Motifs in Natural Products as Useful Scaffolds to Obtain Novel Benzo[d]imidazole-Based Cannabinoid Type 2 (CB2) Receptor Agonists
Abstract
1. Introduction
2. Results
2.1. Design of Compounds
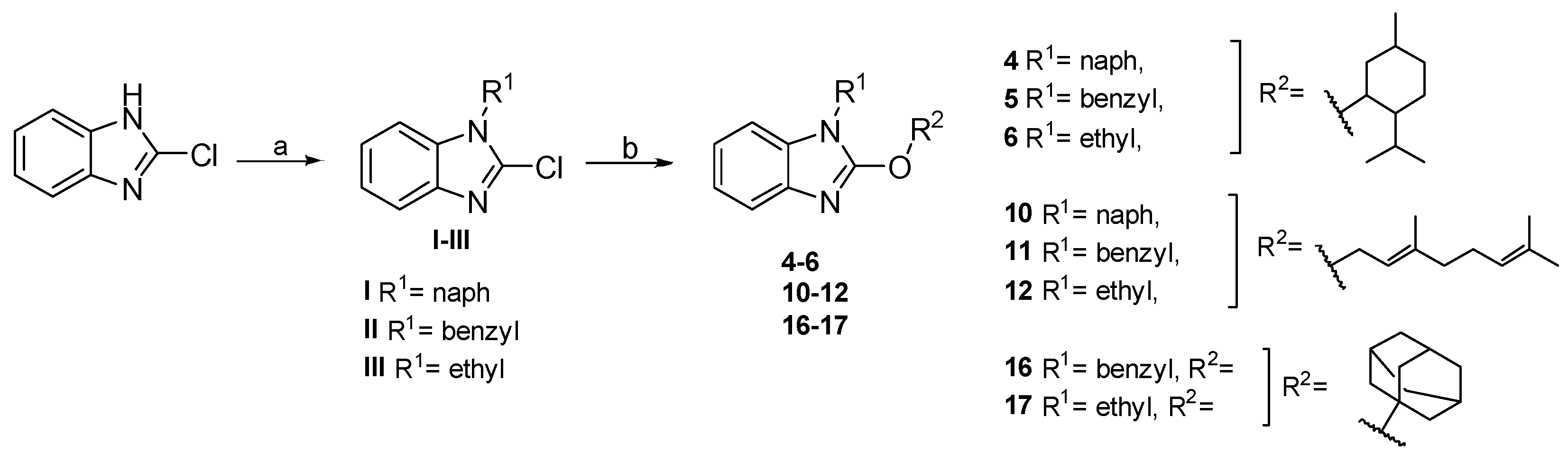
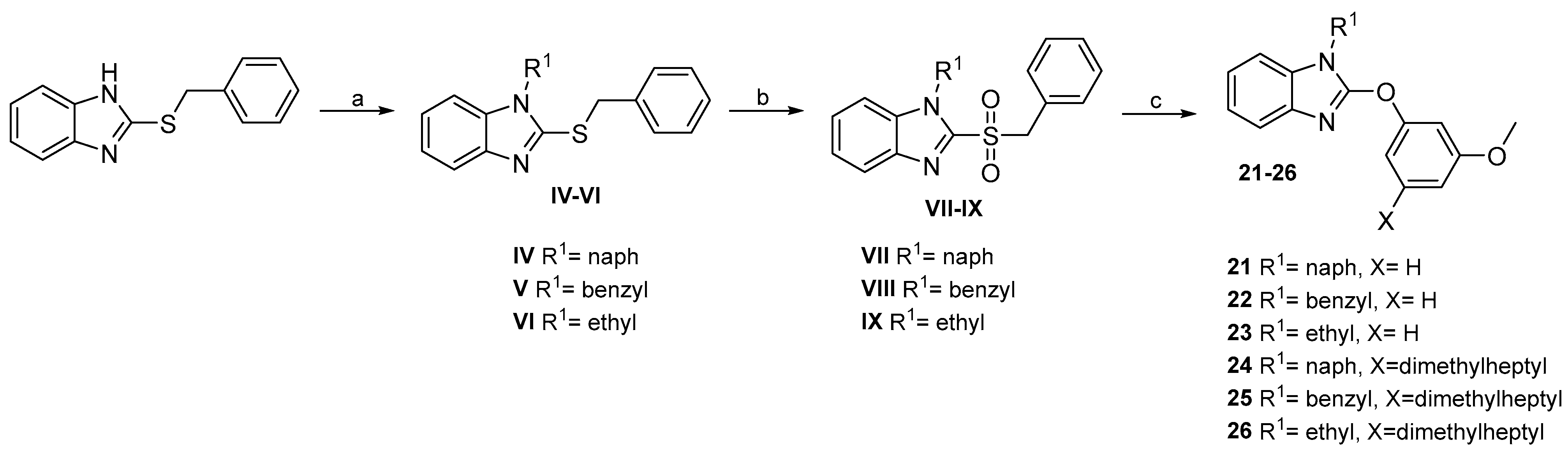
2.2. Chemistry
2.3. Radioligand Displacement Assay
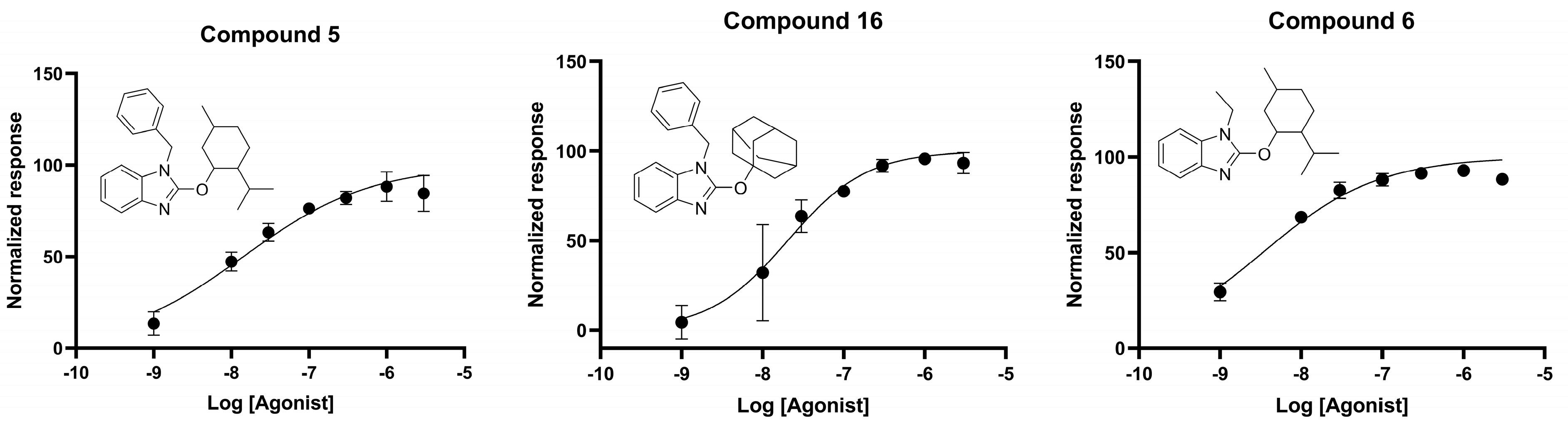
2.4. cAMP Accumulation Assay
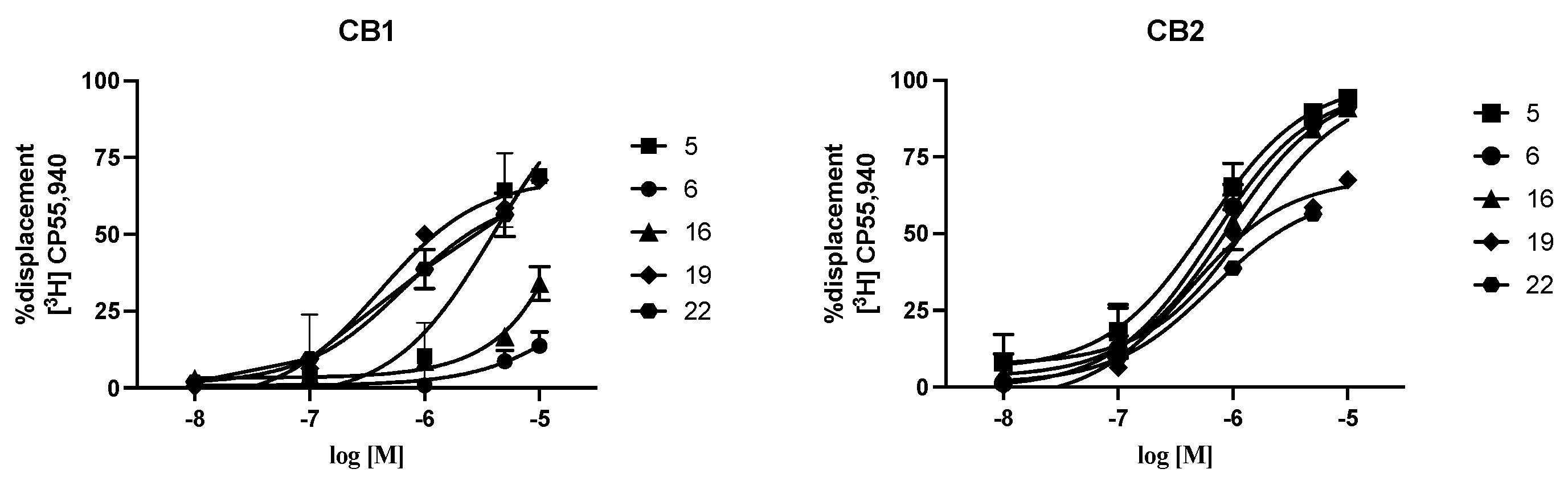
2.5. CB1/CB2 Receptor Selectivity
2.6. Molecular Docking
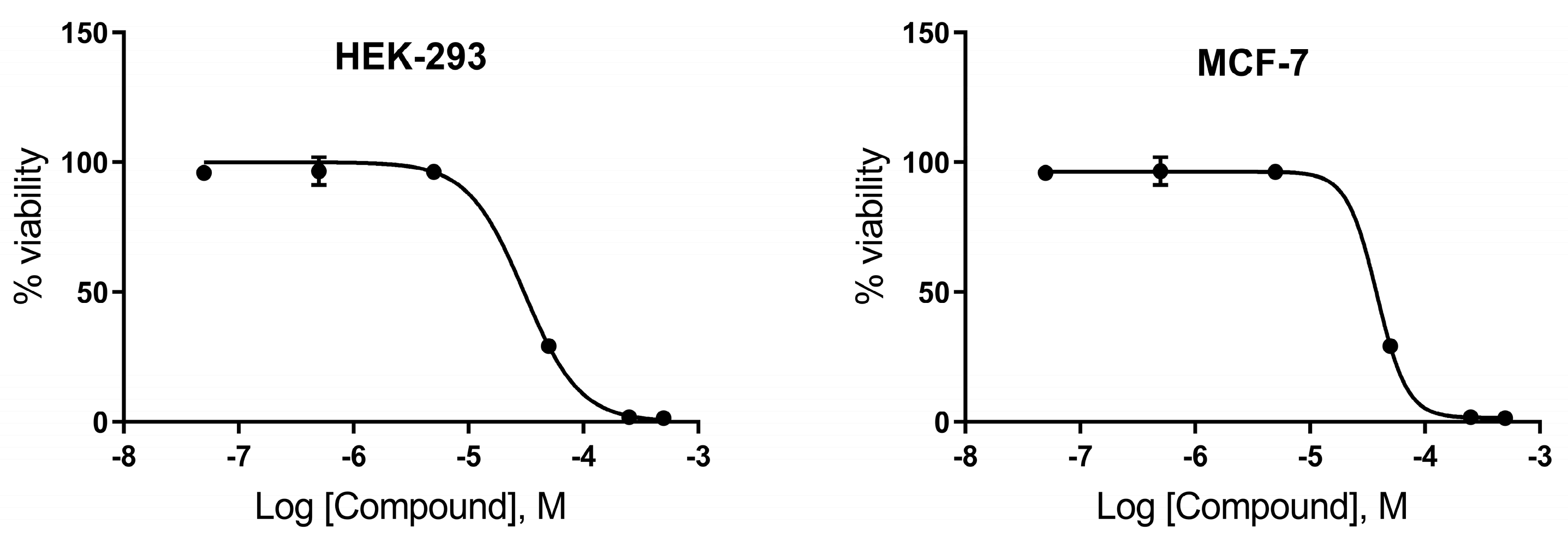
2.7. Neutral Red Uptake Assay
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. General Procedure for the Synthesis of Compounds I–III
- 2-chloro-1-(naphthalen-1-ylmethyl)-1H-benzo[d]imidazole (I). Yield: 98%. White solid. 1H NMR (400 MHz, Chloroform-d) δ 8.16 (d, J = 8.4 Hz, 1H), 8.06 (dd, J = 8.1, 1.5 Hz, 1H), 7.96–7.90 (m, 1H), 7.81–7.68 (m, 1H), 7.47–7.38 (m, 1H), 7.32 (ddd, J = 8.3, 7.2, 1.1 Hz, 1H), 7.22 (d, J = 8.1 Hz, 1H), 6.80 (dd, J = 7.2, 1.3 Hz, 1H), 5.96 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 141.34, 141.16, 135.26, 133.73, 130.27, 129.88, 129.21, 128.67, 126.95, 126.31, 125.49, 123.70, 123.24, 123.21, 121.99, 119.44, 110.15, 45.76. Purified by column chromatography using DCM:AcOEt (4:1).
- 1-benzyl-2-chloro-1H-benzo[d]imidazole (II). Yield: 92%. White solid. 1H NMR (400 MHz, Chloroform-d) δ 7.70–7.63 (m, 1H), 7.28–7.18 (m, 4H), 7.18–7.14 (m, 2H), 7.13–7.07 (m, 2H), 5.28 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 141.85, 140.80, 135.18, 135.06, 129.02, 128.20, 126.81, 123.39, 122.87, 119.53, 109.92, 47.90. Recrystallized in H2O:EtOH.
