, K. Interleukin-37 in Endometriosis. Immunol. Lett. 2017, 185, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, A.; Salmeri, F.M.; Ardita, F.V.; Sofo, V.; Tripepi, M.; Marsico, S. Behaviour of Cytokine Levels in Serum and Peritoneal Fluid of Women with Endometriosis. Gynecol. Obstet. Investig. 2003, 54, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Chegini, N.; Roberts, M.; Ripps, B. Differential Expression of Interleukins (IL)-13 and IL-15 in Ectopic and Eutopic Endometrium of Women with Endometriosis and Normal Fertile Women. Am. J. Reprod. Immunol. 2003, 49, 75–83. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.-K.L.; Flavell, R.A. Transforming Growth Factor-Β Regulation of Immune Responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- Young, V.J.; Ahmad, S.F.; Duncan, W.C.; Horne, A.W. The Role of TGF-β in the Pathophysiology of Peritoneal Endometriosis. Hum. Reprod. Update 2017, 23, 548–559. [Google Scholar] [CrossRef]
- Chegini, N. TGF-β System: The Principal Profibrotic Mediator of Peritoneal Adhesion Formation. Semin. Reprod. Med. 2008, 26, 298–312. [Google Scholar] [CrossRef]
- Sikora, J.; Smycz-Kubańska, M.; Mielczarek-Palacz, A.; Bednarek, I.; Kondera-Anasz, Z. The Involvement of Multifunctional TGF-β and Related Cytokines in Pathogenesis of Endometriosis. Immunol. Lett. 2018, 201, 31–37. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Abrão, M.S.; Mechsner, S. Can Chemokines Be Used as Biomarkers for Endometriosis? A Systematic Review. Hum. Reprod. 2014, 29, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Xavier, P.; Belo, L.; Beires, J.; Rebelo, I.; Martinez-de-Oliveira, J.; Lunet, N.; Barros, H. Serum Levels of VEGF and TNF-α and Their Association with C-Reactive Protein in Patients with Endometriosis. Arch. Gynecol. Obstet. 2006, 273, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.-F.; Yang, H.-M.; Zhou, W.-D.; Zhang, L.-R.; Bai, J.-B.; Lin, D.-C.; Ng, T.-W.; Dai, S.-J.; Chen, Q.-H.; Chen, Q.-X. Effect of Interleukin-1β and Lipoxin A4 in Human Endometriotic Stromal Cells: Proteomic Analysis. J. Obstet. Gynaecol. Res. 2017, 43, 308–319. [Google Scholar] [CrossRef]
- Mei, J.; Xie, X.-X.; Li, M.-Q.; Wei, C.-Y.; Jin, L.-P.; Li, D.-J.; Zhu, X.-Y. Indoleamine 2,3-Dioxygenase-1 (IDO1) in Human Endometrial Stromal Cells Induces Macrophage Tolerance through Interleukin-33 in the Progression of Endometriosis. Int. J. Clin. Exp. Pathol. 2014, 7, 2743–2757. [Google Scholar]
- Mei, J.; Zhou, W.-J.; Zhu, X.-Y.; Lu, H.; Wu, K.; Yang, H.-L.; Fu, Q.; Wei, C.-Y.; Chang, K.-K.; Jin, L.-P.; et al. Suppression of Autophagy and HCK Signaling Promotes PTGS2high FCGR3− NK Cell Differentiation Triggered by Ectopic Endometrial Stromal Cells. Autophagy 2018, 14, 1376–1397. [Google Scholar] [CrossRef] [PubMed]
- Višnić, A.; Jurešić, G.; Domitrović, R.; Klarić, M.; Šepić, T.S.; Barišić, D. Proteins in urine—Possible biomarkers of endometriosis. J. Reprod. Immunol. 2023, 157, 103941. [Google Scholar] [CrossRef]
- ENG—Endoglin—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P17813/entry (accessed on 4 May 2023).
- Nolan-Stevaux, O.; Zhong, W.; Culp, S.; Shaffer, K.; Hoover, J.; Wickramasinghe, D.; Ruefli-Brasse, A. Endoglin Requirement for BMP9 Signaling in Endothelial Cells Reveals New Mechanism of Action for Selective Anti-Endoglin Antibodies. PLoS ONE 2012, 7, e50920. [Google Scholar] [CrossRef]
- Castonguay, R.; Werner, E.D.; Matthews, R.G.; Presman, E.; Mulivor, A.W.; Solban, N.; Sako, D.; Pearsall, R.S.; Underwood, K.W.; Seehra, J.; et al. Soluble Endoglin Specifically Binds Bone Morphogenetic Proteins 9 and 10 via Its Orphan Domain, Inhibits Blood Vessel Formation, and Suppresses Tumor Growth. J. Biol. Chem. 2011, 286, 30034–30046. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Tu, F.; Yang, Q.; Wang, D.; Zhu, Q. Endoglin Promotes Cell Migration and Invasion in Endometriosis by Regulating EMT. Ginekol. Pol. 2021, 1–16. [Google Scholar] [CrossRef]
- LUM—Lumican—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P51884/entry (accessed on 4 May 2023).
- Sahar, T.; Nigam, A.; Anjum, S.; Waziri, F.; Jain, S.K.; Wajid, S. Differential Expression of Lumican, Mimecan, Annexin A5 and Serotransferrin in Ectopic and Matched Eutopic Endometrium in Ovarian Endometriosis: A Case-Control Study. Gynecol. Endocrinol. 2021, 37, 56–60. [Google Scholar] [CrossRef]
- TGFBR2—TGF-Beta Receptor Type-2—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P37173/entry (accessed on 4 May 2023).
