Paradoxical Induction of ALOX15/15B by Cortisol in Human Amnion Fibroblasts: Implications for Inflammatory Responses of the Fetal Membranes at Parturition
Abstract
1. Introduction
2. Results
2.1. Parallel Increases in 15(S)-HETE and Cortisol in Human Amnion at Parturition
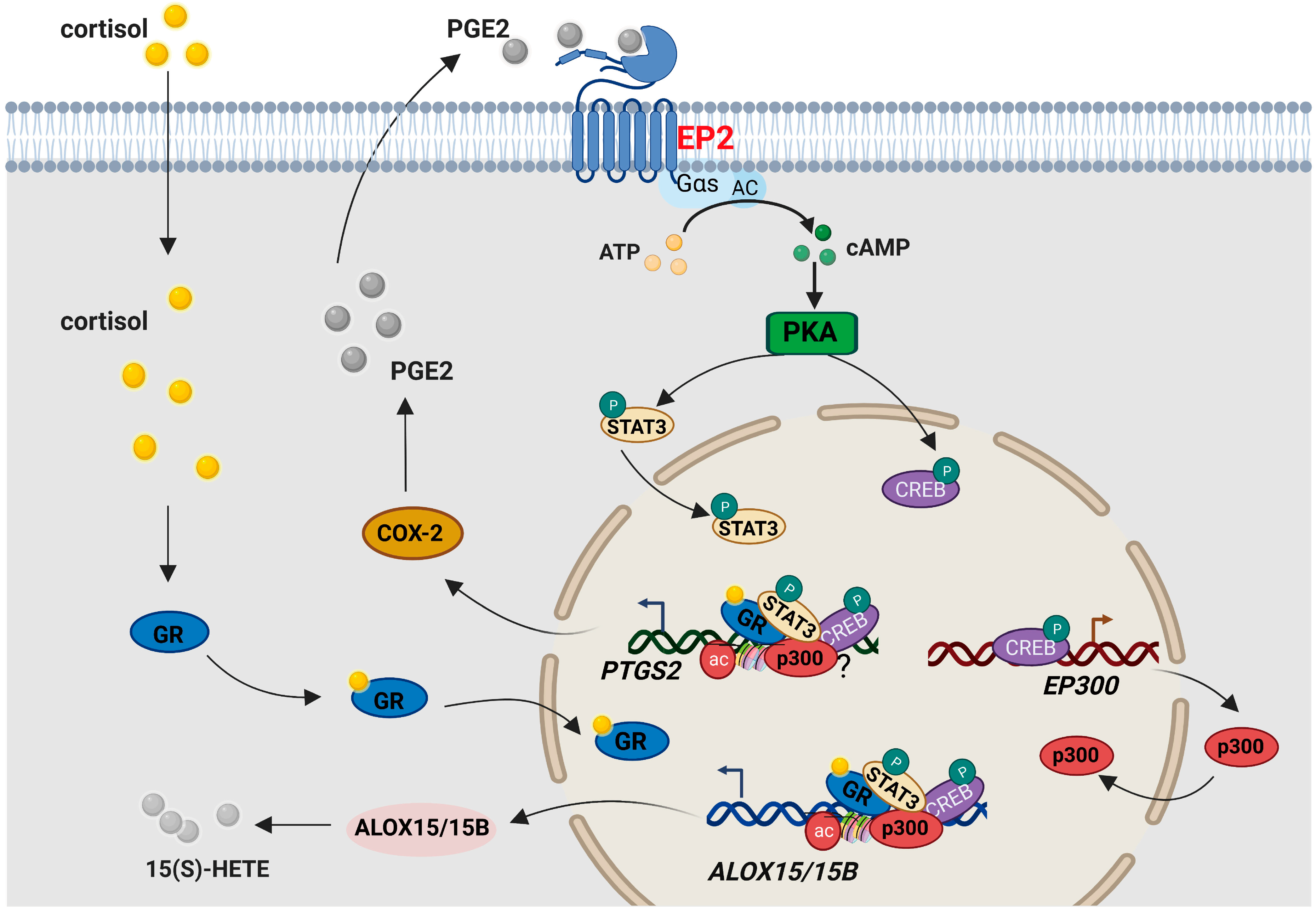
2.2. Effect of Cortisol on ALOX15/15B Expression and 15(S)-HETE Synthesis in the Human Amnion
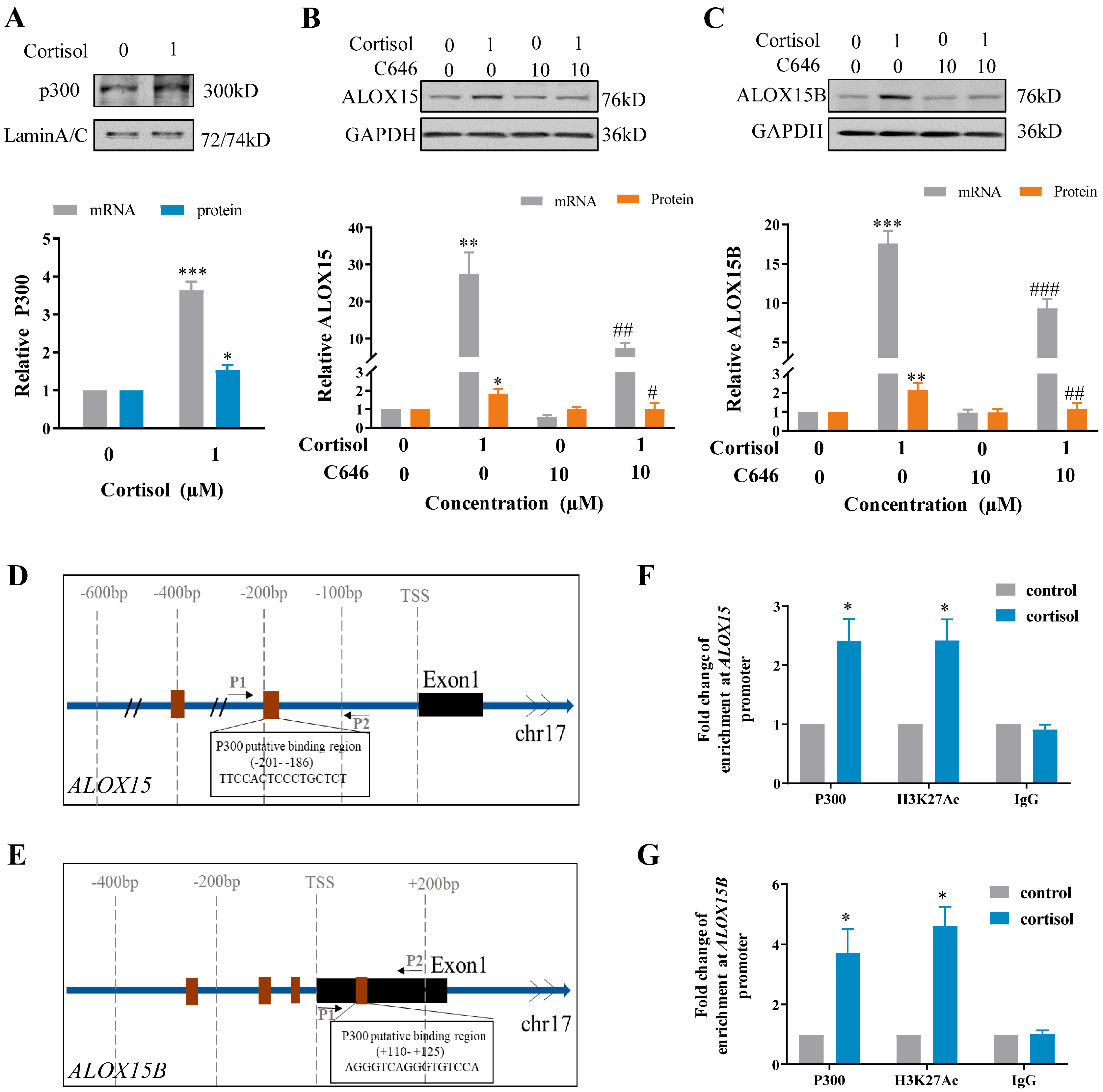
2.3. Involvement of p300-Mediated Histone Acetylation in the Induction of ALOX15/15B by Cortisol in Human Amnion Fibroblasts
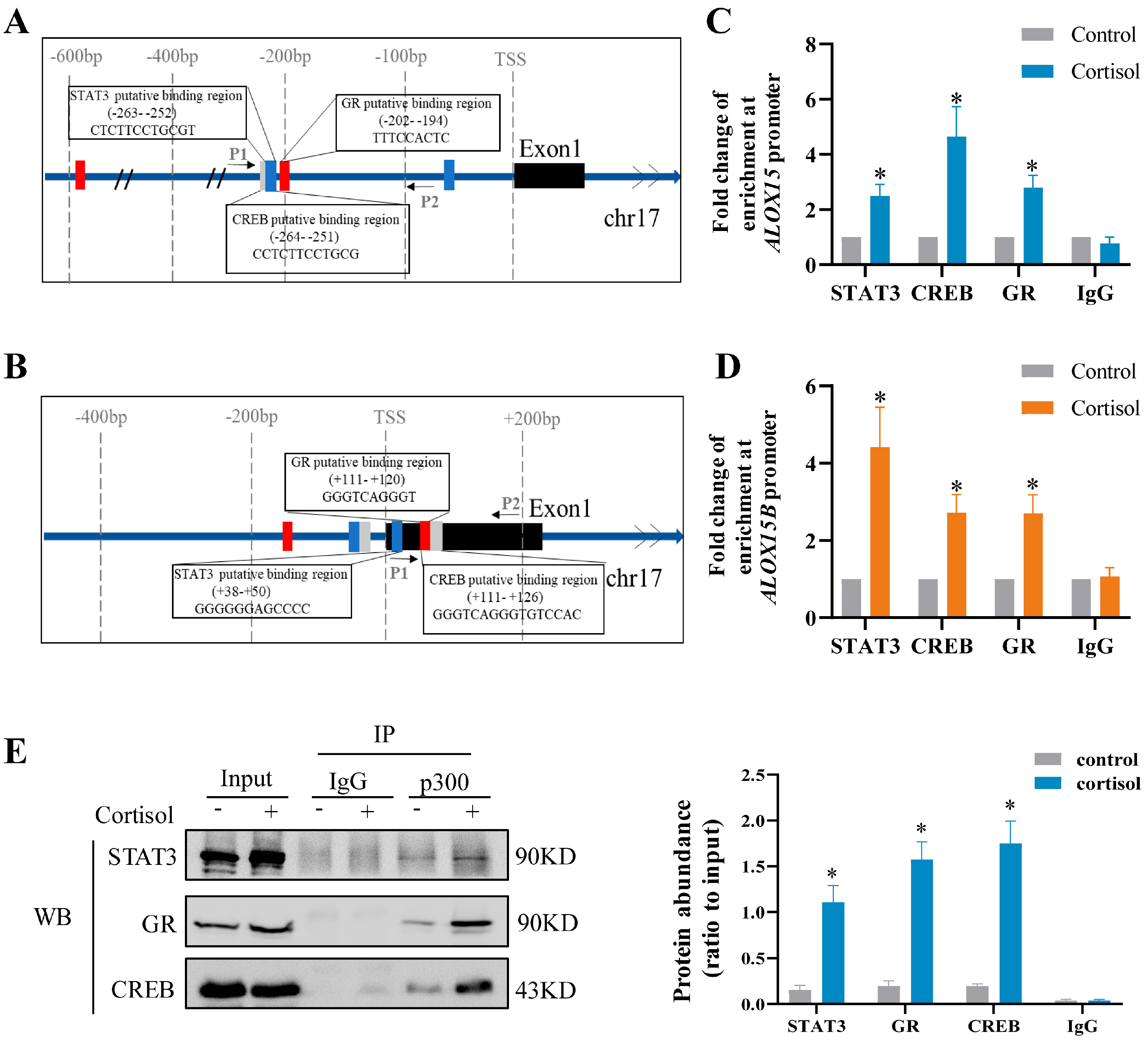
2.4. Role of GR, CREB, and STAT3 in the Induction of ALOX15/15B by Cortisol in Human Amnion Fibroblasts
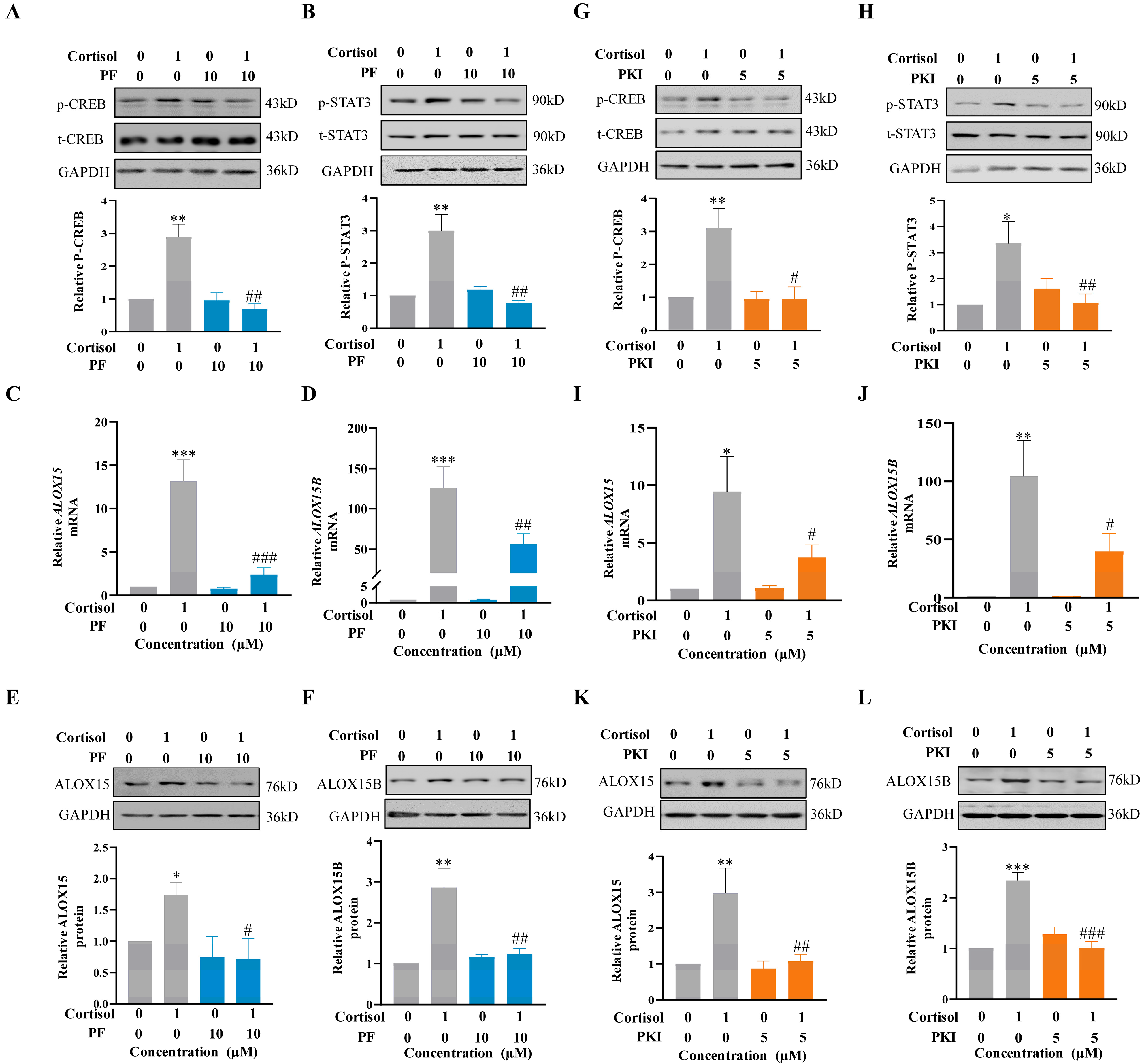
2.5. Role of the cAMP/PKA Pathway Coupled with the EP2 Receptor of PGE2 in the Induction of ALOX15/15B Expression by Cortisol in Human Amnion Fibroblasts
2.6. Role of the cAMP/PKA Pathway Coupled with the EP2 Receptor of PGE2 in the Induction of p300 Expression by Cortisol in Human Amnion Fibroblasts
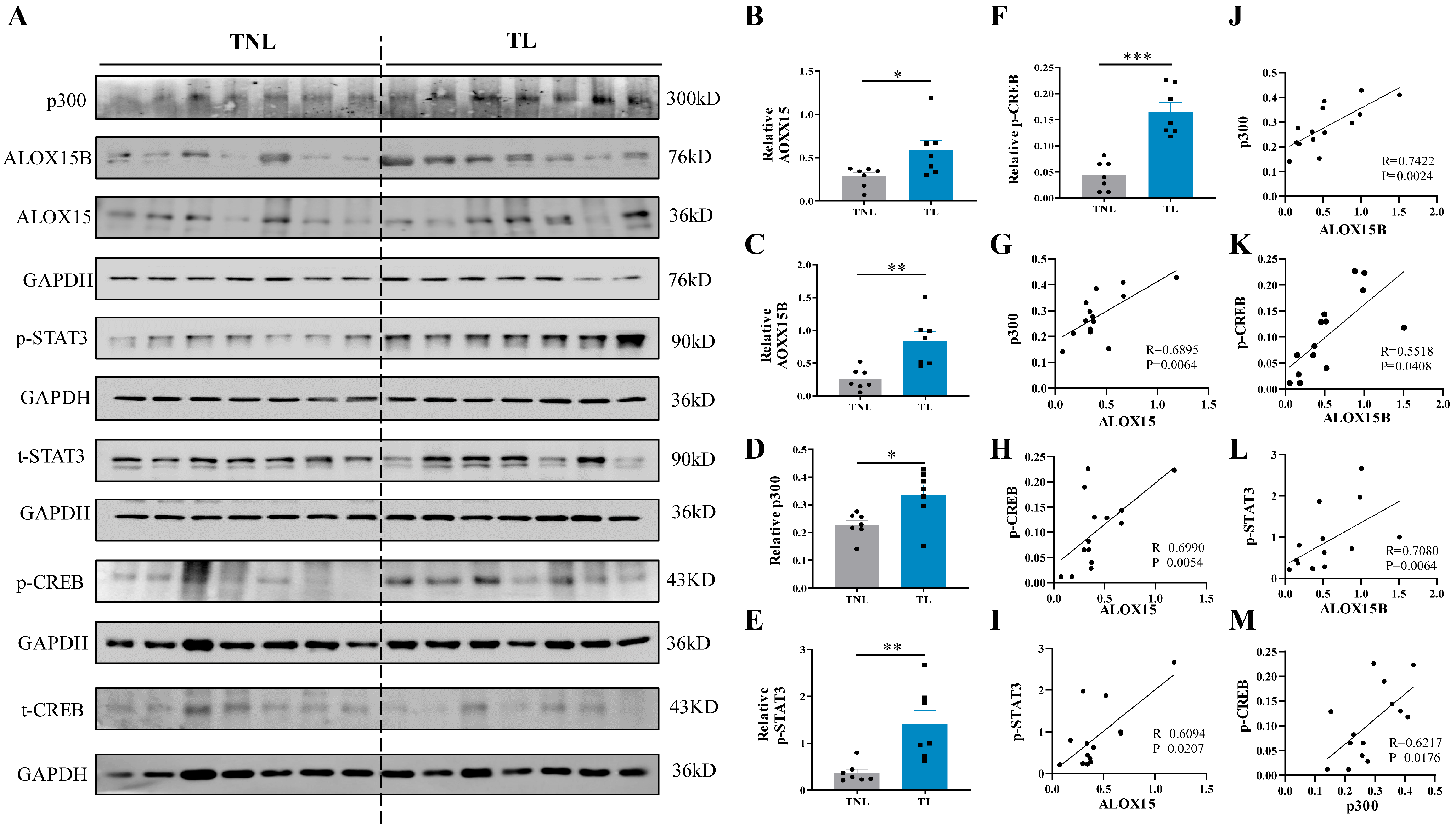
2.7. Correlation among ALOX15/15B, p300, p-STAT3 and p-CREB Abundance in the Human Amnion at Parturition
3. Discussion
4. Materials and Methods
4.1. Collection of Human Fetal Membranes
4.2. Measurement of Cortisol and 15(S)-HETE in Human Amnion with ELISA
4.3. Isolation and Culture of Human Amnion Fibroblasts
4.4. Treatment of Human Amnion Fibroblasts
4.5. Extraction of RNA and Analysis with qRT-PCR
4.6. Extraction of Protein and Analysis with Western Blotting
4.7. Immunofluorescence Staining
4.8. ChIP Assay
4.9. CoIP Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Challis, J.R.; Lockwood, C.J.; Myatt, L.; Norman, J.E.; Strauss, J.F., 3rd; Petraglia, F. Inflammation and pregnancy. Reprod. Sci. 2009, 16, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, R.; Sadovsky, Y.; Goodarzi, H.; Zhang, H.; Biggio, J.R.; Varner, M.; Parry, S.; Xiao, F.; Esplin, S.M.; Andrews, W.; et al. Onset of human preterm and term birth is related to unique inflammatory transcriptome profiles at the maternal fetal interface. PeerJ 2017, 5, e3685. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Espinoza, J.; Goncalves, L.F.; Kusanovic, J.P.; Friel, L.A.; Nien, J.K. Inflammation in preterm and term labour and delivery. Semin. Fetal Neonatal Med. 2006, 11, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Espinoza, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.; Erez, O.; Chaiworapongsa, T.; Mazor, M. The preterm parturition syndrome. BJOG 2006, 113 (Suppl. S3), 17–42. [Google Scholar] [CrossRef]
