The Nature of the Enthalpy–Entropy Compensation and “Exotic” Arrhenius Parameters in the Denaturation Kinetics of Proteins
Abstract
1. Introduction
2. Results
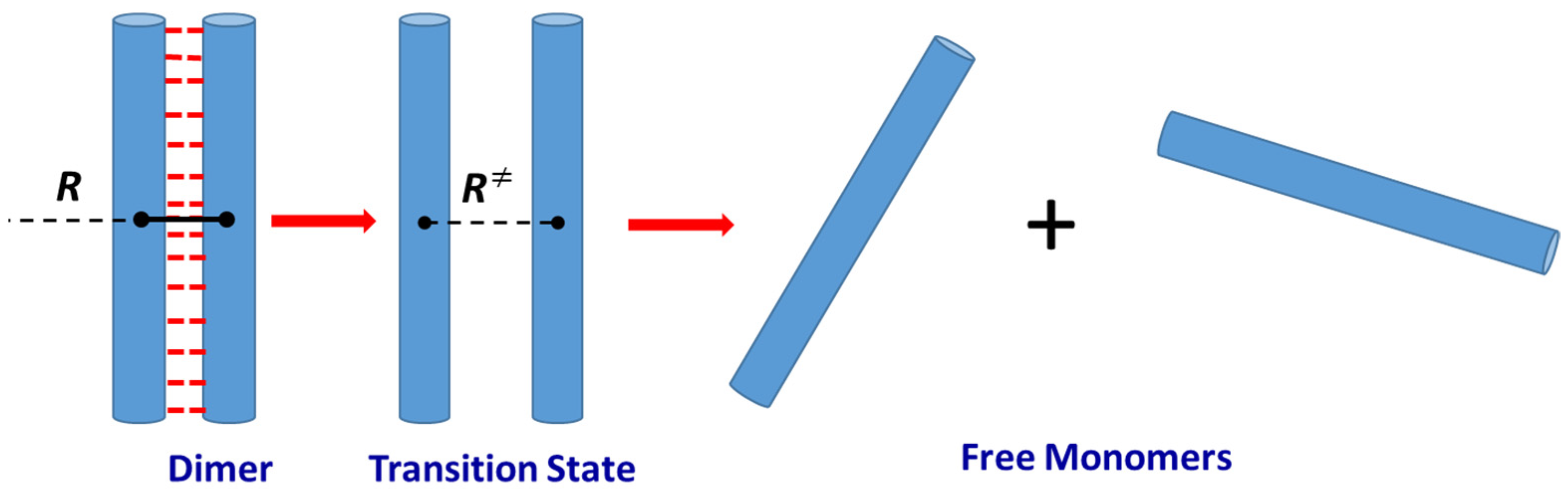
2.1. “Completely Loose” Transitionj State Theory Model
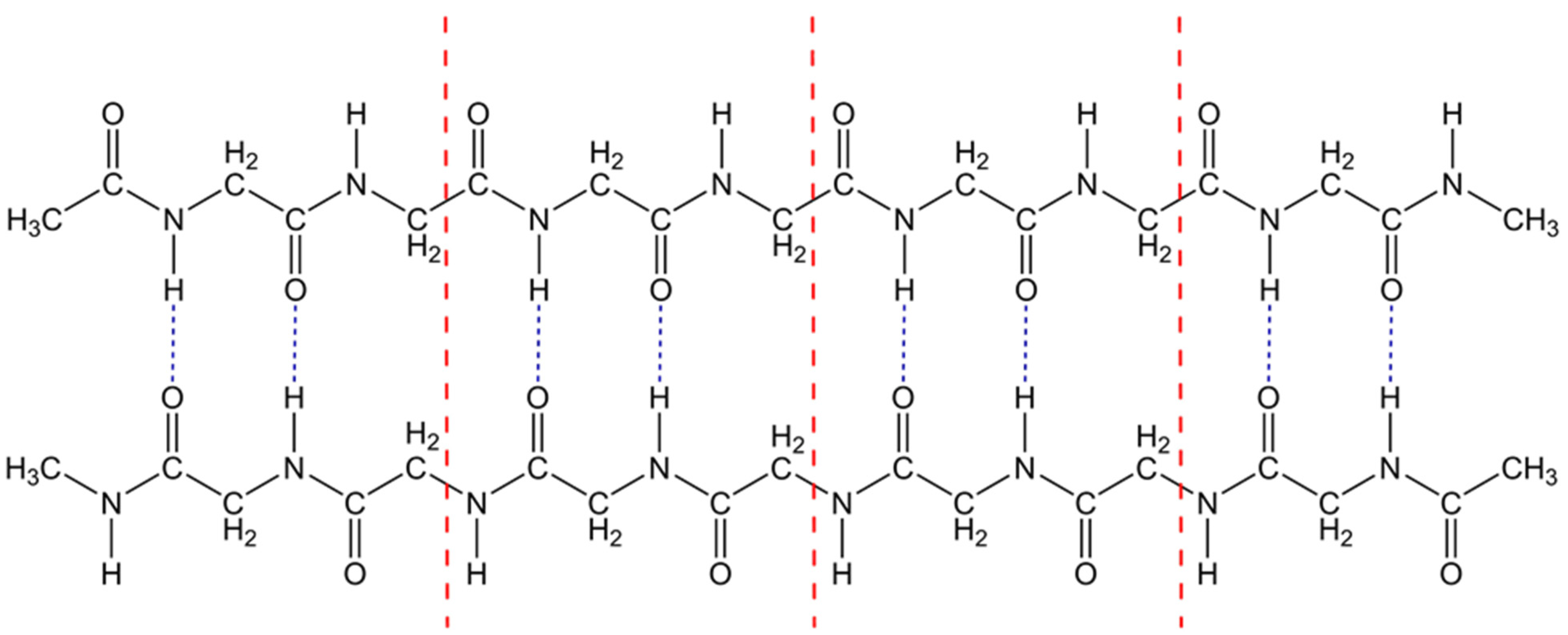
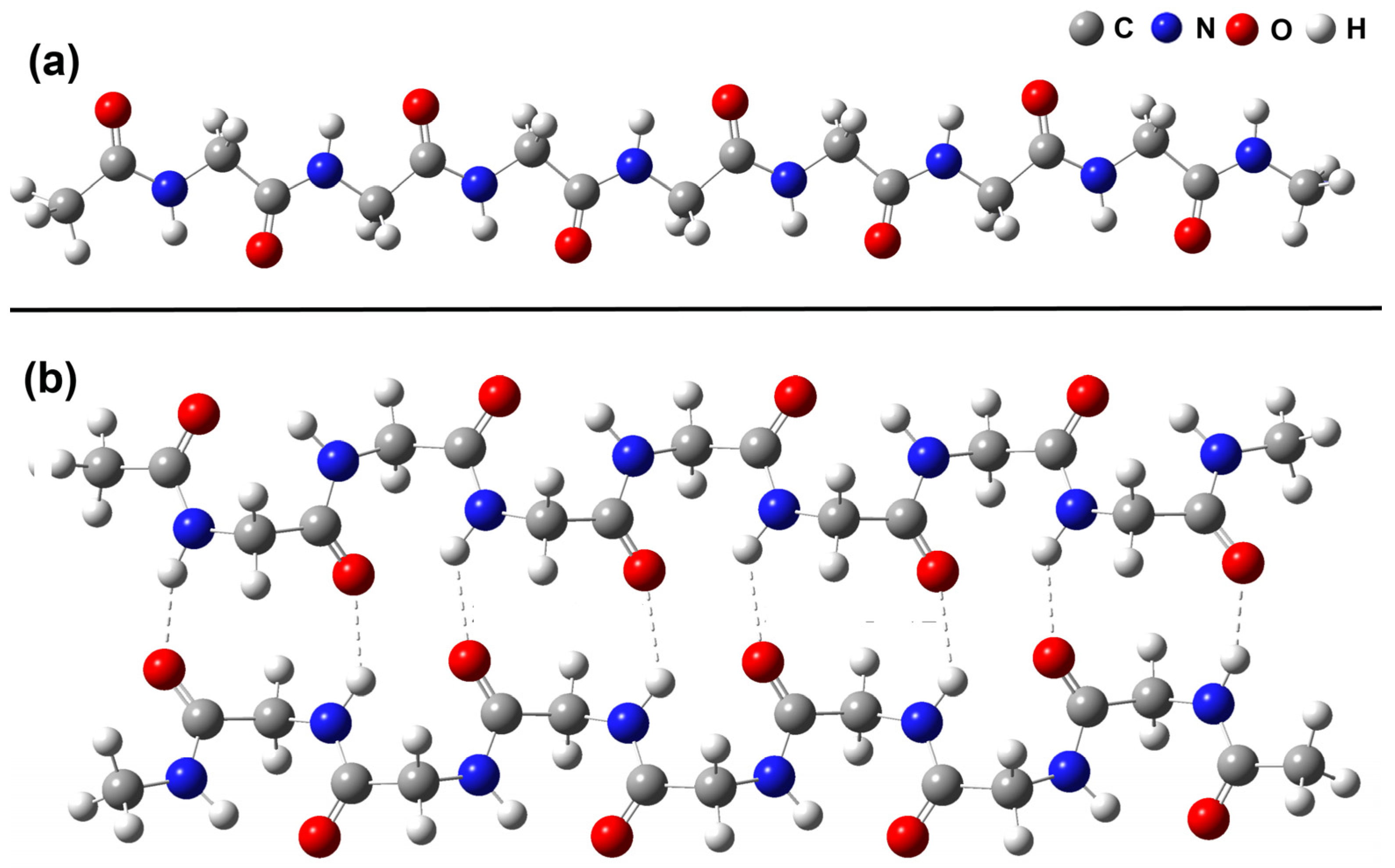
2.2. Polyglycine Monomer and Dimer Structure
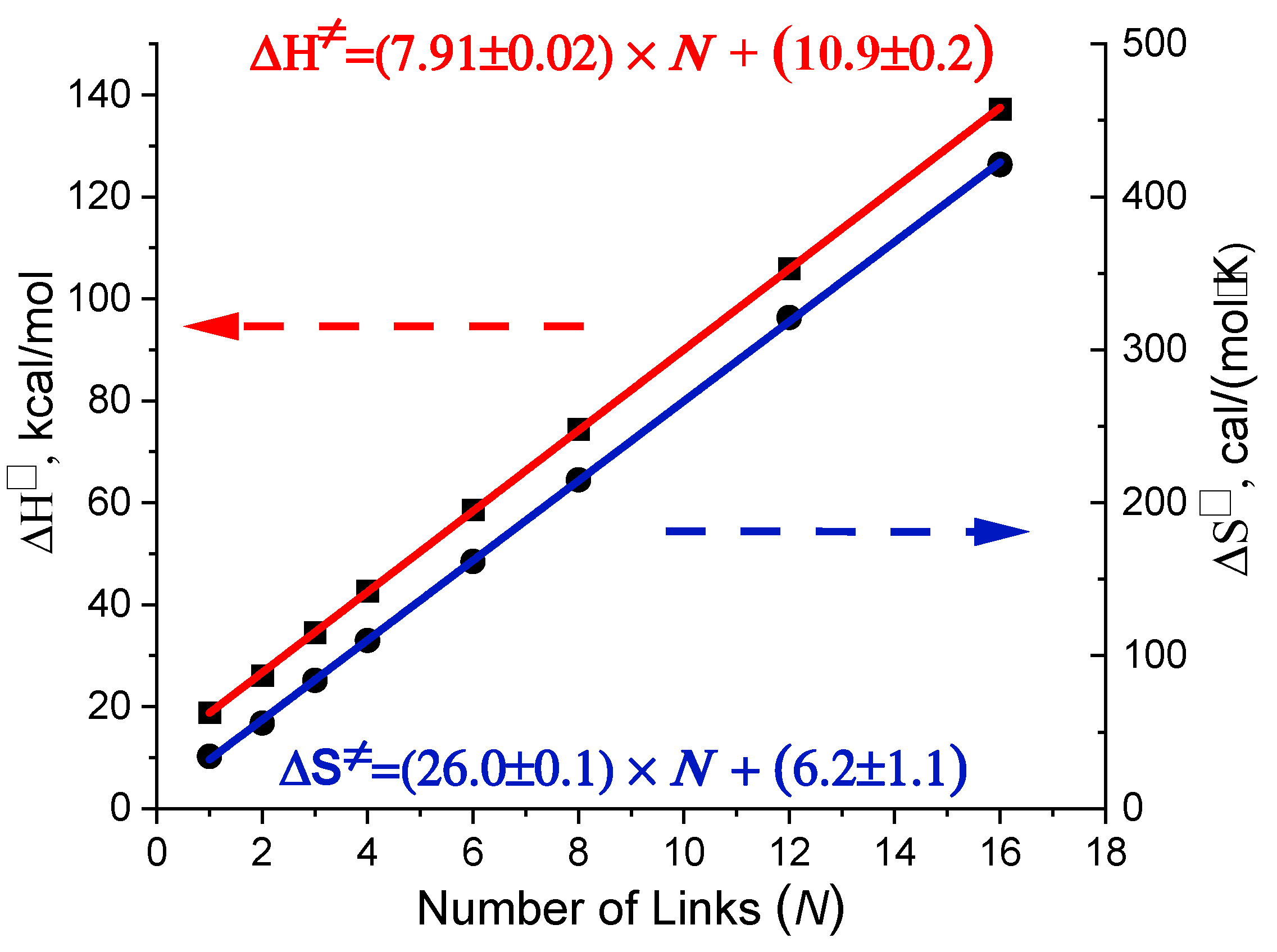
2.3. The Enthalpy–Entropy Compensation
3. Discussion
3.1. The Enthalpy–Entropy Compensation
3.2. “Exotic” Values of Pre-Exponential Factor
3.3. Extrapolation of the Results of the Unfolding of Polypeptides and Proteins with Other Amino Acid Composition
3.4. Extrapolation of the Results of the Unfolding of Polypeptides and Proteins in Water and Other Solvents
4. Materials and Methods
4.1. Quantum-Chemical Calculations
4.2. Transition State Theory Calculations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Eyring, H.; Stearn, A.E. The application of the theory of absolute reaction rates to proteins. Chem. Rev. 1939, 24, 253–270. [Google Scholar] [CrossRef]
- Bahar, I.; Jernigan, R.L.; Dill, K. Protein Actions: Principles and Modeling; Garland Science: New York, NY, USA, 2017. [Google Scholar]
- Rosenberg, B.; Kemeny, G.; Switzer, R.C.; Hamilton, T.C. Quantitative evidence for protein denaturation as the cause of thermal death. Nature 1971, 232, 471–473. [Google Scholar] [CrossRef]
- Sukhorukov, B.I.; Likhtenshtein, G.I. Kinetics and mechanism of biopolimers denaturation. Biofizika 1965, 10, 935–942. [Google Scholar]