- 2-chloro-1-ethyl-1H-benzo[d]imidazole (III). Yield: 97%. White solid. 1H NMR (400 MHz, DMSO-d6) δ 7.60 (d, J = 8.0 Hz, 2H), 7.38–7.16 (m, 2H), 4.27 (q, J = 7.2 Hz, 2H), 1.30 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 141.69, 139.83, 134.97, 123.35, 122.78, 119.09, 110.81, 39.43, 14.89. Purified by column chromatography using DCM:AcOEt (4:1).
4.1.2. General Procedure for the Synthesis of Compounds IV–VI
- 2-(benzylthio)-1-(naphthalen-1-ylmethyl)-1H-benzo[d]imidazole (IV). Yield: 70%. Beige solid. 1H NMR (400 MHz, Chloroform-d) δ 7.91 (d, J = 7.9 Hz, 1H), 7.86 (d, J = 8.0 Hz, 2H), 7.72 (d, J = 8.4 Hz, 1H), 7.58–7.46 (m, 2H), 7.45–7.34 (m, 2H), 7.31–7.15 (m, 5H), 7.09 (t, J = 7.7 Hz, 1H), 6.97 (d, J = 8.1 Hz, 1H), 6.61 (d, J = 7.2 Hz, 1H), 5.60 (s, 2H), 4.65 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 152.24, 143.84, 136.84, 136.57, 133.73, 130.65, 130.46, 129.21, 129.15, 128.74, 128.37, 127.77, 126.76, 126.18, 125.60, 123.39, 122.44, 122.33, 122.24, 118.67, 109.55, 45.33, 37.50. Purified via column chromatography using Hexane:AcOEt (6:1), then recrystallized in EtOH:AcOEt.
- 1-benzyl-2-(benzylthio)-1H-benzo[d]imidazole (V). Yield: 83%. Beige solid. 1H NMR (400 MHz, Chloroform-d) δ 7.77 (d, J = 8.0 Hz, 1H), 7.42 (dd, J = 7.8, 1.8 Hz, 2H), 7.34–7.22 (m, 7H), 7.17 (d, J = 4.1 Hz, 2H), 7.10 (dd, J = 7.2, 2.4 Hz, 2H), 5.23 (s, 2H), 4.65 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 151.77, 143.76, 136.77, 136.28, 135.70, 129.20, 128.90, 128.75, 127.96, 127.75, 126.94, 122.29, 122.16, 118.57, 109.38, 47.64, 37.58. Purified via column chromatography using Hexane:AcOEt (3:1), then recrystallized in EtOH:H2O.
