Bilirubin Concentration in Follicular Fluid Is Increased in Infertile Females, Correlates with Decreased Antioxidant Levels and Increased Nitric Oxide Metabolites, and Negatively Affects Outcome Measures of In Vitro Fertilization
Abstract
1. Introduction
2. Results
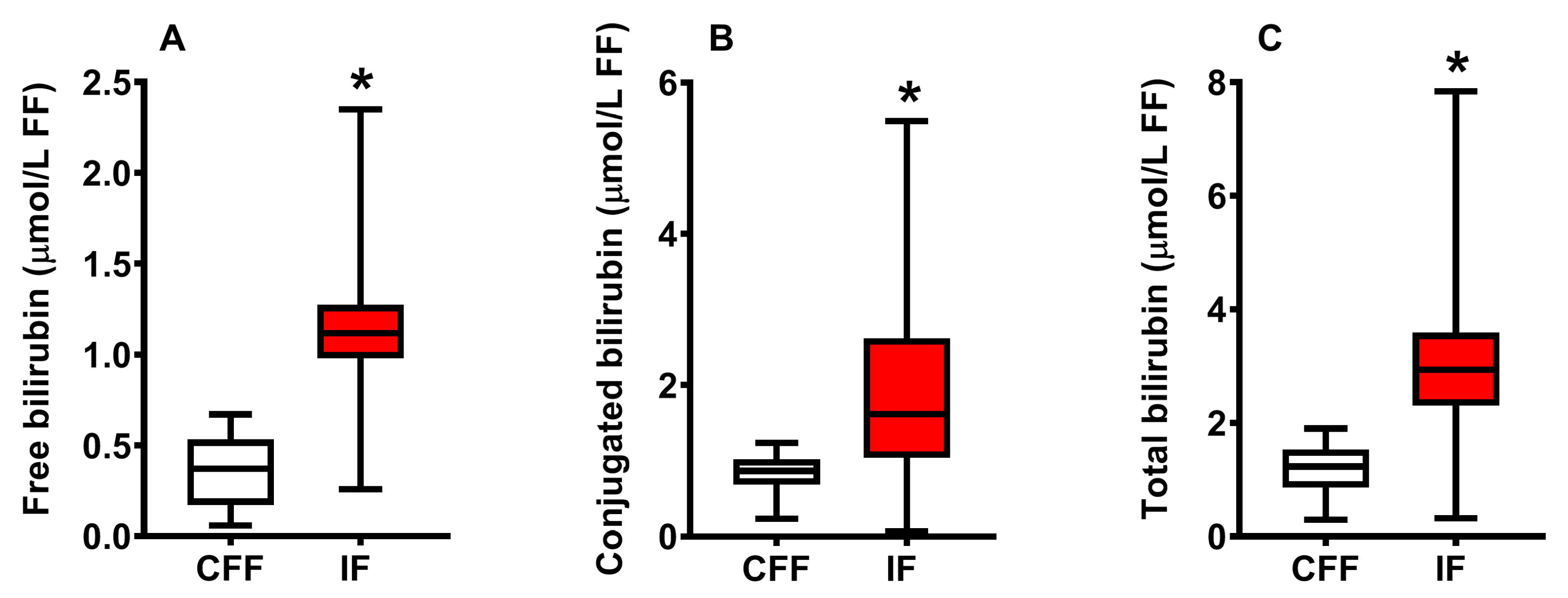
2.1. Concentration of Bilirubin in FF of Control Fertile and Pooled Infertile Females
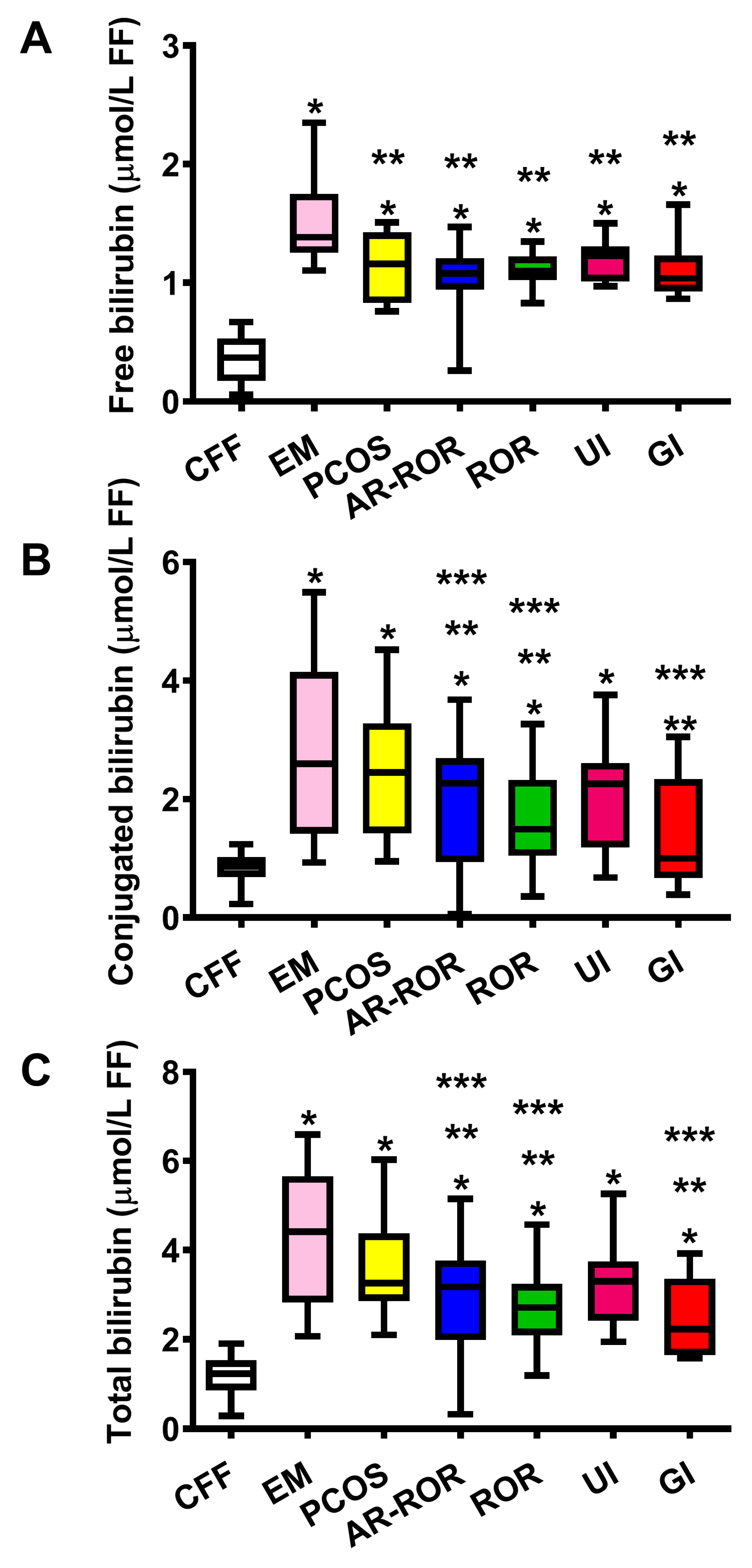
2.2. Concentration of Bilirubin in FF of CFF and IF Categorized According to Their Clinical Diagnosis of Infertility
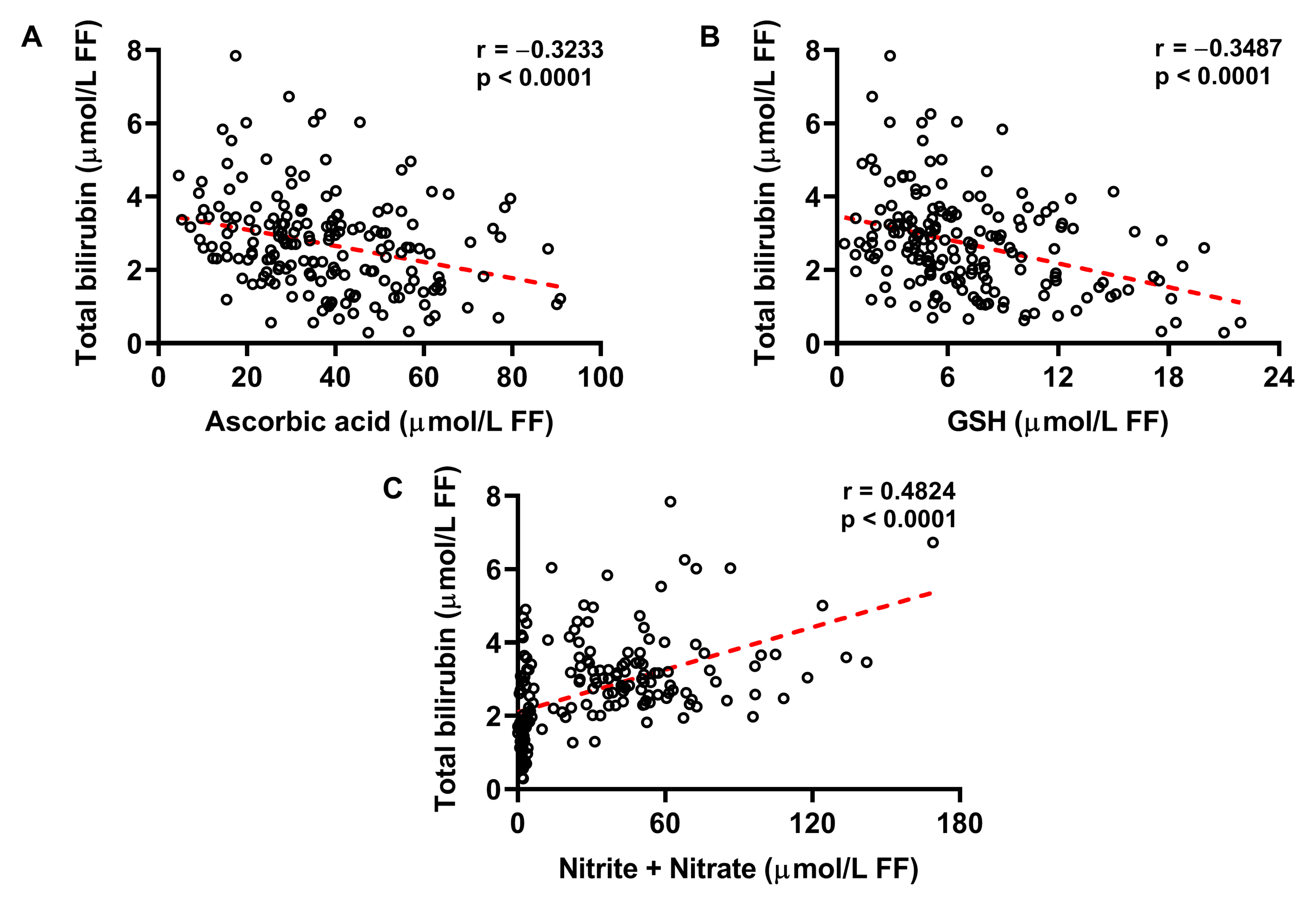
2.3. Increased Concentration of Bilirubin in FF Correlates with Decreased Ascorbic Acid and GSH, and Increased Nitrite + Nitrate
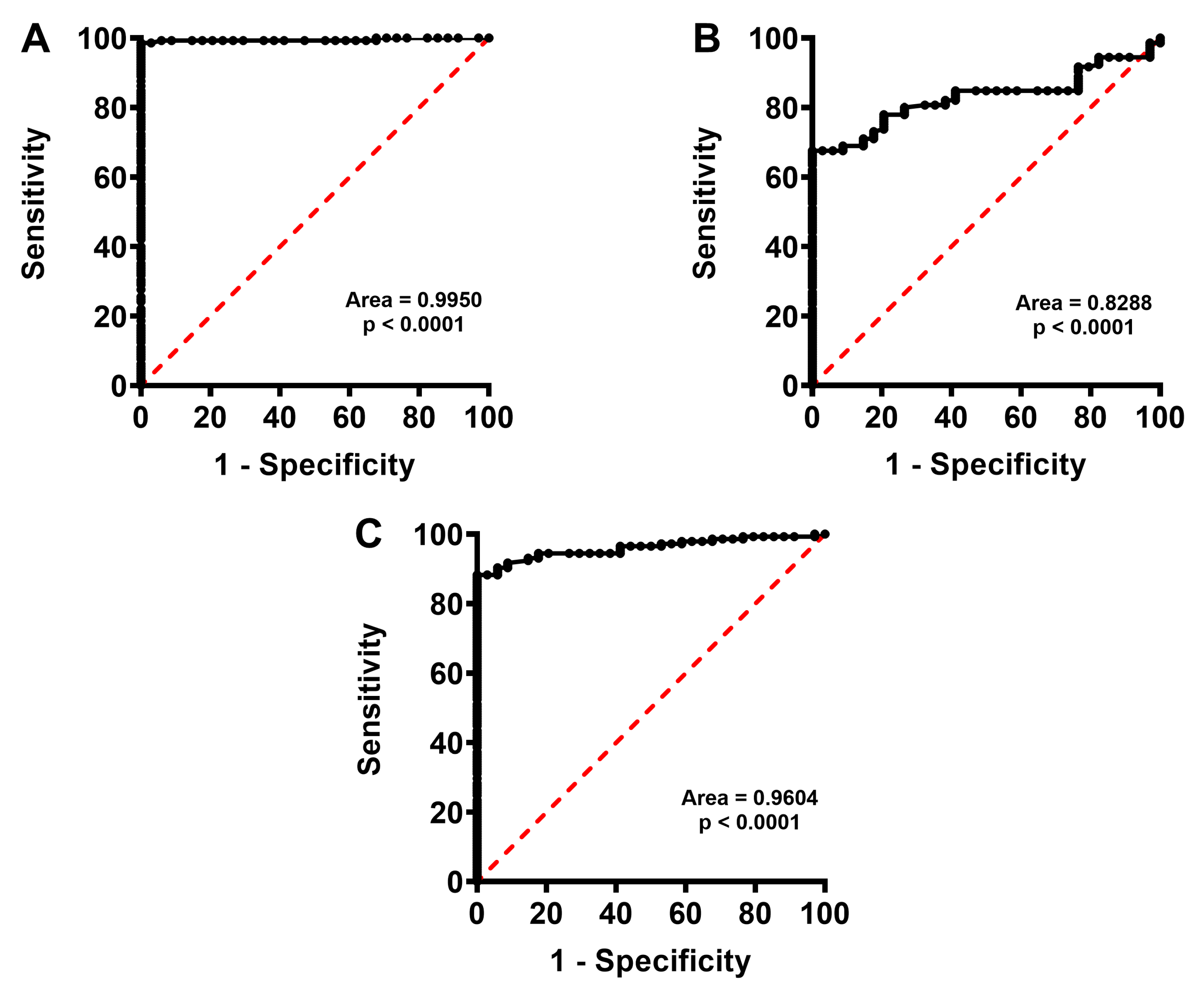
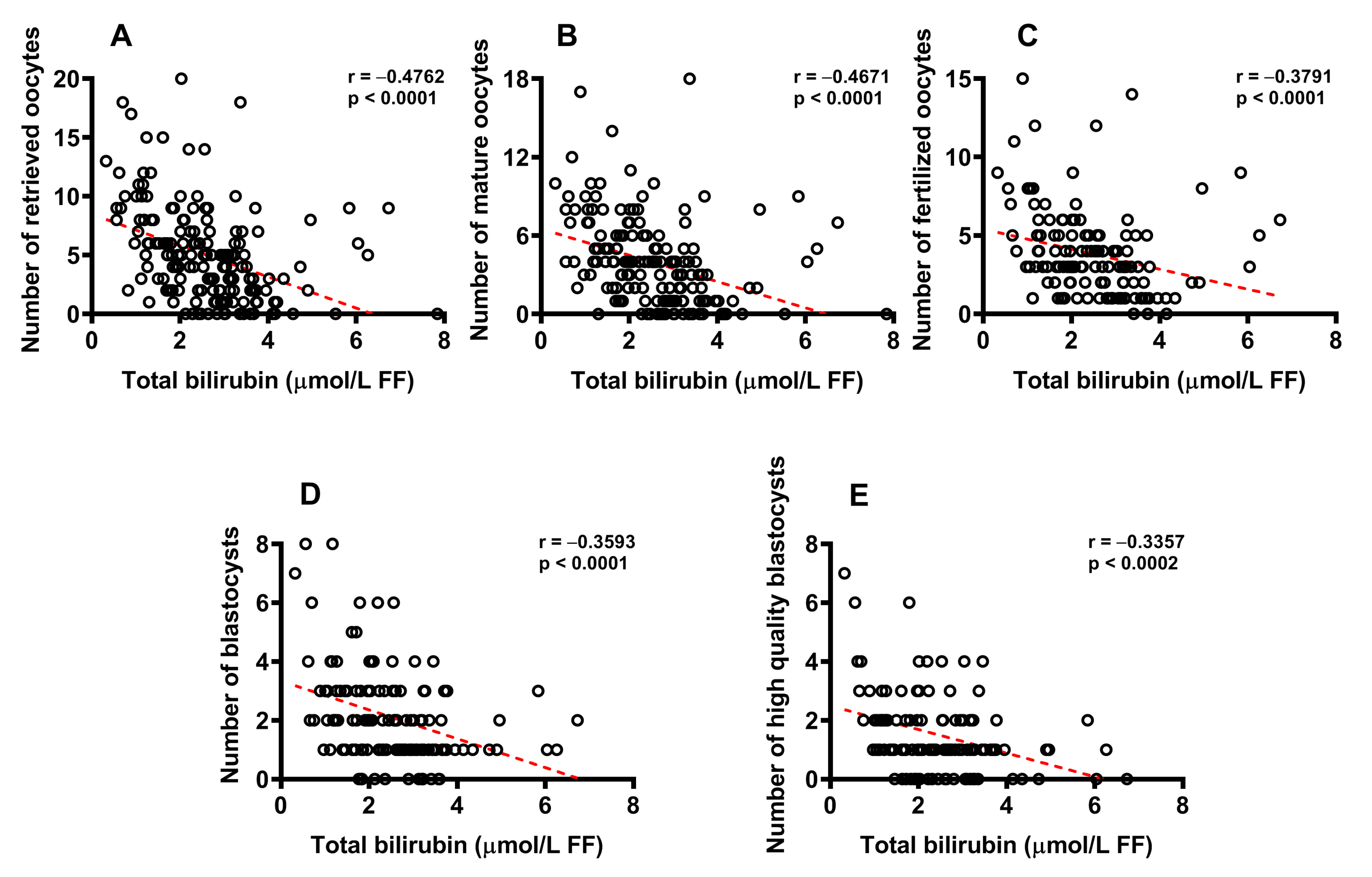
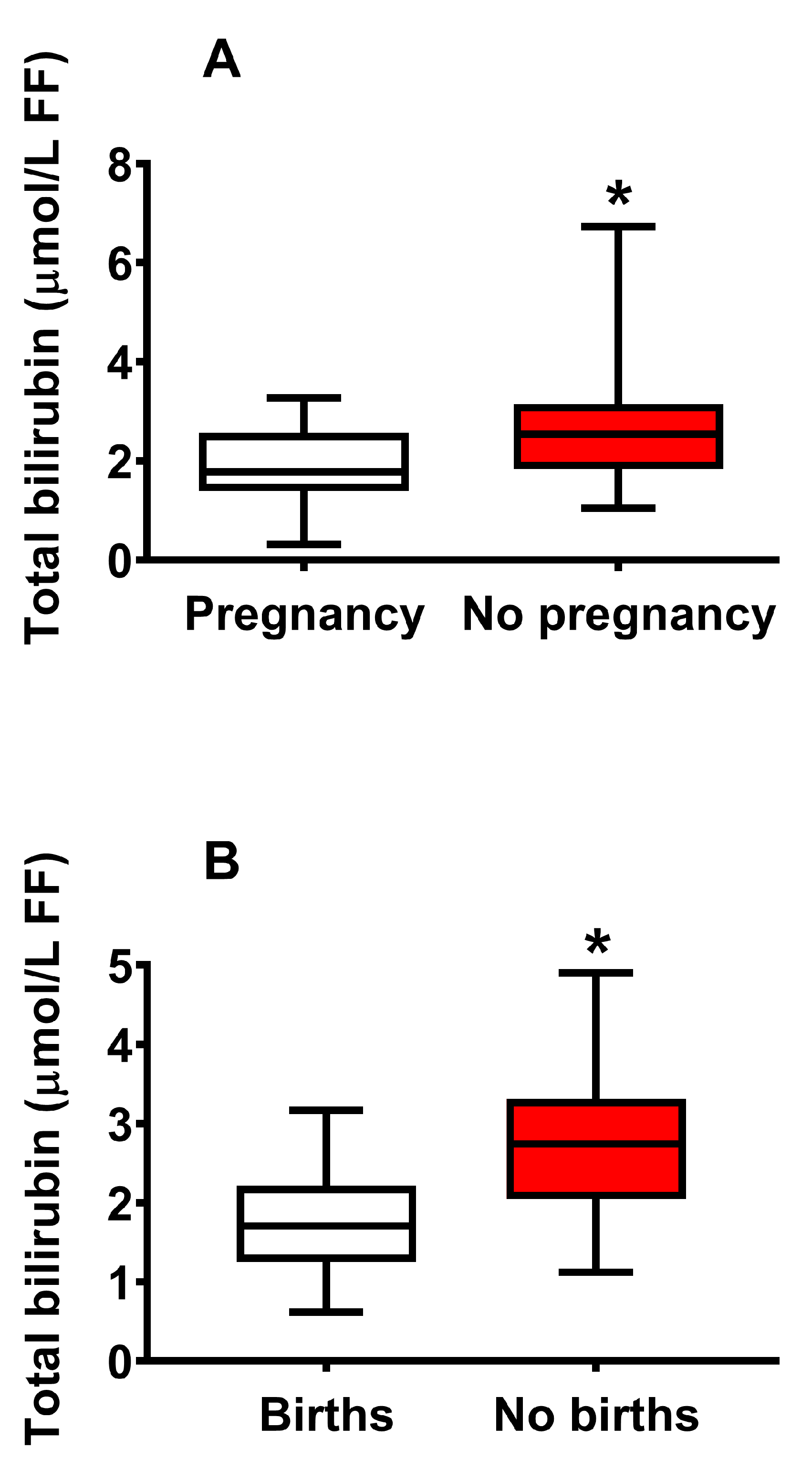
2.4. Concentration of Bilirubin in FF Correlates with Biological and Clinical Parameters of IVF and Successful Pregnancy
3. Discussion
4. Materials and Methods
4.1. Patients’ Characteristics and Protocols for Ovarian Stimulation
4.2. Follicle Recovery, FF Preparation, Embryo Culture and Assessment of Successful Fertilization
4.3. Samples Processing and HPLC Assay of Bilirubin in the Fat-Soluble Fraction of FF Samples
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brinca, A.T.; Ramalhinho, A.C.; Sousa, Â.; Oliani, A.H.; Breitenfeld, L.; Passarinha, L.A.; Gallardo, E. Follicular Fluid: A Powerful Tool for the Understanding and Diagnosis of Polycystic Ovary Syndrome. Biomedicines 2022, 10, 1254. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.; Deshmukh, H.; Atkin, S.L.; Sathyapalan, T. miRNAs as a novel clinical biomarker and therapeutic targets in polycystic ovary syndrome (PCOS): A review. Life Sci. 2020, 259, 118174. [Google Scholar] [CrossRef]