- TSPAN1—Tetraspanin-1—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/O60635/entry (accessed on 4 May 2023).
- Shin, H.; Yang, W.; Chay, D.B.; Lee, E.; Chung, J.; Kim, H.; Kim, J. Tetraspanin 1 Promotes Endometriosis Leading to Ovarian Clear Cell Carcinoma. Mol. Oncol. 2021, 15, 987–1004. [Google Scholar] [CrossRef] [PubMed]
- CD44—CD44 Antigen—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P16070/entry (accessed on 4 May 2023).
- Yoshida, T.; Matsuda, Y.; Naito, Z.; Ishiwata, T. CD44 in Human Glioma Correlates with Histopathological Grade and Cell Migration. Pathol. Int. 2012, 62, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Pazhohan, A.; Amidi, F.; Akbari-Asbagh, F.; Seyedrezazadeh, E.; Aftabi, Y.; Abdolalizadeh, J.; Khodarahmian, M.; Khanlarkhani, N.; Sobhani, A. Expression and Shedding of CD44 in the Endometrium of Women with Endometriosis and Modulating Effects of Vitamin D: A Randomized Exploratory Trial. J. Steroid Biochem. Mol. Biol. 2018, 178, 150–158. [Google Scholar] [CrossRef] [PubMed]
- TNC—Tenascin—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P24821/entry (accessed on 4 May 2023).
- Tan, O.; Ornek, T.; Seval, Y.; Sati, L.; Arici, A. Tenascin Is Highly Expressed in Endometriosis and Its Expression Is Upregulated by Estrogen. Fertil. Steril. 2008, 89, 1082–1089. [Google Scholar] [CrossRef]
- CTSG—Cathepsin G—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P08311/entry (accessed on 4 May 2023).
- Thorpe, M.; Fu, Z.; Chahal, G.; Akula, S.; Kervinen, J.; de Garavilla, L.; Hellman, L. Extended Cleavage Specificity of Human Neutrophil Cathepsin G: A Low Activity Protease with Dual Chymase and Tryptase-Type Specificities. PLoS ONE 2018, 13, e0195077. [Google Scholar] [CrossRef]
- Grzywa, R.; Gorodkiewicz, E.; Burchacka, E.; Lesner, A.; Laudański, P.; Łukaszewski, Z.; Sieńczyk, M. Determination of Cathepsin G in Endometrial Tissue Using a Surface Plasmon Resonance Imaging Biosensor with Tailored Phosphonic Inhibitor. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 182, 38–42. [Google Scholar] [CrossRef]
- DSP—Desmoplakin—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P15924/entry (accessed on 4 May 2023).
- Baranov, V.; Malysheva, O.; Yarmolinskaya, M. Pathogenomics of Endometriosis Development. Int. J. Mol. Sci. 2018, 19, 1852. [Google Scholar] [CrossRef]
- THBS1—Thrombospondin 1—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/A8MZG1/entry (accessed on 4 May 2023).
- Manero, M.G.; Olartecoechea, B.; Royo, P.; Alcázar, J.L. Thrombospondin-1 Serum Levels Do Not Correlate with Pelvic Pain in Patients with Ovarian Endometriosis. J. Ovarian Res. 2009, 2, 18. [Google Scholar] [CrossRef]
- Tan, X.-J.; Lang, J.-H.; Zheng, W.-M.; Leng, J.-H.; Zhu, L. Ovarian Steroid Hormones Differentially Regulate Thrombospondin-1 Expression in Cultured Endometrial Stromal Cells: Implications for Endometriosis. Fertil. Steril. 2010, 93, 328–331. [Google Scholar] [CrossRef]
- PCDH1—Protocadherin-1—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/Q08174/entry (accessed on 4 May 2023).
- Li, M.; Xin, X.-Y.; Jin, Z.-S.; Hua, T.; Wang, H.-B.; Wang, H.-B. Transcriptomic Analysis of Stromal Cells from Patients with Endometrial Carcinoma. Int. J. Clin. Exp. Pathol. 2017, 10, 9853–9857. [Google Scholar]
- SPARCL1—SPARC-like Protein 1—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/Q14515/entry (accessed on 4 May 2023).
- Yusuf, N.; Inagaki, T.; Kusunoki, S.; Okabe, H.; Yamada, I.; Matsumoto, A.; Terao, Y.; Takeda, S.; Kato, K. SPARC Was Overexpressed in Human Endometrial Cancer Stem-like Cells and Promoted Migration Activity. Gynecol. Oncol. 2014, 134, 356–363. [Google Scholar] [CrossRef] [PubMed]
- AZGP1—Zinc-Alpha-2-Glycoprotein—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P25311/entry#function (accessed on 4 May 2023).
