Spermidine Attenuates High Glucose-Induced Oxidative Damage in Retinal Pigment Epithelial Cells by Inhibiting Production of ROS and NF-κB/NLRP3 Inflammasome Pathway
Abstract
1. Introduction
2. Results
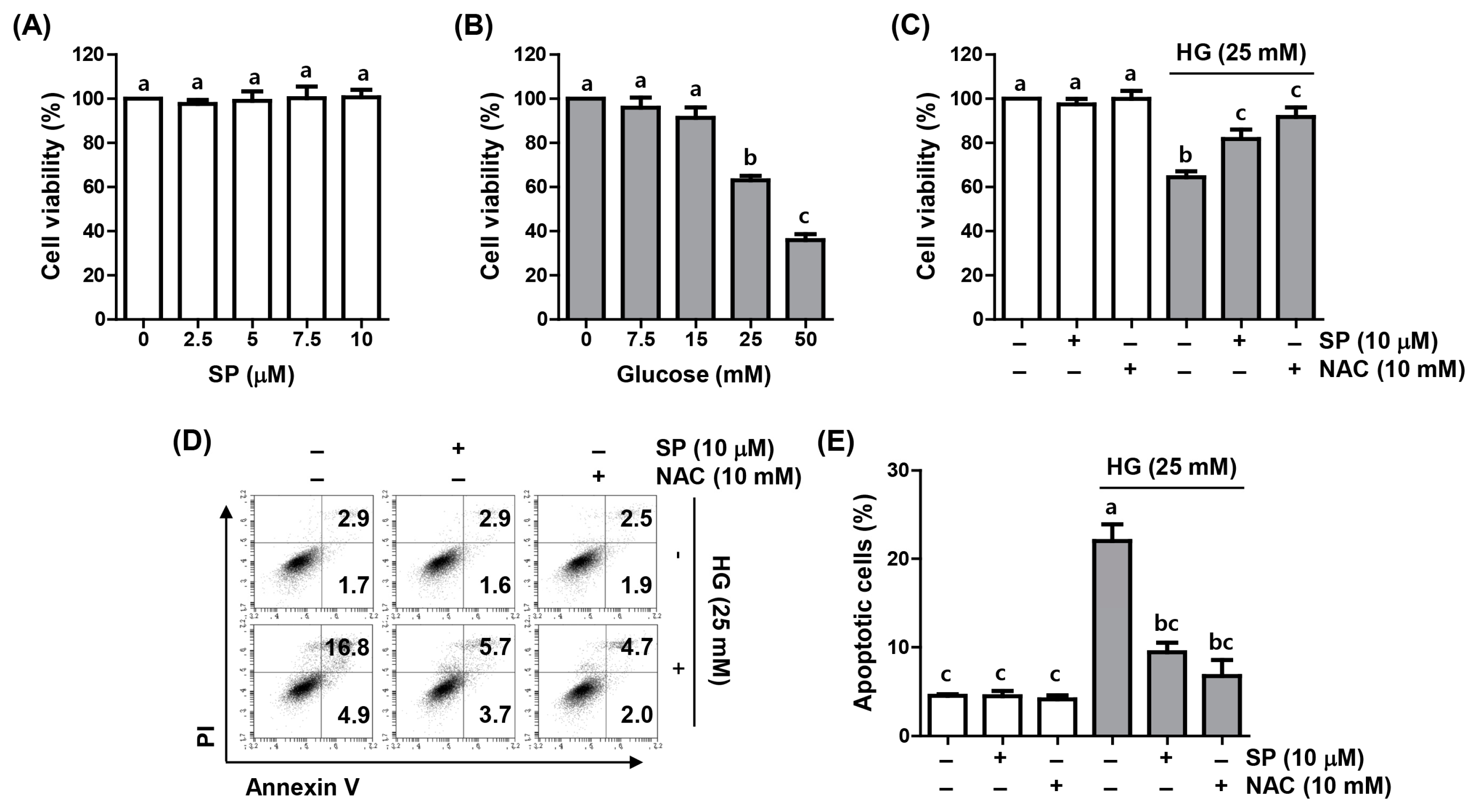
2.1. Spermidine Reduced HG-Induced Decrease in Cell Viability and Apoptosis
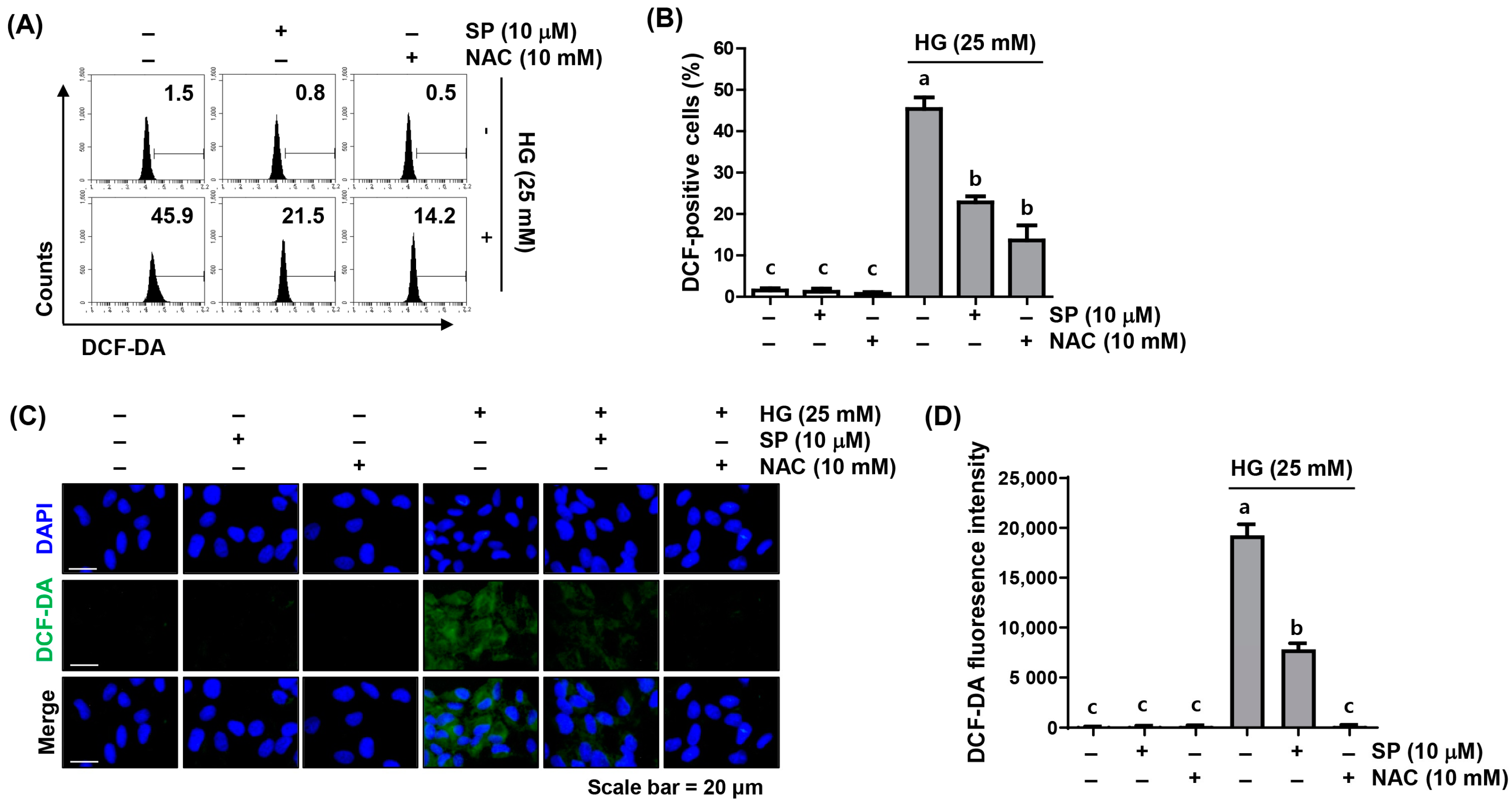
2.2. Spermidine Ameliorated HG-Induced Intracellular ROS Generation
2.3. Spermidine Attenuated HG-Induced Mitochondrial ROS Generation
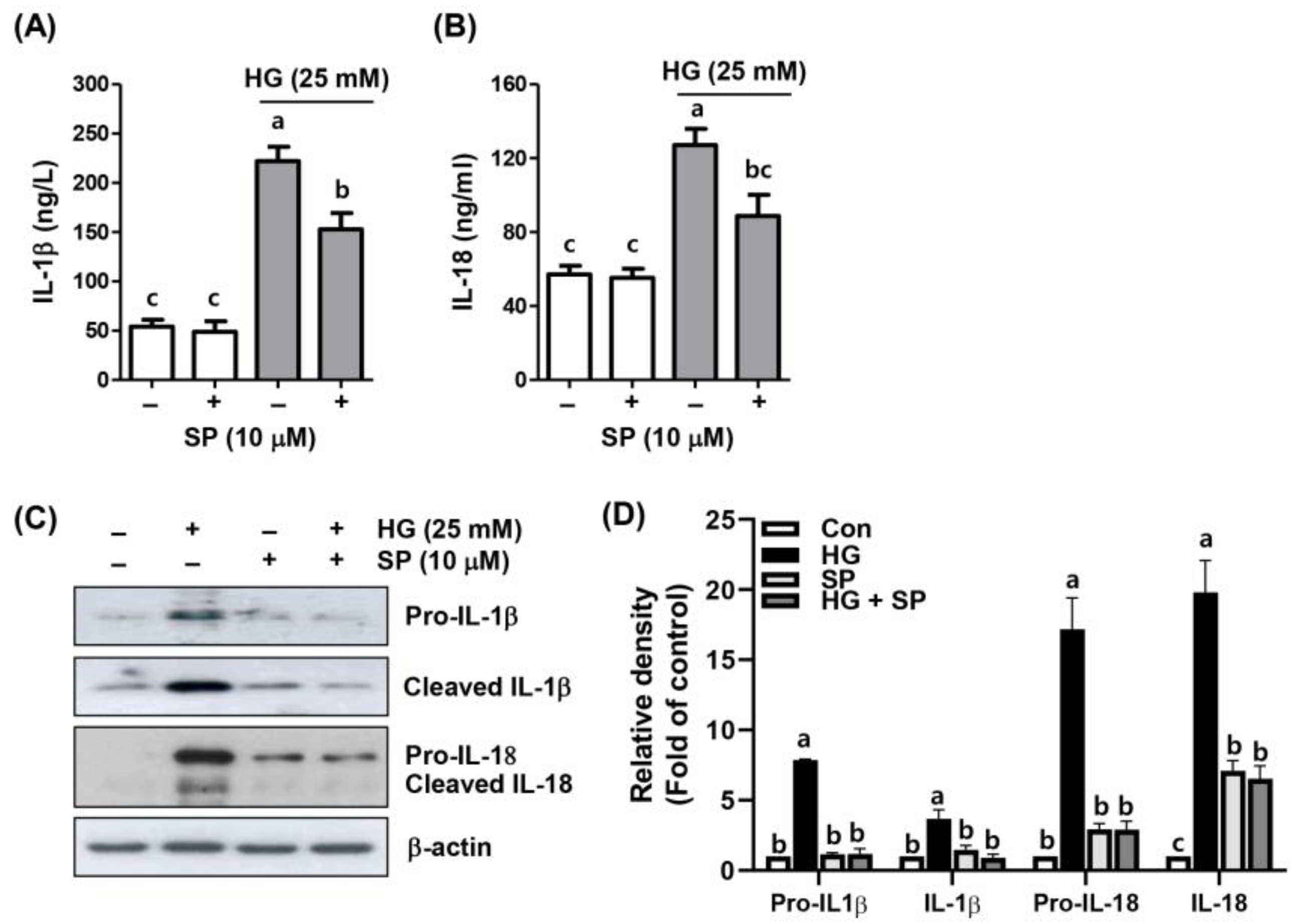
2.4. Spermidine Alleviated HG-Induced Inflammatory Response
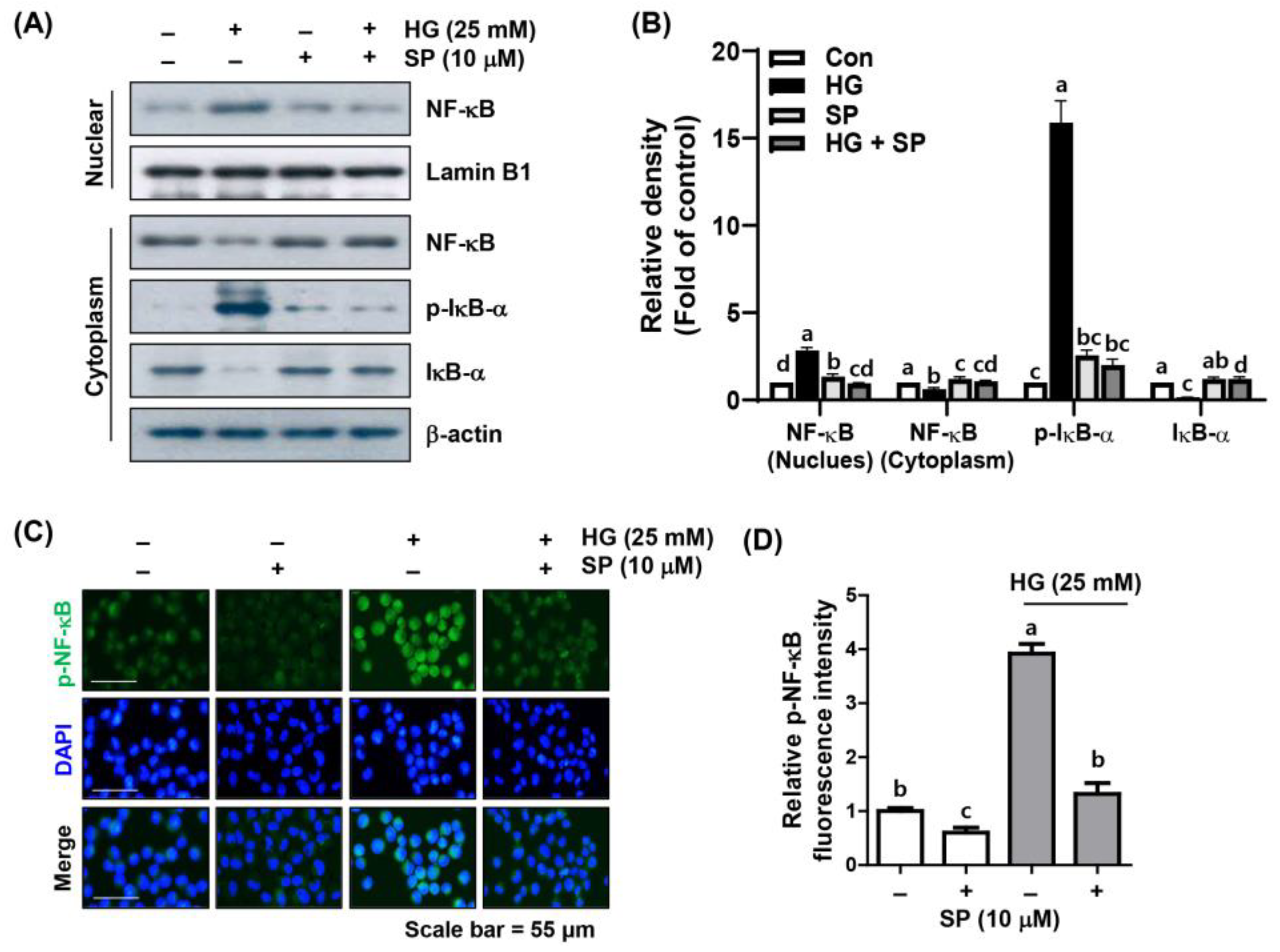
2.5. Spermidine Mitigated HG-Induced NF-κB Signaling Activation
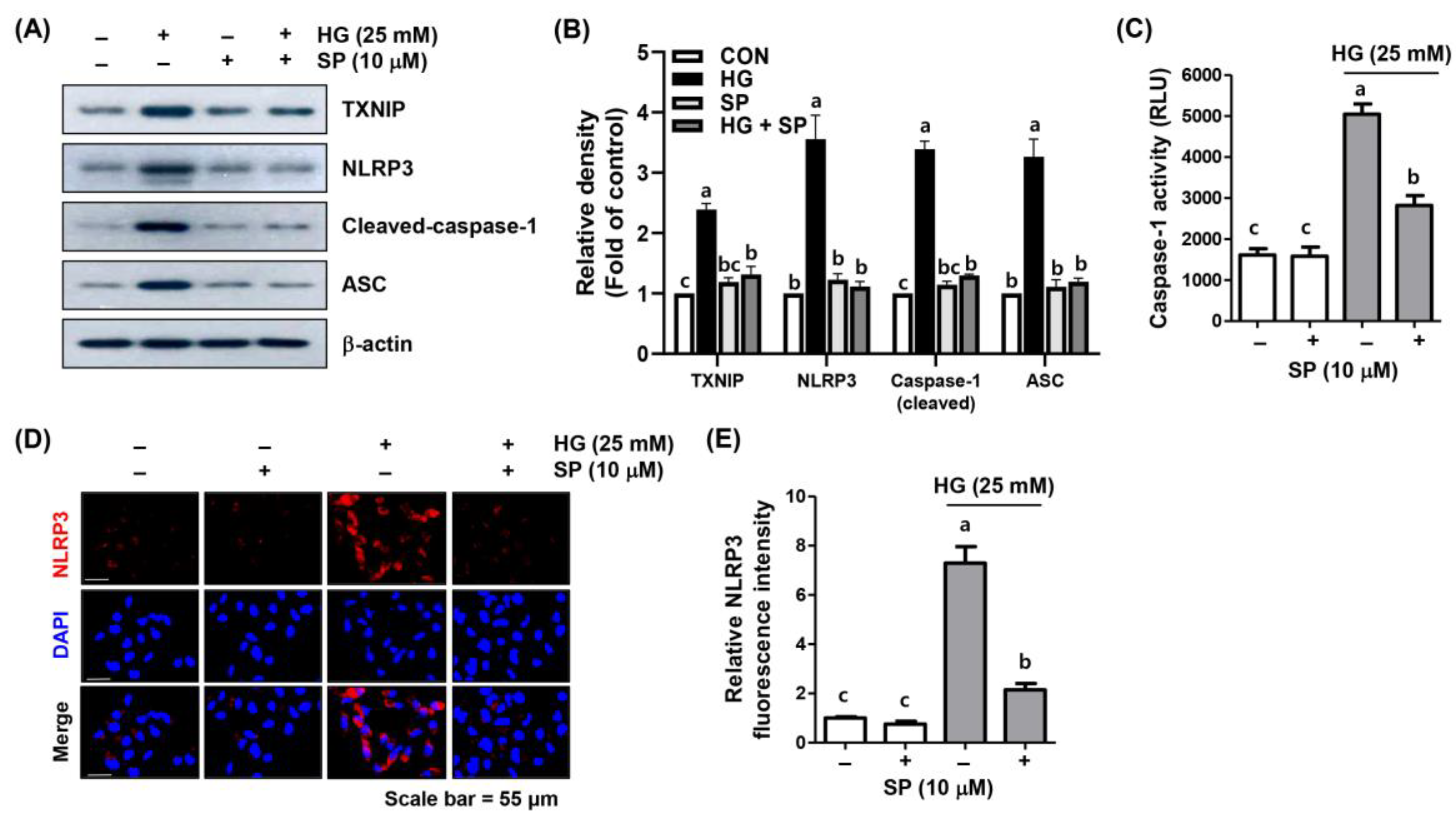
2.6. Spermidine Attenuated HG-Induced NLRP3 Inflammasome Activation
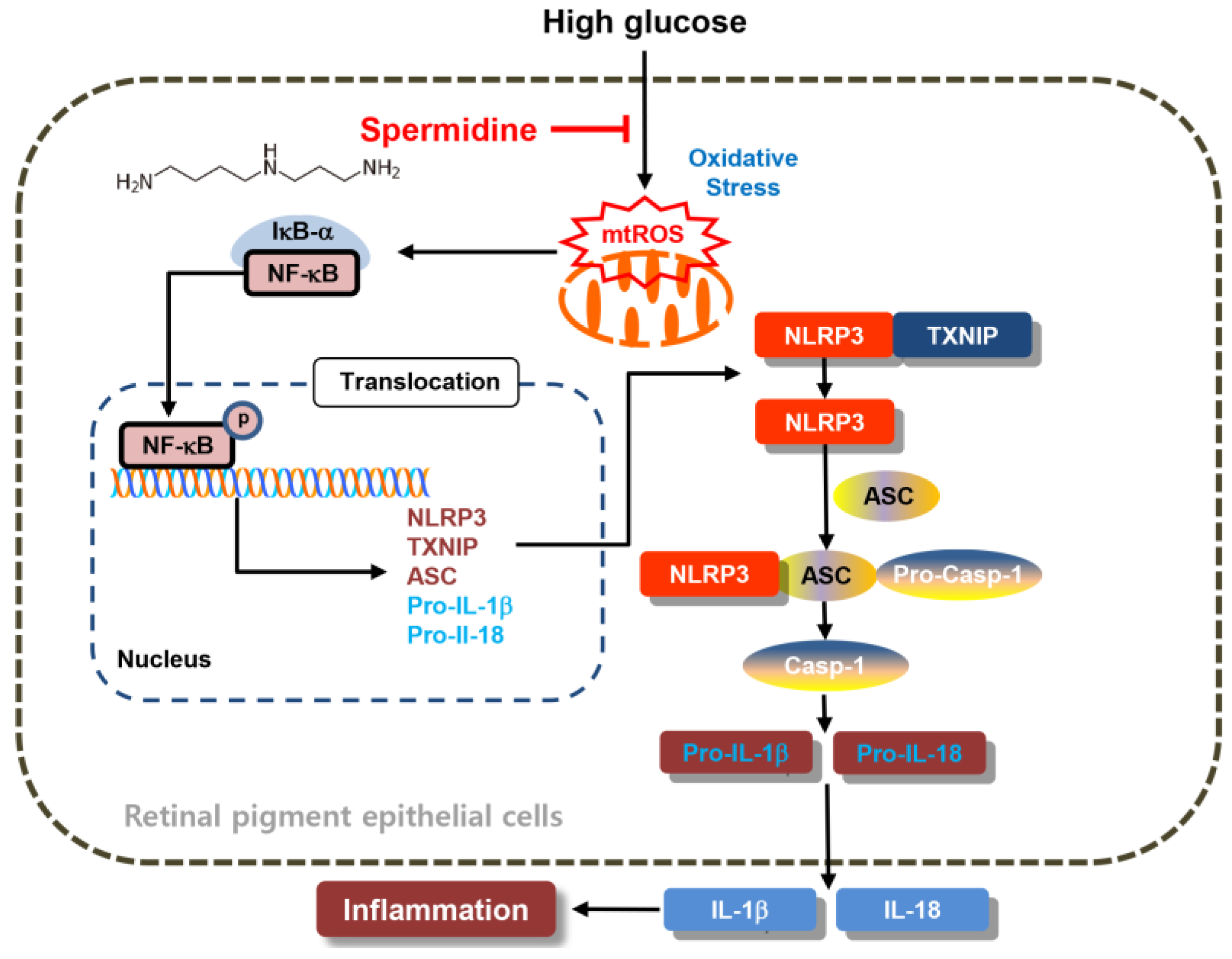
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Cell Proliferation Assay
4.3. Apoptosis Assay
4.4. Measurement of ROS Generation
4.5. Analysis of Cytokine Levels
4.6. Western Blot Analysis
4.7. Immunofluorescence for NF-κB and NLRP3
4.8. Caspase-1 Activity Measurement
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, T.E.; Wong, T.Y. Diabetic retinopathy: Looking forward to 2030. Front. Endocrinol. 2023, 13, 1077669. [Google Scholar] [CrossRef]
- Trott, M.; Driscoll, R.; Pardhan, S. Associations between diabetic retinopathy, mortality, disease, and mental health: An umbrella review of observational meta-analyses. BMC Endocr. Disord. 2022, 22, 311. [Google Scholar] [CrossRef]
