Resistance to Chemotherapeutic 5-Fluorouracil Conferred by Modulation of Heterochromatic Integrity through Ino80 Function in Fission Yeast
Abstract
1. Introduction
2. Results
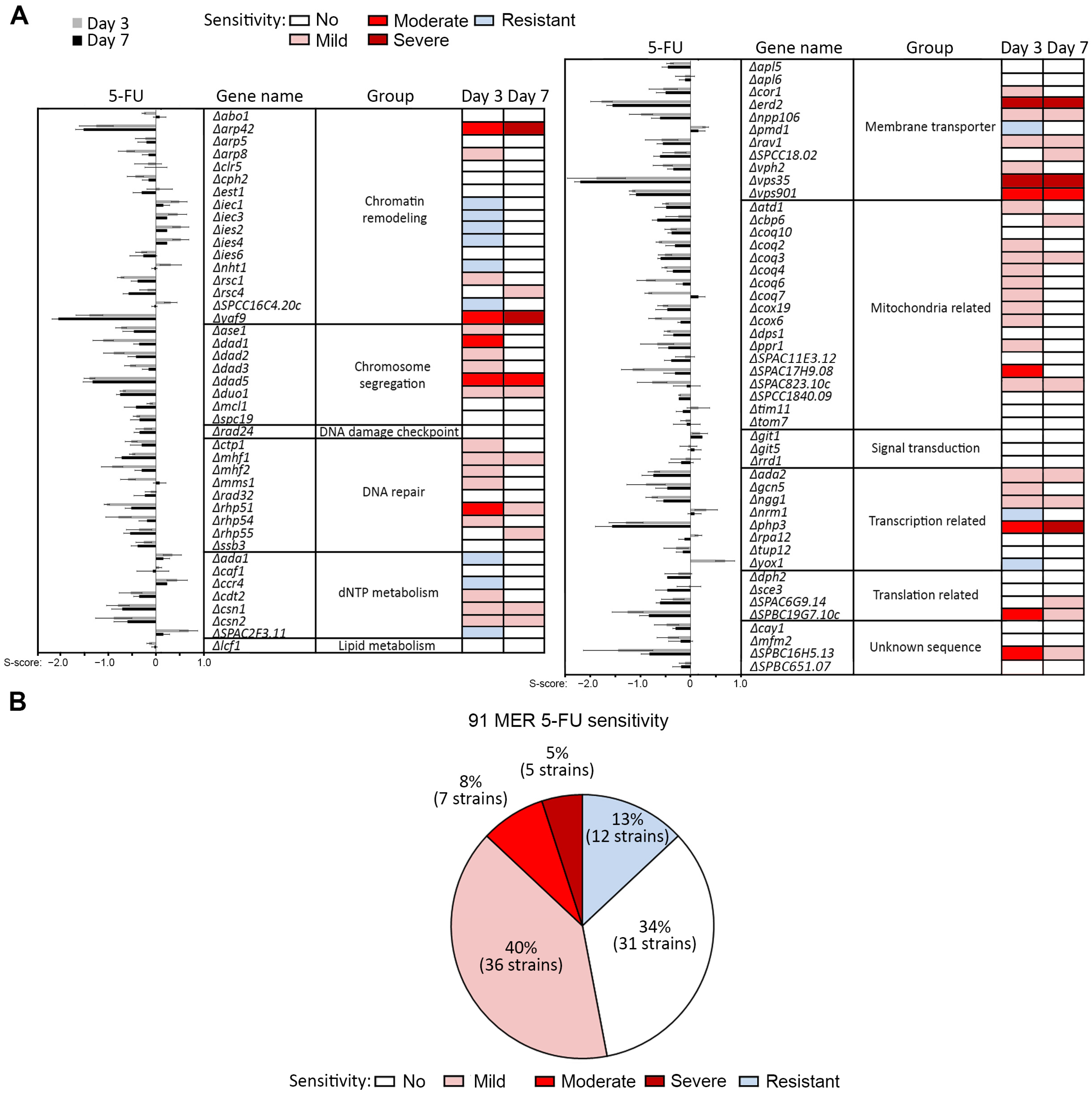
2.1. Cell Growth Assay of MER Gene Mutants to Identify Genes Involved in 5-FU Sensitivity and Resistance
2.2. Mutants Involved in Centromere Stability Are Sensitive to 5-FU
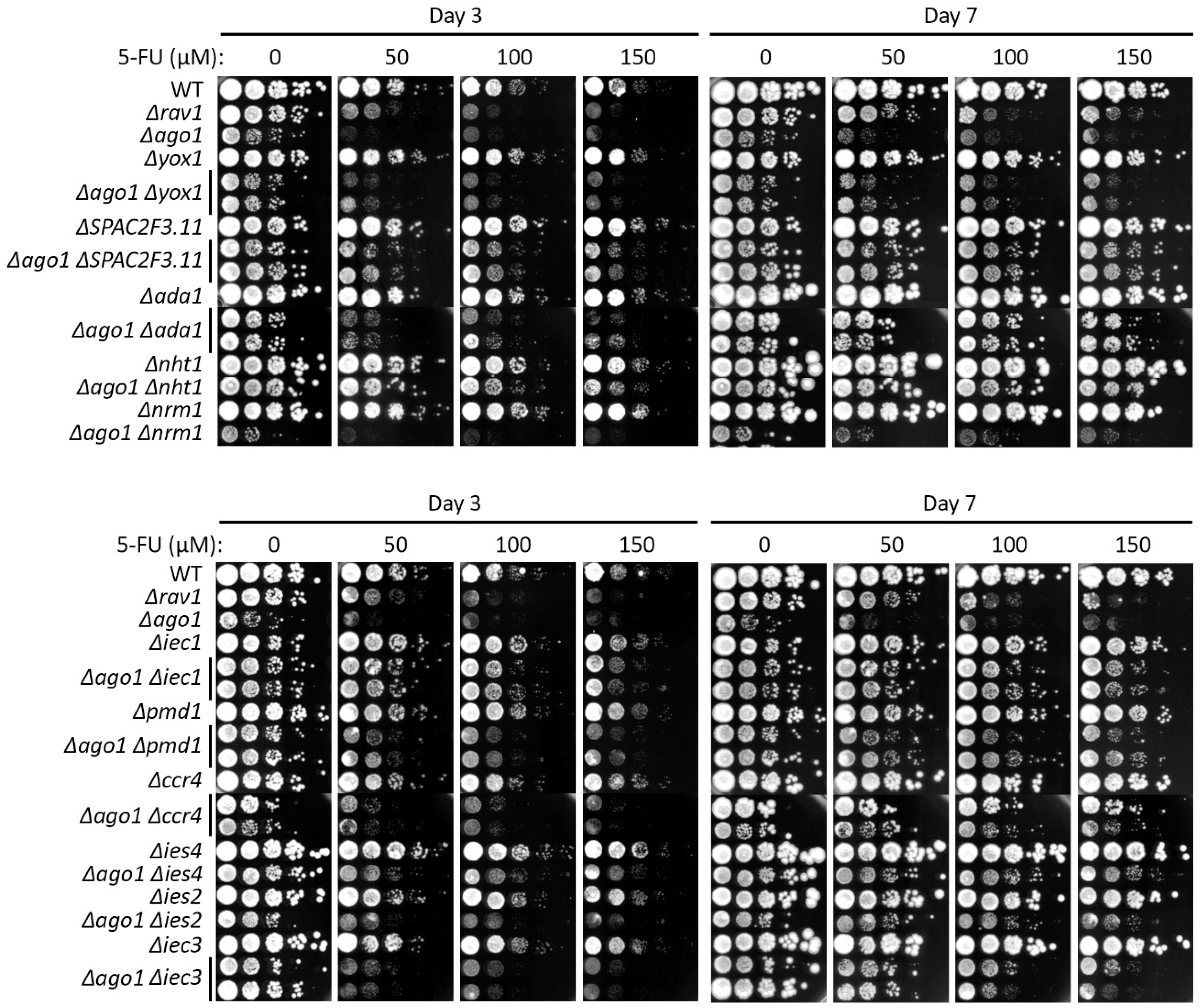
2.3. Mutants of Certain Ino80 Gene Family Members Partially Suppress 5-FU Sensitivity in Heterochromatin Mutants
2.4. 5-FU-Resistant Mutations Also Suppressed TBZ Sensitivity of Δago1
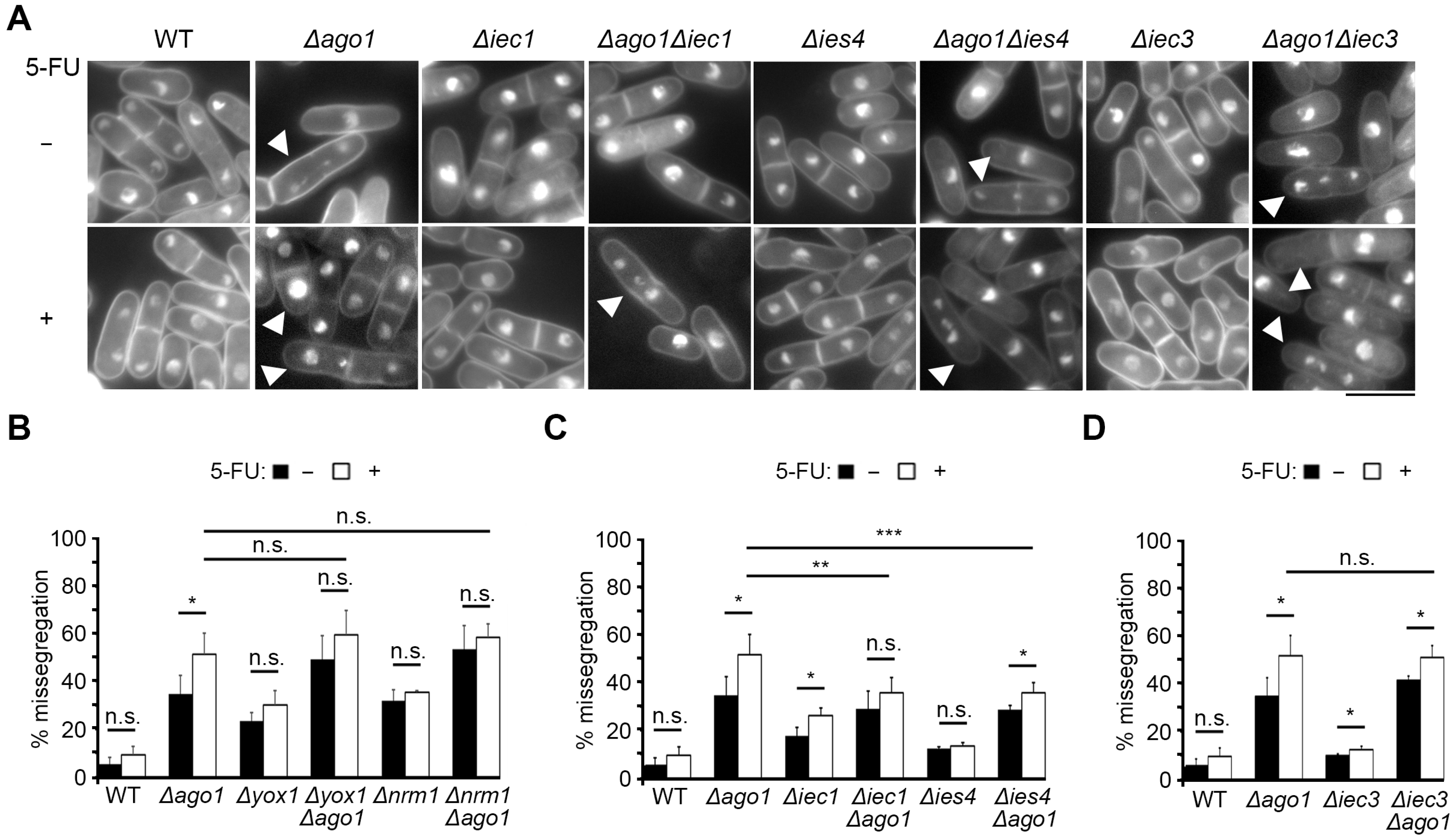
2.5. Loss of Ino80 Function Promotes Chromosome-Segregation Fidelity of Δago1
2.6. Ino80 Disruption Restores Heterochromatic Silencing Defect of Δago1 at Pericentromere
3. Discussion
3.1. Susceptibility to 5-FU Results from Disruption to dNTP Metabolism, Mitochondrial Functions and Membrane Transport Genes
3.2. The Roles of Chromatin Organization and Chromosomal Segregation in 5-FU Sensitivity
3.3. Loss of Ino80 Function Conferred 5-FU Resistance by Counteracting Loss of Heterochromatin Integrity
4. Materials and Methods
4.1. Fission Yeast Manipulation
4.2. Serial Dilution Spotting Cell Growth Assay
4.3. Fluorescence Microscopy
4.4. Statistical Analysis
4.5. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| MER | Multi-environmental factors responsiveness |
| H3K9 | Histone H3 lysine 9 |
| H3K9me | Methylated histone H3 lysine 9 |
| dNTP | Deoxyribonucleotide triphosphate |
| TBZ | Thiabendazole |
| WT | Wild-type |
| otr | Outer centromere |
| FUTP | Fluorouridine triphosphate |
| FdUMP | Fluorodeoxyuridine monophosphate |
| FdUTP | Fluorodeoxyuridine triphosphate |
| dUTP | Deoxyuridine triphosphate |
| h | hour |
| CoQ10 | Coenzyme Q10 |
| RNAi | RNA interference |
| RT-PCR | Reverse transcription polymerase chain reaction |
| RT-qPCR | Reverse transcription quantitative polymerase chain reaction |
| DAPI | 4′,6-diamidino-2-phenylindole |
References
- Pai, C.; Kearsey, S.E. A Critical Balance: dNTPs and the Maintenance of Genome Stability. Genes 2017, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, A.; DePamphilis, M.L. Links between DNA Replication, Stem Cells and Cancer. Genes 2017, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Harwood, F.G.; Frazier, M.W.; Krajewski, S.; Reed, J.C.; Houghton, J.A. Acute and delayed apoptosis induced by thymidine deprivation correlates with expression of p53 and p53-regulated genes in colon carcinoma cells. Oncogene 1996, 12, 2057–2067. [Google Scholar]
- Ahmad, S.I.; Kirk, S.H.; Eisenstark, A. Thymine metabolism and thymineless death in prokaryotes and eukaryotes. Ann. Rev. Micro. 1998, 52, 591–625. [Google Scholar] [CrossRef]