- Woźny, Ł.A.; Morawiecka-Pietrzak, M.; Jaszczura, M.; Ziora, K.; Grzeszczak, W. The new adipokine zinc-α2-glycoprotein (ZAG) as a link between adipose tissue and kidney? [Czy nowa adipocytokina cynkowa α2-glikoproteina (ZAG) stanowi ogniwo między tkanką tłuszczową a nerkami?]. Endokrynol. Pol. 2019, 70, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Anastasiu, C.V.; Moga, M.A.; Elena Neculau, A.; Bălan, A.; Scârneciu, I.; Dragomir, R.M.; Dull, A.-M.; Chicea, L.-M. Biomarkers for the Noninvasive Diagnosis of Endometriosis: State of the Art and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 1750. [Google Scholar] [CrossRef]
- Kovács, Z.; Glover, L.; Reidy, F.; MacSharry, J.; Saldova, R. Novel Diagnostic Options for Endometriosis—Based on the Glycome and Microbiome. J. Adv. Res. 2021, 33, 167–181. [Google Scholar] [CrossRef]
- ANXA2—Annexin A2—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P07355/entry (accessed on 4 May 2023).
- Deng, L.; Gao, Y.; Li, X.; Cai, M.; Wang, H.; Zhuang, H.; Tan, M.; Liu, S.; Hao, Y.; Lin, B. Expression and Clinical Significance of Annexin A2 and Human Epididymis Protein 4 in Endometrial Carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 96. [Google Scholar] [CrossRef] [PubMed]
- Sansone, A.M.; Hisrich, B.V.; Young, R.B.; Abel, W.F.; Bowens, Z.; Blair, B.B.; Funkhouser, A.T.; Schammel, D.P.; Green, L.J.; Lessey, B.A.; et al. Evaluation of BCL6 and SIRT1 as Non-Invasive Diagnostic Markers of Endometriosis. Curr. Issues Mol. Biol. 2021, 43, 1350–1360. [Google Scholar] [CrossRef]
- Jiang, I.; Yong, P.J.; Allaire, C.; Bedaiwy, M.A. Intricate Connections between the Microbiota and Endometriosis. Int. J. Mol. Sci. 2021, 22, 5644. [Google Scholar] [CrossRef]
- Qin, R.; Tian, G.; Liu, J.; Cao, L. The Gut Microbiota and Endometriosis: From Pathogenesis to Diagnosis and Treatment. Front. Cell. Infect. Microbiol. 2022, 12, 1069557. [Google Scholar] [CrossRef]
- Proestling, K.; Wenzl, R.; Yotova, I.; Hauser, C.; Husslein, H.; Kuessel, L. Investigating Selected Adhesion Molecules as Urinary Biomarkers for Diagnosing Endometriosis. Reprod. BioMedicine Online 2020, 40, 555–558. [Google Scholar] [CrossRef]
- Kuessel, L.; Jaeger-Lansky, A.; Pateisky, P.; Rossberg, N.; Schulz, A.; Schmitz, A.A.P.; Staudigl, C.; Wenzl, R. Cytokeratin-19 as a Biomarker in Urine and in Serum for the Diagnosis of Endometriosis–a Prospective Study. Gynecol. Endocrinol. 2014, 30, 38–41. [Google Scholar] [CrossRef]
- Chen, X.; Liu, H.; Sun, W.; Guo, Z.; Lang, J. Elevated Urine Histone 4 Levels in Women with Ovarian Endometriosis Revealed by Discovery and Parallel Reaction Monitoring Proteomics. J. Proteom. 2019, 204, 103398. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Oh, Y.J.; Nam, A.; Kim, H.Y.; Park, J.H.; Kim, J.H.; Park, K.H.; Cho, D.J.; Lee, B.S. Evaluation of Serum and Urinary Angiogenic Factors in Patients with Endometriosis. Am. J. Reprod. Immunol. 2007, 58, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Tokushige, N.; Markham, R.; Crossett, B.; Ahn, S.B.; Nelaturi, V.L.; Khan, A.; Fraser, I.S. Discovery of a Novel Biomarker in the Urine in Women with Endometriosis. Fertil. Steril. 2011, 95, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Muñoz, S.; Morcillo, I.; Puchades-Carrasco, L.; Payá, V.; Pellicer, A.; Pineda-Lucena, A. Nuclear Magnetic Resonance Metabolomic Profiling of Urine Provides a Noninvasive Alternative to the Identification of Biomarkers Associated with Endometriosis. Fertil. Steril. 2015, 104, 1202–1209. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, C.; Zhang, A.; Zhang, J.; Wang, X.; Sun, X.; Sun, Z.; Wang, X. High-Throughput Metabolomics Used to Identify Potential Therapeutic Targets of Guizhi Fuling Wan against Endometriosis of Cold Coagulation and Blood Stasis. RSC Adv. 2018, 8, 19238–19250. [Google Scholar] [CrossRef]
- Othman, E.R.; Markeb, A.A.; Khashbah, M.Y.; Abdelaal, I.I.; ElMelegy, T.T.; Fetih, A.N.; Van der Houwen, L.E.; Lambalk, C.B.; Mijatovic, V. Markers of Local and Systemic Estrogen Metabolism in Endometriosis. Reprod. Sci. 2021, 28, 1001–1011. [Google Scholar] [CrossRef]
- Le, N.; Cregger, M.; Brown, V.; Mola, J.L.D.; Bremer, P.; Nguyen, L.; Groesch, K.; Wilson, T.; Diaz-Sylvester, P.; Braundmeier-Fleming, A. Association of Microbial Dynamics with Urinary Estrogens and Estrogen Metabolites in Patients with Endometriosis. PLoS ONE 2021, 16, e0261362. [Google Scholar] [CrossRef]
- Ser, H.-L.; Au Yong, S.-J.; Shafiee, M.N.; Mokhtar, N.M.; Ali, R.A.R. Current Updates on the Role of Microbiome in Endometriosis: A Narrative Review. Microorganisms 2023, 11, 360. [Google Scholar] [CrossRef]
