Chitosan-Based Biomaterials for Hemostatic Applications: A Review of Recent Advances
Abstract
1. Introduction
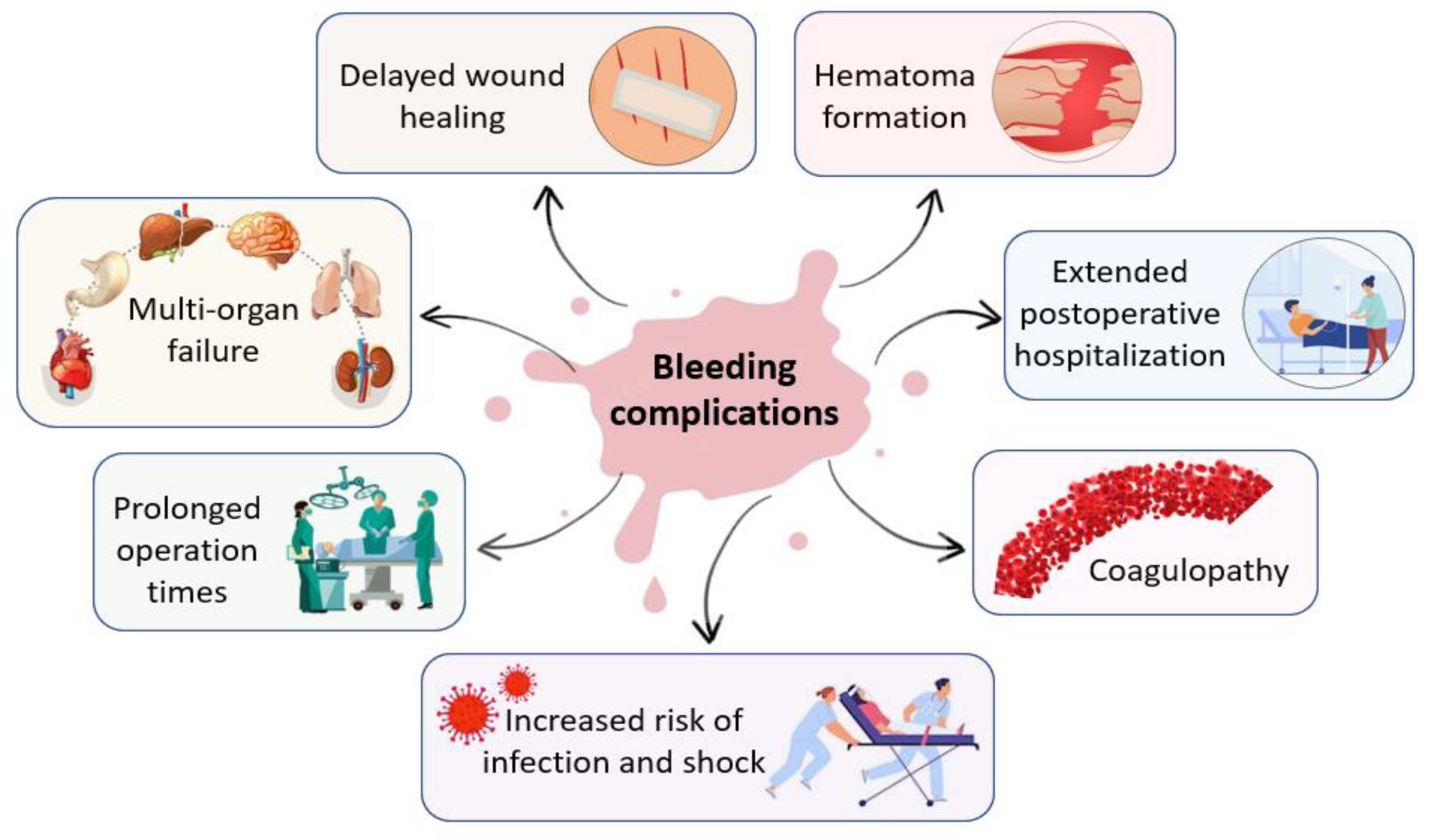
1.1. Hemorrhage in Surgical and Trauma Setting
1.2. Achieving Hemostasis
2. Chitosan Properties and Hemostasis Efficiency
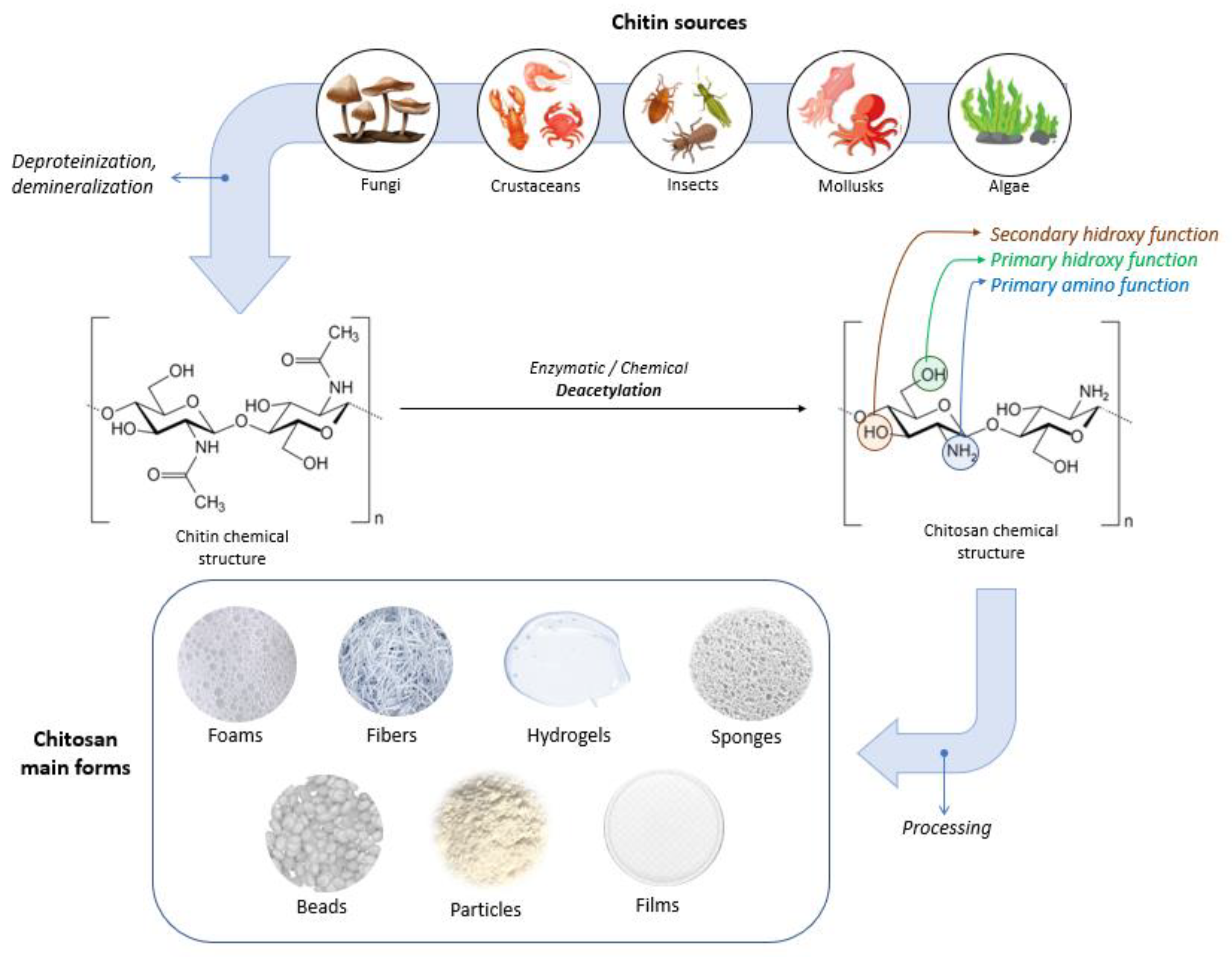
2.1. Chitosan Source and Structure
2.2. Chitosan Properties
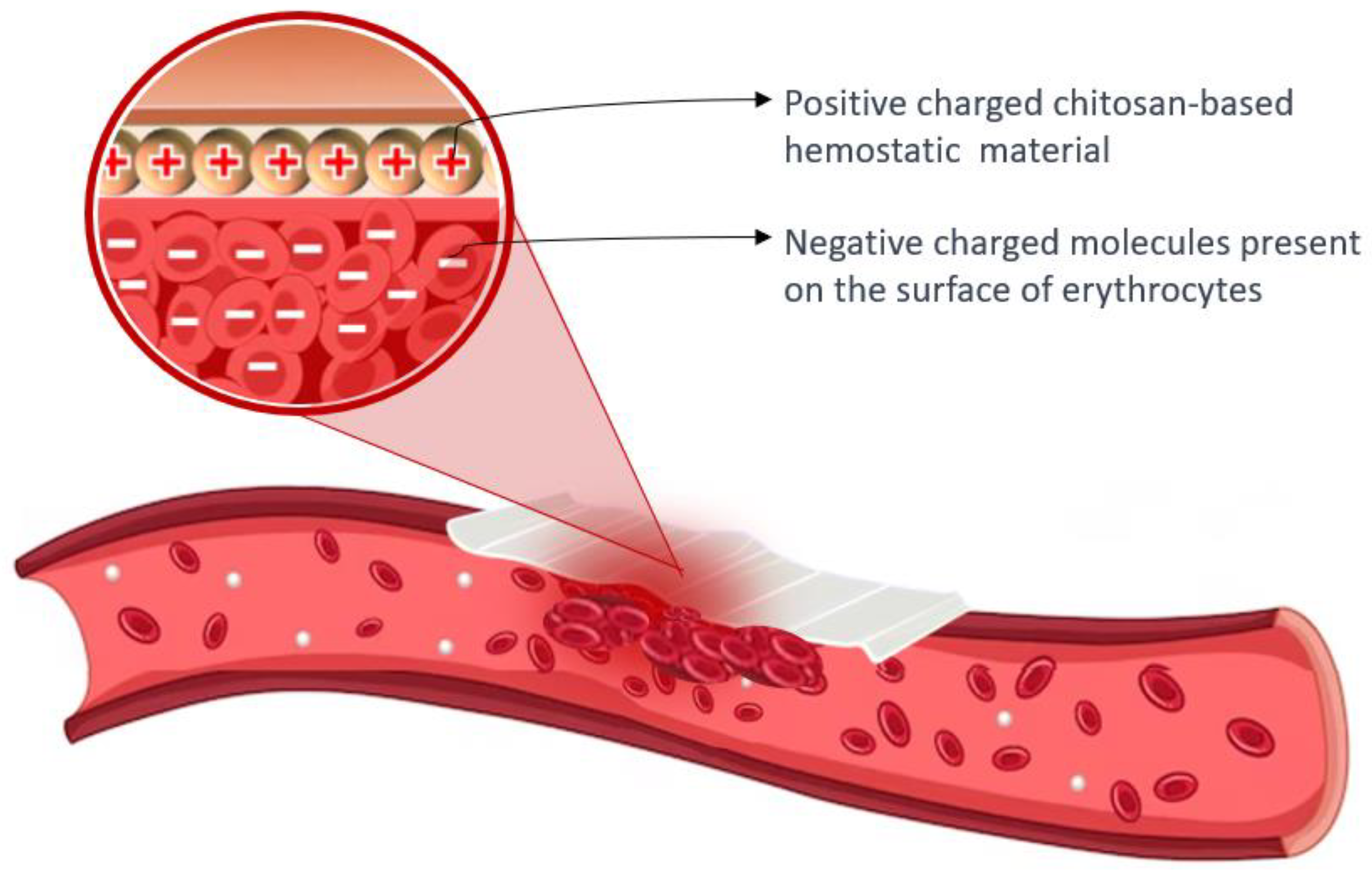
2.3. Hemostatic Application of Chitosan
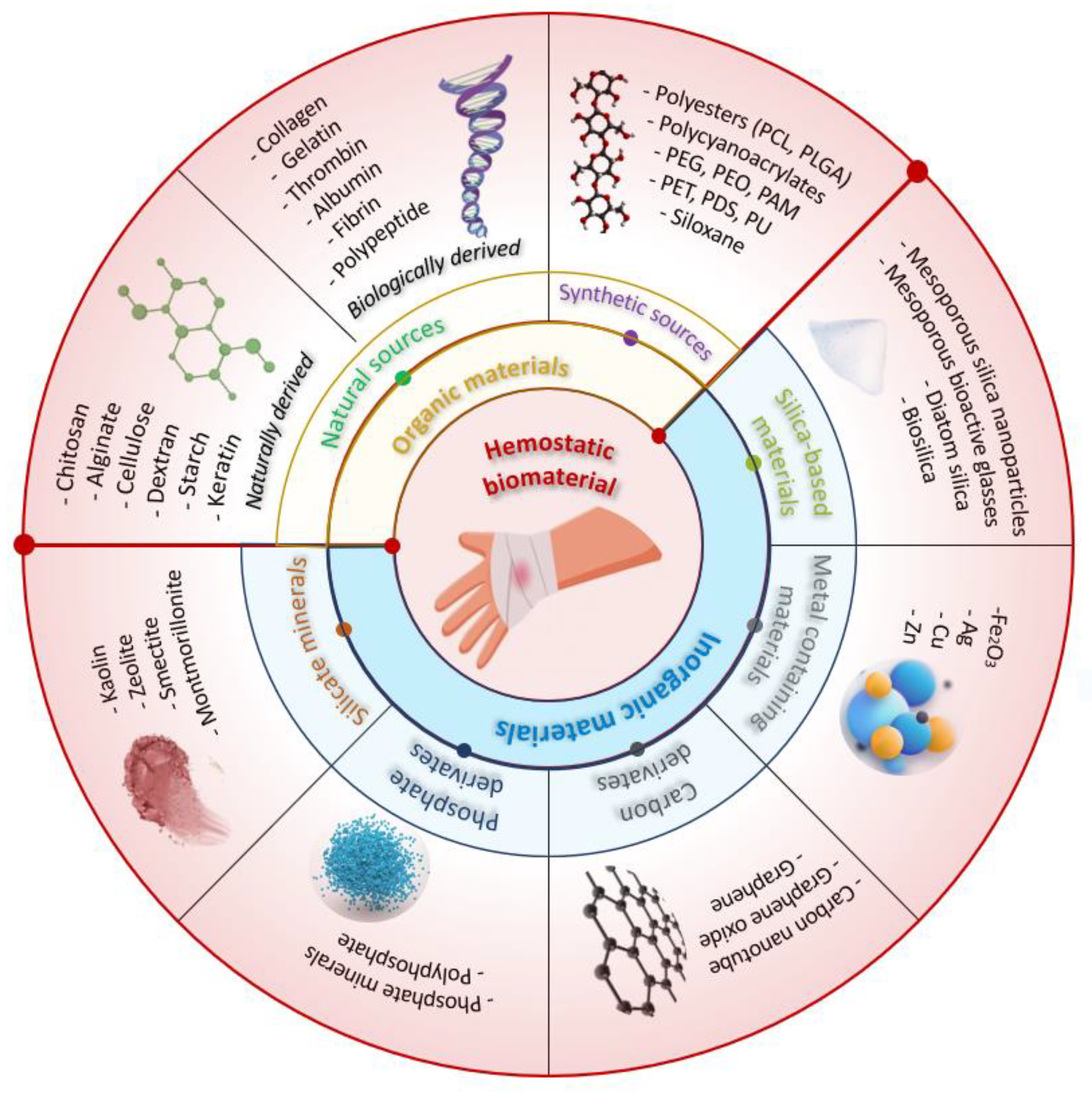
3. Current Trends in Chitosan-Based Hemostatics
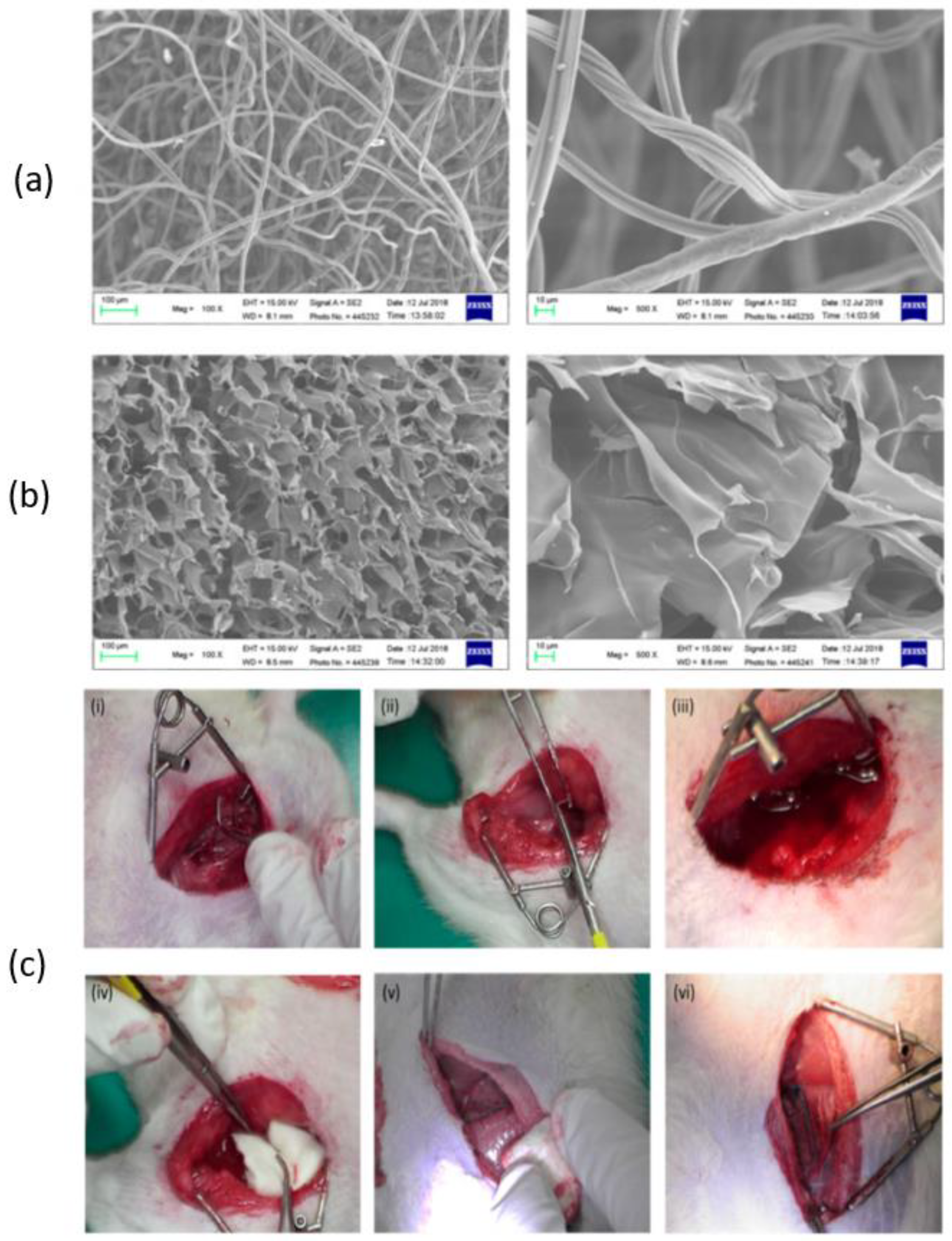
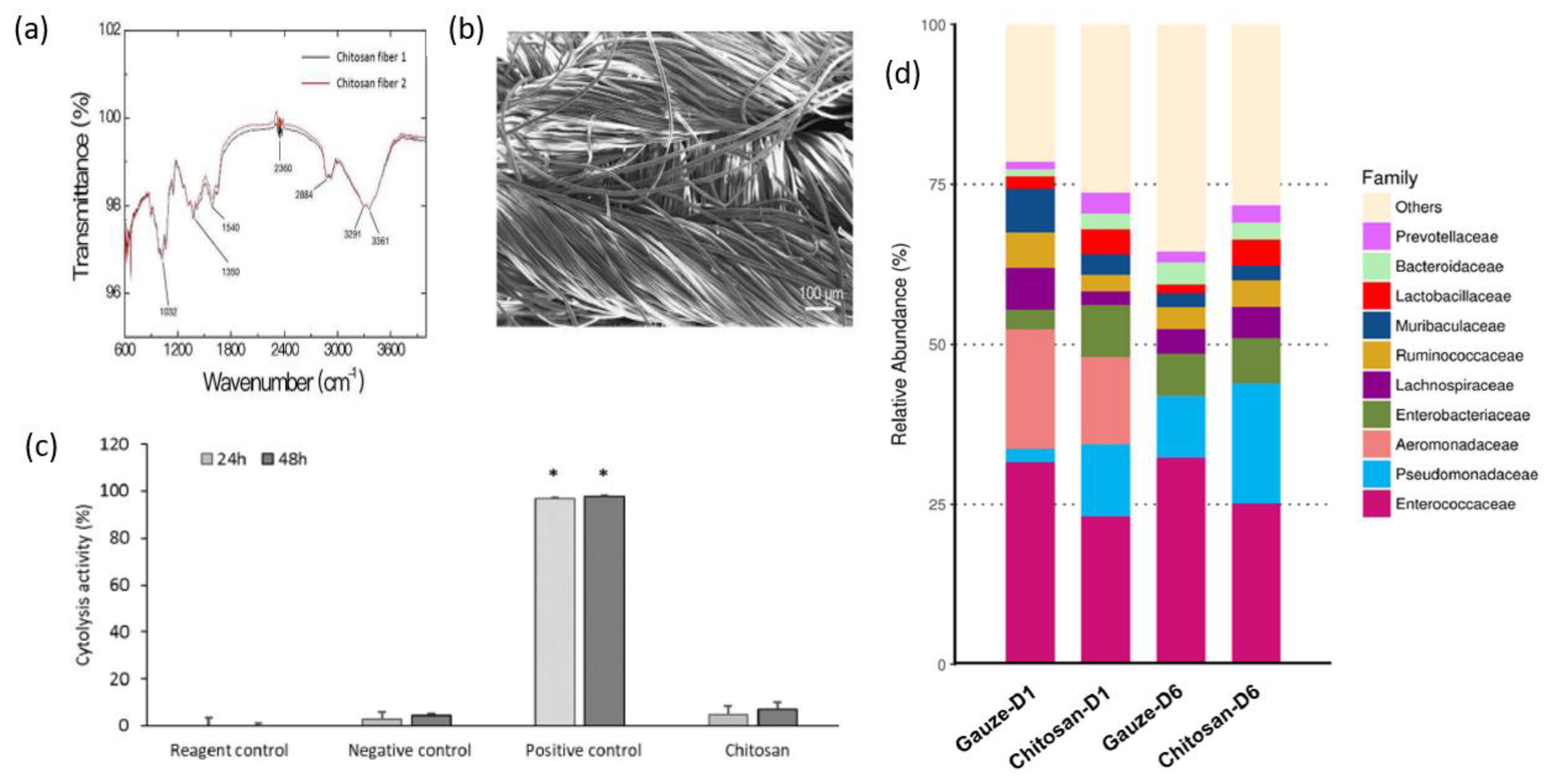
3.1. Dressing
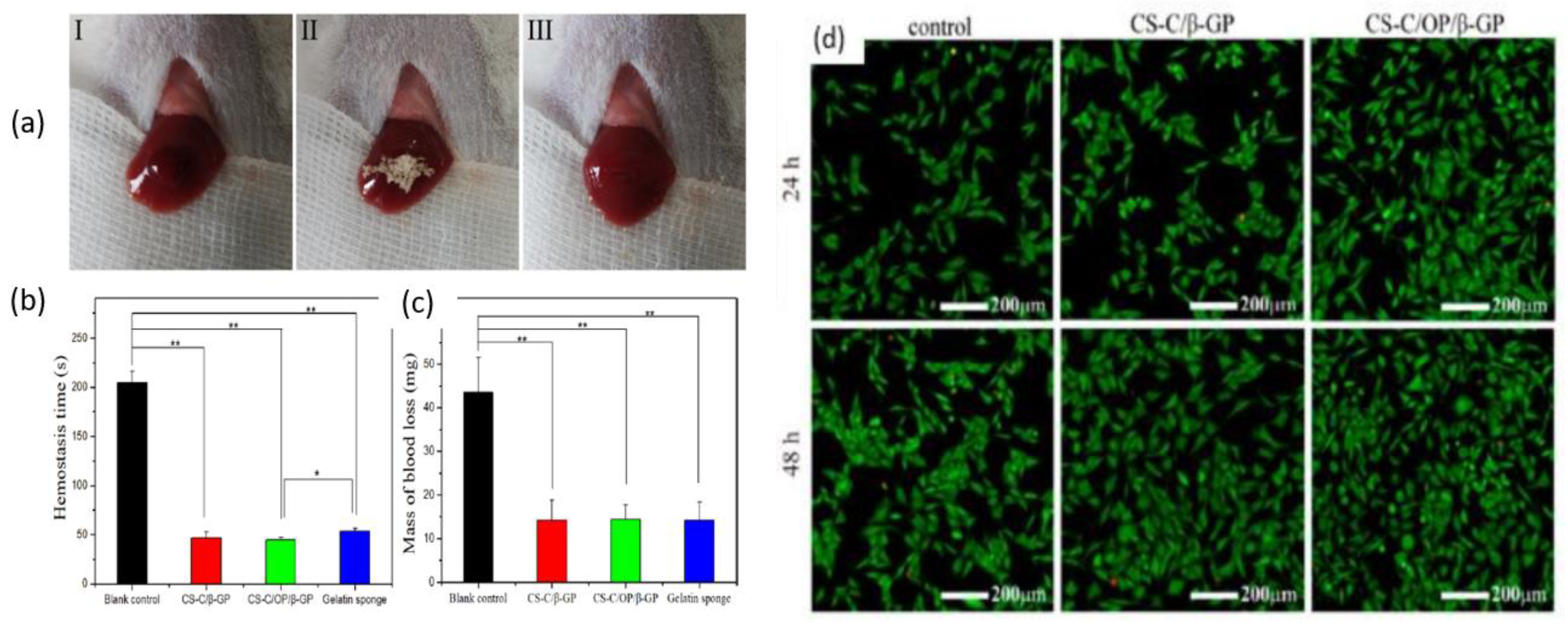
3.2. Hydrogel
3.3. Sponge
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malik, A.; Rehman, F.U.; Shah, K.U.; Naz, S.S.; Qaisar, S. Hemostatic Strategies for Uncontrolled Bleeding: A Comprehensive Update. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.; Fitzgerald, A.; Tsuzuki, T. The Role of Nanoscale Structures in the Development of Topical Hemostatic Agents. Mater. Today Nano 2021, 16, 100137. [Google Scholar] [CrossRef]
- Moore, E.E.; Moore, H.B.; Kornblith, L.Z.; Neal, M.D.; Hoffman, M.; Mutch, N.J.; Schöchl, H.; Hunt, B.J.; Sauaia, A. Trauma-Induced Coagulopathy. Nat. Rev. Dis. Prim. 2021, 7, 30. [Google Scholar] [CrossRef]
- Yesudasan, S.; Averett, R.D. Recent Advances in Computational Modeling of Fibrin Clot Formation: A Review. Comput. Biol. Chem. 2019, 83, 107148. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Palmer, A.J.R.; Klein, A.A. Strategies to Minimize Intraoperative Blood Loss during Major Surgery. Br. J. Surg. 2020, 107, e26–e38. [Google Scholar] [CrossRef] [PubMed]
- Renkens, K.L.; Payner, T.D.; Leipzig, T.J.; Feuer, H.; Morone, M.A.; Koers, J.M.; Lawson, K.J.; Lentz, R.; Shuey, H.; Conaway, G.L.; et al. A Multicenter, Prospective, Randomized Trial Evaluating a New Hemostatic Agent for Spinal Surgery. Spine 2001, 26, 1645–1650. [Google Scholar] [CrossRef]
- Moldovan, H.; Antoniac, I.; Gheorghiță, D.; Safta, M.S.; Preda, S.; Broască, M.; Badilă, E.; Fronea, O.; Scafa-Udrişte, A.; Cacoveanu, M.; et al. Biomaterials as Haemostatic Agents in Cardiovascular Surgery: Review of Current Situation and Future Trends. Polymers 2022, 14, 1189. [Google Scholar] [CrossRef]
- Han, W.; Wang, S. Advances in Hemostatic Hydrogels That Can Adhere to Wet Surfaces. Gels 2022, 9, 2. [Google Scholar] [CrossRef]
- Dang, N.C.; Ardehali, A.; Bruckner, B.A.; Parrino, P.E.; Gillen, D.L.; Hoffman, R.W.; Spotnitz, R.; Cavoores, S.; Shorn, I.J.; Manson, R.J.; et al. Prospective, Multicenter, Randomized, Controlled Trial Evaluating the Performance of a Novel Combination Powder vs. Hemostatic Matrix in Cardiothoracic Operations. J. Card. Surg. 2020, 35, 313–319. [Google Scholar] [CrossRef]
- Agarwal, R.; Niezgoda, J.; Niezgoda, J.; Madetipati, N.; Gopalakrishnan, S. Advances in Hemostatic Wound Dressings: Clinical Implications and Insight. Adv. Skin Wound Care 2022, 35, 113–121. [Google Scholar] [CrossRef]
- Moldovan, H.; Gheorghita, D.; Antoniac, I.; Gheorghe, D.; Fiori, F.; Mohan, A.; Raftu, G.; Ionel, C.; Costache, V. Bioadhesives Used in Cardiovascular Surgery. Rev. Chim. 2018, 69, 2799–2803. [Google Scholar] [CrossRef]
- Park, S.M.; Kang, D.R.; Lee, J.H.; Jeong, Y.H.; Shin, D.A.; Yi, S.; Ha, Y.; Kim, K.N. Efficacy and Safety of a Thrombin-Containing Collagen-Based Hemostatic Agent in Spinal Surgery: A Randomized Clinical Trial. World Neurosurg. 2021, 154, e215–e221. [Google Scholar] [CrossRef]