- Heidelberger, C.; Chaudhuri, N.; Danneberg, P.; Mooren, D.; Griesbach, L.; Duschinsky, R.; Schnitzer, R.J.; Pleven, E.; Schiener, J. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature 1957, 179, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef]
- Miura, K.; Kinouchi, M.; Ishida, K.; Fujibuchi, W.; Naitoh, T.; Ogawa, H.; Ando, T.; Yazaki, N.; Watanabe, K.; Haneda, S.; et al. 5-FU Metabolism in Cancer and Orally-Administrable 5-FU Drugs. Cancers 2010, 2, 1717–1730. [Google Scholar] [CrossRef]
- Peters, G.J.; van der Wilt, C.L.; van Groeningen, C.J.; Smid, K.; Meijer, S.; Pinedo, H.M. Thymidylate synthase inhibition after administration of fluorouracil with or without leucovorin in colon cancer patients: Implications for treatment with fluorouracil. J. Clin. Oncol. 1994, 12, 2035–2042. [Google Scholar] [CrossRef]
- Li, M.H.; Ito, D.; Sanada, M.; Odani, T.; Hatori, M.; Iwase, M.; Nagumo, M. Effect of 5-fluorouracil on G1 phase cell cycle regulation in oral cancer cell lines. Oral Oncol. 2004, 40, 63–70. [Google Scholar] [CrossRef]
- Liao, Z.Y.; Sordet, O.; Zhang, H.L.; Kohlhagen, G.; Antony, S.; Gmeiner, W.H.; Pommier, Y. A Novel Polypyrimidine Antitumor Agent FdUMP[10] Induces Thymineless Death with Topoisomerase I-DNA Complexes. Cancer Res. 2005, 65, 4844–4851. [Google Scholar] [CrossRef]
- He, X.; Huang, C.; Xie, B. Autophagy inhibition enhanced 5-FU-induced cell death in human gastric carcinoma BGC-823 cells. Mol. Med. Rep. 2018, 17, 6768–6776. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, J.; Scott Butler, J. Evidence for distinct DNA- and RNA-based mechanisms of 5-fluorouracil cytotoxicity in Saccharomyces cerevisiae. Yeast 2007, 24, 861–870. [Google Scholar] [CrossRef]
- Silverstein, R.A.; González de Valdivia, E.; Visa, N. The incorporation of 5-fluorouracil into RNA affects the ribonucleolytic activity of the exosome subunit Rrp6. Mol. Cancer Res. 2011, 9, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Becker, E.; Stuparevic, I.; Wery, M.; Szachnowski, U.; Morillon, A.; Primig, M. The anti-cancer drug 5-fluorouracil affects cell cycle regulators and potential regulatory long non-coding RNAs in yeast. RNA Biol. 2019, 16, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Mojardín, L.; Botet, J.; Moreno, S.; Salas, M. Chromosome segregation and organization are targets of 5′-Fluorouracil in eukaryotic cells. Cell Cycle 2015, 14, 206–218. [Google Scholar] [CrossRef]
- Cam, H.P.; Sugiyama, T.; Chen, E.S.; Chen, X.; FitzGerald, P.C.; Grewal, S.I. Comprehensive analysis of heterochromatin- and RNAi-mediated epigenetic control of the fission yeast genome. Nat. Genet. 2005, 37, 809–819. [Google Scholar] [CrossRef]
- Cam, H.P.; Chen, E.S.; Grewal, S.I. Transcriptional scaffolds for heterochromatin assembly. Cell 2009, 136, 610–614. [Google Scholar] [CrossRef]
- Djupedal, I.; Portoso, M.; Spåhr, H.; Bonilla, C.; Gustafsson, C.M.; Allshire, R.C.; Ekwall, K. RNA Pol II subunit Rpb7 promotes centromeric transcription and RNAi-directed chromatin silencing. Genes Dev. 2005, 19, 2301–2306. [Google Scholar] [CrossRef]