- Chadchan, S.B.; Naik, S.K.; Popli, P.; Talwar, C.; Putluri, S.; Ambati, C.R.; Lint, M.A.; Kau, A.L.; Stallings, C.L.; Kommagani, R. Gut Microbiota and Microbiota-Derived Metabolites Promotes Endometriosis. Cell Death Discov. 2023, 9, 28. [Google Scholar] [CrossRef]
- Colonetti, T.; Saggioratto, M.C.; Grande, A.J.; Colonetti, L.; Junior, J.C.D.; Ceretta, L.B.; Roever, L.; Silva, F.R.; da Rosa, M.I. Gut and Vaginal Microbiota in the Endometriosis: Systematic Review and Meta-Analysis. BioMed Res. Int. 2023, 2023, e2675966. [Google Scholar]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The Microbiota Continuum along the Female Reproductive Tract and Its Relation to Uterine-Related Diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef] [PubMed]
- Green, K.A.; Zarek, S.M.; Catherino, W.H. Gynecologic Health and Disease in Relation to the Microbiome of the Female Reproductive Tract. Fertil. Steril. 2015, 104, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.S.; Bayatibojakhi, S. Exploring Preterm Birth as a Polymicrobial Disease: An Overview of the Uterine Microbiome. Front. Immunol. 2014, 5, 595. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.M.; Werner, M.D.; Juneau, C.R.; Tao, X.; Landis, J.; Zhan, Y.; Treff, N.R.; Scott, R.T. Endometrial Microbiome at the Time of Embryo Transfer: Next-Generation Sequencing of the 16S Ribosomal Subunit. J. Assist. Reprod. Genet. 2016, 33, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Davies, J. In a Map for Human Life, Count the Microbes, Too. Science 2001, 291, 2316. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Simon, C. Deciphering the Effect of Reproductive Tract Microbiota on Human Reproduction. Reprod. Med. Biol. 2019, 18, 40–50. [Google Scholar] [CrossRef]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence That the Endometrial Microbiota Has an Effect on Implantation Success or Failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef]
- Ma, B.; Forney, L.J.; Ravel, J. Vaginal Microbiome: Rethinking Health and Disease. Annu. Rev. Microbiol. 2012, 66, 371–389. [Google Scholar] [CrossRef]
- Muzny, C.A.; Laniewski, P.; Schwebke, J.R.; Herbst-Kralovetz, M.M. Host–Vaginal Microbiota Interactions in the Pathogenesis of Bacterial Vaginosis. Curr. Opin. Infect. Dis. 2020, 33, 59. [Google Scholar] [CrossRef]
- Nunn, K.L.; Clair, G.C.; Adkins, J.N.; Engbrecht, K.; Fillmore, T.; Forney, L.J. Amylases in the Human Vagina. mSphere 2020, 5, e00943-20. [Google Scholar] [CrossRef]
- Yarbrough, V.L.; Winkle, S.; Herbst-Kralovetz, M.M. Antimicrobial Peptides in the Female Reproductive Tract: A Critical Component of the Mucosal Immune Barrier with Physiological and Clinical Implications. Hum. Reprod. Update 2015, 21, 353–377. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The Gut Microbiome in Health and in Disease. Curr. Opin. Gastroenterol. 2015, 31, 69. [Google Scholar] [CrossRef] [PubMed]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The Role of Gut Microbiota in Intestinal Inflammation with Respect to Diet and Extrinsic Stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef]
- Muhleisen, A.L.; Herbst-Kralovetz, M.M. Menopause and the Vaginal Microbiome. Maturitas 2016, 91, 42–50. [Google Scholar] [CrossRef]
- Smith, S.B.; Ravel, J. The Vaginal Microbiota, Host Defence and Reproductive Physiology. J. Physiol. 2017, 595, 451–463. [Google Scholar] [CrossRef]
- Farage, M.; Maibach, H. Lifetime Changes in the Vulva and Vagina. Arch. Gynecol. Obstet. 2006, 273, 195–202. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Haick, A.; Nkwopara, E.; Garcia, R.; Rendi, M.; Agnew, K.; Fredricks, D.N.; Eschenbach, D. Colonization of the Upper Genital Tract by Vaginal Bacterial Species in Nonpregnant Women. Am. J. Obstet. Gynecol. 2015, 212, 611.e1–611.e9. [Google Scholar] [CrossRef]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Fadrosh, D.; Chang, K.; Silver, M.I.; Viscidi, R.P.; Burke, A.E.; Ravel, J.; Gravitt, P.E. Association between the Vaginal Microbiota, Menopause Status, and Signs of Vulvovaginal Atrophy. Menopause 2014, 21, 450. [Google Scholar] [CrossRef]
- Verstraelen, H.; Vilchez-Vargas, R.; Desimpel, F.; Jauregui, R.; Vankeirsbilck, N.; Weyers, S.; Verhelst, R.; Sutter, P.D.; Pieper, D.H.; Wiele, T.V.D. Characterisation of the Human Uterine Microbiome in Non-Pregnant Women through Deep Sequencing of the V1-2 Region of the 16S RRNA Gene. PeerJ 2016, 4, e1602. [Google Scholar] [CrossRef] [PubMed]
