Lung Surfactant Protein B Peptide Mimics Interact with the Human ACE2 Receptor
Abstract
1. Introduction
2. Results
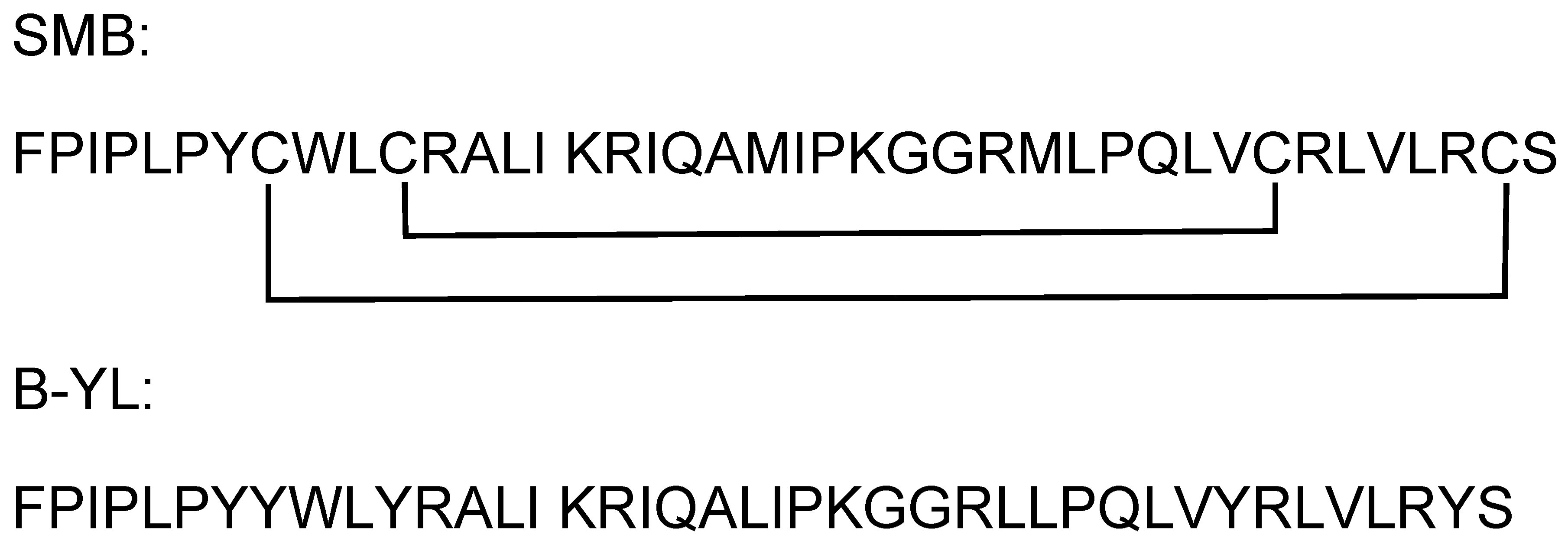
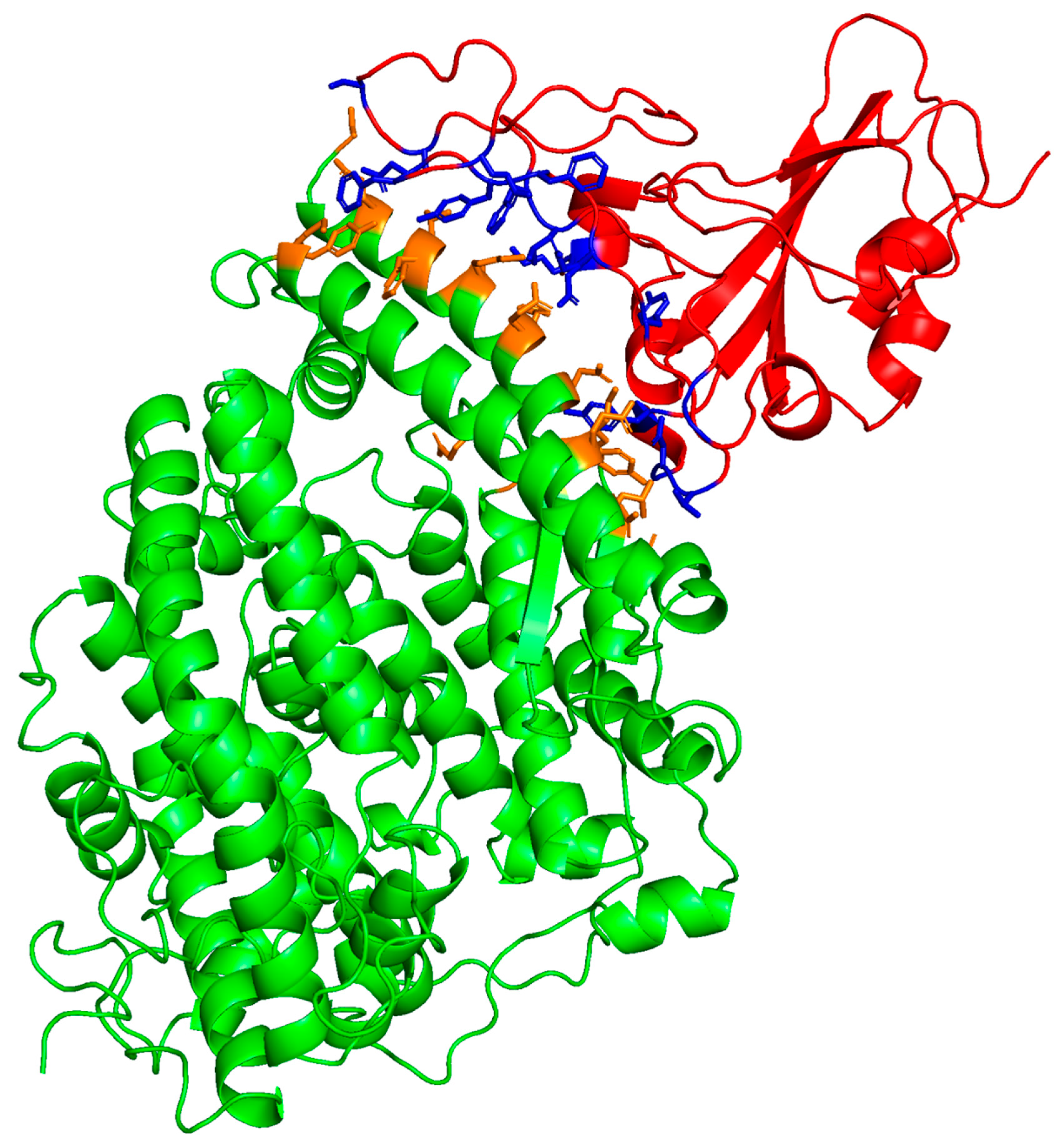
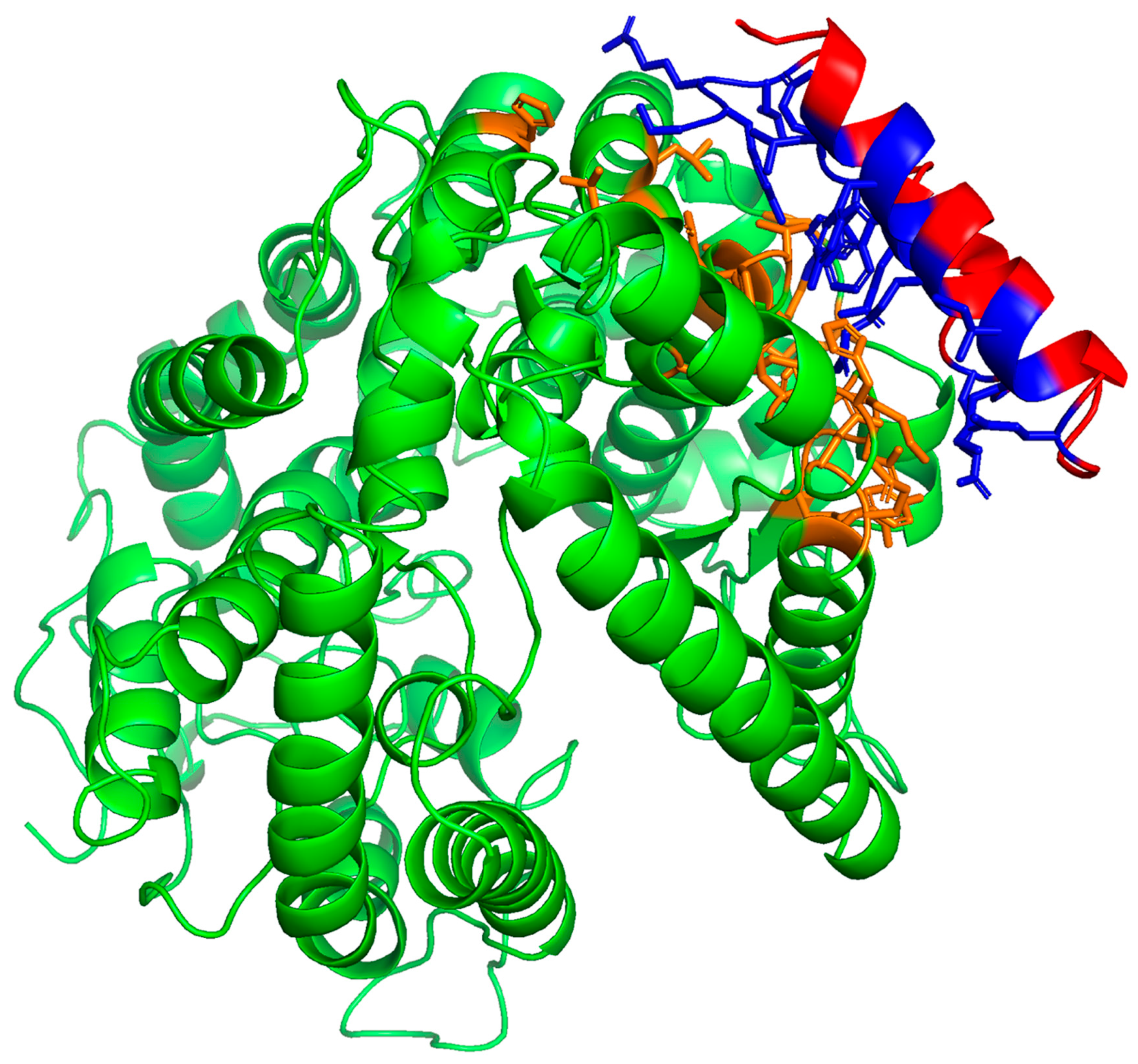
2.1. Determination of the Potential SMB and B-YL Peptides to Form Complexes with Human ACE2 Receptor Using Molecular Docking
2.2. Surface Plasmon Resonance (SPR) Measurements of SMB and B-YL Peptide Binding to ACE-2 Domain
3. Discussion
4. Materials and Methods
4.1. Protein–Peptide Docking and Prediction of Binding Affinities
4.2. Protein and Peptide Constructs
4.3. Surface Plasmon Resonance (SPR) Measurements of the Binding of SMB and B-YL Surfactant Peptides to Lung Receptor ACE-2 Domain
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walther, F.J.; Waring, A.J. Synthetic lung surfactant treatment for COVID-19 pneumonia. Coronaviruses 2021, 2, 8–10. [Google Scholar] [CrossRef]
- National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; National Institutes of Health: Bethesda, MD, USA, 2023.