- Kato, H.; Goto, D.B.; Martienssen, R.A.; Urano, T.; Furukawa, K.; Murakami, Y. RNA polymerase II is required for RNAi-dependent heterochromatin assembly. Science 2005, 309, 467–469. [Google Scholar] [CrossRef]
- Chen, E.S.; Zhang, K.; Nicolas, E.; Cam, H.P.; Zofall, M.; Grewal, S.I. Cell cycle control of centromeric repeat transcription and heterochromatin assembly. Nature 2008, 451, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Cam, H.; Verdel, A.; Moazed, D.; Grewal, S.I. RNA-dependent RNA polymerase is an essential component of a self-enforcing loop coupling heterochromatin assembly to siRNA production. Proc. Natl. Acad. Sci. USA 2005, 102, 152–157. [Google Scholar] [CrossRef]
- Noma, K.; Sugiyama, T.; Cam, H.; Verdel, A.; Zofall, M.; Jia, S.; Moazed, D.; Grewal, S.I. RITS acts in cis to promote RNA interference-mediated transcriptional and post-transcriptional silencing. Nat. Genet. 2004, 36, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Verdel, A.; Jia, S.; Gerber, S.; Sugiyama, T.; Gygi, S.; Grewel, S.I.; Moazed, D. RNAi-mediated targeting of heterochromatin by the RITS complex. Science 2004, 303, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Colmenares, S.U.; Buker, S.M.; Buhler, M.; Dlakić, M.; Moazed, D. Coupling of double-stranded RNA synthesis and siRNA generation in fission yeast RNAi. Mol. Cell 2007, 27, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Buker, S.M.; Iida, T.; Bühler, M.; Villén, J.; Gygi, S.P.; Nakayama, J.; Moazed, D. Two different Argonaute complexes are required for siRNA generation and heterochromatin assembly in fission yeast. Nat. Struct. Mol. Biol. 2007, 14, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Holoch, D.; Moazed, D. RNA-mediated epigenetic regulation of gene expression. Nat. Rev. Genet. 2015, 16, 71–84. [Google Scholar] [CrossRef]
- Sugiyama, T.; Cam, H.P.; Sugiyama, R.; Noma, K.; Zofall, M.; Kobayashi, R.; Grewal, S.I. SHREC, an effector complex for heterochromatic transcriptional silencing. Cell 2007, 128, 491–504. [Google Scholar] [CrossRef]
- Grewal, S.I.; Jia, S. Heterochromatin revisited. Nat. Rev. Genet. 2007, 8, 35–46. [Google Scholar] [CrossRef]
- Zhang, K.; Mosch, K.; Fischle, W.; Grewal, S.I. Roles of the Clr4 methyltransferase complex in nucleation, spreading and maintenance of heterochromatin. Nat. Struct. Mol. Biol. 2008, 15, 381–388. [Google Scholar] [CrossRef]
- Tay, Z.; Eng, R.J.; Sajiki, K.; Lim, K.K.; Tang, M.Y.; Yanagida, M.; Chen, E.S. Cellular robustness conferred by genetic crosstalk underlies resistance against chemotherapeutic drug doxorubicin in fission yeast. PLoS ONE 2013, 8, e55041. [Google Scholar] [CrossRef] [PubMed]