- Winters, A.D.; Romero, R.; Gervasi, M.T.; Gomez-Lopez, N.; Tran, M.R.; Garcia-Flores, V.; Pacora, P.; Jung, E.; Hassan, S.S.; Hsu, C.-D.; et al. Does the Endometrial Cavity Have a Molecular Microbial Signature? Sci. Rep. 2019, 9, 9905. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; He, F.; Lin, Z.; Liu, S.; Tang, L.; Huang, Y.; Hu, Z. Dysbiosis of the endometrial microbiota and its association with inflammatory cytokines in endometrial cancer. Int. J. Cancer 2021, 148, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine Microbiota: Residents, Tourists, or Invaders? Front. Immunol. 2018, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Zervomanolakis, I.; Ott, H.; Hadziomerovic, D.; Mattle, V.; Seeber, B.E.; Virgolini, I.; Heute, D.; Kissler, S.; Leyendecker, G.; Wildt, L. Physiology of Upward Transport in the Human Female Genital Tract. Ann. N. Y. Acad. Sci. 2007, 1101, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kissler, S.; Hamscho, N.; Zangos, S.; Wiegratz, I.; Schlichter, S.; Menzel, C.; Doebert, N.; Gruenwald, F.; Vogl, T.; Gaetje, R.; et al. Uterotubal Transport Disorder in Adenomyosis and Endometriosis—A Cause for Infertility. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 902–908. [Google Scholar] [CrossRef]
- Fang, R.-L.; Chen, L.-X.; Shu, W.-S.; Yao, S.-Z.; Wang, S.-W.; Chen, Y.-Q. Barcoded Sequencing Reveals Diverse Intrauterine Microbiomes in Patients Suffering with Endometrial Polyps. Am. J. Transl. Res. 2016, 8, 1581–1592. [Google Scholar]
- Ata, B.; Yildiz, S.; Turkgeldi, E.; Brocal, V.P.; Dinleyici, E.C.; Moya, A.; Urman, B. The Endobiota Study: Comparison of Vaginal, Cervical and Gut Microbiota between Women with Stage 3/4 Endometriosis and Healthy Controls. Sci. Rep. 2019, 9, 2204. [Google Scholar] [CrossRef]
- Walther-António, M.R.S.; Chen, J.; Multinu, F.; Hokenstad, A.; Distad, T.J.; Cheek, E.H.; Keeney, G.L.; Creedon, D.J.; Nelson, H.; Mariani, A.; et al. Potential Contribution of the Uterine Microbiome in the Development of Endometrial Cancer. Genome Med. 2016, 8, 122. [Google Scholar] [CrossRef]
- Le, N.; Cregger, M.; Fazleabas, A.; Braundmeier-Fleming, A. Effects of Endometriosis on Immunity and Mucosal Microbial Community Dynamics in Female Olive Baboons. Sci. Rep. 2022, 12, 1590. [Google Scholar] [CrossRef]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.E.; Zhong, X.; Koenig, S.S.K.; Fu, L.; Ma, Z.; Zhou, X.; et al. Temporal Dynamics of the Human Vaginal Microbiota. Sci. Transl. Med. 2012, 4, 132ra52. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Cregger, M.A.; Lenz, K.; Leary, E.; Leach, R.; Fazleabas, A.; White, B.; Braundmeier, A. Reproductive Microbiomes: Using the Microbiome as a Novel Diagnostic Tool for Endometriosis. Reprod. Immunol. Open Access 2017, 2, 36. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the Microbiota and the Immune System. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Spurbeck, R.R.; Arvidson, C.G. Inhibition of Neisseria gonorrhoeae Epithelial Cell Interactions by Vaginal Lactobacillus Species. Infect. Immun. 2008, 76, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Benner, M.; Ferwerda, G.; Joosten, I.; van der Molen, R.G. How Uterine Microbiota Might Be Responsible for a Receptive, Fertile Endometrium. Hum. Reprod. Update 2018, 24, 393–415. [Google Scholar] [CrossRef]
- Li, Z.; Quan, G.; Jiang, X.; Yang, Y.; Ding, X.; Zhang, D.; Wang, X.; Hardwidge, P.R.; Ren, W.; Zhu, G. Effects of metabolites derived from gut microbiota and hosts on pathogens. Front. Cell. Infect. Microbiol. 2018, 8, 314. [Google Scholar] [CrossRef]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Fu, H.; Shi, Y.Q.; Mo, S.J. Effect of short-chain fatty acids on the proliferation and differentiation of the human colonic adenocarcinoma cell line Caco-2. Chin. J. Dig. Dis. 2004, 5, 115–117. [Google Scholar] [CrossRef]
- Semaan, J.; El-Hakim, S.; Ibrahim, J.-N.; Safi, R.; Elnar, A.A.; El Boustany, C. Comparative effect of sodium butyrate and sodium propionate on proliferation, cell cycle and apoptosis in human breast cancer cells MCF-7. Breast Cancer 2020, 27, 696–705. [Google Scholar] [CrossRef]
- Bindels, L.B.; Porporato, P.; Dewulf, E.M.; Verrax, J.; Neyrinck, A.M.; Martin, J.C.; Scott, K.P.; Calderon, P.B.; Feron, O.; Muccioli, G.G.; et al. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br. J. Cancer 2012, 107, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Bhatt, B.; Zeng, P.; Zhu, H.; Sivaprakasam, S.; Li, S.; Xiao, H.; Dong, L.; Shiao, P.; Kolhe, R.; Patel, N.; et al. Gpr109a Limits Microbiota-Induced IL-23 Production to Constrain ILC3-Mediated Colonic Inflammation. J. Immunol. 2018, 200, 2905–2914. [Google Scholar] [CrossRef]