- Van Uden, N.; Abranches, P.; Cabeca-Silva, C. Temperature functions of thermal death of yeasts and their relation to the maximum temperature for growth. Arch. Mikrobiol. 1968, 61, 381–393. [Google Scholar] [CrossRef]
- Munblit, V.Y.; Tal’rose, V.L.; Trofimov, V.I. Thermal Inactivation of Microorganisms; Nauka: Moscow, Russia, 1985. [Google Scholar]
- He, X.; Bischof, J.C. Quantification of temperature and injury response in thermal therapy and cryosurgery. Crit. Rev. Biomed. Eng. 2003, 31, 355–421. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.T. On a relationship between the Arrhenius parameters from thermal damage studies. J. Biomech. Eng. 2003, 125, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Balasubramanian, S.K.; Wolkers, W.F.; Pearce, J.A.; Bischof, J.C. Correlated Parameter Fit of Arrhenius Model for Thermal Denaturation of Proteins and Cells. Ann. Biomed. Eng. 2014, 42, 2392–2404. [Google Scholar] [CrossRef] [PubMed]
- Yap, F.; Liu, Z.; Shveda, R.A.; Preston, D.J. A predictive model of the temperature-dependent inactivation of coronaviruses. Appl. Phys. Lett. 2020, 117, 060601. [Google Scholar] [CrossRef]
- Robinson, P.J.; Holbrook, K.A. Unimolecular Reactions; Wiley: London, UK, 1972. [Google Scholar]
- Lumry, R.; Rajender, S. Enthalpy-entropy compensation phenomena in water solutions of proteins and small molecules: A ubiquitous property of water. Biopolymers 1970, 9, 1125–1227. [Google Scholar] [CrossRef]
- Krug, R.R.; Hunter, W.G.; Grieger, R.A. Statistical interpretation of enthalpy–entropy compensation. Nature 1976, 261, 566–567. [Google Scholar] [CrossRef]