- Tay, Z.; Koo, S.H.; Nguyen, T.T.; Tan, T.S.; Chen, M.L.; Chin, C.F.; Lim, K.K.; Ang, W.H.; Bay, B.H.; Lee, E.J.; et al. P-glycoprotein and vacuolar ATPase synergistically confer anthracycline resistance to fission yeast and human cells. Curr. Med. Chem. 2014, 21, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.Y.; Guo, H.; Nguyen, T.T.; Low, L.S.; Jackson, R.A.; Yamada, T.; Chen, E.S. Two fission yeast high mobility group box proteins in the maintenance of genomic integrity following doxorubicin insult. Gene 2015, 562, 70–75. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Chua, J.K.; Seah, K.S.; Koo, S.H.; Yee, J.Y.; Yang, E.G.; Lim, K.K.; Pang, S.Y.W.; Yuen, A.; Zhang, L.; et al. Predicting chemotherapeutic drug combinations through gene network profiling. Sci. Rep. 2016, 5, 18658. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Lim, Y.J.; Fan, M.H.; Jackson, R.A.; Lim, K.K.; Ang, W.H.; Ban, K.H.; Chen, E.S. Calcium modulation of doxorubicin cytotoxicity in yeast and human cells. Genes Cells 2016, 21, 226–240. [Google Scholar] [CrossRef]
- Liu, X.; McLeod, I.; Anderson, S.; Yates, J.R., 3rd; He, X. Molecular analysis of kinetochore architecture in fission yeast. EMBO J. 2005, 24, 2919–2930. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Saitoh, S.; Ogiyama, Y.; Soejima, S.; Takahashi, K. The fission yeast DASH complex is essential for satisfying the spindle assembly checkpoint induced by defects in the inner-kinetochore proteins. Genes Cells 2007, 12, 311–328. [Google Scholar] [CrossRef]
- Danenberg, P.V.; Malli, H.; Swenson, S. Thymidylate synthase inhibitors. Semin. Oncol. 1999, 26, 621–631. [Google Scholar]
- Sampath, D.; Rao, V.A.; Plunkett, W. Mechanisms of apoptosis induction by nucleoside analogs. Oncogene 2003, 22, 9063–9074. [Google Scholar] [CrossRef]
- Lee, S.Q.E.; Tan, T.S.; Kawamukai, M.; Chen, E.S. Cellular factories for coenzyme Q10 production. Microb. Cell Fact. 2017, 16, 39. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Jiang, G.; Chen, S.; Zhou, L.; Sakamoto, N.; Kuno, T.; Fang, Y.; Yao, F. Genome-wide screen reveals important roles for ESCRT proteins in drug/ion resistance of fission yeast. PLoS ONE 2018, 13, e0198516. [Google Scholar] [CrossRef] [PubMed]
- Mojardín, L.; Botet, J.; Quintales, L.; Moreno, S.; Salas, M. New Insights into the RNA-Based Mechanism of Action of the Anticancer Drug 5′-Fluorouracil in Eukaryotic Cells. PLoS ONE 2013, 8, e78172. [Google Scholar] [CrossRef]
- Han, S.; Lee, M.; Chang, H.; Nam, M.; Park, H.O.; Kwak, Y.S.; Ha, H.J.; Kim, D.; Hwang, S.O.; Hoe, K.L.; et al. Construction of the first compendium of chemical-genetic profiles in the fission yeast Schizosaccharomyces pombe and comparative compendium approach. Biochem. Biophys. Res. Commun. 2013, 436, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.M.; Bao, K.; Diedrich, J.; Chen, X.; Lu, C.; Yates, J.R.; Jia, S. The INO80 Complex Regulates Epigenetic Inheritance of Heterochromatin. Cell Rep. 2020, 33, 108561. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, M.R.; Verdel, A.; Colmenares, S.U.; Gerber, S.A.; Gygi, S.P.; Moazed, D. Two RNAi Complexes, RITS and RDRC, Physically Interact and Localize to Noncoding Centromeric RNAs. Cell 2004, 119, 789–802. [Google Scholar] [CrossRef]
