Label-Free Electrochemical Aptasensor for Sensitive Detection of Malachite Green Based on AuNPs/MWCNTs@TiO2 Nanocomposites
Abstract
1. Introduction
2. Results
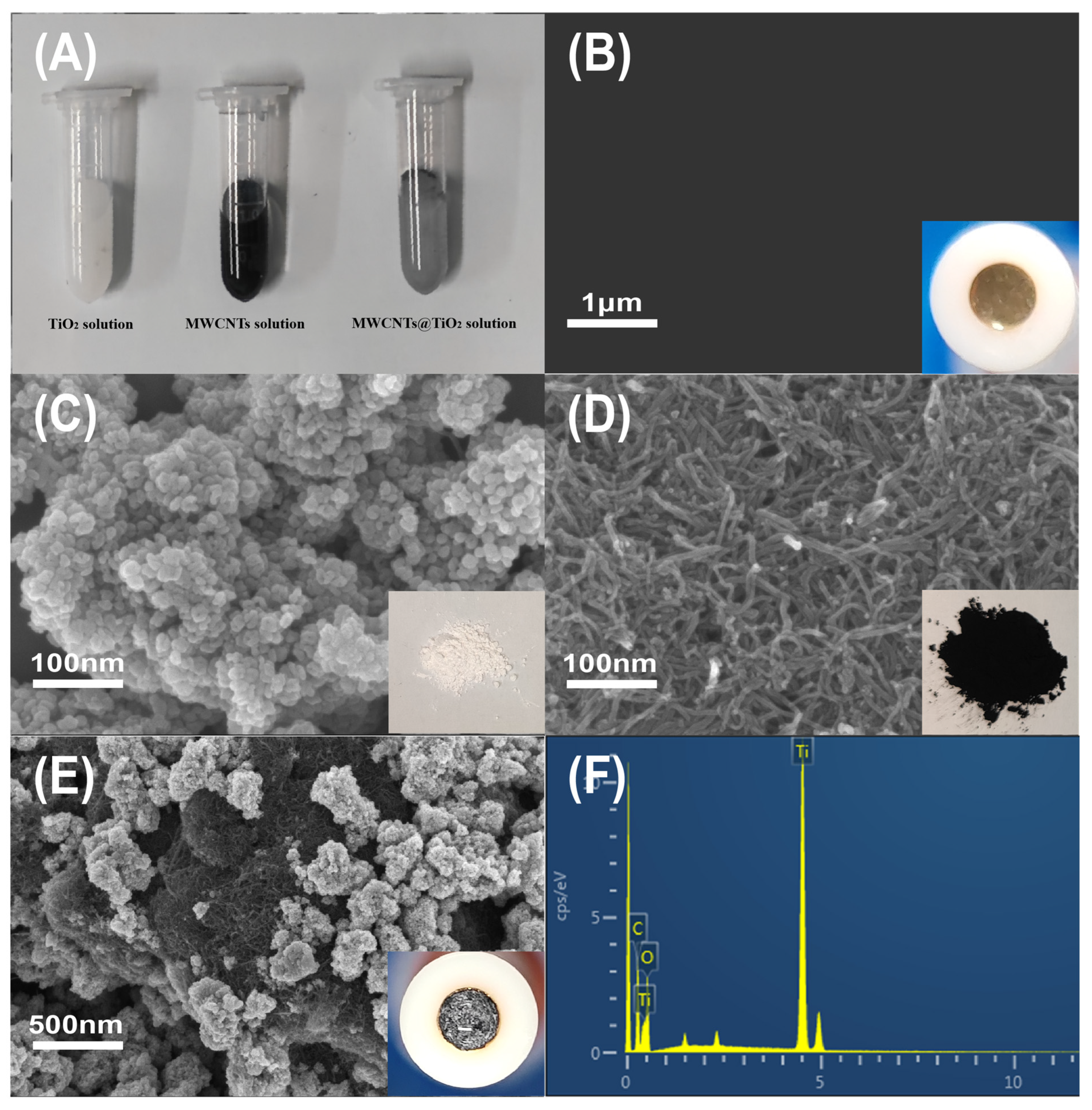
2.1. The Characterization of MWCNTs@TiO2
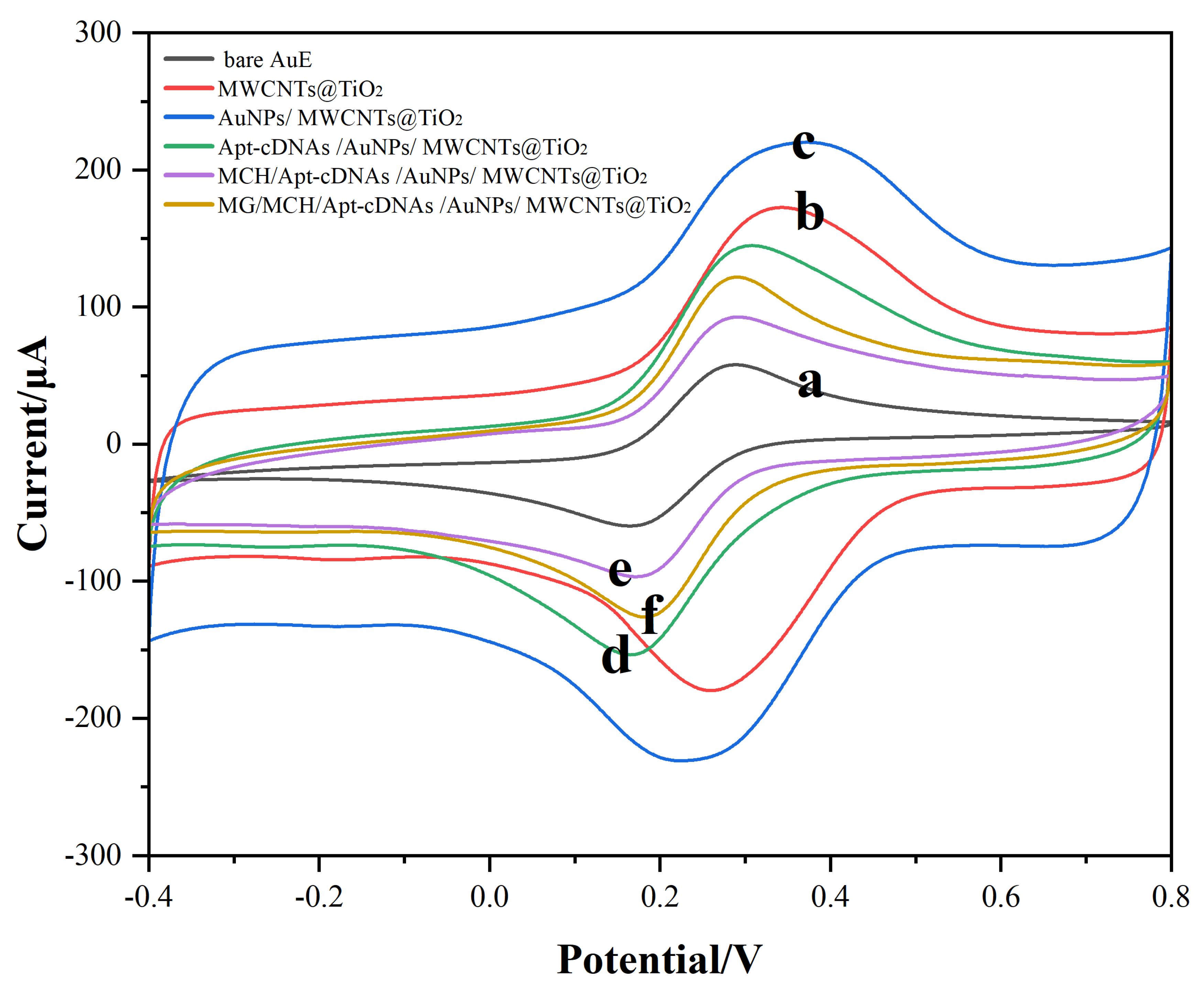
2.2. The Characterization of E-Apt Sensor Fabrication
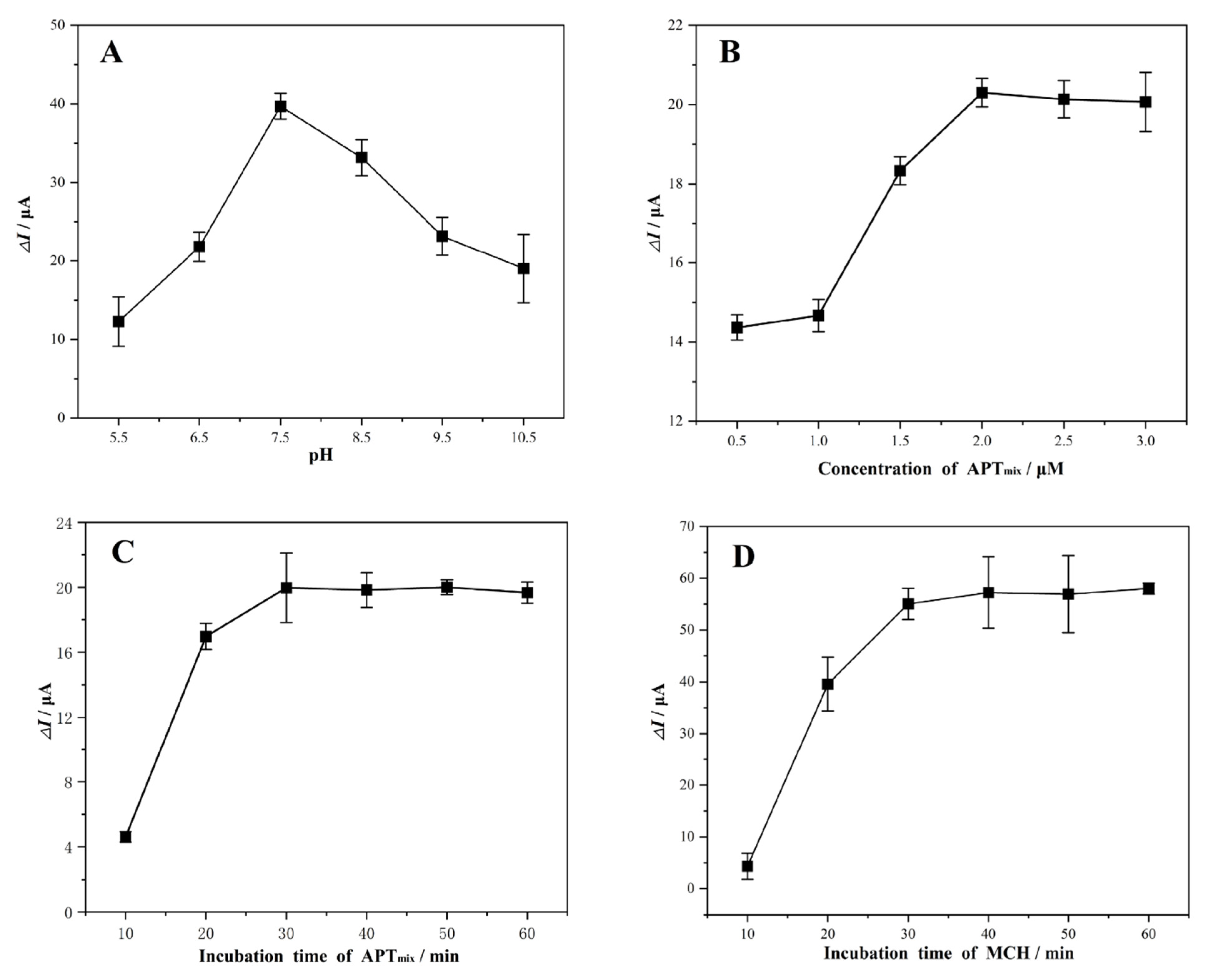
2.3. Optimization of Experimental Conditions
2.4. Analytical Performance of E-Apt Sensor
2.4.1. Linear Response and LOD for MG
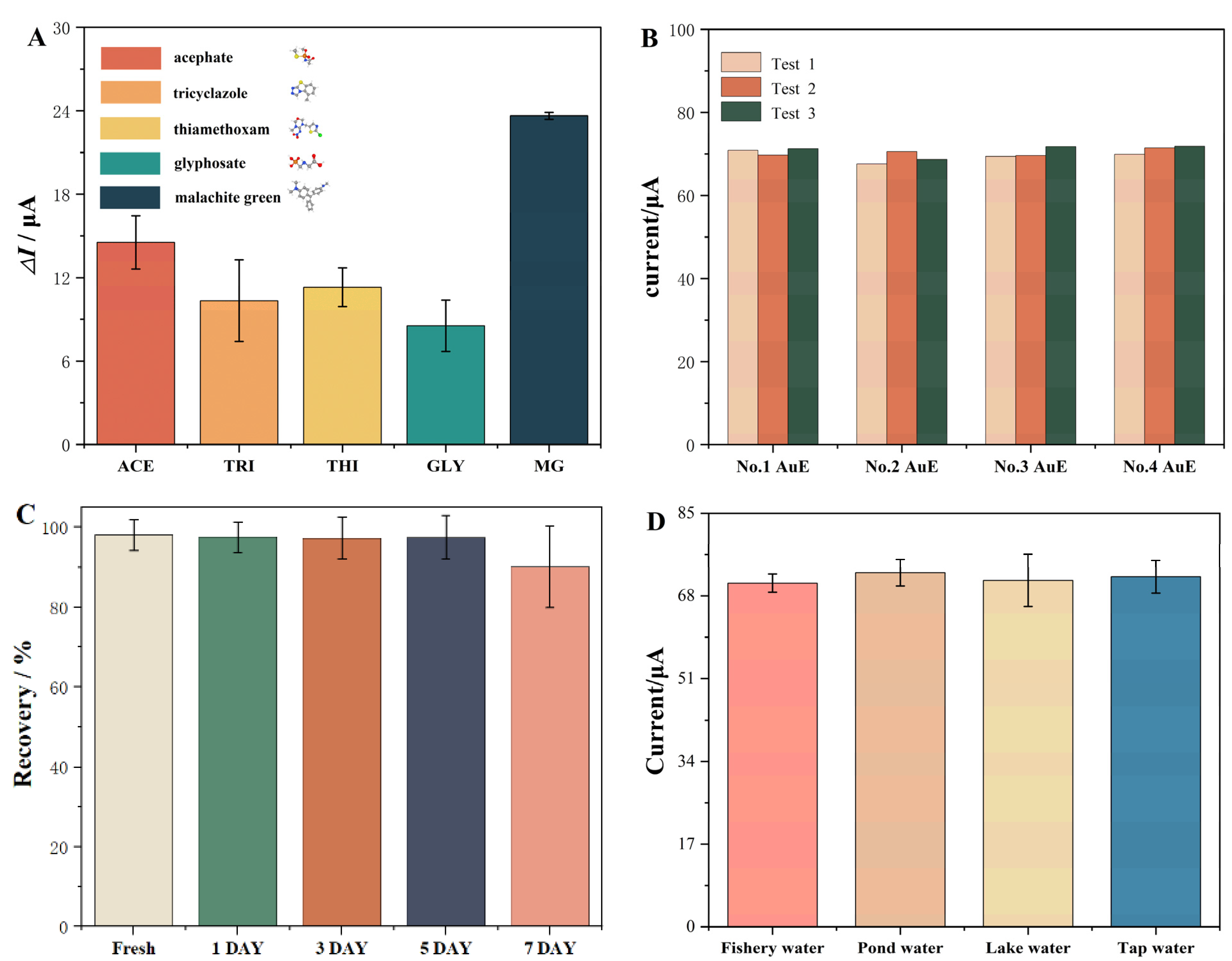
2.4.2. Specificity, Reproducibility, and Stability of Proposed E-Apt Sensor
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Srivastava, S.; Sinha, R.; Roy, D. Toxicological Effects of Malachite Green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Bhange, K.; Bhatt, R.; Verma, P. Biodetoxification of High Amounts of Malachite Green by a Multifunctional Strain of Pseudomonas Mendocina and Its Ability to Metabolize Dye Adsorbed Chicken Feathers. J. Environ. Chem. Eng. 2013, 1, 1205–1213. [Google Scholar] [CrossRef]
- Rao, K.V.K. Inhibition of DNA Synthesis in Primary Rat Hepatocyte Cultures by Malachite Green: A New Liver Tumor Promoter. Toxicol. Lett. 1995, 81, 107–113. [Google Scholar] [CrossRef]
- Ding, F.; Li, X.-N.; Diao, J.-X.; Sun, Y.; Zhang, L.; Ma, L.; Yang, X.-L.; Zhang, L.; Sun, Y. Potential Toxicity and Affinity of Triphenylmethane Dye Malachite Green to Lysozyme. Ecotoxicol. Environ. Saf. 2012, 78, 41–49. [Google Scholar] [CrossRef]