- Yelon, A.; Movaghar, B.; Branz, H. Origin and consequences of the compensation (Meyer–Neldel) law. Phys. Rev. B 1992, 46, 12244–12250. [Google Scholar] [CrossRef] [PubMed]
- Gilli, P.; Ferretti, V.; Gilli, G.; Borea, P.A. Enthalpy-entropy compensation in drug-receptor binding. J. Phys. Chem. 1994, 98, 1515–1518. [Google Scholar] [CrossRef]
- Grunwald, E.; Steel, C. Solvent reorganization and thermodynamic enthalpy-entropy compensation. J. Am. Chem. Soc. 1995, 117, 5687–5692. [Google Scholar] [CrossRef]
- Dunitz, J.D. Win some, lose some: Enthalpy-entropy compensation in weak intermolecular interactions. Chem. Biol. 1995, 2, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Petruska, J.; Goodman, M.F. Enthalpy-entropy compensation in DNA melting thermodynamics. J. Biol. Chem. 1995, 270, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Qian, H. Entropy–enthalpy compensation: Conformational fluctuation and induced fit. J. Chem. Phys. 1998, 109, 10015–10017. [Google Scholar] [CrossRef]
- Gottstein, G.; Shwindlerman, L.S. The compensation effect in thermally activated interface processes. Interface Sci. 1998, 6, 265–276. [Google Scholar]
- Miles, C.A.; Ghelashvili, M. Polymer-in-a-box mechanism for the thermal stabilization of collagen molecules in fibers. Biophys. J. 1999, 76, 3243–3252. [Google Scholar] [CrossRef]
- Bond, G.C.; Keane, M.A.; Kral, H.; Lercher, J.A. Compensation phenomena in heterogeneous catalysis: General principles and a possible explanation. Catal. Rev. Sci. Eng. 2000, 42, 323–383. [Google Scholar] [CrossRef]