- Cheng, Y.; Ren, T.; Wang, N. Biomechanical homeostasis in ocular diseases: A mini-review. Front. Public Health 2023, 11, 1106728. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, L.; Wang, Q.; Li, Y.; Wei, C.; Xie, L. Mechanistic investigations of diabetic ocular surface diseases. Front. Endocrinol. 2022, 13, 1079541. [Google Scholar] [CrossRef]
- Kang, H.; Yin, N.; Lyon, H.; Rupenthal, I.D.; Thakur, S.S.; Mugisho, O.O. The influence of hyperglycemia on the safety of ultrasound in retinal pigment epithelial cells. Cell Biol. Int. 2021, 45, 558–568. [Google Scholar] [CrossRef]
- Karthikkeyan, G.; Nareshkumar, R.N.; Aberami, S.; Sulochana, K.N.; Vedantham, S.; Coral, K. Hyperglycemia induced early growth response-1 regulates vascular dysfunction in human retinal endothelial cells. Microvasc. Res. 2018, 117, 37–43. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, S.; Pan, Y.; Jin, M.; Li, J.; Luo, Y.; Sun, X.; Li, G. Diabetic retinopathy: Involved cells, biomarkers, and treatments. Front. Pharmacol. 2022, 13, 953691. [Google Scholar] [CrossRef]
- Cunha-Vaz, J. The blood-retinal barrier in the management of retinal disease: EURETINA award lecture. Ophthalmologica 2017, 237, 1–10. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Geng, Z.; Khattak, S.; Ji, X.; Wu, D.; Dang, Y. Role of oxidative stress in retinal disease and the early intervention strategies: A review. Oxid. Med. Cell. Longev. 2022, 2022, 7836828. [Google Scholar] [CrossRef]