- Dabaja, M.Z.; Dos Santos, A.A.; Christofolini, D.M.; Barbosa, C.P.; de Oliveira, D.N.; de Oliveira, A.N.; Melo, C.F.O.R.; Guerreiro, T.M.; Catharino, R.R. Comparative metabolomic profiling of women undergoing in vitro fertilization procedures reveals potential infertility-related biomarkers in follicular fluid. Sci. Rep. 2022, 12, 20531. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Wei, Y.; Zhu, P.; Yin, T.; Wan, Q. Metabonomic analysis of follicular fluid in patients with diminished ovarian reserve. Front. Endocrinol. 2023, 14, 1132621. [Google Scholar] [CrossRef] [PubMed]
- Luti, S.; Fiaschi, T.; Magherini, F.; Modesti, P.A.; Piomboni, P.; Governini, L.; Luddi, A.; Amoresano, A.; Illiano, A.; Pinto, G.; et al. Relationship between the metabolic and lipid profile in follicular fluid of women undergoing in vitro fertilization. Mol. Reprod. Dev. 2020, 87, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhao, Y.; Li, T.; Li, M.; Li, J.; Li, R.; Liu, P.; Yu, Y.; Qiao, J. Metabolism alteration in follicular niche: The nexus among intermediary metabolism, mitochondrial function, and classic polycystic ovary syndrome. Free Radic. Biol. Med. 2015, 86, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.L.; Townsend, P.M.; Oehninger, S.; Castora, F.J. Endometriosis may be associated with mitochondrial dysfunction in cumulus cells from subjects undergoing in vitro fertilization-intracytoplasmic sperm injection, as reflected by decreased adenosine triphosphate production. Fertil. Steril. 2015, 103, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Prieto, L.; Quesada, J.F.; Cambero, O.; Pacheco, A.; Pellicer, A.; Codoceo, R.; Garcia-Velasco, J.A. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertil. Steril. 2012, 98, 126–130. [Google Scholar] [CrossRef]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- Tenhunen, R.; Marver, H.S.; Schmid, R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. USA 1968, 61, 748–755. [Google Scholar] [CrossRef]