- Liu, L.; Yang, C.; Guo, Q.X. A study on the enthalpy-entropy compensation in protein unfolding. Biophys. Chem. 2000, 84, 239–251. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Q.X. Isokinetic relationship, isoequilibrium relationship, and enthalpy-entropy compensation. Chem. Rev. 2001, 101, 673–695. [Google Scholar] [CrossRef]
- Sharp, K. Entropy-enthalpy compensation: Fact or artifact? Protein Sci. 2001, 10, 661–667. [Google Scholar] [CrossRef]
- Strazewski, P. Thermodynamic correlation analysis: Hydration and Perturbation Sensitivity of RNA Secondary Structures. J. Am. Chem. Soc. 2002, 124, 3546–3554. [Google Scholar] [CrossRef] [PubMed]
- Fisicaro, E.; Compari, C.; Braibanti, A. Entropy/enthalpy compensation: Hydrophobic effect, micelles, and protein complexes. Phys. Chem. Chem. Phys. 2004, 6, 4156–4166. [Google Scholar] [CrossRef]
- Yelon, A.; Movaghar, B.; Crandall, R.S. Multi-excitation entropy: Its role in thermodynamics and kinetics. Rep. Prog. Phys. 2006, 69, 1145–1194. [Google Scholar] [CrossRef]
- Starikov, E.B.; Norden, B. Enthalpy-entropy compensation: A phantom or something useful? J. Phys. Chem. B 2007, 111, 14431–14435. [Google Scholar] [CrossRef]
- Freed, K.F. Entropy-enthalpy compensation in chemical reactions and adsorption: An exactly solvable model. J. Phys. Chem. B 2011, 115, 1689–1692. [Google Scholar] [CrossRef]
- Barrie, P.J. The mathematical origins of the kinetic compensation effect: 1. The effect of random experimental errors. Phys. Chem. Chem. Phys. 2012, 14, 318–326. [Google Scholar] [CrossRef]
- Pan, A.; Biswas, T.; Rakshit, A.K.; Moulik, S.P. Enthalpy-entropy compensation (EEC) effect: A revisit. J. Phys. Chem. B 2015, 119, 15876–15884. [Google Scholar] [CrossRef]