- Nicolas, E.; Yamada, T.; Cam, H.P.; Fitzgerald, P.C.; Kobayashi, R.; Grewal, S.I. Distinct roles of HDAC complexes in promoter silencing, antisense suppression and DNA damage protection. Nat. Struct. Mol. Biol. 2007, 14, 372–380. [Google Scholar] [CrossRef]
- Hogan, C.J.; Aligianni, S.; Durand-Dubief, M.; Persson, J.; Will, W.R.; Webster, J.; Wheeler, L.; Mathews, C.K.; Elderkin, S.; Oxley, D.; et al. Fission yeast Iec1-ino80-mediated nucleosome eviction regulates nucleotide and phosphate metabolism. Mol. Cell. Biol. 2010, 30, 657–674. [Google Scholar] [CrossRef]
- Lim, K.K.; Teo, H.Y.; Tan, Y.Y.; Zeng, Y.B.; Lam, U.T.F.; Choolani, M.; Chen, E.S. Fission Yeast Methylenetetrahydrofolate Reductase Ensures Mitotic and Meiotic Chromosome Segregation Fidelity. Int. J. Mol. Sci. 2021, 22, 639. [Google Scholar] [CrossRef]
- Hiraoka, Y.; Toda, T.; Yanagida, M. The NDA3 gene of fission yeast encodes β-tubulin: A cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell 1984, 39, 349–358. [Google Scholar] [CrossRef]
- Kunert, F.; Metzner, F.J.; Jung, J.; Höpfler, M.; Woike, S.; Schall, K.; Kostrewa, D.; Moldt, M.; Chen, J.X.; Bantele, S.; et al. Structural mechanism of extranucleosomal DNA readout by the INO80 complex. Sci. Adv. 2022, 8, eadd3189. [Google Scholar] [CrossRef]
- Ekwall, K.; Cranston, G.; Allshire, R.C. Fission yeast mutants that alleviate transcriptional silencing in centromeric flanking repeats and disrupt chromosome segregation. Genetics 1999, 153, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Allshire, R.C.; Ekwall, K. Epigenetic regulation of chromatin states in Schizosaccharomyces pombe. Cold Spring Harb. Pespect. Biol. 2015, 7, a018770. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Chen, E.S. Regulation of centromeric heterochromatin in the cell cycle by phosphorylation of histone H3 tyrosine 41. Curr. Genet. 2019, 65, 829–836. [Google Scholar] [CrossRef]
- Ren, B.; Tan, H.L.; Nguyen, T.T.T.; Sayed, A.M.M.; Li, Y.; Mok, Y.K.; Yang, H.; Chen, E.S. Regulation of transcriptional silencing and chromodomain protein localization at centromeric heterochromatin by histone H3 tyrosine 41 phosphorylation in fission yeast. Nucleic Acids Res. 2018, 46, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Bondar, T.; Ponomarev, A.; Raychaudhuri, P. Ddb1 is required for the proteolysis of the Schizosaccharomyces pombe replication inhibitor Spd1 during S phase and after DNA damage. J. Biol. Chem. 2004, 279, 9937–9943. [Google Scholar] [CrossRef]