- Menon, R.; Richardson, L.S.; Lappas, M. Fetal membrane architecture, aging and inflammation in pregnancy and parturition. Placenta 2019, 79, 40–45. [Google Scholar] [CrossRef]
- Menon, R.; Behnia, F.; Polettini, J.; Richardson, L.S. Novel pathways of inflammation in human fetal membranes associated with preterm birth and preterm pre-labor rupture of the membranes. Semin. Immunopathol. 2020, 42, 431–450. [Google Scholar] [CrossRef]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef]
- Li, W.J.; Lu, J.W.; Zhang, C.Y.; Wang, W.S.; Ying, H.; Myatt, L.; Sun, K. PGE2 vs PGF2alpha in human parturition. Placenta 2021, 104, 208–219. [Google Scholar] [CrossRef]
- Gibb, W. The role of prostaglandins in human parturition. Ann. Med. 1998, 30, 235–241. [Google Scholar] [CrossRef]
- Okazaki, T.; Casey, M.L.; Okita, J.R.; MacDonald, P.C.; Johnston, J.M. Initiation of human parturition. XII. Biosynthesis and metabolism of prostaglandins in human fetal membranes and uterine decidua. Am. J. Obstet. Gynecol. 1981, 139, 373–381. [Google Scholar] [CrossRef]
- Keelan, J.A.; Blumenstein, M.; Helliwell, R.J.; Sato, T.A.; Marvin, K.W.; Mitchell, M.D. Cytokines, prostaglandins and parturition—A review. Placenta 2003, 24 (Suppl. A), S33–S46. [Google Scholar] [CrossRef]
- Li, W.; Wang, W.; Zuo, R.; Liu, C.; Shu, Q.; Ying, H.; Sun, K. Induction of pro-inflammatory genes by serum amyloid A1 in human amnion fibroblasts. Sci. Rep. 2017, 7, 693. [Google Scholar] [CrossRef]
- Sun, K.; Ma, R.; Cui, X.; Campos, B.; Webster, R.; Brockman, D.; Myatt, L. Glucocorticoids induce cytosolic phospholipase A2 and prostaglandin H synthase type 2 but not microsomal prostaglandin E synthase (PGES) and cytosolic PGES expression in cultured primary human amnion cells. J. Clin. Endocrinol. Metab. 2003, 88, 5564–5571. [Google Scholar] [CrossRef]
- Challis, J.R.; Lye, S.J.; Gibb, W. Prostaglandins and parturition. Ann. N. Y. Acad. Sci. 1997, 828, 254–267. [Google Scholar] [CrossRef]
- MacKenzie, I.Z.; Embrey, M.P. A comparison of PGE2 and PGF2 alpha vaginal gel for ripening the cervix before induction of labour. Br. J. Obstet. Gynaecol. 1979, 86, 167–170. [Google Scholar] [CrossRef]