- Ang, Z.; Ding, J.L. GPR41 and GPR43 in Obesity and Inflammation—Protective or Causative? Front. Immunol. 2016, 7, 28. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.; Jannasch, A.; Cooper, B.; Patterson, J.; Kim, C. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 2014, 8, 80–93. [Google Scholar] [CrossRef]
- Licciardi, P.V.; Ververis, K.; Karagiannis, T.C. Histone Deacetylase Inhibition and Dietary Short-Chain Fatty Acids. ISRN Allergy 2011, 2011, 869647. [Google Scholar] [CrossRef]
- Chadchan, S.B.; Popli, P.; Ambati, C.R.; Tycksen, E.; Han, S.J.; E Bulun, S.; Putluri, N.; Biest, S.W.; Kommagani, R. Gut microbiota–derived short-chain fatty acids protect against the progression of endometriosis. Life Sci. Alliance 2021, 4, e202101224. [Google Scholar] [CrossRef] [PubMed]
- Vallvé-Juanico, J.; Santamaria, X.; Vo, K.C.; Houshdaran, S.; Giudice, L.C. Macrophages Display Proinflammatory Phenotypes in the Eutopic Endometrium of Women with Endometriosis with Relevance to an Infectious Etiology of the Disease. Fertil. Steril. 2019, 112, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Sun, S.; Bi, Y.; Ding, J.; Cheng, W.; Yu, J.; Zhou, L.; Li, M.; Yu, C. Correlation of Fecal Metabolomics and Gut Microbiota in Mice with Endometriosis. Am. J. Reprod. Immunol. 2020, 84, e13307. [Google Scholar] [CrossRef] [PubMed]
- Meroni, M.; Longo, M.; Dongiovanni, P. Alcohol or Gut Microbiota: Who Is the Guilty? Int. J. Mol. Sci. 2019, 20, 4568. [Google Scholar] [CrossRef]
- Harada, T.; Iwabe, T.; Terakawa, N. Role of Cytokines in Endometriosis. Fertil. Steril. 2001, 76, 1–10. [Google Scholar] [CrossRef]
- Nothnick, W.B. Treating Endometriosis as an Autoimmune Disease. Fertil. Steril. 2001, 76, 223–231. [Google Scholar] [CrossRef]
- Wanderley, C.W.; Colón, D.F.; Luiz, J.P.M.; Oliveira, F.F.; Viacava, P.R.; Leite, C.A.; Pereira, J.A.; Silva, C.M.; Silva, C.R.; Silva, R.L.; et al. Paclitaxel Reduces Tumor Growth by Reprogramming Tumor-Associated Macrophages to an M1 Profile in a TLR4-Dependent Manner. Cancer Res. 2018, 78, 5891–5900. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Paster, B.J.; Boches, S.K.; Galvin, J.L.; Ericson, R.E.; Lau, C.N.; Levanos, V.A.; Sahasrabudhe, A.; Dewhirst, F.E. Bacterial Diversity in Human Subgingival Plaque. J. Bacteriol. 2001, 183, 3770–3783. [Google Scholar] [CrossRef]
- Zaura, E.; Keijser, B.J.; Huse, S.M.; Crielaard, W. Defining the Healthy “Core Microbiome” of Oral Microbial Communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef]
- Segata, N.; Haake, S.K.; Mannon, P.; Lemon, K.P.; Waldron, L.; Gevers, D.; Huttenhower, C.; Izard, J. Composition of the Adult Digestive Tract Bacterial Microbiome Based on Seven Mouth Surfaces, Tonsils, Throat and Stool Samples. Genome Biol. 2012, 13, R42. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zeng, X.; Ning, K.; Liu, K.-L.; Lo, C.-C.; Wang, W.; Chen, J.; Wang, D.; Huang, R.; Chang, X.; et al. Saliva Microbiomes Distinguish Caries-Active from Healthy Human Populations. ISME J. 2012, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, Y.; Cao, Y.; Xue, J.; Zhou, X. The Oral Microbiome Diversity and Its Relation to Human Diseases. Folia Microbiol. 2015, 60, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, F. The Microbiota in Inflammatory Bowel Disease: Friend, Bystander, and Sometime-Villain. Nutr. Rev. 2012, 70, S31–S37. [Google Scholar] [CrossRef]
- Docktor, M.J.; Paster, B.J.; Abramowicz, S.; Ingram, J.; Wang, Y.E.; Correll, M.; Jiang, H.; Cotton, S.L.; Kokaras, A.S.; Bousvaros, A. Alterations in Diversity of the Oral Microbiome in Pediatric Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2012, 18, 935–942. [Google Scholar] [CrossRef]
- Seoudi, N.; Bergmeier, L.A.; Drobniewski, F.; Paster, B.; Fortune, F. The Oral Mucosal and Salivary Microbial Community of Behçet’s Syndrome and Recurrent Aphthous Stomatitis. J. Oral Microbiol. 2015, 7, 27150. [Google Scholar] [CrossRef]
- Slocum, C.; Kramer, C.; Genco, C.A. Immune Dysregulation Mediated by the Oral Microbiome: Potential Link to Chronic Inflammation and Atherosclerosis. J. Intern. Med. 2016, 280, 114–128. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The Oral and Gut Microbiomes Are Perturbed in Rheumatoid Arthritis and Partly Normalized after Treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The Subgingival Microbiome in Health and Periodontitis and Its Relationship with Community Biomass and Inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef]