- Scridon, A. Platelets and Their Role in Hemostasis and Thrombosis—From Physiology to Pathophysiology and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 12772. [Google Scholar] [CrossRef]
- Wang, L.; Hao, F.; Tian, S.; Dong, H.; Nie, J.; Ma, G. Targeting Polysaccharides Such as Chitosan, Cellulose, Alginate and Starch for Designing Hemostatic Dressings. Carbohydr. Polym. 2022, 291, 119574. [Google Scholar] [CrossRef]
- Li, L.; Du, Y.; Yin, Z.; Li, L.; Peng, H.; Zheng, H.; Yang, A.; Li, H.; Lv, G. Preparation and the Hemostatic Property Study of Porous Gelatin Microspheres Both in Vitro and in Vivo. Colloids Surf. B. Biointerfaces 2020, 187, 110641. [Google Scholar] [CrossRef]
- Singh Chandel, A.K.; Ohta, S.; Taniguchi, M.; Yoshida, H.; Tanaka, D.; Omichi, K.; Shimizu, A.; Isaji, M.; Hasegawa, K.; Ito, T. Balance of Antiperitoneal Adhesion, Hemostasis, and Operability of Compressed Bilayer Ultrapure Alginate Sponges. Biomater. Adv. 2022, 137, 212825. [Google Scholar] [CrossRef]
- Hajosch, R.; Suckfuell, M.; Oesser, S.; Ahlers, M.; Flechsenhar, K.; Schlosshauer, B. A Novel Gelatin Sponge for Accelerated Hemostasis. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 372–379. [Google Scholar] [CrossRef]
- Antoniac, I. (Ed.) Biologically Responsive Biomaterials for Tissue Engineering; Springer: New York, NY, USA, 2013; Volume 1, ISBN 978-1-4614-4327-8. [Google Scholar]
- Zheng, Y.; Wu, J.; Zhu, Y.; Wu, C. Inorganic-Based Biomaterials for Rapid Hemostasis and Wound Healing. Chem. Sci. 2022, 14, 29–53. [Google Scholar] [CrossRef]
- Baharlouei, P.; Rahman, A. Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing. Mar. Drugs 2022, 20, 460. [Google Scholar] [CrossRef]
- Mecwan, M.; Li, J.; Falcone, N.; Ermis, M.; Torres, E.; Morales, R.; Hassani, A.; Haghniaz, R.; Mandal, K.; Sharma, S.; et al. Recent Advances in Biopolymer-Based Hemostatic Materials. Regen. Biomater. 2022, 9, rbac063. [Google Scholar] [CrossRef]
- Feng, Y.; He, Y.; Lin, X.; Xie, M.; Liu, M.; Lvov, Y. Assembly of Clay Nanotubes on Cotton Fibers Mediated by Biopolymer for Robust and High-Performance Hemostatic Dressing. Adv. Health Mater. 2023, 12, 2202265. [Google Scholar] [CrossRef]
- Shakiba-Marani, R.; Ehtesabi, H. A Flexible and Hemostatic Chitosan, Polyvinyl Alcohol, Carbon Dot Nanocomposite Sponge for Wound Dressing Application. Int. J. Biol. Macromol. 2023, 224, 831–839. [Google Scholar] [CrossRef]
- Patil, G.; Torris, A.; Suresha, P.R.; Jadhav, S.; Badiger, M.V.; Ghormade, V. Design and Synthesis of a New Topical Agent for Halting Blood Loss Rapidly: A Multimodal Chitosan-Gelatin Xerogel Composite Loaded with Silica Nanoparticles and Calcium. Colloids Surf. B Biointerfaces 2021, 198, 111454. [Google Scholar] [CrossRef]
- Sun, X.; Fang, Y.; Tang, Z.; Wang, Z.; Liu, X.; Liu, H. Mesoporous Silica Nanoparticles Carried on Chitosan Microspheres for Traumatic Bleeding Control. Int. J. Biol. Macromol. 2019, 127, 311–319. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, M.; Liu, Q.; Liu, G.; Wang, S.; Li, J. Recent Advances in the Medical Applications of Hemostatic Materials. Theranostics 2023, 13, 161–196. [Google Scholar] [CrossRef]
- Behrens, A.M.; Sikorski, M.J.; Kofinas, P. Hemostatic Strategies for Traumatic and Surgical Bleeding. J. Biomed. Mater. Res. A 2014, 102, 4182–4194. [Google Scholar] [CrossRef]