- Challis, J.R.; Sloboda, D.M.; Alfaidy, N.; Lye, S.J.; Gibb, W.; Patel, F.A.; Whittle, W.L.; Newnham, J.P. Prostaglandins and mechanisms of preterm birth. Reproduction 2002, 124, 1–17. [Google Scholar] [CrossRef]
- Murphy, B.E. Chorionic membrane as an extra-adrenal source of foetal cortisol in human amniotic fluid. Nature 1977, 266, 179–181. [Google Scholar] [CrossRef]
- Murphy, B.E. Ontogeny of cortisol-cortisone interconversion in human tissues: A role for cortisone in human fetal development. J. Steroid Biochem. 1981, 14, 811–817. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Z.J.; Myatt, L.; Sun, K. 11beta-HSD1 in Human Fetal Membranes as a Potential Therapeutic Target for Preterm Birth. Endocr. Rev. 2018, 39, 241–260. [Google Scholar] [CrossRef]
- Alfaidy, N.; Li, W.; MacIntosh, T.; Yang, K.; Challis, J. Late gestation increase in 11beta-hydroxysteroid dehydrogenase 1 expression in human fetal membranes: A novel intrauterine source of cortisol. J. Clin. Endocrinol. Metab. 2003, 88, 5033–5038. [Google Scholar] [CrossRef]
- Lu, J.W.; Wang, W.S.; Zhou, Q.; Ling, L.J.; Ying, H.; Sun, Y.; Myatt, L.; Sun, K. C/EBPdelta drives key endocrine signals in the human amnion at parturition. Clin. Transl. Med. 2021, 11, e416. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, L.; Wang, Y.; Duan, T.; Myatt, L.; Sun, K. Enhancement of cortisol-induced 11beta-hydroxysteroid dehydrogenase type 1 expression by interleukin 1beta in cultured human chorionic trophoblast cells. Endocrinology 2006, 147, 2490–2495. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Myatt, L. Enhancement of glucocorticoid-induced 11beta-hydroxysteroid dehydrogenase type 1 expression by proinflammatory cytokines in cultured human amnion fibroblasts. Endocrinology 2003, 144, 5568–5577. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, Q.; Lu, J.W.; Wang, W.S.; Sun, K. Involvement of STAT3 in the synergistic induction of 11beta-HSD1 by SAA1 and cortisol in human amnion fibroblasts. Am. J. Reprod. Immunol. 2019, 82, e13150. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.; Kuitert, L.M.; Slater, D.M.; Adcock, I.M.; Barnes, P.J. Cytokine induction of cytosolic phospholipase A2 and cyclooxygenase-2 mRNA is suppressed by glucocorticoids in human epithelial cells. Life Sci. 1997, 60, 67–78. [Google Scholar] [CrossRef]