- Dammak, A.; Huete-Toral, F.; Carpena-Torres, C.; Martin-Gil, A.; Pastrana, C.; Carracedo, G. From oxidative stress to inflammation in the posterior ocular diseases: Diagnosis and treatment. Pharmaceutics 2021, 13, 1376. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef]
- Patergnani, S.; Bouhamida, E.; Leo, S.; Pinton, P.; Rimessi, A. Mitochondrial oxidative stress and “Mito-Inflammation”: Actors in the diseases. Biomedicines 2021, 9, 216. [Google Scholar] [CrossRef]
- Naik, E.; Dixit, V.M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 2011, 208, 417–420. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Maran, J.J.; Jamieson, E.G.; Rupenthal, I.D.; Murphy, R.; Mugisho, O.O. Characterization of NLRP3 inflammasome activation in the onset of diabetic retinopathy. Int. J. Mol. Sci. 2022, 23, 14471. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Liao, N.; Mi, L.; Peng, Y.; Liu, B.; Zhang, S.; Wen, F. Enhanced expression of NLRP3 inflammasome-related inflammation in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 978–985. [Google Scholar] [CrossRef]
- Loukovaara, S.; Piippo, N.; Kinnunen, K.; Hytti, M.; Kaarniranta, K.; Kauppinen, A. NLRP3 inflammasome activation is associated with proliferative diabetic retinopathy. Acta Ophthalmol. 2017, 95, 803–808. [Google Scholar] [CrossRef]
- Kinoshita, T.; Imamura, R.; Kushiyama, H.; Suda, T. NLRP3 mediates NF-κB activation and cytokine induction in microbially induced and sterile inflammation. PLoS ONE 2015, 10, e0119179. [Google Scholar] [CrossRef]