- Liu, C.; Powell, K.A.; Mundt, K.; Wu, L.; Carr, A.M.; Caspari, T. Cop9/signalosome subunits and Pcu4 regulate ribonucleotide reductase by both checkpoint-dependent and -independent mechanisms. Genes Dev. 2003, 17, 1130–1140. [Google Scholar] [CrossRef]
- Liu, C.; Poitelea, M.; Watson, A.; Yoshida, S.; Shimoda, C.; Holmberg, C.; Nielsen, O.; Carr, A.M. Transactivation of Schizosaccharomyces pombe cdt2+ stimulates a Pcu4–Ddb1–CSN ubiquitin ligase. EMBO J. 2005, 24, 3940–3951. [Google Scholar] [CrossRef]
- Zou, S.; Qin, B.; Yang, Z.; Wang, W.; Zhang, J.; Zhang, Y.; Meng, M.; Feng, J.; Xie, Y.; Fang, L.; et al. CSN6 Mediates Nucleotide Metabolism to Promote Tumor Development and Chemoresistance in Colorectal Cancer. Cancer Res. 2023, 83, 414–427. [Google Scholar] [CrossRef]
- Hu, L.; Yao, F.; Ma, Y.; Liu, Q.; Chen, S.; Hayafuji, T.; Kuno, T.; Fang, Y. Genetic evidence for involvement of membrane trafficking in the action of 5-fluorouracil. Fungal Genet. Biol. 2016, 93, 17–24. [Google Scholar] [CrossRef]
- Seaman, M.N. The retromer complex–endosomal protein recycling and beyond. J. Cell Sci. 2012, 125, 4693–4702. [Google Scholar] [CrossRef]
- Iwaki, T.; Hosomi, A.; Tokudomi, S.; Kusunoki, Y.; Fujita, Y.; Giga-Hama, Y.; Tanaka, N.; Takegawa, K. Vacuolar protein sorting receptor in Schizosaccharomyces pombe. Microbiology 2006, 152, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C.; Hardwick, K.G.; Dean, N.; Pelham, H.R. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell 1990, 61, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Townsley, F.M.; Frigerio, G.; Pelham, H.R. Retrieval of HDEL proteins is required for growth of yeast cells. J. Cell Biol. 1994, 127, 21–28. [Google Scholar] [CrossRef]
- Saudek, V. Cystinosin, MPDU1, SWEETs and KDELR belong to a well-defined protein family with putative function of cargo receptors involved in vesicle trafficking. PLoS ONE 2012, 7, e30876. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.H.; Gomez, T.S.; Osborne, D.G.; Phillips-Krawczak, C.A.; Zhang, J.S.; Billadeau, D.D. Nuclear FAM21 participates in NF-κB-dependent gene regulation in pancreatic cancer cells. J. Cell Sci. 2015, 128, 373–384. [Google Scholar] [CrossRef]
- Lee, G.H.; Kim, D.S.; Kim, H.T.; Lee, J.W.; Chung, C.H.; Ahn, T.; Lim, J.M.; Kim, I.K.; Chae, H.J.; Kim, H.R. Enhanced lysosomal activity is involved in Bax inhibitor-1-induced regulation of the endoplasmic reticulum (ER) stress response and cell death against ER stress: Involvement of vacuolar H+-ATPase (V-ATPase). J. Biol. Chem. 2011, 286, 24743–24753. [Google Scholar] [CrossRef] [PubMed]
- Guida, M.C.; Hermle, T.; Graham, L.A.; Hauser, V.; Ryan, M.; Stevens, T.H.; Simons, M. ATP6AP2 functions as a V-ATPase assembly factor in the endoplasmic reticulum. Mol. Biol. Cell 2018, 29, 2156–2164. [Google Scholar] [CrossRef]
- Dawson, K.; Toone, W.M.; Jones, N.; Wilkinson, C.R. Loss of regulators of vacuolar ATPase function and ceramide synthesis results in multidrug sensitivity in Schizosaccharomyces pombe. Eukaryot. Cell 2008, 7, 926–937. [Google Scholar] [CrossRef]