- Shibahara, S.; Kitamuro, T.; Takahashi, K. Heme degradation and human disease: Diversity is the soul of life. Antioxid. Redox Signal. 2002, 4, 593–602. [Google Scholar] [CrossRef]
- Sferrazzo, G.; Di Rosa, M.; Barone, E.; Li Volti, G.; Musso, N.; Tibullo, D.; Barbagallo, I. Heme Oxygenase-1 in Central Nervous System Malignancies. J. Clin. Med. 2020, 9, 1562. [Google Scholar] [CrossRef]
- Bianco, A.; Tiribelli, C.; Bellarosa, C. Translational Approach to the Protective Effect of Bilirubin in Diabetic Kidney Disease. Biomedicines 2022, 10, 696. [Google Scholar] [CrossRef]
- Li, S.; Huang, H.; Zhang, Y.; Li, L.; Hua, Z. Bilirubin Induces A1-Like Reactivity of Astrocyte. Neurochem. Res. 2023, 48, 804–815. [Google Scholar] [CrossRef]
- Rauti, R.; Qaisiya, M.; Tiribelli, C.; Ballerini, L.; Bellarosa, C. Bilirubin disrupts calcium homeostasis in neonatal hippocampal neurons: A new pathway of neurotoxicity. Arch. Toxicol. 2020, 94, 845–855. [Google Scholar] [CrossRef]
- Costa Silva, R.C.M.; Correa, L.H.T. Heme Oxygenase 1 in Vertebrates: Friend and Foe. Cell Biochem. Biophys. 2022, 80, 97–113. [Google Scholar] [CrossRef]
- Li Volti, G.; Tibullo, D.; Vanella, L.; Giallongo, C.; Di Raimondo, F.; Forte, S.; Di Rosa, M.; Signorelli, S.S.; Barbagallo, I. The Heme Oxygenase System in Hematological Malignancies. Antioxid. Redox Signal. 2017, 27, 363–377. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Lei, H.; Cai, Y.; Shen, J.; Zhu, P.; He, Q.; Zhao, M. The Nrf-2/HO-1 Signaling Axis: A Ray of Hope in Cardiovascular Diseases. Cardiol. Res. Pract. 2020, 2020, 5695723. [Google Scholar] [CrossRef]
- Menon, R.; Peltier, M.R. Novel Insights into the Regulatory Role of Nuclear Factor (Erythroid-Derived 2)-Like 2 in Oxidative Stress and Inflammation of Human Fetal Membranes. Int. J. Mol. Sci. 2020, 21, 6139. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.J.; Hwangbo, H.; Ji, S.Y.; Kim, D.H.; Kim, M.Y.; Bang, E.; Hong, S.H.; Kim, S.O.; Jeong, S.J.; et al. Phloroglucinol Inhibits Oxidative-Stress-Induced Cytotoxicity in C2C12 Murine Myoblasts through Nrf-2-Mediated Activation of HO-1. Int. J. Mol. Sci. 2023, 24, 4637. [Google Scholar] [CrossRef]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.D.; Tosaki, A. Heme Degradation in Pathophysiology of and Countermeasures to Inflammation-Associated Disease. Int. J. Mol. Sci. 2020, 21, 9698. [Google Scholar] [CrossRef] [PubMed]
- Rostami, S.; Alyasin, A.; Saedi, M.; Nekoonam, S.; Khodarahmian, M.; Moeini, A.; Amidi, F. Astaxanthin ameliorates inflammation, oxidative stress, and reproductive outcomes in endometriosis patients undergoing assisted reproduction: A randomized, triple-blind placebo-controlled clinical trial. Front. Endocrinol. 2023, 14, 1144323. [Google Scholar] [CrossRef] [PubMed]
- Chełchowska, M.; Jurczewska, J.; Gajewska, J.; Mazur, J.; Szostak-Węgierek, D.; Rudnicka, E.; Ambroszkiewicz, J. Antioxidant Defense Expressed as Glutathione Status and Keap1-Nrf2 System Action in Relation to Anthropometric Parameters and Body Composition in Young Women with Polycystic Ovary Syndrome. Antioxidants 2023, 12, 730. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Nisenblat, V.; Lu, C.; Li, R.; Qiao, J.; Zhen, X.; Wang, S. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: A randomized controlled trial. Reprod. Biol. Endocrinol. 2018, 16, 29. [Google Scholar] [CrossRef]
- Nishimoto-Kakiuchi, A.; Sato, I.; Nakano, K.; Ohmori, H.; Kayukawa, Y.; Tanimura, H.; Yamamoto, S.; Sakamoto, Y.; Nakamura, G.; Maeda, A.; et al. A long-acting anti-IL-8 antibody improves inflammation and fibrosis in endometriosis. Sci. Transl. Med. 2023, 15, eabq5858. [Google Scholar] [CrossRef]
- Xie, Q.; Hong, W.; Li, Y.; Ling, S.; Zhou, Z.; Dai, Y.; Wu, W.; Weng, R.; Zhong, Z.; Tan, J.; et al. Chitosan oligosaccharide improves ovarian granulosa cells inflammation and oxidative stress in patients with polycystic ovary syndrome. Front. Immunol. 2023, 14, 1086232. [Google Scholar] [CrossRef]