- Wei, Z.X.; Cai, L.; Zhao, X.M.; Jiang, X.R.; Li, X.L. Effects of spermidine on cell proliferation, migration, and inflammatory response in porcine enterocytes. Front. Biosci. 2022, 27, 194. [Google Scholar] [CrossRef]
- Larqué, E.; Sabater-Molina, M.; Zamora, S. Biological significance of dietary polyamines. Nutrition 2007, 23, 87–95. [Google Scholar] [CrossRef]
- Park, I.H.; Kim, M.M. Inhibitory effect of spermidine with antioxidant activity on oxidative stress in human dermal fibroblasts. J. Life Sci. 2011, 21, 693–699. [Google Scholar]
- Chen, Z.; Lin, C.X.; Song, B.; Li, C.C.; Qiu, J.X.; Li, S.X.; Lin, S.P.; Luo, W.Q.; Fu, Y.; Fang, G.B.; et al. Spermidine activates RIP1 deubiquitination to inhibit TNF-α-induced NF-κB/p65 signaling pathway in osteoarthritis. Cell Death Dis. 2020, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Al-Kharashi, A.S. Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi J. Ophthalmol. 2018, 32, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Araki, E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 2007, 9, 343–353. [Google Scholar] [CrossRef]
- Yildirim, Z.; Uçgun, N.I.; Kiliç, N.; Gürsel, E.; Sepici-Dinçel, A. Antioxidant enzymes and diabetic retinopathy. Ann. N. Y. Acad. Sci. 2007, 1100, 199–206. [Google Scholar]
- Rider, J.E.; Hacker, A.; Mackintosh, C.A.; Pegg, A.E.; Woster, P.M.; Casero, R.A., Jr. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 2007, 33, 231–240. [Google Scholar] [CrossRef]