- 2-(benzylthio)-1-ethyl-1H-benzo[d]imidazole (VI). Yield: 85%. Yellow oil. 1H NMR (400 MHz, Chloroform-d) δ 7.76–7.68 (m, 1H), 7.43–7.37 (m, 2H), 7.32–7.18 (m, 6H), 4.62 (s, 2H), 4.06 (q, J = 7.3 Hz, 2H), 1.30 (t, J = 7.3 Hz, 4H). 13C NMR (101 MHz, CDCl3) δ 151.37, 144.08, 137.17, 136.06, 129.49, 129.08, 128.06, 122.31, 122.19, 118.81, 109.07, 39.27, 37.59, 14.93. Purified via column chromatography using a gradient from DCM to DCM:MeOH 5%.
4.1.3. General Procedure for the Synthesis of Compounds VII–IX
- 2-(benzylsulfonyl)-1-(naphthalen-1-ylmethyl)-1H-benzo[d]imidazole (VII). Yield: 88%. Beige solid. 1H NMR (400 MHz, Chloroform-d) δ 7.94 (d, J = 8.3, 1.0 Hz, 1H), 7.80 (t, J = 8.0, 1.6 Hz, 2H), 7.66 (d, J = 8.3 Hz, 1H), 7.49 (qd, J = 14.8, 8.3, 6.9, 1.5 Hz, 2H), 7.37–7.31 (m, 2H), 7.28–7.19 (m, 3H), 7.18–7.13 (m, 2H), 7.08 (t, J = 8.2, 7.2 Hz, 1H), 6.97 (d, J = 8.3, 1.0 Hz, 1H), 6.29 (dd, J = 7.2, 1.2 Hz, 1H), 5.76 (s, 2H), 4.74 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 147.07, 141.27, 135.74, 133.55, 131.48, 130.58, 130.05, 129.30, 129.03, 128.87, 128.26, 126.73, 126.52, 126.30, 126.10, 125.45, 124.38, 122.86, 122.00, 121.95, 111.57, 61.70, 46.09. Recrystallized in EtOH:DCM
- 1-benzyl-2-(benzylsulfonyl)-1H-benzo[d]imidazole (VIII). Yield: 82%. Yellow solid. 1H NMR (400 MHz, Chloroform-d) δ 7.97–7.93 (m, 1H), 7.43–7.34 (m, 3H), 7.32–7.19 (m, 8H), 7.06–6.97 (m, 2H), 5.41 (s, 2H), 4.77 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 146.64, 141.26, 135.53, 135.26, 131.43, 129.27, 128.86, 128.85, 128.08, 126.95, 126.41, 126.20, 124.27, 121.92, 111.57, 61.62, 48.51. Recrystallized in Hexane:AcOEt.
- 2-(benzylsulfonyl)-1-ethyl-1H-benzo[d]imidazole (IX). Yield: 93%. Yellow solid. 1H NMR (400 MHz, Chloroform-d) δ 7.90 (d, J = 7.9 Hz, 1H), 7.53–6.98 (m, 8H), 4.81 (s, 2H), 4.17 (q, J = 7.1 Hz, 2H), 1.19 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 146.26, 141.26, 134.89, 131.30, 129.19, 128.79, 126.56, 125.87, 124.04, 121.94, 110.67, 61.62, 40.22, 15.27. Purified via column chromatography using Hexane:AcOEt (3:1).
4.1.4. General Procedure for the Synthesis of Derivatives XII–XIII and XV
- 2-((2-isopropyl-5-methylcyclohexyl)thio)-1H-benzo[d]imidazole (XII). Yield: 46%. White solid. 1H NMR (400 MHz, DMSO-d6) δ 12.46 (s, 1H), 7.46–7.39 (m, 2H), 7.14–7.06 (m, 2H), 4.53 (dq, J = 4.4, 2.7 Hz, 1H), 2.02 (dq, J = 13.4, 2.9 Hz, 1H), 1.89–1.70 (m, 3H), 1.57 (dp, J = 9.6, 6.6 Hz, 1H), 1.40 (ddd, J = 13.7, 11.7, 3.1 Hz, 1H), 1.32–1.21 (m, 1H), 1.09–0.93 (m, 2H), 0.92 (d, J = 3.5 Hz, 3H), 0.90 (d, J = 3.5 Hz, 3H), 0.85 (d, J = 6.5 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 150.80, 121.67, 48.68, 47.89, 41.66, 35.01, 30.78, 27.66, 27.07, 22.37, 21.30, 20.91. Recrystallized in AcOEt:EtOH.
- (E)-2-((3,7-dimethylocta-2,6-dien-1-yl)thio)-1H-benzo[d]imidazole (XIII). Yield: 44%. Yellow oil. 1H NMR (400 MHz, Chloroform-d) δ 9.59 (s, 1H), 7.66 (s, 1H), 7.36 (s, 1H), 7.23–7.14 (m, 2H), 5.41 (t, J = 8.0 Hz, 1H), 5.05 (t, J = 6.5 Hz, 1H), 3.97 (d, J = 7.9 Hz, 2H), 2.11–1.98 (m, 4H), 1.67 (s, 3H), 1.66 (s, 3H), 1.58 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 150.36, 141.63, 131.92, 123.72, 122.32, 118.37, 39.51, 31.18, 26.31, 25.66, 17.73, 16.19. Purified via column chromatography using Hexane:AcOEt (3:1).
- 2-((4-methoxybenzyl)thio)-1H-benzo[d]imidazole (XV). Yield: 85%. White solid. 1H NMR (400 MHz, DMSO-d6) δ 12.55 (s, 1H), 7.55 (s, 1H), 7.37 (d, J = 8.5 Hz, 3H), 7.16–7.09 (m, 2H), 6.86 (d, J = 8.6 Hz, 2H), 4.52 (s, 2H), 3.71 (s, 3H). 13C NMR (101 MHz, DMSO) δ 158.54, 149.86, 143.67, 135.43, 130.07, 129.37, 121.62, 121.14, 117.41, 113.88, 110.31, 55.03, 39.52, 34.78. Recrystallized in H2O:EtOH.
4.1.5. Synthesis of Compound XIV
- 2-(adamantan-1-ylthio)-1H-benzo[d]imidazole (XIV). Yield: 65%. White solid. 1H NMR (400 MHz, DMSO-d6) δ 7.66–7.36 (m, 2H), 7.30–6.97 (m, 2H), 1.98 (s, 9H), 1.60 (s, 6H). 13C NMR (101 MHz, DMSO) δ 144.83, 121.94, 50.56, 43.36, 39.52, 35.49, 29.49. Recrystallized in H2O:EtOH.
4.1.6. General Procedure for the Synthesis of Derivatives 1–3, 7–9, 13–15, and 18–20
- 2-((2-isopropyl-5-methylcyclohexyl)thio)-1-(naphthalen-1-ylmethyl)-1H-benzo[d]imidazole (1). Yield: 75%. white solid. 1H NMR (400 MHz, Chloroform-d) δ 8.33 (d, J = 8.3 Hz, 2H), 8.15 (d, J = 8.0 Hz, 1H), 8.00 (t, J = 8.7 Hz, 2H), 7.82 (dt, J = 21.1, 7.1 Hz, 2H), 7.55–7.48 (m, 1H), 7.44 (t, J = 7.5 Hz, 1H), 7.35–7.21 (m, 2H), 6.92 (d, J = 7.2 Hz, 1H), 6.04 (s, 2H), 4.95 (s, 1H), 2.47 (dq, J = 14.0, 3.1 Hz, 1H), 2.08–1.87 (m, 4H), 1.84–1.73 (m, 1H), 1.72–1.62 (m, 1H), 1.54–1.43 (m, 1H), 1.32–1.16 (m, 3H), 1.12 (d, J = 6.7 Hz, 3H), 1.08 (d, J = 6.4 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 153.31, 143.88, 136.43, 133.70, 130.78, 130.51, 129.07, 128.18, 126.57, 126.01, 125.49, 123.36, 122.28, 121.89, 121.82, 118.34, 109.20, 50.01, 48.28, 45.35, 41.48, 35.10, 30.68, 27.53, 26.89, 22.05, 21.02, 20.83. Purified via preparative plate using Hexane:AcOEt (6:1) and then recrystallized in MeOH.