- Gerard, L.; Lecocq, M.; Bouzin, C.; Hoton, D.; Schmit, G.; Pereira, J.P.; Montiel, V.; Plante-Bordeneuve, T.; Laterre, P.F.; Pilette, C. Increased Angiotensin-Converting Enzyme 2 and Loss of Alveolar Type II Cells in COVID-19-related acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2021, 204, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Piva, S.; DiBlasi, R.M.; Slee, A.E.; Jobe, A.H.; Roccaro, A.M.; Filippini, M.; Latronico, N.; Bertoni, M.; Marshall, J.C.; Portman, M.A. Surfactant therapy for COVID-19 related ARDS: A retrospective case-control pilot study. Respir. Res. 2021, 22, 20. [Google Scholar] [CrossRef]
- Numata, M.; Kandasamy, P.; Voelker, D.R. The anti-inflammatory and antiviral properties of anionic pulmonary surfactant phospholipids. Immunol. Rev. 2023. [Google Scholar] [CrossRef] [PubMed]
- Cattel, F.; Giordano, S.; Bertiond, C.; Lupia, T.; Corcione, S.; Scaldaferri, M.; Angelone, L.; De Rosa, F.G. Use of exogenous pulmonary surfactant in acute respiratory distress syndrome (ARDS): Role in SARS-CoV-2-related lung injury. Respir. Physiol. Neurobiol. 2021, 288, 103645. [Google Scholar] [CrossRef]
- Walther, F.J.; Gordon, L.M.; Waring, A.J. Advances in synthetic lung surfactant protein technology. Expert Rev. Respir. Med. 2019, 13, 499–501. [Google Scholar] [CrossRef]
- Walther, F.J.; Waring, A.J.; Hernandez-Juviel, J.M.; Gordon, L.M.; Wang, Z.; Jung, C.-L.; Ruchala, P.; Clark, A.P.; Smith, W.M.; Sharma, S.; et al. Critical structural and functional roles for the N-terminal insertion sequence in surfactant protein B analogs. PLoS ONE 2010, 5, e8672. [Google Scholar] [CrossRef]
- Walther, F.J.; Gupta, M.; Gordon, L.M.; Waring, A.J. A sulfur-free peptide mimic of surfactant protein B (B-YL) exhibits high in vitro and in vivo surface activities. Gates Open Res. 2018, 2, 13. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Benton, D.J.; Wrobel, A.G.; Xu, P.; Roustan, C.; Martin, S.R.; Rosenthal, P.B.; Skehel, J.J.; Gamblin, S.J. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature 2020, 588, 327–330. [Google Scholar] [CrossRef]
- Cao, L.; Goreshnik, I.; Coventry, B.; Case, J.B.; Miller, L.; Kozodoy, L.; Chen, R.E.; Carter, L.; Walls, A.C.; Park, Y.J.; et al. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science 2020, 370, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Peter, E.K.; Schug, A. The inhibitory effect of a coronavirus spike protein fragment with ACE2. Biophys. J. 2021, 120, 1001–1010. [Google Scholar] [CrossRef]
- Struck, A.W.; Axmann, M.; Pfefferle, S.; Drosten, C.; Meyer, B. A hexapeptide of the receptor-binding domain of SARS corona virus spike protein blocks viral entry into host cells via the human receptor ACE2. Antiviral Res. 2012, 94, 288–296. [Google Scholar] [CrossRef] [PubMed]