- Collodel, G.; Gambera, L.; Stendardi, A.; Nerucci, F.; Signorini, C.; Pisani, C.; Marcheselli, M.; Vellucci, F.L.; Pizzasegale, S.E.; Micheli, L.; et al. Follicular Fluid Components in Reduced Ovarian Reserve, Endometriosis, and Idiopathic Infertility. Int. J. Mol. Sci. 2023, 24, 2589. [Google Scholar] [CrossRef]
- Lazzarino, G.; Pallisco, R.; Bilotta, G.; Listorti, I.; Mangione, R.; Saab, M.W.; Caruso, G.; Amorini, A.M.; Brundo, M.V.; Lazzarino, G.; et al. Altered Follicular Fluid Metabolic Pattern Correlates with Female Infertility and Outcome Measures of In Vitro Fertilization. Int. J. Mol. Sci. 2021, 22, 8735. [Google Scholar] [CrossRef]
- Bayer, S.R.; Armant, D.R.; Dlugi, A.M.; Seibel, M.M. Spectrophotometric absorbance of follicular fluid: A predictor of oocyte fertilizing capability. Fertil. Steril. 1988, 49, 442–446. [Google Scholar] [CrossRef]
- Bayer, S.R.; Zeind, A.J.; Turksoy, R.N.; Emmi, A.M.; Reindollar, R.H. Further study and characterization of the yellow pigments in follicular fluid that are related to oocyte quality. Fertil. Steril. 1992, 58, 964–969. [Google Scholar] [CrossRef]
- Hood, R.B.; Liang, D.; Tan, Y.; Ford, J.; Souter, I.; Jones, D.P.; Hauser, R.; Gaskins, A.J. Characterizing the follicular fluid metabolome: Quantifying the correlation across follicles and differences with the serum metabolome. Fertil. Steril. 2022, 118, 970–979. [Google Scholar] [CrossRef]
- Němeček, D.; Dvořáková, M.; Sedmíková, M. Heme oxygenase/carbon monoxide in the female reproductive system: An overlooked signalling pathway. Int. J. Biochem. Mol. Biol. 2017, 8, 1–12. [Google Scholar]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/3163 (accessed on 3 June 2023).
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/3162 (accessed on 3 June 2023).
- Raffaele, M.; Li Volti, G.; Barbagallo, I.A.; Vanella, L. Therapeutic Efficacy of Stem Cells Transplantation in Diabetes: Role of Heme Oxygenase. Front. Cell Dev. Biol. 2016, 4, 80. [Google Scholar] [CrossRef]