- 1-benzyl-2-((2-isopropyl-5-methylcyclohexyl)thio)-1H-benzo[d]imidazole (2). Yield: 39%. Yellow solid. 1H NMR (400 MHz, DMSO-d6) δ 7.60–7.53 (m, 1H), 7.46–7.40 (m, 1H), 7.32–7.28 (m, 2H), 7.26 (d, J = 7.1 Hz, 1H), 7.18–7.12 (m, 4H), 5.41 (d, J = 2.2 Hz, 2H), 2.08–1.96 (m, 1H), 1.83 (d, J = 12.2 Hz, 1H), 1.75–1.67 (m, 2H), 1.54–1.47 (m, 1H), 1.44–1.36 (m, 1H), 1.33–1.25 (m, 1H), 1.08–0.91 (m, 2H), 0.89 (dd, J = 2.8, 6.6 Hz, 6H), 0.82 (d, J = 6.3 Hz, 3H), 0.76 (dd, J = 6.4, 10.6 Hz, 1H). 13C NMR (101 MHz, DMSO) δ 152.30, 143.58, 136.91, 136.48, 129.08, 128.03, 127.28, 122.09, 122.06, 118.11, 110.25, 50.26, 47.81, 47.03, 41.45, 34.92, 30.81, 27.74, 27.16, 22.33, 21.26, 20.83. Purified via preparative plate using Hexane:AcOEt (6:1).
- 1-ethyl-2-((2-isopropyl-5-methylcyclohexyl)thio)-1H-benzo[d]imidazole (3). Yield: 68%. Yellow oil. 1H NMR (400 MHz, Chloroform-d) δ 7.77–7.69 (m, 1H), 7.33–7.26 (m, 1H), 7.26–7.17 (m, 2H), 4.78–4.65 (m, 1H), 4.20 (q, J = 7.3 Hz, 2H), 2.32–2.19 (m, 1H), 2.01–1.89 (m, 2H), 1.88–1.78 (m, 1H), 1.76–1.60 (m, 1H), 1.53–1.44 (m, 1H), 1.43 (t, J = 7.7, 7.3 Hz, 3H), 1.25–1.12 (m, 2H), 1.09–1.01 (m, 1H), 0.99 (t, J = 5.9 Hz, 6H), 0.92 (d, J = 6.5 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 152.11, 143.91, 135.81, 121.59, 121.54, 118.33, 108.46, 77.16, 49.56, 48.43, 41.63, 38.85, 35.32, 30.88, 27.77, 27.16, 22.18, 21.18, 20.96, 14.67. Purified via preparative plate using Hexane:AcOEt (20:1).
- (E)-2-((3,7-dimethylocta-2,6-dien-1-yl)thio)-1-(naphthalen-1-ylmethyl)-1H-benzo[d]imidazole (7). Yield: 54%. Yellow oil. 1H NMR (400 MHz, Chloroform-d) δ 8.23 (d, J = 8.3 Hz, 1H), 8.09 (d, J = 7.8 Hz, 1H), 7.95 (d, J = 8.2 Hz, 2H), 7.83–7.69 (m, 2H), 7.49–7.36 (m, 2H), 7.28 (t, J = 7.6 Hz, 1H), 7.22 (d, J = 7.9 Hz, 1H), 6.87 (dd, J = 7.2, 1.2 Hz, 1H), 5.96 (s, 2H), 5.62–5.52 (m, 1H), 5.26–5.17 (m, 1H), 4.26 (d, J = 7.9 Hz, 2H), 2.28–2.13 (m, 4H), 1.88 (s, 3H), 1.81 (s, 3H), 1.74 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 152.97, 143.80, 141.89, 136.45, 133.70, 131.76, 130.67, 130.45, 129.09, 128.29, 126.66, 126.07, 125.52, 123.78, 123.34, 122.17, 122.14, 122.09, 118.43, 117.79, 109.29, 45.38, 39.57, 31.31, 26.39, 25.67, 17.71, 16.29. Purified via preparative plate using Hexane:AcOEt (6:1).
- (E)-1-benzyl-2-((3,7-dimethylocta-2,6-dien-1-yl)thio)-1H-benzo[d]imidazole (8). Yield: 50%. Yellow oil. 1H NMR (400 MHz, Chloroform-d) δ 7.77 (d, J = 7.9 Hz, 1H), 7.41–7.30 (m, 3H), 7.32–7.18 (m, 5H), 5.48 (t, J = 7.9 Hz, 1H), 5.36 (s, 2H), 5.12 (t, J = 6.5 Hz, 1H), 4.13 (d, J = 7.9 Hz, 2H), 2.21–2.03 (m, 4H), 1.79 (s, 3H), 1.73 (s, 3H), 1.65 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 152.45, 143.72, 141.83, 136.16, 135.77, 131.76, 128.83, 127.88, 126.90, 123.78, 122.03, 121.96, 118.34, 117.84, 109.15, 47.62, 39.57, 31.36, 26.37, 25.68, 17.72, 16.28. Purified via preparative plate using Hexane:AcOEt (6:1).
- (E)-2-((3,7-dimethylocta-2,6-dien-1-yl)thio)-1-ethyl-1H-benzo[d]imidazole (9). Yield: 68%. Yellow oil. 1H NMR (400 MHz, Chloroform-d) δ 7.31 (d, J = 7.8 Hz, 1H), 7.07–7.01 (m, 1H), 6.97–6.90 (m, 2H), 6.31 (dd, J = 10.8, 17.6 Hz, 1H), 5.33–5.15 (m, 2H), 5.04 (t, J = 7.2 Hz, 1H), 3.90 (q, J = 7.1 Hz, 2H), 2.32–2.14 (m, 2H), 2.13–2.00 (m, 1H), 1.90–1.77 (m, 4H), 1.59 (s, 3H), 1.45 (s, 3H), 1.31 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 144.16, 131.80, 129.47, 123.66, 120.66, 120.06, 112.95, 112.47, 106.94, 63.36, 38.24, 35.45, 25.62, 25.15, 22.84, 17.47, 13.44. Purified via preparative plate using Hexane:AcOEt (6:1).
- 2-(adamantan-1-ylthio)-1-(naphthalen-1-ylmethyl)-1H-benzo[d]imidazole (13). Yield: 58%. White solid. 1H NMR (400 MHz, Chloroform-d) δ 8.26 (d, J = 8.4 Hz, 1H), 8.04 (dd, J = 8.1, 18.7 Hz, 2H), 7.90 (d, J = 8.4 Hz, 1H), 7.74 (dt, J = 7.4, 26.0 Hz, 2H), 7.45–7.37 (m, 2H), 7.37–7.26 (m, 1H), 7.22 (d, J = 8.2 Hz, 1H), 6.69 (d, J = 7.1 Hz, 1H), 6.17 (s, 2H), 2.24 (s, 6H), 2.19 (s, 3H), 1.81 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 148.13, 143.72, 135.67, 133.65, 131.30, 130.34, 129.08, 128.09, 126.59, 126.03, 125.46, 123.26, 123.01, 122.35, 122.27, 119.83, 110.36, 52.87, 45.81, 43.92, 36.00, 30.25. Recrystallized in EtOH:AcOEt.
- 2-(adamantan-1-ylthio)-1-benzyl-1H-benzo[d]imidazole (14). Yield: 92%. White solid. 1H NMR (400 MHz, Chloroform-d) δ 7.88 (d, J = 7.9 Hz, 1H), 7.37–7.28 (m, 4H), 7.25 (d, J = 4.2 Hz, 2H), 7.18 (d, J = 6.5 Hz, 2H), 5.61 (s, 2H), 2.20–2.09 (m, 9H), 1.73 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 147.58, 143.64, 136.31, 135.40, 128.75, 127.72, 126.75, 122.92, 122.25, 119.78, 110.29, 52.94, 48.11, 43.92, 36.00, 30.25. Recrystallized in H2O:EtOH.
- 2-(adamantan-1-ylthio)-1-ethyl-1H-benzo[d]imidazole (15). Yield: 77%. White solid. 1H NMR (200 MHz, DMSO-d6) δ 7.64 (d, J = 6.9 Hz, 1H), 7.55 (d, J = 8.5 Hz, 1H), 7.33–7.13 (m, 2H), 4.32 (q, J = 7.2 Hz, 2H), 2.16–1.91 (m, 9H), 1.63 (s, 6H), 1.27 (t, J = 7.2 Hz, 3H). 13C NMR (50 MHz, DMSO) δ 146.21, 143.07, 134.56, 122.32, 121.70, 118.66, 110.19, 51.75, 43.12, 35.48, 29.52, 14.92. Recrystallized in H2O:EtOH.