- VanPatten, S.; He, M.; Altiti, A.; FCheng, K.; Ghanem, M.H.; Al-Abed, Y. Evidence supporting the use of peptides and peptidomimetics as potential SARS-CoV-2 (COVID-19) therapeutics. Future Med. Chem. 2020, 12, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Sadremomtaz, A.; Al-Dahmani, Z.M.; Ruiz-Moreno, A.J.; Monti, A.; Wang, C.; Azad, T.; Bell, J.C.; Doti, N.; Velasco-Velázquez, M.A.; de Jong, D.; et al. Synthetic peptides that antagonize the angiotensin-converting enzyme-2 (ACE-2) interaction with SARS-CoV-2 receptor binding spike protein. J. Med. Chem. 2022, 65, 2836–2847. [Google Scholar] [CrossRef]
- Xue, L.C.; Rodrigues, J.P.; Kastritis, P.L.; Bonvin, A.M.; Vangone, A. PRODIGY: A web server for predicting the binding affinity of protein-protein complexes. Bioinformatics 2016, 32, 3676–3678. [Google Scholar] [CrossRef] [PubMed]
- van Zundert, G.C.P.; Rodrigues, J.P.G.L.M.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; van Dijk, M.; de Vries, S.J.; Bonvin, A.M.J.J. The HADDOCK2.2 web server: User-friendly integrative modeling of biomolecular complexes. J. Mol. Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef]
- Patching, S.G. Surface plasmon resonance spectroscopy for characterisation of membrane protein-ligand interactions and its potential for drug discovery. Biochim. Biophys. Acta 2014, 1838 (Pt A), 43–55. [Google Scholar] [CrossRef]
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 12, CD015477. [Google Scholar] [CrossRef]
- Day, C.J.; Bailly, B.; Guillon, P.; Dirr, L.; Jen, F.E.; Spillings, B.L.; Mak, J.; von Itzstein, M.; Haselhorst, T.; Jennings, M.P. Multidisciplinary approaches identify compounds that bind to human ACE2 or SARS-CoV-2 spike protein as candidates to block SARS-CoV-2-ACE2 receptor interactions. mBio 2021, 12, e03681-20. [Google Scholar] [CrossRef]
- Das, A.; Vishvakarma, V.; Dey, A.; Dey, S.; Gupta, A.; Das, M.; Vishwakarma, K.K.; Roy, D.S.; Yadav, S.; Kesarwani, S.; et al. Biophysical properties of the isolated spike protein binding helix of human ACE2. Biophys. J. 2021, 120, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Walther, F.J.; Sharma, S.; Gordon, L.M.; Waring, A.J. Structural and functional stability of the sulfur-free surfactant protein B peptide mimic B-YL in synthetic surfactant lipids. BMC Pulm. Med. 2021, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Waring, A.J.; Whitelegge, J.P.; Sharma, S.K.; Gordon, L.M.; Walther, F.J. Emulation of the structure of the Saposin protein fold by a lung surfactant peptide construct of surfactant Protein B. PLoS ONE 2022, 17, e0276787. [Google Scholar] [CrossRef] [PubMed]
- Rozak, H.; Nihonyanagi, S.; Myalitsin, A.; Roy, S.; Ahmed, M.; Tahara, T.; Rzeznicka, I.I. Adsorption of SARS-CoV-2 Spike (N501Y) RBD to Human Angiotensin-Converting Enzyme 2 at a Lipid/Water Interface. J. Phys. Chem. B 2023, 127, 4406–4414. [Google Scholar] [CrossRef] [PubMed]