- Camiolo, G.; Tibullo, D.; Giallongo, C.; Romano, A.; Parrinello, N.L.; Musumeci, G.; Di Rosa, M.; Vicario, N.; Brundo, M.V.; Amenta, F.; et al. α-Lipoic Acid Reduces Iron-induced Toxicity and Oxidative Stress in a Model of Iron Overload. Int. J. Mol. Sci. 2019, 20, 609. [Google Scholar] [CrossRef]
- Bergandi, L.; Basso, G.; Evangelista, F.; Canosa, S.; Dalmasso, P.; Aldieri, E.; Revelli, A.; Benedetto, C.; Ghigo, D. Inducible nitric oxide synthase and heme oxygenase 1 are expressed in human cumulus cells and may be used as biomarkers of oocyte competence. Reprod. Sci. 2014, 21, 1370–1377. [Google Scholar] [CrossRef]
- Canosa, S.; Bergandi, L.; Macrì, C.; Charrier, L.; Paschero, C.; Carosso, A.; Di Segni, N.; Silvagno, F.; Gennarelli, G.; Benedetto, C.; et al. Morphokinetic analysis of cleavage stage embryos and assessment of specific gene expression in cumulus cells independently predict human embryo development to expanded blastocyst: A preliminary study. J. Assist. Reprod. Genet. 2020, 37, 1409–1420. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef]
- Kanwugu, O.N.; Glukhareva, T.V. Activation of Nrf2 pathway as a protective mechanism against oxidative stress-induced diseases: Potential of astaxanthin. Arch. Biochem. Biophys. 2023, 741, 109601. [Google Scholar] [CrossRef]
- Li, D.; Yuan, X.; Dong, S.; Al-Dhamin, Z.; Du, J.; Fu, N.; Nan, Y. Heme oxygenase-1 prevents non-alcoholic steatohepatitis through modulating mitochondrial quality control. Acta Physiol. 2023, 237, e13918. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wen, Q.; Xu, X.; Yu, D.; Liu, Z.; Zhang, C.; Zhang, X.; Ma, J.; Zhao, H.; Song, L. Heme oxygenase-1 protects against PM2.5 induced endothelial dysfunction through inhibition of HIF1α. Environ. Toxicol. Pharmacol. 2023, 97, 104024. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Chisholm, K.M.; Zhao, H.; Kalish, F.; Yang, Y.; Wong, R.J.; Stevenson, D.K. Heme oxygenase-1 confers protection and alters T-cell populations in a mouse model of neonatal intestinal inflammation. Pediatr. Res. 2015, 77, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Castilho, Á.; Aveleira, C.A.; Leal, E.C.; Simões, N.F.; Fernandes, C.R.; Meirinhos, R.I.; Baptista, F.I.; Ambrósio, A.F. Heme oxygenase-1 protects retinal endothelial cells against high glucose- and oxidative/nitrosative stress-induced toxicity. PLoS ONE 2012, 7, e42428. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Jetté, M.; Sidney, K.; Blümchen, G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin. Cardiol. 1990, 13, 555–565. [Google Scholar] [CrossRef]
- Lazzarino, G.; Longo, S.; Amorini, A.M.; Di Pietro, V.; D’Urso, S.; Lazzarino, G.; Belli, A.; Tavazzi, B. Single-step preparation of selected biological fluids for the high performance liquid chromatographic analysis of fat-soluble vitamins and antioxidants. J. Chromatogr. A 2017, 1527, 43–52. [Google Scholar] [CrossRef]
- Lazzarino, G.; Listorti, I.; Bilotta, G.; Capozzolo, T.; Amorini, A.M.; Longo, S.; Caruso, G.; Lazzarino, G.; Tavazzi, B.; Bilotta, P. Water- and Fat-Soluble Antioxidants in Human Seminal Plasma and Serum of Fertile Males. Antioxidants 2019, 8, 96. [Google Scholar] [CrossRef]
- Lazzarino, G.; Listorti, I.; Muzii, L.; Amorini, A.M.; Longo, S.; Di Stasio, E.; Caruso, G.; D’Urso, S.; Puglia, I.; Pisani, G.; et al. Low-molecular weight compounds in human seminal plasma as potential biomarkers of male infertility. Hum. Reprod. 2018, 33, 1817–1828. [Google Scholar] [CrossRef]
- Lazzarino, G.; Mangione, R.; Saab, M.W.; Tavazzi, B.; Pittalà, A.; Signoretti, S.; Di Pietro, V.; Lazzarino, G.; Amorini, A.M. Traumatic Brain Injury Alters Cerebral Concentrations and Redox States of Coenzymes Q9 and Q10 in the Rat. Antioxidants 2023, 12, 985. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangione, R.; Pallisco, R.; Bilotta, G.; Marroni, F.; Di Pietro, V.; Capoccia, E.; Lazzarino, G.; Tavazzi, B.; Lazzarino, G.; Bilotta, P.; et al. Bilirubin Concentration in Follicular Fluid Is Increased in Infertile Females, Correlates with Decreased Antioxidant Levels and Increased Nitric Oxide Metabolites, and Negatively Affects Outcome Measures of In Vitro Fertilization. Int. J. Mol. Sci. 2023, 24, 10707. https://doi.org/10.3390/ijms241310707
Mangione R, Pallisco R, Bilotta G, Marroni F, Di Pietro V, Capoccia E, Lazzarino G, Tavazzi B, Lazzarino G, Bilotta P, et al. Bilirubin Concentration in Follicular Fluid Is Increased in Infertile Females, Correlates with Decreased Antioxidant Levels and Increased Nitric Oxide Metabolites, and Negatively Affects Outcome Measures of In Vitro Fertilization. International Journal of Molecular Sciences. 2023; 24(13):10707. https://doi.org/10.3390/ijms241310707
Chicago/Turabian StyleMangione, Renata, Romina Pallisco, Gabriele Bilotta, Francesca Marroni, Valentina Di Pietro, Elena Capoccia, Giuseppe Lazzarino, Barbara Tavazzi, Giacomo Lazzarino, Pasquale Bilotta, and et al. 2023. "Bilirubin Concentration in Follicular Fluid Is Increased in Infertile Females, Correlates with Decreased Antioxidant Levels and Increased Nitric Oxide Metabolites, and Negatively Affects Outcome Measures of In Vitro Fertilization" International Journal of Molecular Sciences 24, no. 13: 10707. https://doi.org/10.3390/ijms241310707
APA StyleMangione, R., Pallisco, R., Bilotta, G., Marroni, F., Di Pietro, V., Capoccia, E., Lazzarino, G., Tavazzi, B., Lazzarino, G., Bilotta, P., & Amorini, A. M. (2023). Bilirubin Concentration in Follicular Fluid Is Increased in Infertile Females, Correlates with Decreased Antioxidant Levels and Increased Nitric Oxide Metabolites, and Negatively Affects Outcome Measures of In Vitro Fertilization. International Journal of Molecular Sciences, 24(13), 10707. https://doi.org/10.3390/ijms241310707