- Sagar, K.; Smyth, M.R.; Wilson, J.G.; McLaughlin, K. High-Performance Liquid Chromatographic Determination of the Triphenylmethane Dye, Malachite Green, Using Amperometric Detection at a Carbon Fibre Microelectrode. J. Chromatogr. A 1994, 659, 329–336. [Google Scholar] [CrossRef]
- Halme, K.; Lindfors, E.; Peltonen, K. Determination of Malachite Green Residues in Rainbow Trout Muscle with Liquid Chromatography and Liquid Chromatography Coupled with Tandem Mass Spectrometry. Food Addit. Contam. 2004, 21, 641–648. [Google Scholar] [CrossRef]
- Nebot, C.; Iglesias, A.; Barreiro, R.; Miranda, J.M.; Vázquez, B.; Franco, C.M.; Cepeda, A. A Simple and Rapid Method for the Identification and Quantification of Malachite Green and Its Metabolite in Hake by HPLC–MS/MS. Food Control 2013, 31, 102–107. [Google Scholar] [CrossRef]
- Bilandžić, N.; Varenina, I.; Kolanović, B.S.; Oraić, D.; Zrnčić, S. Malachite Green Residues in Farmed Fish in Croatia. Food Control 2012, 26, 393–396. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Kang, Y.; Miao, J.; Lai, K. Selective Recognition and Determination of Malachite Green in Fish Muscles via Surface-Enhanced Raman Scattering Coupled with Molecularly Imprinted Polymers. Food Control 2021, 130, 108367. [Google Scholar] [CrossRef]
- Lin, Z.; Li, W.-J.; Chen, Q.-C.; Peng, A.; Huang, Z. Rapid Detection of Malachite Green in Fish with a Fluorescence Probe of Molecularly Imprinted Polymer. Int. J. Polym. Anal. Charact. 2019, 24, 121–131. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Jia, J.; Yan, S.; Lai, X.; Xu, Y.; Liu, T.; Xiang, Y. Colorimetric Aptasensor for Detection of Malachite Green in Fish Sample Based on RNA and Gold Nanoparticles. Food Anal. Methods 2018, 11, 1668–1676. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, D.; Gong, J.; Liu, C.; Chen, J. Highly Sensitive Aptasensor for Trace Arsenic(III) Detection Using DNAzyme as the Biocatalytic Amplifier. Anal. Chem. 2019, 91, 1724–1727. [Google Scholar] [CrossRef]
- Zhou, Y.; Mahapatra, C.; Chen, H.; Peng, X.; Ramakrishna, S.; Nanda, H.S. Recent Developments in Fluorescent Aptasensors for Detection of Antibiotics. Curr. Opin. Biomed. Eng. 2020, 13, 16–24. [Google Scholar] [CrossRef]
- Bang, G.S.; Cho, S.; Kim, B.-G. A Novel Electrochemical Detection Method for Aptamer Biosensors. Biosens. Bioelectron. 2005, 21, 863–870. [Google Scholar] [CrossRef]
- Abu-Ali, H.; Nabok, A.; Smith, T. Development of Novel and Highly Specific SsDNA-Aptamer-Based Electrochemical Biosensor for Rapid Detection of Mercury (II) and Lead (II) Ions in Water. Chemosensors 2019, 7, 27. [Google Scholar] [CrossRef]
- Ribes, À.; Aznar, E.; Bernardos, A.; Marcos, M.D.; Amorós, P.; Martínez-Máñez, R.; Sancenón, F. Fluorogenic Sensing of Carcinogenic Bisphenol A Using Aptamer-Capped Mesoporous Silica Nanoparticles. Chem. Eur. J. 2017, 23, 8581–8584. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; He, S.; Gao, Z.; Di, Y.; Lu, K.; Li, K.; Wang, J. Aptamer-DNA Concatamer-Quantum Dots Based Electrochemical Biosensing Strategy for Green and Ultrasensitive Detection of Tumor Cells via Mercury-Free Anodic Stripping Voltammetry. Biosens. Bioelectron. 2019, 126, 261–268. [Google Scholar] [CrossRef]