- Shaik-Dasthagirisaheb, Y.B.; Huang, N.; Gibson, F.C. Inflammatory Response to Porphyromonas Gingivalis Partially Requires Interferon Regulatory Factor (IRF) 3. Innate Immun. 2014, 20, 312–319. [Google Scholar] [CrossRef]
- Eyster, K.M. The Estrogen Receptors: An Overview from Different Perspectives. Estrogen Recept. Methods Protoc. 2016, 1366, 1–10. [Google Scholar] [CrossRef]
- Chantalat, E.; Valera, M.-C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; et al. Estrogen Receptors and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2815. [Google Scholar] [CrossRef] [PubMed]
- Laux-Biehlmann, A.; d’Hooghe, T.; Zollner, T.M. Menstruation Pulls the Trigger for Inflammation and Pain in Endometriosis. Trends Pharmacol. Sci. 2015, 36, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Galvankar, M.; Singh, N.; Modi, D. Estrogen Is Essential but Not Sufficient to Induce Endometriosis. J. Biosci. 2017, 42, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shen, Q.; Celestino, J.; Milam, M.R.; Westin, S.N.; Lacour, R.A.; Meyer, L.A.; Shipley, G.L.; Davies, P.J.A.; Deng, L.; et al. Enhanced Estrogen-Induced Proliferation in Obese Rat Endometrium. Am. J. Obstet. Gynecol. 2009, 200, 186.e1–186.e8. [Google Scholar] [CrossRef]
- Reis, F.M.; Petraglia, F.; Taylor, R.N. Endometriosis: Hormone Regulation and Clinical Consequences of Chemotaxis and Apoptosis. Hum. Reprod. Update 2013, 19, 406–418. [Google Scholar] [CrossRef] [PubMed]
- van Weelden, W.J.; Massuger, L.F.A.G.; Enitec; Pijnenborg, J.M.A.; Romano, A. Anti-Estrogen Treatment in Endometrial Cancer: A Systematic Review. Front. Oncol. 2019, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen Signaling in Endometrial Cancer: A Key Oncogenic Pathway with Several Open Questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Stafford, J.M. Role of Estrogens in the Regulation of Liver Lipid Metabolism. In Sex and Gender Factors Affecting Metabolic Homeostasis, Diabetes and Obesity; Mauvais-Jarvis, F., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; pp. 227–256. ISBN 978-3-319-70178-3. [Google Scholar]
- Parida, S.; Sharma, D. The Microbiome–Estrogen Connection and Breast Cancer Risk. Cells 2019, 8, 1642. [Google Scholar] [CrossRef]
- The Gut Microbiome and the Estrobolome: How Gut Microbes Affect Estrogen Metabolism—Vibrant Wellness. Available online: https://www.vibrant-wellness.com/the-gut-microbiome-and-the-estrobolome-how-gut-microbes-affect-estrogen-metabolism/ (accessed on 4 May 2023).
- Sui, Y.; Wu, J.; Chen, J. The Role of Gut Microbial β-Glucuronidase in Estrogen Reactivation and Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 631552. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–Gut Microbiome Axis: Physiological and Clinical Implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Ervin, S.M.; Li, H.; Lim, L.; Roberts, L.R.; Liang, X.; Mani, S.; Redinbo, M.R. Gut Microbial β-Glucuronidases Reactivate Estrogens as Components of the Estrobolome That Reactivate Estrogens. J. Biol. Chem. 2019, 294, 18586–18599. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal Microbial Determinants of Fecal and Systemic Estrogens and Estrogen Metabolites: A Cross-Sectional Study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Looijer-van Langen, M.; Hotte, N.; Dieleman, L.A.; Albert, E.; Mulder, C.; Madsen, K.L. Estrogen Receptor-β Signaling Modulates Epithelial Barrier Function. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G621–G626. [Google Scholar] [CrossRef]
- Insenser, M.; Murri, M.; del Campo, R.; Martínez-García, M.Á.; Fernández-Durán, E.; Escobar-Morreale, H.F. Gut Microbiota and the Polycystic Ovary Syndrome: Influence of Sex, Sex Hormones, and Obesity. J. Clin. Endocrinol. Metab. 2018, 103, 2552–2562. [Google Scholar] [CrossRef]
- Beaud, D.; Tailliez, P.; Anba-Mondoloni, J. Genetic Characterization of the β-Glucuronidase Enzyme from a Human Intestinal Bacterium, Ruminococcus Gnavus. Microbiology 2005, 151, 2323–2330. [Google Scholar] [CrossRef]
- Kitawaki, J.; Kado, N.; Ishihara, H.; Koshiba, H.; Kitaoka, Y.; Honjo, H. Endometriosis: The Pathophysiology as an Estrogen-Dependent Disease. J. Steroid Biochem. Mol. Biol. 2002, 83, 149–155. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. The Gut Microbiota: A Puppet Master in the Pathogenesis of Endometriosis? Am. J. Obstet. Gynecol. 2016, 215, 68.e1–68.e4. [Google Scholar] [CrossRef]
- Hakansson, A.; Molin, G. Gut Microbiota and Inflammation. Nutrients 2011, 3, 637–682. [Google Scholar] [CrossRef]
- Morotti, M.; Vincent, K.; Becker, C.M. Mechanisms of Pain in Endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 8–13. [Google Scholar] [CrossRef]