- Honorato, R.V.; Koukos, P.I.; Jiménez-García, B.; Tsaregorodtsev, A.; Verlato, M.; Giachetti, A.; Rosato, A.; Bonvin, A.M.J.J. Structural biology in the clouds: The WeNMR-EOSC ecosystem. Front. Mol. Biosci. 2021, 8, 729513. [Google Scholar] [CrossRef]
- Vangone, A.; Bonvin, A.M. Contacts-based prediction of binding affinity in protein-protein complexes. Elife 2015, 4, e07454. [Google Scholar] [CrossRef]
- Tam, J.P.; Dong, X.C.; Wu, C.R. Solvent assistance in regiospecific disulfide formation in dimethylsulfoxide. Lett. Peptide Sci. 1999, 6, 265–273. [Google Scholar] [CrossRef]
- Anthis, N.J.; Clore, G.M. Sequence-specific determination of protein and peptide concentrations by absorbance at 205 nm. Protein Sci. 2013, 22, 851–858. [Google Scholar] [CrossRef]
- Kauppinen, J.K.; Moffatt, D.J.; Mantsch, H.H.; Cameron, D.G. Fourier self-deconvolution: A method for resolving intrinsically overlapped bands. Appl. Spectr. 1981, 35, 271–276. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Examination of the secondary structure of protein by deconvolved FTIR spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef]

| ACE2-RBD | ACE2–B-YL | ACE2-SMB |
|---|---|---|
| Contact Residues | Contact Residues | Contact Residues |
| LEU 45 A TYR 446 B | ARG 340 A ARG 27 B | PRO 218 A ALA 13 B |
| TYR 83 A ASN 487 B | VAL 370 A LEU 5 B | THR 214 A PRO 4 B |
| TYR 83 A PHE 486 B | ARG 376 A ARG 12 B | GLU 466 A ARG 12 B |
| ARG 357 A THR 500 B | VAL 370 A TRP 9 B | LEU 219 A LYS 16 B |
| HIS 34 A LEU 455 B | ALA 367 A TYR 40 B | LYS 217 A PRO 4 B |
| LYS 31 A LEU 455 B | HIS 17 A TYR 8 B | THR 591 A ARG 12 B |
| ASP 355 A ASN 501 B | LEU 538 A ARG 39 B | LYS 441 A PRO 2 B |
| LYS 31 A PHE 490 B | LEU 543 A SER 41 B | ASP 592 A TRP 9 B |
| ALA 386 A TYR 505 B | ASP 338 A ARG 27 B | GLN 581 A MET 21 B |
| GLN 24 A TYR 489 B | PRO 372 A TYR 8 B | TRP 589 A CYS 8 B |
| ARG 393 A TYR 505 B | ARG 376 A LEU 28 B | LYS 459 A PHE 1 B |
| LEU 45 A GLN 498 B | ALA 369 A GLN 31 B | THR 214 A TYR 7 B |
| LYS 353 A GLN 498 B | ASN 16 A TYR 8 B | GLU 466 A PRO 4 B |
| ASP 30 A LYS 417 B | THR 542 A ARG 39 B | VAL 587 A GLY 25 B |
| LYS 353 A GLY 502 B | LEU 538 A SER 41 B | MET 463 A PHE 1 B |
| ASP 355 A GLY 502 B | GLN 371 A LEU 5 B | LEU 219 A GLN 19 B |
| GLY 354 A THR 500 B | PHE 339 A GLN 31 B | PHE 586 A ARG 27 B |
| LYS 31 A GLN 493 B | VAL 370 A GLN 31 B | LYS 441 A PHE 1 B |
| ASN 330 A THR 500 B | HIS 555 A SER 41 B | THR 591 A TRP 9 B |
| ASP 30 A LEU 455 B | HIS 17 A LYS 16 B | SER 585 A GLY 26 B |
| TYR 41 A GLN 498 B | GLY 337 A GLN 31 B | ASN 582 A MET 21 B |
| THR 27 A TYR 473 B | ASN 305 A TYR 34 B | ASP 592 A GLN 31 B |
| GLU 35 A GLN 493 B | GLN 539 A ARG 39 B | TYR 437 A ILE 3 B |