- Yu, T.; Jhun, B.S.; Yoon, Y. High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxid. Redox Signal. 2011, 14, 425–437. [Google Scholar] [CrossRef]
- Ceriello, A.; dello Russo, P.; Amstad, P.; Cerutti, P. High glucose induces antioxidant enzymes in human endothelial cells in culture. Evidence linking hyperglycemia and oxidative stress. Diabetes 1996, 45, 471–477. [Google Scholar] [CrossRef]
- Oliveira-Marques, V.; Marinho, H.S.; Cyrne, L.; Antunes, F. Role of hydrogen peroxide in NF-kappaB activation: From inducer to modulator. Antioxid. Redox Signal. 2009, 11, 2223–2243. [Google Scholar] [CrossRef]
- Lazzara, F.; Conti, F.; Platania, C.B.M.; Eandi, C.M.; Drago, F.; Bucolo, C. Effects of vitamin D3 and meso-zeaxanthin on human retinal pigmented epithelial cells in three integrated in vitro paradigms of age-related macular degeneration. Front. Pharmacol. 2021, 12, 778165. [Google Scholar] [CrossRef]
- Kane Kane, L.P.; Shapiro, V.S.; Stokoe, D.; Weiss, A. Induction of NF-kappaB by the Akt/PKB kinase. Curr. Biol. 1999, 9, 601–604. [Google Scholar] [CrossRef]
- Lazzara, F.; Fidilio, A.; Platania, C.B.M.; Giurdanella, G.; Salomone, S.; Leggio, G.M.; Tarallo, V.; Cicatiello, V.; De Falco, S.; Eandi, C.M.; et al. Aflibercept regulates retinal inflammation elicited by high glucose via the PlGF/ERK pathway. Biochem. Pharmacol. 2019, 168, 341–351. [Google Scholar] [CrossRef]