- Symons, L.K.; Miller, J.E.; Kay, V.R.; Marks, R.M.; Liblik, K.; Koti, M.; Tayade, C. The Immunopathophysiology of Endometriosis. Trends Mol. Med. 2018, 24, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nicholes, K.; Shih, I.-M. The Origin and Pathogenesis of Endometriosis. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Molina, N.M.; Sola-Leyva, A.; Saez-Lara, M.J.; Plaza-Diaz, J.; Tubić-Pavlović, A.; Romero, B.; Clavero, A.; Mozas-Moreno, J.; Fontes, J.; Altmäe, S. New Opportunities for Endometrial Health by Modifying Uterine Microbial Composition: Present or Future? Biomolecules 2020, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Bodke, H.; Jogdand, S. Role of Probiotics in Human Health. Cureus 2022, 14, e31313. [Google Scholar] [CrossRef]
- George Kerry, R.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of Probiotics for Human Health: A Review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef]
- Hashem, N.M.; Gonzalez-Bulnes, A. The Use of Probiotics for Management and Improvement of Reproductive Eubiosis and Function. Nutrients 2022, 14, 902. [Google Scholar] [CrossRef]
- Khodaverdi, S.; Mohammadbeigi, R.; Khaledi, M.; Mesdaghinia, L.; Sharifzadeh, F.; Nasiripour, S.; Gorginzadeh, M. Beneficial Effects of Oral Lactobacillus on Pain Severity in Women Suffering from Endometriosis: A Pilot Placebo-Controlled Randomized Clinical Trial. Int. J. Fertil. Steril. 2019, 13, 178–183. [Google Scholar]
- Itoh, H.; Sashihara, T.; Hosono, A.; Kaminogawa, S.; Uchida, M. Lactobacillus Gasseri OLL2809 Inhibits Development of Ectopic Endometrial Cell in Peritoneal Cavity via Activation of NK Cells in a Murine Endometriosis Model. Cytotechnology 2011, 63, 205–210. [Google Scholar] [CrossRef]
- Uchida, M.; Kobayashi, O. Effects of Lactobacillus Gasseri OLL2809 on the Induced Endometriosis in Rats. Biosci. Biotechnol. Biochem. 2013, 77, 1879–1881. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The Promotion Mechanism of Prebiotics for Probiotics: A Review. Front. Nutr. 2022, 9, 2223. [Google Scholar] [CrossRef] [PubMed]

| Gene | Name of Protein | Protein ID | Amino Acids | Mass (kDa) | Function | References |
|---|---|---|---|---|---|---|
| ENG | Endoglin | P17813 | 658 | 70.578 |
| [98,99,100,101] |
| LUM | Lumican | P51884 | 338 | 38.429 |
| [102,103] |
| TGFB2 | Transforming Growth Factor Beta Receptor 2 | P37173 | 567 | 64.568 |
| [88,104] |
| TSPAN1 | Tetraspanin-1 | O60635 | 241 | 26.301 |
| [105,106] |
| CD44 | CD44 Antigen | P16070 | 742 | 81.538 |
| [107,108,109] |
| TNC | Tenascin | P24821 | 2201 | 240.853 |
| [110,111] |
| CatG | Cathepsin G | P08311 | 255 | 28.837 |
| [112,113,114] |
| DSP | Desmoplakin | P15924 | 2871 | 331.774 |
| [115,116] |
| THBS1 | Thrombospondin 1 | A8MZG1 | 94 | 10.053 |
| [117,118,119] |
| PCDH1 | Protocadherin-1 | Q08174 | 1060 | 114.743 |
| [120,121] |
| SPARCL1 | SPARC-like Protein 1 | Q14515 | 664 | 75.208 |
| [122,123] |
| AZGP1 | Zinc-alpha-2-glycoprotein | P25311 | 298 | 34.259 |
| [124,125,126,127] |
| ANXA2 | Annexin A2 | P07355 | 339 | 38.604 |
| [96,128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobstyl, A.; Chałupnik, A.; Mertowska, P.; Grywalska, E. How Do Microorganisms Influence the Development of Endometriosis? Participation of Genital, Intestinal and Oral Microbiota in Metabolic Regulation and Immunopathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 10920. https://doi.org/10.3390/ijms241310920
Sobstyl A, Chałupnik A, Mertowska P, Grywalska E. How Do Microorganisms Influence the Development of Endometriosis? Participation of Genital, Intestinal and Oral Microbiota in Metabolic Regulation and Immunopathogenesis of Endometriosis. International Journal of Molecular Sciences. 2023; 24(13):10920. https://doi.org/10.3390/ijms241310920
Chicago/Turabian StyleSobstyl, Anna, Aleksandra Chałupnik, Paulina Mertowska, and Ewelina Grywalska. 2023. "How Do Microorganisms Influence the Development of Endometriosis? Participation of Genital, Intestinal and Oral Microbiota in Metabolic Regulation and Immunopathogenesis of Endometriosis" International Journal of Molecular Sciences 24, no. 13: 10920. https://doi.org/10.3390/ijms241310920
APA StyleSobstyl, A., Chałupnik, A., Mertowska, P., & Grywalska, E. (2023). How Do Microorganisms Influence the Development of Endometriosis? Participation of Genital, Intestinal and Oral Microbiota in Metabolic Regulation and Immunopathogenesis of Endometriosis. International Journal of Molecular Sciences, 24(13), 10920. https://doi.org/10.3390/ijms241310920