| SER 19 A ALA 475 B | VAL 370 A TYR 40 B | PHE 586 A MET 28 B |
| GLN 42 A TYR 449 B | VAL 370 A ARG 35 B | ASN 590 A GLN 31 B |
| GLY 354 A VAL 503 B | LYS 336 A ARG 39 B | VAL 587 A PRO 23 B |
| TYR 41 A ASN 501 B | LYS 536 A ARG 39 B | ARG 583 A MET 21 B |
| THR 27 A ALA 475 B | LYS 535 A ARG 39 B | TRP 589 A MET 28 B |
| GLN 24 A SER 477 B | LYS 336 A TRP 9 B | SER 585 A LYS 24 B |
| ASP 38 A GLN 498 B | GLU 20 A LEU 28 B | PHE 586 A GLY 26 B |
| HIS 34 A TYR 453 B | LEU 538 A LEU 38 B | VAL 587 A MET 21 B |
| GLN 24 A PHE 486 B | PRO 304 A LEU 38 B | HIS 222 A MET 21 B |
| ASP 38 A TYR 449 B | LYS 336 A GLN 31 B | THR 214 A LEU 14 B |
| LYS 353 A PHE 497 B | GLU 21 A LYS 16 B | TRP 589 A ARG 12 B |
| TYR 41 A THR 500 B | GLN 371 A TYR 8 B | HIS 222 A GLN 19 B |
| HIS 34 A LYS 417 B | HIS 17 A ARG 12 B | GLU 462 A PHE 1 B |
| GLY 354 A GLY 502 B | LEU 538 A TYR 40 B | GLU 210 A ILE 3 B |
| TYR 83 A TYR 489 B | ALA 369 A TRP 9 B | LYS 217 A LEU 5 B |
| LEU 79 A PHE 486 B | THR 542 A SER 41 B | PHE 575 A GLN 19 B |
| LYS 353 A ASN 501 B | ARG 376 A GLN 31 B | ASN 590 A TRP 9 B |
| LYS 31 A TYR 489 B | TYR 24 A GLY 25 B | GLN 221 A LYS 16 B |
| ASP 24 A GLY 496 B | GLN 539 A SER 41 B | LYS 217 A TYR 7 B |
| GLN 24 A ASN 487 B | GLU 20 A ARG 12 B | PRO 218 A LEU 14 B |
| GLY 354 A TYR 505 B | LYS 336 A GLY 25 B | GLU 466 A TRP 9 B |
| GLN 42 A GLY 447 B | GLY 337 A LEU 28 B | GLU 210 A ILE 3 B |
| PHE 28 A ASN 487 B | HIS 17 A ILE 15 B | LYS 217 A LEU 5 B |
| ASP 355 A THR 500 B | VAL 370 A TYR 8 B | PHE 575 A GLN 19 B |
| GLY 354 A ASN 501 B | TYR 24 A ARG 27 B | VAL 587 A LYS 16 B |
| GLN 42 A GLY 446 B | MET 366 A TYR 34 B | HIS 222 A LYS 16 B |
| LEU 45 A THR 500 B | GLY 302 A LEU 38 B | GLN 221 A LEU 14 B |
| GLN 24 A GLY 476 B | GLU 20 A LYS 24 B | LYS 217 A CYS 8 B |
| LYS 353 A TYR 495 B | HIS 17 A GLN 19 B | GLU 462 A SER 41 B |
| LYS 31 A GLU 484 B | PRO 304 A TYR 34 B | GLU 466 A SER 41 B |
| GLU 37 A TYR 505 B | GLN 371 A SER 41 B | GLU 210 A PRO 4 B |
| LYS 31 A PHE 456 B | LYS 336 A LEU 28 B | LYS 441 A ILE 3 B |
| HIS 34 A GLN 493 B | THR 542 A TYR 40 B | PRO 218 A GLN 19 B |
| PHE 28 A TYR 489 B | THR 307 A TYR 34 B | THR 591 A SER 41 B |
| LYS 353 A GLY 496 B | GLN 363 A TYR 34 B | MET 445 A PRO 2 B |
| GLN 42 A GLN 498 B | MET 366 A GLN 31 B | GLN 221 A ARG 12 B |
| THR 27 A TYR 489 B | ARG 376 A TRP 9 B | TYR 437 A PRO 4 B |
| GLN 24 A ALA 475 B | MET 366 A TYR 40 B | THR 242 A ARG 27 B |
| ASP 30 A PHE 456 B | VAL 370 A SER 41 B | GLU 466 A TYR 7 B |
| MET 82 A PHE 486 B | PRO 372 A ARG 12 B | VAL 587 A GLY 26 B |
| LYS 353 A TYR 505 B | PHE 373 A ARG 12 B | VAL 587 A ARG 12 B |
| GLN 371 A TRP 9 B | LYS 579 A MET 21 B | |
| LYS 336 A LYS 24 B | SER 585 A GLY 25 B | |
| TYR 368 A TYR 40 B | GLU 466 A CYS 8 B | |
| GLU 21 A ARG 12 B | GLN 221 A LYS 24 B | |