- Koumentakou, I.; Terzopoulou, Z.; Michopoulou, A.; Kalafatakis, I.; Theodorakis, K.; Tzetzis, D.; Bikiaris, D. Chitosan Dressings Containing Inorganic Additives and Levofloxacin as Potential Wound Care Products with Enhanced Hemostatic Properties. Int. J. Biol. Macromol. 2020, 162, 693–703. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef]
- Ouyang, Q.; Hou, T.; Li, C.; Hu, Z.; Liang, L.; Li, S.; Zhong, Q.; Li, P. Construction of a composite sponge containing tilapia peptides and chitosan with improved hemostatic performance. Int. J. Biol. Macromol. 2019, 139, 719–729. [Google Scholar] [CrossRef]
- Uranues, S.; Fingerhut, A.; Levin, E.; Spazierer, D.; Rahimi, N.; Baumgartner, B. Effectiveness of Hemopatch® versus Surgicel® Original to Control Mild and Moderate Liver Bleeding. BMC Surg. 2022, 22, 316. [Google Scholar] [CrossRef]
- Ponsen, A.C.; Proust, R.; Soave, S.; Mercier-Nomé, F.; Garcin, I.; Combettes, L.; Lataillade, J.J.; Uzan, G. A New Hemostatic Agent Composed of Zn2+-Enriched Ca2+ Alginate Activates Vascular Endothelial Cells in Vitro and Promotes Tissue Repair in Vivo. Bioact. Mater. 2022, 18, 368–382. [Google Scholar] [CrossRef]
- Kliuk-Ben Bassat, O.; Schwartz, D.; Zubkov, A.; Gal-Oz, A.; Gorevoy, A.; Romach, I.; Grupper, A. WoundClot® Hemostatic Gauze Reduces Bleeding Time after Arterial Venous Fistula Decannulation. Blood Purif. 2021, 50, 952–958. [Google Scholar] [CrossRef]
- Mena-Álvarez, J.; Quispe-López, N.; Zubizarreta-Macho, Á.; Rico-Romano, C.; Rodero-Villanueva, R.; Fernández-Aceñero, M.J. Histological Analysis of Different Local Haemostatic Agents Used for Periapical Surgery: An Experimental Study with Sprague-Dawley Rats. Aust. Endod. J. 2019, 45, 357–364. [Google Scholar] [CrossRef]
- Rao, K.; Gomati, A.; Yuen Hao Tong, E.; Ah-See, K.W.; Shakeel, M. Use of PerClot® in Head and Neck Surgery: A Scottish Centre Experience. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 1965–1969. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Wang, H.J.; Raza, A.; Liu, C.; Yu, J.; Wang, J.Y. Preparation and Evaluation of Chitosan/Polyvinylpyrrolidone/Zein Composite Hemostatic Sponges. Int. J. Biol. Macromol. 2022, 205, 110–117. [Google Scholar] [CrossRef]
- Capella-Monsonís, H.; Shridhar, A.; Chirravuri, B.; Figucia, M.; Learn, G.; Greenawalt, K.; Badylak, S.F. A Comparative Study of the Resorption and Immune Response for Two Starch-Based Hemostat Powders. J. Surg. Res. 2023, 282, 210–224. [Google Scholar] [CrossRef]
- Huang, W.; Wu, J.; Huang, Z.; Zhang, D.; Chen, F.; Liu, C. A Self-Gelling Starch-Based Sponge for Hemostasis. J. Mater. Chem. B 2022, 11, 1331–1343. [Google Scholar] [CrossRef]
- Karimi, N.; Amooee, A.; SafiDahaj, F. Comparison of the Effect of PerClot® Powder and a Chitosan Derivative on Postoperative Intra-Abdominal Adhesions in Rat Animal Models. Pol. Przegląd Chir. 2021, 94, 27–31. [Google Scholar] [CrossRef]
- Goldis, A.; Goldis, R.; Chirila, T.V. Biomaterials in Gastroenterology: A Critical Overview. Medicina 2019, 55, 734. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, N.; Sun, H.; Hou, J.; Zhang, Y.; Wang, D.; Zhang, W.; Chen, Z. Advances in the Development and Optimization Strategies of the Hemostatic Biomaterials. Front. Bioeng. Biotechnol. 2023, 10, 1062676. [Google Scholar] [CrossRef]
- Cziperle, D.J. AviteneTM Microfibrillar Collagen Hemostat for Adjunctive Hemostasis in Surgical Procedures: A Systematic Literature Review. Med. Devices Evid. Res. 2021, 14, 155–163. [Google Scholar] [CrossRef]