- 2-((4-methoxybenzyl)thio)-1-(naphthalen-1-ylmethyl)-1H-benzo[d]imidazole (18). Yield: 83%. White solid. 1H NMR (400 MHz, Chloroform-d) δ 7.95 (d, J = 8.1 Hz, 1H), 7.87 (d, J = 7.3 Hz, 1H), 7.80 (d, J = 8.0 Hz, 1H), 7.72 (d, J = 8.4 Hz, 1H), 7.58–7.46 (m, 2H), 7.27 (d, J = 8.6 Hz, 2H), 7.21 (dd, J = 7.8, 16.0 Hz, 2H), 7.09 (t, J = 7.7 Hz, 1H), 7.00 (d, J = 8.0 Hz, 1H), 6.77 (d, J = 8.2 Hz, 2H), 6.58 (d, J = 7.2 Hz, 1H), 5.66 (s, 2H), 4.58 (s, 2H), 3.72 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 159.15, 152.32, 143.77, 136.44, 133.66, 130.59, 130.39, 130.33, 129.08, 128.64, 128.26, 126.67, 126.08, 125.51, 123.33, 122.32, 122.20, 122.16, 118.56, 114.07, 109.46, 55.28, 45.31, 37.09. Recrystallized in EtOH:AcOEt.
- 1-benzyl-2-((4-methoxybenzyl)thio)-1H-benzo[d]imidazole (19). Yield: 86%. White solid. 1H NMR (400 MHz, Chloroform-d) δ 7.76 (d, J = 7.9 Hz, 1H), 7.34 (d, J = 8.3 Hz, 2H), 7.30–7.22 (m, 4H), 7.17 (d, J = 4.3 Hz, 2H), 7.12–7.07 (m, 2H), 6.83 (d, J = 8.3 Hz, 2H), 5.24 (s, 2H), 4.61 (s, 2H), 3.79 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 159.16, 151.86, 143.71, 136.16, 135.67, 130.34, 128.82, 128.63, 127.88, 126.89, 122.19, 122.06, 118.47, 114.08, 109.31, 55.28, 47.60, 37.14. Recrystallized in H2O:EtOH.
- 1-ethyl-2-((4-methoxybenzyl)thio)-1H-benzo[d]imidazole (20). Yield: 63%. Yellow oil. 1H NMR (400 MHz, DMSO-d6) δ 7.62–7.56 (m, 1H), 7.51–7.45 (m, 1H), 7.37 (d, J = 8.5 Hz, 2H), 7.22–7.13 (m, 1H), 6.86 (d, J = 8.5 Hz, 2H), 4.56 (s, 2H), 4.11 (q, J = 7.2 Hz, 2H), 3.71 (s, 3H), 1.21 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 158.60, 150.47, 143.06, 135.57, 130.16, 129.10, 121.57, 117.68, 113.87, 109.40, 55.04, 39.52, 38.34, 35.27, 14.42. Purified using preparative plate using Hexane:AcOEt (4:1).
4.1.7. General Procedure for the Synthesis of 4–6, 10–12, and 16–17
- 2-((2-isopropyl-5-methylcyclohexyl)oxy)-1-(naphthalen-1-ylmethyl)-1H-benzo[d]imidazole (4). Yield: 33%. White solid. 1H NMR (400 MHz, Chloroform-d) δ 8.33 (d, J = 8.1 Hz, 1H), 8.13 (d, J = 7.9 Hz, 1H), 8.01 (d, J = 8.3 Hz, 1H), 7.86 (d, J = 7.9 Hz, 1H), 7.78 (p, J = 7.0 Hz, 2H), 7.55 (t, J = 7.8 Hz, 1H), 7.39 (t, J = 7.5 Hz, 1H), 7.30–7.14 (m, 3H), 5.85 (s, 2H), 5.34 (td, J = 10.9, 4.4 Hz, 1H), 2.69 (d, J = 11.0 Hz, 1H), 2.17–2.04 (m, 1H), 2.02–1.80 (m, 3H), 1.68 (t, J = 11.7 Hz, 1H), 1.47–1.23 (m, 2H), 1.16 (d, J = 6.2 Hz, 3H), 1.13–1.05 (m, 1H), 1.02 (d, J = 7.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 157.32, 140.56, 133.89, 133.76, 131.44, 130.78, 129.02, 128.27, 126.45, 125.96, 125.41, 124.24, 122.58, 121.55, 120.76, 117.66, 108.70, 80.70, 47.66, 43.58, 40.71, 34.34, 31.29, 26.38, 23.54, 22.08, 20.74, 16.66. Purified via preparative plate using Hexane:AcOEt (9:1), then recrystallized in MeOH.
- 1-benzyl-2-((2-isopropyl-5-methylcyclohexyl)oxy)-1H-benzo[d]imidazole (5). Yield: 46%. White solid. 1H NMR (400 MHz, Chloroform-d) δ 7.73 (d, J = 7.8 Hz, 1H), 7.50–7.38 (m, 3H), 7.42–7.26 (m, 3H), 7.30–7.19 (m, 2H), 5.37–5.16 (m, 3H), 2.64–2.54 (m, 1H), 2.04 (pd, 1H), 1.94–1.74 (m, 3H), 1.74–1.62 (m, 1H), 1.38–1.16 (m, 2H), 1.14–1.05 (m, 4H), 1.02 (d, J = 7.0 Hz, 3H), 0.93 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 157.16, 140.45, 136.48, 133.61, 128.71, 127.66, 127.03, 121.46, 120.67, 117.59, 108.35, 80.52, 47.84, 45.56, 40.69, 34.35, 31.27, 26.35, 23.53, 22.06, 20.81, 16.64. Purified via preparative plate using Hexane:AcOEt (9:1), then recrystallized in MeOH:H2O.
- 1-ethyl-2-((2-isopropyl-5-methylcyclohexyl)oxy)-1H-benzo[d]imidazole (6). Yield: 35%. Yellow solid. 1H NMR (400 MHz, DMSO-d6) δ 7.41–7.34 (m, 1H), 7.36–7.29 (m, 1H), 7.10–7.02 (m, 2H), 4.94 (td, J = 4.3, 10.8 Hz, 1H), 4.00 (q, J = 7.2 Hz, 2H), 2.28–2.18 (m, 1H), 2.01 (pd, J = 2.7, 6.9 Hz, 1H), 1.75–1.64 (m, 2H), 1.63–1.48 (m, 2H), 1.24 (t, J = 7.2 Hz, 3H), 1.21–1.02 (m, 2H), 0.99–0.91 (m, 1H), 0.90 (dd, J = 1.9, 6.8 Hz, 6H), 0.77 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, DMSO) δ 156.80, 140.36, 133.50, 121.27, 120.67, 117.33, 108.98, 80.15, 47.42, 40.69, 36.69, 34.23, 31.32, 26.70, 23.75, 22.34, 20.89, 17.10, 14.79. Purified via preparative plate using Hexane:AcOEt (9:1).
- (E)-2-((3,7-dimethylocta-2,6-dien-1-yl)oxy)-1-(naphthalen-1-ylmethyl)-1H-benzo[d]imidazole (10). Yield: 29%. Yellow oil. 1H NMR (400 MHz, Chloroform-d) δ 8.11 (d, J = 8.2 Hz, 1H), 7.72 (d, J = 7.6 Hz, 1H), 7.63 (d, J = 8.2 Hz, 1H), 7.48–7.33 (m, 2H), 7.27–7.20 (m, 2H), 7.13 (dd, J = 7.1, 1.2 Hz, 1H), 6.76 (pd, J = 7.5, 1.5 Hz, 2H), 6.68–6.62 (m, 1H), 6.24 (dd, J = 17.6, 10.9 Hz, 1H), 5.47 (d, J = 16.1 Hz, 0H), 5.32 (d, J = 16.1 Hz, 0H), 5.17–5.08 (m, 2H), 5.00–4.94 (m, 1H), 2.20–2.11 (m, 2H), 2.11–1.95 (m, 1H), 1.82 (s, 3H), 1.81–1.71 (m, 1H), 1.50 (s, 3H), 1.35 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 154.50, 144.19, 133.90, 131.95, 131.65, 131.23, 130.03, 129.56, 128.85, 128.41, 126.56, 125.98, 125.27, 125.16, 123.74, 123.26, 120.89, 120.48, 113.15, 112.51, 108.33, 63.64, 42.95, 38.49, 29.77, 25.70, 25.37, 22.95, 17.61.