- Hoeck, W.G.; Ramesha, C.S.; Chang, D.J.; Fan, N.; Heller, R.A. Cytoplasmic phospholipase A2 activity and gene expression are stimulated by tumor necrosis factor: Dexamethasone blocks the induced synthesis. Proc. Natl. Acad. Sci. USA 1993, 90, 4475–4479. [Google Scholar] [CrossRef]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory action of glucocorticoids—New mechanisms for old drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef]
- Duchesne, M.J.; Thaler-Dao, H.; de Paulet, A.C. Prostaglandin synthesis in human placenta and fetal membranes. Prostaglandins 1978, 15, 19–42. [Google Scholar] [CrossRef]
- Zakar, T.; Hirst, J.J.; Mijovic, J.E.; Olson, D.M. Glucocorticoids stimulate the expression of prostaglandin endoperoxide H synthase-2 in amnion cells. Endocrinology 1995, 136, 1610–1619. [Google Scholar] [CrossRef]
- Economopoulos, P.; Sun, M.; Purgina, B.; Gibb, W. Glucocorticoids stimulate prostaglandin H synthase type-2 (PGHS-2) in the fibroblast cells in human amnion cultures. Mol. Cell. Endocrinol. 1996, 117, 141–147. [Google Scholar] [CrossRef]
- Cooper, M.S.; Stewart, P.M. 11Beta-hydroxysteroid dehydrogenase type 1 and its role in the hypothalamus-pituitary-adrenal axis, metabolic syndrome, and inflammation. J. Clin. Endocrinol. Metab. 2009, 94, 4645–4654. [Google Scholar] [CrossRef]
- Zhu, X.O.; Yang, Z.; Guo, C.M.; Ni, X.T.; Li, J.N.; Ge, Y.C.; Myatt, L.; Sun, K. Paradoxical stimulation of cyclooxygenase-2 expression by glucocorticoids via a cyclic AMP response element in human amnion fibroblasts. Mol. Endocrinol. 2009, 23, 1839–1849. [Google Scholar] [CrossRef]
- Wang, W.; Guo, C.; Zhu, P.; Lu, J.; Li, W.; Liu, C.; Xie, H.; Myatt, L.; Chen, Z.J.; Sun, K. Phosphorylation of STAT3 mediates the induction of cyclooxygenase-2 by cortisol in the human amnion at parturition. Sci. Signal. 2015, 8, ra106. [Google Scholar] [CrossRef]
- Lu, J.; Wang, W.; Mi, Y.; Zhang, C.; Ying, H.; Wang, L.; Wang, Y.; Myatt, L.; Sun, K. AKAP95-mediated nuclear anchoring of PKA mediates cortisol-induced PTGS2 expression in human amnion fibroblasts. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, K.; Wang, W.S. Identification of a Feed-Forward Loop Between 15(S)-HETE and PGE2 in Human Amnion at Parturition. J. Lipid Res. 2022, 63, 100294. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, M.; Deng, Y.; Zhao, J.; Zhou, X.; Trudeau, J.B.; Goldschmidt, E.; Moore, J.A.; Chu, H.; Zhang, W.; et al. 15-Lipoxygenase 1 in nasal polyps promotes CCL26/eotaxin 3 expression through extracellular signal-regulated kinase activation. J. Allergy Clin. Immunol. 2019, 144, 1228–1241.e1229. [Google Scholar] [CrossRef]