- Zhong, Y.; Hu, H.; Min, N.; Wei, Y.; Li, X.; Li, X. Application and Outlook of Topical Hemostatic Materials: A Narrative Review. Ann. Transl. Med. 2021, 9, 577. [Google Scholar] [CrossRef]
- Ghimire, S.; Sarkar, P.; Rigby, K.; Maan, A.; Mukherjee, S.; Crawford, K.E.; Mukhopadhyay, K. Polymeric Materials for Hemostatic Wound Healing. Pharmaceutics 2021, 13, 2127. [Google Scholar] [CrossRef]
- Pereira, B.M.; Bortoto, J.B.; Fraga, G.P. Topical Hemostatic Agents in Surgery: Review and Prospects. Rev. Col. Bras. Cir. 2018, 45, 5. [Google Scholar]
- Salama, N.M.; Tabashy, R.H.; Mahmoud, I.H.; El Rahman, A.E.R.M.A.; Mohamed, D.N.E.; El Kassas, H. Does Gelfoam Slurry Embolization Post-Pulmonary Biopsy Reduce Risk of Pneumothorax? A Prospective Randomized Control Study. Egypt. J. Radiol. Nucl. Med. 2023, 54, 4. [Google Scholar] [CrossRef]
- Sekyi-Djan, N.; Chapple, C.; Hammond-Kenny, A.; Hilger, A. The Use of ARTISS Fibrin Sealant in Thyroid Surgery: Case Series and Review of the Literature. Br. J. Surg. 2022, 109, 1130. [Google Scholar] [CrossRef]
- Somani, S.N.; Moshirfar, M.; Shmunes, K.M.; Ronquillo, Y.C. Comparison and Application of Commercially Available Fibrin Sealants in Ophthalmology. Ocul. Surf. 2020, 18, 418–426. [Google Scholar] [CrossRef]
- Jolly, K.; Gupta, K.K.; Egbuji, O.; Naik, P.P.; Ahmed, S.K. Endoscopic Transsphenoidal Surgery Reconstruction Using the Fibrin Sealant Patch Tachosil®. Br. J. Neurosurg. 2021, 1–10. [Google Scholar] [CrossRef]
- Carretta, A.; Epskamp, M.; Ledermann, L.; Staartjes, V.E.; Neidert, M.C.; Regli, L.; Stienen, M.N. Collagen-Bound Fibrin Sealant (TachoSil®) for Dural Closure in Cranial Surgery: Single-Centre Comparative Cohort Study and Systematic Review of the Literature. Neurosurg. Rev. 2022, 45, 3779–3788. [Google Scholar] [CrossRef]
- Eichinger, J.K.; Oldenburg, K.S.; Lin, J.; Wilkie, E.; Mock, L.; Tavana, M.L.; Friedman, R.J. Comparing Dermabond PRINEO versus Dermabond or Staples for Wound Closure: A Randomized Control Trial Following Total Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2022, 31, 2066–2075. [Google Scholar] [CrossRef]
- Mirzaei, Y.; Hagemeister, K.; Tolba, R.H.; Steitz, J. Novel In Vitro Study to Assess Microbial Barrier Properties of Polyurethane-Based Tissue Adhesives in Comparison to the Gold Standard Dermabond®. Biomed. Res. Int. 2022, 2022, 5249214. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Liu, Z.; Kong, J.; Zhao, B.; He, X.; Gu, J.; Su, H. Transcatheter Arterial Embolization Using N-Butyl-2 Cyanoacrylate Glubran® 2 for Acute Massive Pancreati Coduodenal Arterial Hemorrhage. Front. Mater. 2022, 9, 1003539. [Google Scholar] [CrossRef]
- Slezak, P.; Klang, A.; Ferguson, J.; Monforte, X.; Schmidt, P.; Bauder, B.; Url, A.; Osuchowski, M.; Redl, H.; Spazierer, D.; et al. Tissue Reactions to Polyethylene Glycol and Glutaraldehyde-Based Surgical Sealants in a Rabbit Aorta Model. J. Biomater. Appl. 2020, 34, 1330–1340. [Google Scholar] [CrossRef]
- Dhandapani, V.; Ringuette, V.; Desrochers, M.; Sirois, M.; Vermette, P. Composition, Host Responses and Clinical Applications of Bioadhesives. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2779–2797. [Google Scholar] [CrossRef]
- Keskin, E.; Aydin, H.A.; Kalayci, M.; Işik, E.; Özgen, U.; Şimşek, K.; Baklaci, D.; Gökçe, M. The Histopathological Effects of Reabsorbable Polyethylene Glycol Hydrogel (Coseal) on Epidural Fibrosis in an Experimental Postlaminectomy Model in Rats. Turk. J. Med. Sci. 2021, 51, 1512–1520. [Google Scholar] [CrossRef]