- Bai, X.; Qin, C.; Huang, X. Voltammetric Determination of Chloramphenicol Using a Carbon Fiber Microelectrode Modified with Fe3O4 Nanoparticles. Microchim. Acta 2016, 183, 2973–2981. [Google Scholar] [CrossRef]
- Cheng, N.; Song, Y.; Fu, Q.; Du, D.; Luo, Y.; Wang, Y.; Xu, W.; Lin, Y. Aptasensor Based on Fluorophore-Quencher Nano-Pair and Smartphone Spectrum Reader for on-Site Quantification of Multi-Pesticides. Biosens. Bioelectron. 2018, 117, 75–83. [Google Scholar] [CrossRef]
- Kara, P.; De La Escosura-Muñiz, A.; Maltez-da Costa, M.; Guix, M.; Ozsoz, M.; Merkoçi, A. Aptamers Based Electrochemical Biosensor for Protein Detection Using Carbon Nanotubes Platforms. Biosens. Bioelectron. 2010, 26, 1715–1718. [Google Scholar] [CrossRef]
- Yazdanparast, S.; Benvidi, A.; Banaei, M.; Nikukar, H.; Tezerjani, M.D.; Azimzadeh, M. Dual-Aptamer Based Electrochemical Sandwich Biosensor for MCF-7 Human Breast Cancer Cells Using Silver Nanoparticle Labels and a Poly(Glutamic Acid)/MWNT Nanocomposite. Microchim. Acta 2018, 185, 405. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Tavakolian-Ardakani, Z.; Sahraei, N.; Moshtaghioun, S.M. Fabrication of an Ultrasensitive and Selective Electrochemical Aptasensor to Detect Carcinoembryonic Antigen by Using a New Nanocomposite. Biosens. Bioelectron. 2019, 129, 1–6. [Google Scholar] [CrossRef]