- Zhao, J.; O’Donnell, V.B.; Balzar, S.; St Croix, C.M.; Trudeau, J.B.; Wenzel, S.E. 15-Lipoxygenase 1 interacts with phosphatidylethanolamine-binding protein to regulate MAPK signaling in human airway epithelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 14246–14251. [Google Scholar] [CrossRef]
- Michalak, A.; Lewandowska-Polak, A.; Moskwa, S.; Kowalski, M.L.; Grzegorczyk, J.L. IgE-mediated 15-hydroxyeicosatetraenoic acid (15-HETE) generation by peripheral blood leukocytes: Its association with basophil activation. Postepy Dermatol. Alergol. 2015, 32, 262–267. [Google Scholar] [CrossRef]
- Singh, N.K.; Rao, G.N. Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Prog. Lipid Res. 2019, 73, 28–45. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Peng, H.; Liu, H.; Cheng, Q.; Cheng, X.; Zeng, P.; Wu, P.; Chen, H.; Huang, Y.; et al. Glucocorticoids sensitize rat placental inflammatory responses via inhibiting lipoxin A4 biosynthesis. Biol. Reprod. 2014, 90, 74. [Google Scholar] [CrossRef]
- Feng, Y.; Bai, X.; Yang, Q.; Wu, H.; Wang, D. Downregulation of 15-lipoxygenase 2 by glucocorticoid receptor in prostate cancer cells. Int. J. Oncol. 2010, 36, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Park, C.; Shim, M.K.; Lee, Y.H.; Lee, Y.M.; Lee, Y. Glucocorticoids suppress hypoxia-induced COX-2 and hypoxia inducible factor-1alpha expression through the induction of glucocorticoid-induced leucine zipper. Br. J. Pharmacol. 2014, 171, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zeng, J.; Miao, X.; Liu, H.; Ge, L.; Huang, W.; Jiao, J.; Ye, D. Glucocorticoid exposure induces preeclampsia via dampening 1,25-dihydroxyvitamin D(3). Hypertens. Res. 2018, 41, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Laprise, C.; Sladek, R.; Ponton, A.; Bernier, M.C.; Hudson, T.J.; Laviolette, M. Functional classes of bronchial mucosa genes that are differentially expressed in asthma. BMC Genom. 2004, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Carey, M.; Workman, J.L. The role of chromatin during transcription. Cell 2007, 128, 707–719. [Google Scholar] [CrossRef]