- Martínez-Zaguilán, R.; Raghunand, N.; Lynch, R.M.; Bellamy, W.; Martinez, G.M.; Rojas, B.; Smith, D.; Dalton, W.S.; Gillies, R.J. pH and drug resistance. I. Functional expression of plasmalemmal V-type H+-ATPase in drug-resistant human breast carcinoma cell lines. Biochem. Pharmacol. 1999, 57, 1037–1046. [Google Scholar] [CrossRef]
- Perez-Sayans, M.; Garcia-Garcia, A.; Scozzafava, A.; Supuran, C.T. Inhibition of V-ATPase and carbonic anhydrases as interference strategy with tumor acidification processes. Curr. Pharm. Des. 2012, 18, 1407–1413. [Google Scholar] [CrossRef]
- Fan, S.; Niu, Y.; Tan, N.; Wu, Z.; Wang, Y.; You, H.; Ke, R.; Song, J.; Shen, Q.; Wang, W.; et al. LASS2 enhances chemosensitivity of breast cancer by counteracting acidic tumor microenvironment through inhibiting activity of V-ATPase proton pump. Oncogene 2013, 32, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Lu, S.; Huang, L.; Wang, T.; Wan, Y.; Zhou, C.X.; Zhang, C.; Zhang, Z.; Li, X. The expression of V-ATPase is associated with drug resistance and pathology of non-small-cell lung cancer. Diagn. Pathol. 2013, 8, 145. [Google Scholar] [CrossRef]
- Monahan, B.J.; Villén, J.; Marguerat, S.; Bähler, J.; Gygi, S.P.; Winston, F. Fission yeast SWI/SNF and RSC complexes show compositional and functional differences from budding yeast. Nat. Struct. Mol. Biol. 2008, 15, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gundu, A.; Strahl, B.D. Recognition of acetylated histone by Yaf9 regulates metabolic cycling of transcription initiation and chromatin regulatory factors. Genes Dev. 2021, 35, 1678–1692. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, G.; Sim, S.; Leary, A.; Pascariu, M.; Vogel, J.; D’Amours, D. Interphase Microtubules Safeguard Mitotic Progression by Suppressing an Aurora B-Dependent Arrest Induced by DNA Replication Stress. Cell Rep. 2019, 26, 2875–2889.e3. [Google Scholar] [CrossRef] [PubMed]
- Bloom, K.S. Centromeric heterochromatin: The primordial segregation machine. Annu. Rev. Genet. 2014, 48, 457–484. [Google Scholar] [CrossRef]
- Yen, K.; Vinayachandran, V.; Pugh, B.F. SWR-C and INO80 chromatin remodelers recognize nucleosome-free regions near +1 nucleosomes. Cell 2013, 154, 1246–1256. [Google Scholar] [CrossRef]
- Hauer, M.H.; Seeber, A.; Singh, V.; Thierry, R.; Sack, R.; Amitai, A.; Kryzhanovska, M.; Eglinger, J.; Holcman, D.; Owen-Hughes, T.; et al. Histone degradation in response to DNA damage enhances chromatin dynamics and recombination rates. Nat. Struct. Mol. Biol. 2017, 24, 99–107. [Google Scholar] [CrossRef]
- Singh, P.P.; Shukla, M.; White, S.A.; Lafos, M.; Tong, P.; Auchynnikava, T.; Spanos, C.; Rappsilber, J.; Pidoux, A.L.; Allshire, R.C. Hap2-Ino80-facilitated transcription promotes de novo establishment of CENP-A chromatin. Genes Dev. 2020, 34, 226–238. [Google Scholar] [CrossRef]