- Li, X.F.; Lu, P.; Jia, H.R.; Li, G.; Zhu, B.; Wang, X.; Wu, F.G. Emerging Materials for Hemostasis. Coord. Chem. Rev. 2023, 475, 214823. [Google Scholar] [CrossRef]
- Ohlinger, R.; Rutkowski, R.; Kohlmann, T.; Paepke, S.; Alwafai, Z.; Flieger, C.; Moller, S.; Lenz, F.; Zygmunt, M.; Unger, J. Impact of the Lysine-Urethane Adhesive Tissuglu® on Postoperative Complications and Interventions after Drain-Free Mastectomy. Anticancer Res. 2020, 40, 2801–2812. [Google Scholar] [CrossRef]
- Yılmaz, G.; Özdenkaya, Y.; Karatepe, O.; Tanrıkulu, Y.; Kamalı, G.; Yalçın, O. Effects of Polyurethane Membrane on Septic Colon Anastomosis and Intra-Abdominal Adhesions. Turk. J. Trauma Emerg. Surg. 2021, 27, 1–8. [Google Scholar] [CrossRef]
- Kim, K.; Siddiqui, Z.; Acevedo-Jake, A.M.; Roy, A.; Choudhury, M.; Grasman, J.; Kumar, V. Angiogenic Hydrogels to Accelerate Early Wound Healing. Macromol. Biosci. 2022, 22, 2200067. [Google Scholar] [CrossRef]
- Meng, F.C.; Lee, C.Y. Safety and Efficiency of Femoral Artery Access Closure Using QuikClot Combat Gauze in Patients with Severe Arterial Calcification of Access Sites. Quant. Imaging Med. Surg. 2023, 13, 282–292. [Google Scholar] [CrossRef]
- Jia, Y.J.; Du, W.Q.; Zong, Z.W.; Jiang, R.Q.; Zhong, X.; Ye, Z.; Li, T.S.; Yang, H.Y.; Xiao, L.P.; Fan, J. Hemostatic Effects of Bio-Zeolite Gauze and QuikClot Combat Gauze on Major Bleeding in Rabbits Acutely Exposed to High Altitude. Prehosp. Emerg. Care 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wei, X.; Yang, K.; Lin, S.; Tian, F.; Li, F. Ca-Ga Double Doping Strategy to Fabricate Hemostatic Mesoporous Silica Nanoparticles (MSN) with Antibacterial Activity. Silicon 2020, 13, 4033–4045. [Google Scholar] [CrossRef]
- Pogorielov, M.V.; Sikora, V.Z. Chitosan as a Hemostatic Agent: Current State. Eur. J. Medicine. Ser. B 2015, 2, 24–33. [Google Scholar] [CrossRef]
- Biranje, S.S.; Sun, J.; Shi, Y.; Yu, S.; Jiao, H.; Zhang, M.; Wang, Q.; Wang, J.; Liu, J. Polysaccharide-Based Hemostats: Recent Developments, Challenges, and Future Perspectives. Cellulose 2021, 28, 8899–8937. [Google Scholar] [CrossRef]
- Khan, M.A.; Mujahid, M. A Review on Recent Advances in Chitosan Based Composite for Hemostatic Dressings. Int. J. Biol. Macromol. 2019, 124, 138–147. [Google Scholar] [CrossRef]
- Khoshmohabat, H.; Paydar, S.; Kazemi, H.M.; Dalfardi, B. Overview of Agents Used for Emergency Hemostasis. Trauma. Mon. 2016, 21, e26023. [Google Scholar] [CrossRef]
- Milić, M.; Vuković, B.; Barbir, R.; Pem, B.; Milić, M.; Šerić, V.; Frőhlich, E.; Vinković Vrček, I. Effect of Differently Coated Silver Nanoparticles on Hemostasis. Platelets 2021, 32, 651–661. [Google Scholar] [CrossRef]
- Fernando, L.; Chen, W.-T.; Lai, C.-W.; Ye, F.-Y.; Lai, P.-S.; Lin, J.-J.; Yasuda, K.; Song, T.-T.; Song, J.-M. Biocompatibility and Antimicrobial Activity of Copper(II) Oxide Hybridized with Nano Silicate Platelets. Surf. Coat. Technol. 2022, 435, 128253. [Google Scholar] [CrossRef]
- Milić, M.; Cvetić, Ž.; Bendelja, K.; Vuković, B.; Galić, E.; Ćurlin, M.; Dobrošević, B.; Jurak Begonja, A.; Vinković Vrček, I. Response of Platelets to Silver Nanoparticles Designed with Different Surface Functionalization. J. Inorg. Biochem. 2021, 224, 111565. [Google Scholar] [CrossRef]