- (E)-1-benzyl-2-((3,7-dimethylocta-2,6-dien-1-yl)oxy)-1H-benzo[d]imidazole (11). Yield: 33%. Yellow oil. 1H NMR (400 MHz, Chloroform-d) δ 7.32–7.22 (m, 5H), 7.23–7.14 (m, 1H), 6.95–6.83 (m, 2H), 6.84–6.75 (m, 1H), 6.30 (dd, J = 10.8, 17.6 Hz, 1H), 5.25–5.15 (m, 2H), 5.07–4.91 (m, 3H), 2.30–2.14 (m, 2H), 2.08 (dq, J = 5.6, 6.2, 11.8 Hz, 1H), 1.88 (s, 3H), 1.86–1.77 (m, 1H), 1.57 (s, 3H), 1.43 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 154.59, 144.15, 136.66, 131.88, 129.68, 129.48, 128.78, 128.73, 128.49, 127.55, 127.37, 123.68, 120.87, 120.46, 113.10, 112.53, 107.79, 63.55, 44.41, 38.31, 25.68, 25.30, 22.92, 17.55.
- (E)-2-((3,7-dimethylocta-2,6-dien-1-yl)oxy)-1-ethyl-1H-benzo[d]imidazole (12). Yield: 32%. Yellow oil. 1H NMR (400 MHz, Chloroform-d) δ 7.31 (d, J = 7.8 Hz, 1H, H4), 7.07–7.01 (m, 1H, H1), 6.97–6.90 (m, 2H, H2–3), 6.31 (dd, J = 10.8, 17.6 Hz, 1H, H21), 5.33–5.15 (m, 2H), 5.04 (t, J = 7.2 Hz, 1H), 3.90 (q, J = 7.1 Hz, 2H), 2.32–2.14 (m, 2H), 2.13–2.00 (m, 1H), 1.90–1.77 (m, 4H), 1.59 (s, 3H), 1.45 (s, 3H), 1.31 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 144.16, 131.80, 129.47, 123.66, 120.66, 120.06, 112.95, 112.47, 106.94, 63.36, 38.24, 35.45, 25.62, 25.15, 22.84, 17.47, 13.44. Purified via preparative plate using Hexane:AcOEt (9:1).
- 2-(adamantan-1-yloxy)-1-benzyl-1H-benzo[d]imidazole (16). Yield: 5%. White solid. 1H NMR (200 MHz, Chloroform-d) δ 7.56 (dd, J = 7.1, 1.5 Hz, 1H), 7.36–7.17 (m, 6H), 7.12 (dd, J = 7.7, 4.4 Hz, 1H), 7.08–7.02 (m, 2H), 5.13 (s, 2H), 2.26 (s, 9H), 1.69 (s, 6H). 13C NMR (50 MHz, CDCl3) δ 155.16, 140.66, 136.74, 132.70, 128.65, 127.57, 127.16, 121.26, 120.63, 117.86, 108.38, 83.32, 77.64, 77.01, 76.37, 45.73, 41.72, 36.06, 31.09. Purified via preparative plate using Hexane:AcOEt (6:1).
- 2-(adamantan-1-yloxy)-1-ethyl-1H-benzo[d]imidazole (17). Yield: 21%. White solid. 1H NMR (400 MHz, Acetone-d6) δ 7.45–7.35 (m, 1H), 7.30–7.22 (m, 1H), 7.10–7.00 (m, 2H), 4.05 (q, J = 7.2 Hz, 2H), 2.40–2.32 (m, 6H), 2.27–2.19 (m, 3H), 1.82–1.67 (m, 6H), 1.31 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, Acetone) δ 155.62, 141.81, 133.38, 121.55, 121.02, 118.18, 108.95, 82.88, 42.28, 37.35, 36.83, 31.97, 14.70. Purified via preparative plate using Hexane:AcOEt (4:1).
4.1.8. General Procedure for the Synthesis of 21–26
- 2-(3-methoxyphenoxy)-1-(naphthalen-1-ylmethyl)-1H-benzo[d]imidazole (21). Yield: 39%. White solid. 1H NMR (400 MHz, DMSO-d6) δ 8.29 (d, J = 8.9 Hz, 1H), 8.00 (d, J = 7.7 Hz, 1H), 7.89 (d, J = 8.2 Hz, 1H), 7.67–7.56 (m, 2H), 7.50 (d, J = 8.3 Hz, 1H), 7.44 (t, J = 7.7 Hz, 1H), 7.39–7.32 (m, 2H), 7.20–7.07 (m, 2H), 7.02 (dd, J = 7.0, 1.2 Hz, 1H), 6.98–6.92 (m, 2H), 6.86 (ddd, J = 8.3, 2.4, 0.9 Hz, 1H), 5.96 (s, 2H), 3.75 (s, 3H). 13C NMR (101 MHz, DMSO) δ 160.76, 155.88, 154.87, 139.86, 134.01, 133.82, 132.38, 130.78, 130.67, 129.19, 128.56, 127.05, 126.65, 125.98, 124.34, 123.54, 122.23, 121.99, 118.48, 112.72, 111.66, 110.36, 106.74, 55.89, 44.07. Purified via column chromatography using gradient elution from DCM to DCM:MeOH 5%, then recrystallized in AcOEt.
- 1-benzyl-2-(3-methoxyphenoxy)-1H-benzo[d]imidazole (22). Yield: 66%. Yellow oil. 1H NMR (400 MHz, Chloroform-d) δ 7.66–7.58 (m, 1H), 7.38–7.27 (m, 6H), 7.23–7.16 (m, 3H), 6.95 (ddd, J = 8.1, 2.3, 0.9 Hz, 1H), 6.91 (t, J = 2.4 Hz, 1H), 6.81 (ddd, J = 8.3, 2.4, 0.9 Hz, 1H), 5.34 (s, 2H), 3.80 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 160.83, 155.65, 154.63, 139.79, 135.97, 133.34, 130.26, 128.94, 127.99, 127.18, 122.11, 121.75, 118.79, 112.30, 111.52, 109.04, 106.26, 55.48, 46.20. Purified via preparative plate using Hexane:AcOEt (6:1).
- 1-ethyl-2-(3-methoxyphenoxy)-1H-benzo[d]imidazole (23). Yield: 50%. Yellow oil. 1H NMR (400 MHz, Chloroform-d) δ 7.67–7.57 (m, 1H), 7.34 (t, J = 8.2 Hz, 1H), 7.31–7.25 (m, 1H), 7.28–7.17 (m, 2H), 7.01–6.92 (m, 2H), 6.82 (dd, J = 8.2, 2.3 Hz, 1H), 4.21 (q, J = 7.2 Hz, 2H), 3.84 (s, 3H), 1.49 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 160.83, 155.34, 154.80, 139.92, 133.01, 130.24, 121.76, 121.44, 118.79, 112.14, 111.33, 108.44, 106.10, 55.48, 37.43, 14.67. Purified via preparative plate using Hexane:DCM (1:4).
- 2-(3-methoxy-5-(2-methyloctan-2-yl)phenoxy)-1-(naphthalen-1-ylmethyl)-1H-benzo[d]imidazole (24). Yield: 29%. Yellow oil. 1H NMR (200 MHz, Chloroform-d) δ 8.15 (d, J = 7.3 Hz, 1H), 7.92 (dd, J = 7.7, 1.9 Hz, 1H), 7.81 (d, J = 8.3 Hz, 1H), 7.68–7.48 (m, 3H), 7.37 (t, J = 7.7 Hz, 1H), 7.27–7.00 (m, 4H), 6.87–6.70 (m, 3H), 5.83 (s, 2H), 3.77 (s, 3H), 1.62–1.45 (m, 2H), 1.31–1.13 (m, 16H), 0.87–0.72 (m, 4H). 13C NMR (50 MHz, CDCl3) δ 160.27, 155.93, 154.39, 152.99, 140.06, 133.78, 133.68, 131.05, 130.67, 129.07, 128.47, 126.66, 126.08, 125.45, 124.11, 122.42, 122.06, 121.67, 118.84, 110.15, 109.28, 102.39, 77.66, 77.02, 76.39, 65.84, 55.38, 44.45, 44.13, 38.02, 31.75, 29.99, 28.81, 24.61, 22.67, 14.06. Purified via preparative plate using DCM:NEt3 (0.1%).