- Vargas-Lara, F.; Starr, F.W.; Douglas, J.F. Molecular rigidity and enthalpy–entropy compensation in DNA melting. Soft Matter 2017, 13, 8309–8330. [Google Scholar] [CrossRef]
- Gelin, S.; Champagne-Ruel, A.; Mousseau, N. Enthalpy-entropy compensation of atomic diffusion originates from softening of low frequency phonons. Nat. Commun. 2020, 11, 3977–3983. [Google Scholar] [CrossRef] [PubMed]
- Lyon, R.E. A physical basis for kinetic compensation. J. Phys. Chem. A 2023, 127, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Ben-Naim, A. Water and Aqueous Solutions: Introduction to a Molecular Theory; Plenum Press: New York, NY, USA, 1974. [Google Scholar]
- Ben-Naim, A. The Protein Folding Problem and Its Solutions; World Scientific: Singapore, 2013. [Google Scholar]
- Quack, M.; Troe, J. Unimolecular processes V: Maximum free energy criterion for the high pressure limit of dissociation reactions. Ber. Bunsenges. Phys. Chem. 1977, 81, 329–337. [Google Scholar] [CrossRef]
- Vennelakanti, V.; Nazemi, A.; Mehmood, R.; Steeves, A.H.; Kulik, H.J. Harder, better, faster, stronger: Large-scale QM and QM/MM for predictive modeling in enzymes and proteins. Curr. Opin. Struct. Biol. 2022, 72, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.Y.; Yang, D.Y.; Selzle, H.L.; Schlag, E.W. Energetics of hydrogen bonds in peptides. Proc. Natl. Acad. Sci. USA 2003, 100, 12683–12687. [Google Scholar] [CrossRef]
- Robertson, S.H.; Wagner, A.F.; Wardlaw, D.M. Canonical flexible transition state theory revisited. J. Chem. Phys. 1995, 103, 2917–2928. [Google Scholar] [CrossRef]