- Paimard, G.; Shahlaei, M.; Moradipour, P.; Karamali, V.; Arkan, E. Impedimetric Aptamer Based Determination of the Tumor Marker MUC1 by Using Electrospun Core-Shell Nanofibers. Microchim. Acta 2020, 187, 5. [Google Scholar] [CrossRef]
- Kaur, N.; Thakur, H.; Prabhakar, N. Multi Walled Carbon Nanotubes Embedded Conducting Polymer Based Electrochemical Aptasensor for Estimation of Malathion. Microchem. J. 2019, 147, 393–402. [Google Scholar] [CrossRef]
- Pligovka, A.; Lazavenka, A.; Turavets, U.; Hoha, A.; Salerno, M. Two-Level 3D Column-like Nanofilms with Hexagonally–Packed Tantalum Fabricated via Anodizing of Al/Nb and Al/Ta Layers—A Potential Nano-Optical Biosensor. Materials 2023, 16, 993. [Google Scholar] [CrossRef]
- Pligovka, A.; Lazavenka, A.; Gorokh, G. Anodic Niobia Column-like 3-D Nanostructures for Semiconductor Devices. IEEE Trans. Nanotechnol. 2019, 18, 790–797. [Google Scholar] [CrossRef]
- Heydari-Bafrooei, E.; Amini, M.; Ardakani, M.H. An Electrochemical Aptasensor Based on TiO2/MWCNT and a Novel Synthesized Schiff Base Nanocomposite for the Ultrasensitive Detection of Thrombin. Biosens. Bioelectron. 2016, 85, 828–836. [Google Scholar] [CrossRef]
- Cai, G.; Yu, Z.; Ren, R.; Tang, D. Exciton–Plasmon Interaction between AuNPs/Graphene Nanohybrids and CdS Quantum Dots/TiO2 for Photoelectrochemical Aptasensing of Prostate-Specific Antigen. ACS Sens. 2018, 3, 632–639. [Google Scholar] [CrossRef]