- Lazzara, F.; Longo, A.M.; Giurdanella, G.; Lupo, G.; Platania, C.B.M.; Rossi, S.; Drago, F.; Anfuso, C.D.; Bucolo, C. Vitamin D3 preserves blood retinal barrier integrity in an in vitro model of diabetic retinopathy. Front. Pharmacol. 2022, 13, 971164. [Google Scholar] [CrossRef]
- Dasu, M.R.; Devaraj, S.; Zhao, L.; Hwang, D.H.; Jialal, I. High glucose induces toll-like receptor expression in human monocytes: Mechanism of activation. Diabetes 2008, 57, 3090–3098. [Google Scholar] [CrossRef]
- Boaru, S.G.; Borkham-Kamphorst, E.; Van de Leur, E.; Lehnen, E.; Liedtke, C.; Weiskirchen, R. NLRP3 inflammasome expression is driven by NF-κB in cultured hepatocytes. Biochem. Biophys. Res. Commun. 2015, 458, 700–706. [Google Scholar] [CrossRef]
- Hong, S.H.; Park, C.; Hwangbo, B.; Bang, E.J.; Kim, S.O.; Shim, J.H.; Park, S.H.; Lee, H.; Leem, S.H.; Kim, G.Y.; et al. Activation of heme oxygenase-1 is involved in the preventive effect of honokiol against oxidative damage in human retinal pigment epithelial cells. Biotechnol. Bioprocess. Eng. 2022, 27, 975–986. [Google Scholar] [CrossRef]
- Lee, S.H.; Tsutsui, M.; Matsunaga, A.; Oe, T. Lipid hydroperoxide-derived insulin resistance and its inhibition by pyridoxamine in skeletal muscle cells. Toxicol. Res. 2023, 39, 147–156. [Google Scholar] [CrossRef]
- Choi, Y.H. Tacrolimus induces apoptosis in leukemia Jurkat cells through inactivation of the reactive oxygen species-dependent phosphoinositide-3-kinase/Akt signaling pathway. Biotechnol. Bioprocess Eng. 2022, 27, 183–192. [Google Scholar] [CrossRef]