- Beato, M.; Eisfeld, K. Transcription factor access to chromatin. Nucleic Acids Res. 1997, 25, 3559–3563. [Google Scholar] [CrossRef]
- Verdone, L.; Caserta, M.; Di Mauro, E. Role of histone acetylation in the control of gene expression. Biochem. Cell Biol. 2005, 83, 344–353. [Google Scholar] [CrossRef]
- Grunstein, M. Histone acetylation in chromatin structure and transcription. Nature 1997, 389, 349–352. [Google Scholar] [CrossRef]
- Ogryzko, V.V.; Schiltz, R.L.; Russanova, V.; Howard, B.H.; Nakatani, Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 1996, 87, 953–959. [Google Scholar] [CrossRef]
- Eckner, R.; Ewen, M.E.; Newsome, D.; Gerdes, M.; DeCaprio, J.A.; Lawrence, J.B.; Livingston, D.M. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994, 8, 869–884. [Google Scholar] [CrossRef]
- Wang, W.; Guo, C.; Li, W.; Li, J.; Wang, W.; Myatt, L.; Sun, K. Involvement of GR and p300 in the induction of H6PD by cortisol in human amnion fibroblasts. Endocrinology 2012, 153, 5993–6002. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Liu, C.; Wang, W.; Li, W.; Shu, Q.; Chen, Z.J.; Sun, K. Critical role of histone acetylation by p300 in human placental 11beta-HSD2 expression. J. Clin. Endocrinol. Metab. 2013, 98, E1189–E1197. [Google Scholar] [CrossRef]
- Wang, R.; Cherukuri, P.; Luo, J. Activation of Stat3 sequence-specific DNA binding and transcription by p300/CREB-binding protein-mediated acetylation. J. Biol. Chem. 2005, 280, 11528–11534. [Google Scholar] [CrossRef]
- Kino, T.; Nordeen, S.K.; Chrousos, G.P. Conditional modulation of glucocorticoid receptor activities by CREB-binding protein (CBP) and p300. J. Steroid Biochem. Mol. Biol. 1999, 70, 15–25. [Google Scholar] [CrossRef]
- Lu, J.W.; Wang, W.S.; Zhou, Q.; Gan, X.W.; Myatt, L.; Sun, K. Activation of prostaglandin EP4 receptor attenuates the induction of cyclooxygenase-2 expression by EP2 receptor activation in human amnion fibroblasts: Implications for parturition. FASEB J. 2019, 33, 8148–8160. [Google Scholar] [CrossRef]
- Wang, W.; Liu, C.; Sun, K. Induction of Amnion Epithelial Apoptosis by Cortisol via tPA/Plasmin System. Endocrinology 2016, 157, 4487–4498. [Google Scholar] [CrossRef]
- Lu, J.W.; Lei, W.J.; Ling, L.J.; Wang, L.Y.; Lin, Y.K.; Zhang, F.; Li, M.D.; Pan, F.; Wang, W.S.; Sun, K. Cortisol Stimulates Local Progesterone Withdrawal through Induction of AKR1C1 in Human Amnion Fibroblasts at Parturition. Endocrinology 2022, 163, bqac148. [Google Scholar] [CrossRef]