- Xie, M.; Chen, Z.; Zhao, F.; Lin, Y.; Zheng, S.; Han, S. Selection and Application of SsDNA Aptamers for Fluorescence Biosensing Detection of Malachite Green. Foods 2022, 11, 801. [Google Scholar] [CrossRef]
- Lian, Y.; He, F.; Mi, X.; Tong, F.; Shi, X. Lysozyme Aptamer Biosensor Based on Electron Transfer from SWCNTs to SPQC-IDE. Sens. Actuators B Chem. 2014, 199, 377–383. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.; Hong, C.; Lin, Z.; Huang, Z. Rapid Detection of Malachite Green in Fish and Water Based on the Peroxidase-like Activity of Fe3O4NPs Enhanced with Aptamer. J. Food Compos. Anal. 2021, 104, 104162. [Google Scholar] [CrossRef]
- Gui, W.; Wang, H.; Liu, Y.; Ma, Q. Ratiometric Fluorescent Sensor with Molecularly Imprinted Mesoporous Microspheres for Malachite Green Detection. Sens. Actuators B Chem. 2018, 266, 685–691. [Google Scholar] [CrossRef]
- Wang, J.; Hong, C.; Lin, Z.; Huang, Z.-Y. Fluorescence Detection of Malachite Green in Fish Based on Aptamer and SYBR Green I. J. Food Drug Anal. 2022, 30, 369–381. [Google Scholar] [CrossRef]
- Feng, X.; Gan, N.; Zhang, H.; Yan, Q.; Li, T.; Cao, Y.; Hu, F.; Yu, H.; Jiang, Q. A Novel “Dual-Potential” Electrochemiluminescence Aptasensor Array Using CdS Quantum Dots and Luminol-Gold Nanoparticles as Labels for Simultaneous Detection of Malachite Green and Chloramphenicol. Biosens. Bioelectron. 2015, 74, 587–593. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Pan, Z.; Ye, B.; Xu, M. Determination of Malachite Green in Fish by a Modified MOF-Based Electrochemical Sensor. Food Anal. Methods 2019, 12, 1246–1254. [Google Scholar] [CrossRef]