- Moore, W.H.; Krimm, S. Vibrational analysis of peptides, polypeptides, and proteins. I. Polyglycine I. Biopolymers 1976, 15, 2439–2464. [Google Scholar] [CrossRef]
- Klotz, I.M. Solvent water and protein behavior: View through a retroscope. Protein Sci. 1993, 2, 1992–1999. [Google Scholar] [CrossRef]
- Fersht, A.R.; Shi, J.P.; Knilljones, J.; Lowe, D.M.; Wilkinson, A.J.; Blow, D.M.; Brick, P.; Carter, P.; Waye, M.M.Y.; Winter, G. Hydrogen-bonding and biological specificity analyzed by protein engineering. Nature 1985, 314, 235–238. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baklanov, A.V.; Kiselev, V.G. The Nature of the Enthalpy–Entropy Compensation and “Exotic” Arrhenius Parameters in the Denaturation Kinetics of Proteins. Int. J. Mol. Sci. 2023, 24, 10630. https://doi.org/10.3390/ijms241310630
Baklanov AV, Kiselev VG. The Nature of the Enthalpy–Entropy Compensation and “Exotic” Arrhenius Parameters in the Denaturation Kinetics of Proteins. International Journal of Molecular Sciences. 2023; 24(13):10630. https://doi.org/10.3390/ijms241310630
Chicago/Turabian StyleBaklanov, Alexey V., and Vitaly G. Kiselev. 2023. "The Nature of the Enthalpy–Entropy Compensation and “Exotic” Arrhenius Parameters in the Denaturation Kinetics of Proteins" International Journal of Molecular Sciences 24, no. 13: 10630. https://doi.org/10.3390/ijms241310630
APA StyleBaklanov, A. V., & Kiselev, V. G. (2023). The Nature of the Enthalpy–Entropy Compensation and “Exotic” Arrhenius Parameters in the Denaturation Kinetics of Proteins. International Journal of Molecular Sciences, 24(13), 10630. https://doi.org/10.3390/ijms241310630