- Mi, Y.; Wang, W.; Lu, J.; Zhang, C.; Wang, Y.; Ying, H.; Sun, K. Proteasome-mediated degradation of collagen III by cortisol in amnion fibroblasts. J. Mol. Endocrinol. 2018, 60, 45–54. [Google Scholar] [CrossRef]
- Liu, C.; Guo, C.; Wang, W.; Zhu, P.; Li, W.; Mi, Y.; Myatt, L.; Sun, K. Inhibition of Lysyl Oxidase by Cortisol Regeneration in Human Amnion: Implications for Rupture of Fetal Membranes. Endocrinology 2016, 157, 4055–4065. [Google Scholar] [CrossRef]
- Guller, S.; Kong, L.; Wozniak, R.; Lockwood, C.J. Reduction of extracellular matrix protein expression in human amnion epithelial cells by glucocorticoids: A potential role in preterm rupture of the fetal membranes. J. Clin. Endocrinol. Metab. 1995, 80, 2244–2250. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wang, W.S.; Wang, Y.W.; Lu, J.W.; Lu, Y.; Zhang, C.Y.; Li, W.J.; Sun, K.; Ying, H. Drastic induction of MMP-7 by cortisol in the human amnion: Implications for membrane rupture at parturition. FASEB J. 2019, 33, 2770–2781. [Google Scholar] [CrossRef] [PubMed]
- Casey, M.L.; MacDonald, P.C.; Mitchell, M.D. Despite a massive increase in cortisol secretion in women during parturition, there is an equally massive increase in prostaglandin synthesis. A paradox? J. Clin. Investig. 1985, 75, 1852–1857. [Google Scholar] [CrossRef] [PubMed]
- Chrivia, J.C.; Kwok, R.P.; Lamb, N.; Hagiwara, M.; Montminy, M.R.; Goodman, R.H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature 1993, 365, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Harlow, E.; Whyte, P.; Franza, B.R., Jr.; Schley, C. Association of adenovirus early-region 1A proteins with cellular polypeptides. Mol. Cell. Biol. 1986, 6, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Bedford, D.C.; Brindle, P.K. Is histone acetylation the most important physiological function for CBP and p300? Aging (Albany NY) 2012, 4, 247–255. [Google Scholar] [CrossRef]
- Li, J.; Hsu, A.; Hua, Y.; Wang, G.; Cheng, L.; Ochiai, H.; Yamamoto, T.; Pertsinidis, A. Single-gene imaging links genome topology, promoter-enhancer communication and transcription control. Nat. Struct. Mol. Biol. 2020, 27, 1032–1040. [Google Scholar] [CrossRef]
- Dancy, B.M.; Cole, P.A. Protein lysine acetylation by p300/CBP. Chem. Rev. 2015, 115, 2419–2452. [Google Scholar] [CrossRef]