| ASN 16 A ARG 12 B | LYS 579 A ALA 20 B | |
| THR 242 A GLY 26 B | ||
| PHE 213 A PRO 4 B | ||
| THR 591 A CYS 8 B | ||
| GLU 580 A MET 21 B | ||
| MET 445 A PHE 1 B | ||
| ILE 467 A PHE 1 B | ||
| GLY 588 A ARG 12 B | ||
| VAL 587 A LYS 24 B | ||
| ARG 583 A ILE 22 B | ||
| GLU 210 A PHE 1 B | ||
| ASP 592 A MET 28 B | ||
| PHE 586 A GLY 25 B | ||
| LYS 217 A ARG 12 B | ||
| ASN 590 A MET 28 B | ||
| PRO 218 A ILE 15 B | ||
| TYR 437 A PHE 1 B | ||
| GLY 588 A LYS 24 B | ||
| LEU 578 A MET 21 B | ||
| ASN 590 A ARG 12 B | ||
| ARG 465 A ARG 12 B | ||
| ARG 583 A ALA 20 B | ||
| THR 591 A MET 28 B | ||
| GLU 450 A PRO 2 B |
| Protein-Protein Interaction | ΔG (kcal mol−1) | KD (M) |
|---|---|---|
| Binding-Free Energies and Affinities of the binding of ACE2–SARS-CoV-2 RBD from Experimental SPR Measurements compared with that using structure-based prediction mythology | ||
| ACE2–RBD (PDB: 6m0j.pdb) | −11.90 | 1.90 × 10−9 |
| ACE2–RBD (SPR Data) * | −11.82 | 4.67 × 10−9 |
| Binding-Free Energies and Affinities Predicted from Docking of Molecular Complexes (HADDOCK) | ||
| ACE2–SMB | −13.5 | 3.10 × 10−10 |
| ACE2–B-YL | −11.1 | 5.20 × 10−9 |
| Experimentally Determined Binding-Free Energies and Affinities from SPR experimental measurements | ||
| ACE2–SMB-expt.data-SPR | −11.4 | 9.87 × 10−9 |
| ACE2–B-YL-expt.data-SPR | −12.6 | 1.27 × 10−9 |
| ka (1/Ms) | kd (1/s) | Rmax (RU) | RI (RU) | Conc of Analyte | KA (1/M) | KD (M) | Req (RU) | Kobs (1/s) | |
|---|---|---|---|---|---|---|---|---|---|
| 0.5 µg/mL SMB to hACE2 | 6.2 × 103 | 6.12 × 10−5 | 6.48 × 103 | 5.89 | 1.05 × 10−7 | 1.01 × 108 | 9.87 × 10−9 | 5.93 × 103 | 7.12 × 10−4 |
| 0.5 µg/mL B-YL to hACE2 | 2.19 × 104 | 2.79 × 10−5 | 258 | 2.12 | 1.05 × 10−7 | 7.85 × 108 | 1.27 × 10−9 | 255 | 2.33 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waring, A.J.; Jung, G.C.-L.; Sharma, S.K.; Walther, F.J. Lung Surfactant Protein B Peptide Mimics Interact with the Human ACE2 Receptor. Int. J. Mol. Sci. 2023, 24, 10837. https://doi.org/10.3390/ijms241310837
Waring AJ, Jung GC-L, Sharma SK, Walther FJ. Lung Surfactant Protein B Peptide Mimics Interact with the Human ACE2 Receptor. International Journal of Molecular Sciences. 2023; 24(13):10837. https://doi.org/10.3390/ijms241310837
Chicago/Turabian StyleWaring, Alan J., Grace C.-L. Jung, Shantanu K. Sharma, and Frans J. Walther. 2023. "Lung Surfactant Protein B Peptide Mimics Interact with the Human ACE2 Receptor" International Journal of Molecular Sciences 24, no. 13: 10837. https://doi.org/10.3390/ijms241310837
APA StyleWaring, A. J., Jung, G. C.-L., Sharma, S. K., & Walther, F. J. (2023). Lung Surfactant Protein B Peptide Mimics Interact with the Human ACE2 Receptor. International Journal of Molecular Sciences, 24(13), 10837. https://doi.org/10.3390/ijms241310837