- Forsburg, S.L.; Rhind, N. Basic methods for fission yeast. Yeast 2006, 23, 173–183. [Google Scholar] [CrossRef]
- Moreno, S.; Klar, A.; Nurse, P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991, 194, 795–823. [Google Scholar] [CrossRef]
- Lim, K.K.; Nguyen, T.T.T.; Li, A.Y.; Yeo, Y.P.; Chen, E.S. Histone H3 lysine 36 methyltransferase mobilizes NER factors to regulate tolerance against alkylation damage in fission yeast. Nucleic Acids Res. 2018, 46, 5061–5074. [Google Scholar] [CrossRef]
- Tan, H.L.; Lim, K.K.; Yang, Q.; Fan, J.S.; Sayed, A.M.M.; Low, L.S.; Ren, B.; Lim, T.K.; Lin, Q.; Mok, Y.K.; et al. Prolyl isomerization of the CENP-A N-terminus regulates centromeric integrity in fission yeast. Nucleic Acids Res. 2018, 46, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.K.; Ong, T.Y.; Tan, Y.R.; Yang, E.G.; Ren, B.; Seah, K.S.; Yang, Z.; Tan, T.S.; Dymock, B.W.; Chen, E.S. Mutation of histone H3 serine 86 disrupts GATA factor Ams2 expression and precise chromosome segregation in fission yeast. Sci. Rep. 2015, 5, 14064. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Chen, E.S. GRANT Motif Regulates CENP-A Incorporation and Restricts RNA Polymerase II Accessibility at Centromere. Genes 2022, 13, 1697. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Lim, J.S.; Tang, R.M.; Zhang, L.; Chen, E.S. Fitness profiling links topoisomerase II regulation of centromeric integrity to doxorubicin resistance in fission yeast. Sci. Rep. 2015, 5, 8400. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, K.K.; Koh, N.Z.H.; Zeng, Y.B.; Chuan, J.K.; Raechell, R.; Chen, E.S. Resistance to Chemotherapeutic 5-Fluorouracil Conferred by Modulation of Heterochromatic Integrity through Ino80 Function in Fission Yeast. Int. J. Mol. Sci. 2023, 24, 10687. https://doi.org/10.3390/ijms241310687
Lim KK, Koh NZH, Zeng YB, Chuan JK, Raechell R, Chen ES. Resistance to Chemotherapeutic 5-Fluorouracil Conferred by Modulation of Heterochromatic Integrity through Ino80 Function in Fission Yeast. International Journal of Molecular Sciences. 2023; 24(13):10687. https://doi.org/10.3390/ijms241310687
Chicago/Turabian StyleLim, Kim Kiat, Nathaniel Zhi Hao Koh, Yi Bing Zeng, Jun Kai Chuan, Raechell Raechell, and Ee Sin Chen. 2023. "Resistance to Chemotherapeutic 5-Fluorouracil Conferred by Modulation of Heterochromatic Integrity through Ino80 Function in Fission Yeast" International Journal of Molecular Sciences 24, no. 13: 10687. https://doi.org/10.3390/ijms241310687
APA StyleLim, K. K., Koh, N. Z. H., Zeng, Y. B., Chuan, J. K., Raechell, R., & Chen, E. S. (2023). Resistance to Chemotherapeutic 5-Fluorouracil Conferred by Modulation of Heterochromatic Integrity through Ino80 Function in Fission Yeast. International Journal of Molecular Sciences, 24(13), 10687. https://doi.org/10.3390/ijms241310687