- 1-benzyl-2-(3-methoxy-5-(2-methyloctan-2-yl)phenoxy)-1H-benzo[d]imidazole (25). Yield: 66%. Yellow oil. 1H NMR (200 MHz, Chloroform-d) δ 7.66–7.56 (m, 1H), 7.42–7.24 (m, 5H), 7.25–7.07 (m, 3H), 6.82 (t, J = 1.9 Hz, 1H), 6.77 (d, J = 1.8 Hz, 2H), 5.34 (s, 2H), 3.79 (s, 3H), 1.65–1.50 (m, 2H), 1.33–1.17 (m, 12H), 1.15–1.02 (m, 2H), 0.96–0.79 (m, 3H). 13C NMR (50 MHz, CDCl3) δ 160.43, 155.84, 154.62, 153.15, 140.13, 136.19, 133.51, 129.02, 128.05, 127.29, 122.10, 121.71, 118.93, 110.29, 110.24, 109.10, 102.52, 77.80, 77.16, 76.53, 55.53, 46.30, 44.59, 38.17, 31.90, 30.14, 28.97, 24.76, 22.81, 14.20. Purified using preparative plate using Hexane:AcOEt (6:1).
- 1-ethyl-2-(3-methoxy-5-(2-methyloctan-2-yl)phenoxy)-1H-benzo[d]imidazole (26). Yield: 49%. 1H NMR (200 MHz, Chloroform-d) δ 7.64–7.51 (m, 1H), 7.30–7.11 (m, 3H), 6.85 (t, J = 1.9 Hz, 1H), 6.81–6.71 (m, 2H), 4.20 (q, J = 7.2 Hz, 2H), 3.79 (s, 3H), 1.65–1.52 (m, 2H), 1.47 (t, J = 7.2 Hz, 3H), 1.24 (d, J = 11.3 Hz, 12H), 1.10 (dt, J = 7.7, 4.0 Hz, 2H), 0.85 (q, J = 6.1, 4.9 Hz, 3H). 13C NMR (50 MHz, CDCl3) δ 160.45, 155.54, 154.68, 153.14, 140.12, 133.15, 121.82, 121.46, 118.88, 110.17, 108.51, 102.39, 77.79, 77.16, 76.52, 55.53, 44.60, 38.18, 37.52, 31.90, 30.14, 28.99, 24.76, 22.81, 14.81, 14.20.
4.1.9. General Procedure for the Synthesis of Compounds IVa–b
4.2. Molecular Docking
4.3. cAMP Accumulation Assay
4.4. Cannabinoid Binding Assay
4.5. CB1/CB2 Receptor Binding Assay
4.6. Cell Culture
4.7. Neutral Red Uptake Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kingston, D.G.I. Modern natural products drug discovery and its relevance to biodiversity conservation. J. Nat. Prod. 2011, 74, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The endocannabinoid system: A potential target for the treatment of various diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Sobocińska, A.A.; Czarnecka, A.M.; Król, M.; Botta, B.; Szczylik, C. The Therapeutic Aspects of the Endocannabinoid System (ECS) for Cancer and their Development: From Nature to Laboratory. Curr. Pharm. Des. 2016, 22, 1756–1766. [Google Scholar] [CrossRef]
- Lu, H.-C.; Mackie, K.; Gonsiorek, W.; Lunn, C.; Fan, X.; Narula, S.; Lundell, D.; Hipkin, R.W.; Luk, T.; Jin, W.; et al. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Endocannabinoids and Their Pharmacological Actions. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2015; Volume 231, pp. 1–37. [Google Scholar] [CrossRef]
- Mackie, K. Cannabinoid receptors: Where they are and what they do. J. Neuroendocrinol. 2008, 20, 10–14. [Google Scholar] [CrossRef]
- An, D.; Peigneur, S.; Hendrickx, L.A.; Tytgat, J. Targeting cannabinoid receptors: Current status and prospects of natural products. Int. J. Mol. Sci. 2020, 21, 5064. [Google Scholar] [CrossRef]
- Dhopeshwarkar, A.; Mackie, K. CB2cannabinoid receptors as a therapeutic target-what does the future hold? Mol. Pharmacol. 2014, 86, 430–437. [Google Scholar] [CrossRef]
- Hashiesh, H.M.; Sharma, C.; Goyal, S.N.; Jha, N.K.; Ojha, S. Pharmacological Properties, Therapeutic Potential and Molecular Mechanisms of JWH133, a CB2 Receptor-Selective Agonist. Front. Pharmacol. 2021, 12, 1818. [Google Scholar] [CrossRef]
- Espinosa-Bustos, C.; Lagos, C.F.; Romero-Parra, J.; Zárate, A.M.; Mella-Raipán, J.; Pessoa-Mahana, H.; Recabarren-Gajardo, G.; Iturriaga-Vásquez, P.; Tapia, R.A.; Pessoa-Mahana, C.D. Design, synthesis, biological evaluation and binding mode modeling of benzimidazole derivatives targeting the cannabinoid receptor type 1. Arch. Pharm. 2015, 348, 81–88. [Google Scholar] [CrossRef]
- Faúndez-Parraguez, M.; Alarcón-Miranda, C.; Cho, Y.H.; Pessoa-Mahana, H.; Gallardo-Garrido, C.; Chung, H.; Faúndez, M.; Pessoa-Mahana, D. New pyridone-based derivatives as cannabinoid receptor type 2 agonists. Int. J. Mol. Sci. 2021, 22, 1212. [Google Scholar] [CrossRef] [PubMed]
- Mella-Raipán, J.; Hernández-Pino, S.; Morales-Verdejo, C.; Pessoa-Mahana, D. 3D-QSAR/CoMFA-Based Structure-Affinity/Selectivity Relationships of Aminoalkylindoles in the Cannabinoid CB1 and CB2 Receptors. Molecules 2014, 19, 2842–2861. [Google Scholar] [CrossRef] [PubMed]
- Romero-Parra, J.; Mella-Raipán, J.; Palmieri, V.; Allarà, M.; Torres, M.J.; Pessoa-Mahana, H.; Iturriaga-Vásquez, P.; Escobar, R.; Faúndez, M.; Di Marzo, V.; et al. Synthesis, binding assays, cytotoxic activity and docking studies of benzimidazole and benzothiophene derivatives with selective affinity for the CB2 cannabinoid receptor. Eur. J. Med. Chem. 2016, 124, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Lorca, M.; Valdes, Y.; Chung, H.; Romero-Parra, J.; Pessoa-Mahana, C.D.; Mella, J. Three-dimensional quantitative structure-activity relationships (3D-QSAR) on a series of piperazine-carboxamides fatty acid amide hydrolase (FAAH) inhibitors as a useful tool for the design of new cannabinoid ligands. Int. J. Mol. Sci. 2019, 20, 2510. [Google Scholar] [CrossRef] [PubMed]
- Romero-Parra, J.; Chung, H.; Tapia, R.A.; Faúndez, M.; Morales-Verdejo, C.; Lorca, M.; Lagos, C.F.; Di Marzo, V.; David Pessoa-Mahana, C.; Mella, J. Combined CoMFA and CoMSIA 3D-QSAR study of benzimidazole and benzothiophene derivatives with selective affinity for the CB2 cannabinoid receptor. Eur. J. Pharm. Sci. 2017, 101, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tahlan, S.; Kumar, S.; Narasimhan, B. Pharmacological significance of heterocyclic 1H-benzimidazole scaffolds: A review. BMC Chem. 2019, 13, 101. [Google Scholar] [CrossRef]
- Keri, R.S.; Hiremathad, A.; Budagumpi, S.; Nagaraja, B.M. Comprehensive Review in Current Developments of Benzimidazole-Based Medicinal Chemistry. Chem. Biol. Drug Des. 2015, 86, 19–65. [Google Scholar] [CrossRef]
- Maruthamuthu, S.R.; Christina, R.S.P.; Bharathi Dileepan, A.G.; Ranjith, R. The chemistry and biological significance of imidazole, benzimidazole, benzoxazole, tetrazole and quinazolinone nucleus. J. Chem. Pharm. Res. 2016, 8, 505–526. [Google Scholar]
- Bansal, Y.; Silakari, O. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem. 2012, 20, 6208–6236. [Google Scholar] [CrossRef]