- Danesh, N.M.; Lavaee, P.; Ramezani, M.; Alibolandi, M.; Kianfar, M.; Alinezhad Nameghi, M.; Abnous, K.; Taghdisi, S.M. An Electrochemical Sensing Method Based on an Oligonucleotide Structure for Ultrasensitive Detection of Malachite Green. Microchem. J. 2021, 160, 105598. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, X.; Geng, L.; Wang, Y. Label-Free Electrochemical Aptasensor for Sensitive Detection of Malachite Green Based on Au Nanoparticle/Graphene Quantum Dots/Tungsten Disulfide Nanocomposites. Nanomaterials 2019, 9, 229. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Liu, S.; Yu, J.; Xu, W.; Guo, Y.; Huang, J. An RNA Aptamer-Based Electrochemical Biosensor for Sensitive Detection of Malachite Green. RSC Adv. 2014, 4, 60987–60994. [Google Scholar] [CrossRef]




| Method | Linear Range (ng/mL) | LOD (ng/mL) | Real Sample | Ref. |
|---|---|---|---|---|
| Colorimetric | 21.9–868.7 | 16.7 | Fishery water | [32] |
| Colorimetric | 7.3–109.5 | 5.82 | Fish | [12] |
| Fluorescence | 4–1.2 × 103 | 1.82 | Fishery water | [30] |
| Fluorescence | 10–50 | 6.205 | River water | [33] |
| Fluorescence | 7.3–365 | 0.67 | Fish | [34] |
| ECL | 3.7 × 10−2–37 | 0.012 | Fish | [35] |
| DPV | 3.65–51.1 | 0.803 | Fish | [36] |
| DPV | 0.292–146 | 0.049 | Tap water and serum | [37] |
| DPV | 3.65–3.65 × 103 | 1.233 | Fish | [38] |
| DPV | 0.1–1 × 104 | 0.016 | Fishery water | [39] |
| DPV | 0.01–1 × 103 | 0.008 | Fishery water | This work |