- Metwally, W.M.; El-Habashy, S.E.; El-Nikhely, N.A.; Mahmoud, H.E.; Eltaher, H.M.; El-Khordagui, L. Nano Zinc Oxide-Functionalized Nanofibrous Microspheres: A Bioactive Hybrid Platform with Antimicrobial, Regenerative and Hemostatic Activities. Int. J. Pharm. 2023, 638, 122920. [Google Scholar] [CrossRef]
- Shefa, A.A.; Taz, M.; Hossain, M.; Kim, Y.S.; Lee, S.Y.; Lee, B.-T. Investigation of Efficiency of a Novel, Zinc Oxide Loaded TEMPO-Oxidized Cellulose Nanofiber Based Hemostat for Topical Bleeding. Int. J. Biol. Macromol. 2019, 126, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Lee, J.; Lee, S.Y.; Lee, H.; Chathuranga, K.; Lee, J.; Park, W. Silk Fibroin/Tannin/ZnO Nanocomposite Hydrogel with Hemostatic Activities. Gels 2022, 8, 650. [Google Scholar] [CrossRef] [PubMed]
- Allotey, J.K.; King, A.H.; Kumins, N.H.; Wong, V.L.; Harth, K.C.; Cho, J.S.; Kashyap, V.S. Systematic Review of Hemostatic Agents Used in Vascular Surgery. J. Vasc. Surg. 2021, 73, 2189–2197. [Google Scholar] [CrossRef]
- Tompeck, A.J.; Gajdhar, A.u.R.; Dowling, M.; Johnson, S.B.; Barie, P.S.; Winchell, R.J.; King, D.; Scalea, T.M.; Britt, L.D.; Narayan, M. A Comprehensive Review of Topical Hemostatic Agents: The Good, the Bad, and the Novel. J. Trauma Acute Care Surg. 2020, 88, e1–e21. [Google Scholar] [CrossRef] [PubMed]
- Baxter. Coseal. Available online: https://globaladvancedsurgery.baxter.com/coseal (accessed on 6 June 2023).
- Esutures. Available online: https://www.Esutures.Com/Product/0-in-Date/84-Baxter/739-Coseal/29008-Baxter-Coseal-Surgical-Sealant-4ml-934071/ (accessed on 6 June 2023).
- Sorbus. Celox Haemostatic Granules. Available online: https://Www.Sorbus-Intl.Co.Uk/Celox-Haemostatic-Granules-15g-Pack (accessed on 6 June 2023).
- Chen, Y.; Wu, L.; Li, P.; Hao, X.; Yang, X.; Xi, G.; Liu, W.; Feng, Y.; He, H.; Shi, C. Polysaccharide Based Hemostatic Strategy for Ultrarapid Hemostasis. Macromol. Biosci. 2020, 20, 1900370. [Google Scholar] [CrossRef]
- Global Hemostats Market—Industry Trends and Forecast to 2029. Available online: Https://Www.Databridgemarketresearch.Com/Reports/Global-Hemostats-Market (accessed on 6 June 2023).
- Iannitti, D.A.; Kim, C.; Ito, D.; Epstein, J. Impact of an Active Hemostatic Product Treatment Approach on Bleeding-Related Complications and Hospital Costs among Inpatient Surgeries in the United States. J. Med. Econ. 2021, 24, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Pennington, Z.; Ehresman, J.; Westbroek, E.M.; Lubelski, D.; Cottrill, E.; Sciubba, D.M. Interventions to Minimize Blood Loss and Transfusion Risk in Spine Surgery: A Narrative Review. Clin. Neurol. Neurosurg. 2020, 196, 106004. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R.; Daniel, E.-A.-K. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411. [Google Scholar]
- Al-Rooqi, M.M.; Hassan, M.M.; Moussa, Z.; Obaid, R.J.; Suman, N.H.; Wagner, M.H.; Natto, S.S.A.; Ahmed, S.A. Advancement of Chitin and Chitosan as Promising Biomaterials. J. Saudi Chem. Soc. 2022, 26, 101561. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Truşcă, R.D.; Ilie, C.I.; Oprea, O.C.; Andronescu, E. Innovative Antimicrobial Chitosan/Zno/Ag Nps/Citronella Essential Oil Nanocomposite—Potential Coating for Grapes. Foods 2020, 9, 1801. [Google Scholar] [CrossRef]