- Ajani, O.O.; Aderohunmu, D.V.; Ikpo, C.O.; Adedapo, A.E.; Olanrewaju, I.O. Functionalized Benzimidazole Scaffolds: Privileged Heterocycle for Drug Design in Therapeutic Medicine. Arch. Pharm. 2016, 349, 475–506. [Google Scholar] [CrossRef]
- Devsi, A.; Kiyota, B.; Ouellette, T.; Hegle, A.P.; Rivera-Acevedo, R.E.; Wong, J.; Dong, Y.; Pugsley, M.K.; Fung, T. A pharmacological characterization of Cannabis sativa chemovar extracts. J. Cannabis Res. 2020, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M.; Russo, E.B. Cannabis and Cannabis Extracts. J. Cannabis Ther. 2001, 1, 103–132. [Google Scholar] [CrossRef]
- José Serrano Vega, R.; Campos Xolalpa, N.; Josabad Alonso Castro, A.; Pérez González, C.; Pérez Ramos, J.; Pérez Gutiérrez, S. Terpenes from Natural Products with Potential Anti-Inflammatory Activity. In Terpenes and Terpenoids; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Hahn, D.; Shin, S.H.; Bae, J.S. Natural antioxidant and anti-inflammatory compounds in foodstuff or medicinal herbs inducing heme oxygenase-1 expression. Antioxidants 2020, 9, 1191. [Google Scholar] [CrossRef] [PubMed]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 2114. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Hartung, J.; Hünig, S.; Kneuer, R.; Schwarz, M.; Wenner, H. 1,4-Diazabicyclo[2.2.2]octane (DABCO)—An efficient reagent in the synthesis of alkyl tosylates or sulfenates. Synthesis 1997, 1997, 1433–1438. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Xing, C.; Zhuang, Y.; Xu, T.H.; Feng, Z.; Zhou, X.E.; Chen, M.; Wang, L.; Meng, X.; Xue, Y.; Wang, J.; et al. Cryo-EM Structure of the Human Cannabinoid Receptor CB2-Gi Signaling Complex. Cell 2020, 180, 645–654.e13. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Reddy, C.V.R.; Dubey, P.K. Highly efficient tandem syntheses of unsymmetrically substituted isomeric S,N-disubstituted-2-mercaptobenzimidazoles. Indian J. Chem. Sect. B Org. Med. Chem. 2015, 54B, 829–832. [Google Scholar]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein-ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- Felder, C.C.; Joyce, K.E.; Briley, E.M.; Mansouri, J.; Mackie, K.; Blond, O.; Lai, Y.; Ma, A.L.; Mitchell, R.L. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995, 48, 443–450. [Google Scholar] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Mugnaini, C.; Kostrzewa, M.; Bryk, M.; Mahmoud, A.M.; Brizzi, A.; Lamponi, S.; Giorgi, G.; Ferlenghi, F.; Vacondio, F.; MacCioni, P.; et al. Design, Synthesis, and Physicochemical and Pharmacological Profiling of 7-Hydroxy-5-oxopyrazolo[4,3-b]pyridine-6-carboxamide Derivatives with Antiosteoarthritic Activity in Vivo. J. Med. Chem. 2020, 63, 7369–7391. [Google Scholar] [CrossRef] [PubMed]





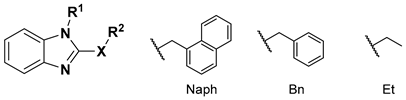 | |||
|---|---|---|---|
| Compound | X | R1 | R2 |
| 1 | S | Naph |  Menthyl (MEN) |
| 2 | Bn | ||
| 3 | Et | ||
| 4 | O | Naph | |
| 5 | Bn | ||
| 6 | Et | ||
| 7 | S | Naph |  Geranyl (GER) |
| 8 | Bn | ||
| 9 | Et | ||
| 10 | O | Naph | |
| 11 | Bn | ||
| 12 | Et | ||
| 13 | S | Naph | 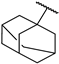 Adamantyl (ADM) |
| 14 | Bn | ||
| 15 | Et | ||
| 16 | O | Bn | |
| 17 | Et | ||
| 18 | S | Naph | 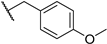 Anisyl (ANS) |
| 19 | Bn | ||
| 20 | Et | ||
| 21 | O | Naph |  O-Methylresorcinyl (RES) |
| 22 | Bn | ||
| 23 | Et | ||
| 24 | O | Naph | 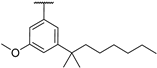 O-methyl-5-(1,1-dimethylheptyl)resorcinyl (DMH) |
| 25 | Bn | ||
| 26 | Et | ||
| Compound | X | R1 | R2 | Inhibition (%) a |
|---|---|---|---|---|
| 1 | S | Naph | MEN | 2 |
| 2 | Bn | 72 | ||
| 3 | Et | 69 | ||
| 4 | O | Naph | 37 | |
| 5 | Bn | 90 | ||
| 6 | Et | 89 | ||
| 7 | S | Naph | GER | 42 |
| 8 | Bn | 71 | ||
| 9 | Et | 54 | ||
| 10 | O | Naph | 40 | |
| 11 | Bn | 35 | ||
| 12 | Et | −3 | ||
| 13 | S | Naph | ADM | 30 |
| 14 | Bn | 69 | ||
| 15 | Et | 63 | ||
| 16 | O | Bn | 86 | |
| 17 | Et | 61 | ||
| 18 | S | Naph | ANS | 46 |
| 19 | Bn | 88 | ||
| 20 | Et | 63 | ||
| 21 | O | Naph | RES | 62 |
| 22 | Bn | 84 | ||
| 23 | Et | 54 | ||
| 24 | O | Naph | DMH | 32 |
| 25 | Bn | 33 | ||
| 26 | Et | 49 |
| Compound | EC50 (nM) |
|---|---|
| 5 | 14 |
| 6 | 3.36 |
| 16 | 20 |
| 19 | ND 1 |
| 22 | ND 1 |
| Compound | cAMP EC50 CB2 (nM) | Ki CB2 (μM) | Ki CB1 (μM) | Ki (CB1)/Ki (CB2) |
|---|---|---|---|---|
| 5 | 14 | 0.40 ± 0.08 | 0.92 ± 1.15 | 2.3 |
| 6 | 3.36 | 0.44 ± 0.10 | >10 | >23 |
| 16 | 20 | 0.63 ± 0.19 | >10 | >16 |
| 19 | ND | 0.93 ± 0.29 | 1.23 ± 0.88 | 1.3 |
| 22 | ND | 0.60 ± 0.52 | 2.04 ± 1.22 | 3.4 |
| Cell Line | IC50 Viability (µM) |
|---|---|
| HEK-293 | 30 |
| MCF-7 | 38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, A.Y.H.; Chung, H.; Romero-Parra, J.; Kumar, P.; Allarà, M.; Ligresti, A.; Gallardo-Garrido, C.; Pessoa-Mahana, H.; Faúndez, M.; Pessoa-Mahana, C.D. Motifs in Natural Products as Useful Scaffolds to Obtain Novel Benzo[d]imidazole-Based Cannabinoid Type 2 (CB2) Receptor Agonists. Int. J. Mol. Sci. 2023, 24, 10918. https://doi.org/10.3390/ijms241310918
Cho AYH, Chung H, Romero-Parra J, Kumar P, Allarà M, Ligresti A, Gallardo-Garrido C, Pessoa-Mahana H, Faúndez M, Pessoa-Mahana CD. Motifs in Natural Products as Useful Scaffolds to Obtain Novel Benzo[d]imidazole-Based Cannabinoid Type 2 (CB2) Receptor Agonists. International Journal of Molecular Sciences. 2023; 24(13):10918. https://doi.org/10.3390/ijms241310918
Chicago/Turabian StyleCho, Analia Young Hwa, Hery Chung, Javier Romero-Parra, Poulami Kumar, Marco Allarà, Alessia Ligresti, Carlos Gallardo-Garrido, Hernán Pessoa-Mahana, Mario Faúndez, and Carlos David Pessoa-Mahana. 2023. "Motifs in Natural Products as Useful Scaffolds to Obtain Novel Benzo[d]imidazole-Based Cannabinoid Type 2 (CB2) Receptor Agonists" International Journal of Molecular Sciences 24, no. 13: 10918. https://doi.org/10.3390/ijms241310918
APA StyleCho, A. Y. H., Chung, H., Romero-Parra, J., Kumar, P., Allarà, M., Ligresti, A., Gallardo-Garrido, C., Pessoa-Mahana, H., Faúndez, M., & Pessoa-Mahana, C. D. (2023). Motifs in Natural Products as Useful Scaffolds to Obtain Novel Benzo[d]imidazole-Based Cannabinoid Type 2 (CB2) Receptor Agonists. International Journal of Molecular Sciences, 24(13), 10918. https://doi.org/10.3390/ijms241310918