- Kim, M.Y.; Bang, E.; Hwangbo, H.; Ji, S.Y.; Kim, D.H.; Lee, H.; Park, C.; Hong, S.H.; Kim, G.Y.; Choi, Y.H. Diallyl trisulfide inhibits monosodium urate-induced NLRP3 inflammasome activation via NOX3/4-dependent mitochondrial oxidative stress in RAW 264.7 and bone marrow-derived macrophages. Phytomedicine 2023, 112, 154705. [Google Scholar] [CrossRef]
- Hwangbo, H.; Ji, S.Y.; Kim, M.Y.; Kim, S.Y.; Lee, H.; Kim, G.Y.; Kim, S.; Cheong, J.; Choi, Y.H. Anti-inflammatory effect of auranofin on palmitic acid and LPS-induced inflammatory response by modulating TLR4 and NOX4-mediated NF-κB signaling pathway in RAW 264.7 macrophages. Int. J. Mol. Sci. 2021, 22, 5920. [Google Scholar] [CrossRef]
- Lee, J.K.; Choi, W.S.; Song, J.Y.; Kwon, O.S.; Lee, Y.J.; Lee, J.S.; Lee, S.; Choi, S.R.; Lee, C.H.; Lee, J.Y. Anti-inflammatory effects of Athyrium yokoscense extract via inhibition of the Erk1/2 and NF-κB pathways in bisphenol A-stimulated A549 cells. Toxicol. Res. 2023, 39, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, C.; Kwon, D.H.; Hwangbo, H.; Kim, S.Y.; Kim, M.Y.; Ji, S.Y.; Kim, D.H.; Jeong, J.W.; Kim, G.Y.; et al. Schisandrae Fructus ethanol extract attenuates particulate matter 2.5-induced inflammatory and oxidative responses by blocking the activation of the ROS-dependent NF-κB signaling pathway. Nutr. Res. Pract. 2021, 15, 686–702. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bang, E.; Park, C.; Hwangbo, H.; Shim, J.-H.; Leem, S.-H.; Hyun, J.W.; Kim, G.-Y.; Choi, Y.H. Spermidine Attenuates High Glucose-Induced Oxidative Damage in Retinal Pigment Epithelial Cells by Inhibiting Production of ROS and NF-κB/NLRP3 Inflammasome Pathway. Int. J. Mol. Sci. 2023, 24, 10550. https://doi.org/10.3390/ijms241310550
Bang E, Park C, Hwangbo H, Shim J-H, Leem S-H, Hyun JW, Kim G-Y, Choi YH. Spermidine Attenuates High Glucose-Induced Oxidative Damage in Retinal Pigment Epithelial Cells by Inhibiting Production of ROS and NF-κB/NLRP3 Inflammasome Pathway. International Journal of Molecular Sciences. 2023; 24(13):10550. https://doi.org/10.3390/ijms241310550
Chicago/Turabian StyleBang, EunJin, Cheol Park, Hyun Hwangbo, Jung-Hyun Shim, Sun-Hee Leem, Jin Won Hyun, Gi-Young Kim, and Yung Hyun Choi. 2023. "Spermidine Attenuates High Glucose-Induced Oxidative Damage in Retinal Pigment Epithelial Cells by Inhibiting Production of ROS and NF-κB/NLRP3 Inflammasome Pathway" International Journal of Molecular Sciences 24, no. 13: 10550. https://doi.org/10.3390/ijms241310550
APA StyleBang, E., Park, C., Hwangbo, H., Shim, J.-H., Leem, S.-H., Hyun, J. W., Kim, G.-Y., & Choi, Y. H. (2023). Spermidine Attenuates High Glucose-Induced Oxidative Damage in Retinal Pigment Epithelial Cells by Inhibiting Production of ROS and NF-κB/NLRP3 Inflammasome Pathway. International Journal of Molecular Sciences, 24(13), 10550. https://doi.org/10.3390/ijms241310550