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of Chitosan and Its Derivatives for Biomedical Applications in the Central Nervous System. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Mar. Drugs 2020, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; El-Zairy, E.M.R. Chitosan as a Biomaterial—Structure, Properties, and Electrospun Nanofibers. In Concepts, Compounds and the Alternatives of Antibacterials; InTech Open: London, UK, 2015. [Google Scholar]
- Janvikul, W.; Uppanan, P.; Thavornyutikarn, B.; Krewraing, J.; Prateepasen, R. In Vitro Comparative Hemostatic Studies of Chitin, Chitosan, and Their Derivatives. J. Appl. Polym. Sci. 2006, 102, 445–451. [Google Scholar] [CrossRef]
- Tiplea, R.E.; Lemnaru, G.M.; Trușcă, R.D.; Holban, A.; Kaya, M.G.A.; Dragu, L.D.; Ficai, D.; Ficai, A.; Bleotu, C. Antimicrobial Films Based on Chitosan, Collagen, and Zno for Skin Tissue Regeneration. Biointerface Res. Appl. Chem. 2021, 11, 11985–11995. [Google Scholar] [CrossRef]
- Radu, E.R.; Pandele, A.M.; Tuncel, C.; Miculescu, F.; Voicu, S.I. Preparation and Characterization of Chitosan/LDH Composite Membranes for Drug Delivery Application. Membranes 2023, 13, 179. [Google Scholar] [CrossRef]
- Okamoto, Y.; Yano, R.; Miyatake, K.; Tomohiro, I.; Shigemasa, Y.; Minami, S. Effects of Chitin and Chitosan on Blood Coagulation. Carbohydr. Polym. 2003, 53, 337–342. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Sustainable Agriculture Reviews 35, Chitin and Chitosan: History, Fundamentals and Innovations; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 978-3-03016-537-6. [Google Scholar]
- Spoială, A.; Ilie, C.I.; Dolete, G.; Croitoru, A.M.; Surdu, V.A.; Trușcă, R.D.; Motelica, L.; Oprea, O.C.; Ficai, D.; Ficai, A.; et al. Preparation and Characterization of Chitosan/TiO2 Composite Membranes as Adsorbent Materials for Water Purification. Membranes 2022, 12, 804. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Skin Tissue Regeneration. In Electrospinning for Tissue Regeneration; Elsevier: Amsterdam, The Netherlands, 2011; pp. 298–316. [Google Scholar]
- Chandel, A.K.S.; Shimizu, A.; Hasegawa, K.; Ito, T. Advancement of Biomaterial-Based Postoperative Adhesion Barriers. Macromol. Biosci. 2021, 21, e2000395. [Google Scholar] [CrossRef]
- Yin, M.; Wang, Y.; Zhang, Y.; Ren, X.; Qiu, Y.; Huang, T.-S. Novel Quaternarized N-Halamine Chitosan and Polyvinyl Alcohol Nanofibrous Membranes as Hemostatic Materials with Excellent Antibacterial Properties. Carbohydr. Polym. 2020, 232, 115823. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, H.; Wang, X. Synthesis and Characterization of an Injectable ε-Polylysine/Carboxymethyl Chitosan Hydrogel Used in Medical Application. Mater. Chem. Phys. 2020, 248, 122902. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.Y.; Lu, S.T.; Li, P.W.; Li, S.D. Chitosan-Based Composite Materials for Prospective Hemostatic Applications. Mar. Drugs 2018, 16, 273. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Chen, Y.C.; Lin, T.H.; Yang, C.Y.; Kuo, Y.W.; Lei, U. Hemostatic Enhancement via Chitosan Is Independent of Classical Clotting Pathways—A Quantitative Study. Polymers 2020, 12, 2391. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Lin, T.H.; Yang, C.Y.; Kuo, Y.W.; Lei, U. Mechanics for the Adhesion and Aggregation of Red Blood Cells on Chitosan. J. Mech. 2018, 34, 725–732. [Google Scholar] [CrossRef]
- Szatmári, V. Chitosan Hemostatic Dressing for Control of Hemorrhage from Femoral Arterial Puncture Site in Dogs. J. Vet. Sci. 2015, 16, 517–523. [Google Scholar] [CrossRef]