| Sample | Addition of MG (ng/mL) | Detection of MG (ng/mL) | Recovery Rate (%) | RSD (%) (n = 3) |
|---|---|---|---|---|
| Fishery water | 0 | 0 | - | - |
| 0.01 | 9.48 × 10−3 | 94.8% | 1.73 | |
| 1 | 0.971 | 97.1% | 1.78 | |
| 100 | 102.4 | 102.4% | 1.04 | |
| Tap water | 0 | 0 | - | - |
| 0.01 | 9.28 × 10−3 | 92.8% | 3.19 | |
| 1 | 1.007 | 100.7% | 2.74 | |
| 100 | 99.90 | 99.9% | 1.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Li, H.; Xie, M.; Zhao, F.; Han, S. Label-Free Electrochemical Aptasensor for Sensitive Detection of Malachite Green Based on AuNPs/MWCNTs@TiO2 Nanocomposites. Int. J. Mol. Sci. 2023, 24, 10594. https://doi.org/10.3390/ijms241310594
Chen Z, Li H, Xie M, Zhao F, Han S. Label-Free Electrochemical Aptasensor for Sensitive Detection of Malachite Green Based on AuNPs/MWCNTs@TiO2 Nanocomposites. International Journal of Molecular Sciences. 2023; 24(13):10594. https://doi.org/10.3390/ijms241310594
Chicago/Turabian StyleChen, Zanlin, Haiming Li, Miaojia Xie, Fengguang Zhao, and Shuangyan Han. 2023. "Label-Free Electrochemical Aptasensor for Sensitive Detection of Malachite Green Based on AuNPs/MWCNTs@TiO2 Nanocomposites" International Journal of Molecular Sciences 24, no. 13: 10594. https://doi.org/10.3390/ijms241310594
APA StyleChen, Z., Li, H., Xie, M., Zhao, F., & Han, S. (2023). Label-Free Electrochemical Aptasensor for Sensitive Detection of Malachite Green Based on AuNPs/MWCNTs@TiO2 Nanocomposites. International Journal of Molecular Sciences, 24(13), 10594. https://doi.org/10.3390/ijms241310594