- Shankaranarayanan, P.; Chaitidis, P.; Kuhn, H.; Nigam, S. Acetylation by histone acetyltransferase CREB-binding protein/p300 of STAT6 is required for transcriptional activation of the 15-lipoxygenase-1 gene. J. Biol. Chem. 2001, 276, 42753–42760. [Google Scholar] [CrossRef]
- Ho, C.F.; Bon, C.P.; Ng, Y.K.; Herr, D.R.; Wu, J.S.; Lin, T.N.; Ong, W.Y. Expression of DHA-Metabolizing Enzyme Alox15 is Regulated by Selective Histone Acetylation in Neuroblastoma Cells. Neurochem. Res. 2018, 43, 540–555. [Google Scholar] [CrossRef]
- Shiama, N. The p300/CBP family: Integrating signals with transcription factors and chromatin. Trends Cell Biol. 1997, 7, 230–236. [Google Scholar] [CrossRef]
- Wang, W.S.; Li, W.J.; Wang, Y.W.; Wang, L.Y.; Mi, Y.B.; Lu, J.W.; Lu, Y.; Zhang, C.Y.; Sun, K. Involvement of serum amyloid A1 in the rupture of fetal membranes through induction of collagen I degradation. Clin. Sci. 2019, 133, 515–530. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Lu, J.-W.; Lei, W.-J.; Li, M.-D.; Pan, F.; Lin, Y.-K.; Wang, W.-S.; Sun, K. Paradoxical Induction of ALOX15/15B by Cortisol in Human Amnion Fibroblasts: Implications for Inflammatory Responses of the Fetal Membranes at Parturition. Int. J. Mol. Sci. 2023, 24, 10881. https://doi.org/10.3390/ijms241310881
Zhang F, Lu J-W, Lei W-J, Li M-D, Pan F, Lin Y-K, Wang W-S, Sun K. Paradoxical Induction of ALOX15/15B by Cortisol in Human Amnion Fibroblasts: Implications for Inflammatory Responses of the Fetal Membranes at Parturition. International Journal of Molecular Sciences. 2023; 24(13):10881. https://doi.org/10.3390/ijms241310881
Chicago/Turabian StyleZhang, Fan, Jiang-Wen Lu, Wen-Jia Lei, Meng-Die Li, Fan Pan, Yi-Kai Lin, Wang-Sheng Wang, and Kang Sun. 2023. "Paradoxical Induction of ALOX15/15B by Cortisol in Human Amnion Fibroblasts: Implications for Inflammatory Responses of the Fetal Membranes at Parturition" International Journal of Molecular Sciences 24, no. 13: 10881. https://doi.org/10.3390/ijms241310881
APA StyleZhang, F., Lu, J.-W., Lei, W.-J., Li, M.-D., Pan, F., Lin, Y.-K., Wang, W.-S., & Sun, K. (2023). Paradoxical Induction of ALOX15/15B by Cortisol in Human Amnion Fibroblasts: Implications for Inflammatory Responses of the Fetal Membranes at Parturition. International Journal of Molecular Sciences, 24(13), 10881. https://doi.org/10.3390/ijms241310881