- Pan, M.; Tang, Z.; Tu, J.; Wang, Z.; Chen, Q.; Xiao, R.; Liu, H. Porous Chitosan Microspheres Containing Zinc Ion for Enhanced Thrombosis and Hemostasis. Mater. Sci. Eng. C 2018, 85, 27–36. [Google Scholar] [CrossRef]
- Karahaliloğlu, Z.; Demirbilek, M.; Ulusoy, İ.; Gümüşkaya, B.; Denkbaş, E.B. Active Nano/Microbilayer Hemostatic Agents for Diabetic Rat Bleeding Model. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1573–1585. [Google Scholar] [CrossRef]
- Wang, C.; Luo, W.; Li, P.; Li, S.; Yang, Z.; Hu, Z.; Liu, Y.; Ao, N. Preparation and Evaluation of Chitosan/Alginate Porous Microspheres/Bletilla Striata Polysaccharide Composite Hemostatic Sponges. Carbohydr. Polym. 2017, 174, 432–442. [Google Scholar] [CrossRef]
- Rodríguez-Acosta, H.; Tapia-Rivera, J.M.; Guerrero-Guzmán, A.; Hernández-Elizarraráz, E.; Hernández-Díaz, J.A.; Garza-García, J.J.O.; Pérez-Ramírez, P.E.; Velasco-Ramírez, S.F.; Ramírez-Anguiano, A.C.; Velázquez-Juárez, G.; et al. Chronic Wound Healing by Controlled Release of Chitosan Hydrogels Loaded with Silver Nanoparticles and Calendula Extract. J. Tissue Viability 2022, 31, 173–179. [Google Scholar] [CrossRef]
- Biranje, S.S.; Madiwale, P.V.; Patankar, K.C.; Chhabra, R.; Dandekar-Jain, P.; Adivarekar, R.V. Hemostasis and Anti-Necrotic Activity of Wound-Healing Dressing Containing Chitosan Nanoparticles. Int. J. Biol. Macromol. 2019, 121, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Fang, Q.Q.; Wang, X.F.; Wang, X.W.; Zhang, T.; Shi, B.H.; Zheng, B.; Zhang, D.D.; Hu, Y.Y.; Ma, L.; et al. Chitosan-Calcium Alginate Dressing Promotes Wound Healing: A Preliminary Study. Wound Repair. Regen. 2020, 28, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Kong, Y.; Shao, C.; Cheng, Y.; Lu, J.; Tao, Y.; Du, J.; Wang, H. Chitosan-Based Multifunctional Flexible Hemostatic Bio-Hydrogel. Acta Biomater. 2021, 136, 170–183. [Google Scholar] [CrossRef]
- Shou, Y.; Zhang, J.; Yan, S.; Xia, P.; Xu, P.; Li, G.; Zhang, K.; Yin, J. Thermoresponsive Chitosan/DOPA-Based Hydrogel as an Injectable Therapy Approach for Tissue-Adhesion and Hemostasis. ACS Biomater. Sci. Eng. 2020, 6, 3619–3629. [Google Scholar] [CrossRef]
- Rao, K.M.; Narayanan, K.B.; Uthappa, U.T.; Park, P.H.; Choi, I.; Han, S.S. Tissue Adhesive, Self-Healing, Biocompatible, Hemostasis, and Antibacterial Properties of Fungal-Derived Carboxymethyl Chitosan-Polydopamine Hydrogels. Pharmaceutics 2022, 14, 1028. [Google Scholar] [CrossRef]
- Xia, L.; Wang, S.; Jiang, Z.; Chi, J.; Yu, S.; Li, H.; Zhang, Y.; Li, L.; Zhou, C.; Liu, W.; et al. Hemostatic Performance of Chitosan-Based Hydrogel and Its Study on Biodistribution and Biodegradability in Rats. Carbohydr. Polym. 2021, 264, 117965. [Google Scholar] [CrossRef]
- Zhou, P.; Xia, Z.; Qi, C.; He, M.; Yu, T.; Shi, L. Construction of Chitosan/Ag Nanocomposite Sponges and Their Properties. Int. J. Biol. Macromol. 2021, 192, 272–277. [Google Scholar] [CrossRef]
- Fan, X.; Li, Y.; Li, N.; Wan, G.; Ali, M.A.; Tang, K. Rapid Hemostatic Chitosan/Cellulose Composite Sponge by Alkali/Urea Method for Massive Haemorrhage. Int. J. Biol. Macromol. 2020, 164, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.H. Antimicrobial and Wound Healing Properties of Feo Fabricated Chitosan/Pva Nanocomposite Sponge. Antibiotics 2021, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Gordienko, M.G.; Palchikova, V.V.; Kalenov, S.V.; Lebedev, E.A.; Belov, A.A.; Menshutina, N.V. The Alginate–Chitosan Composite Sponges with Biogenic Ag Nanoparticles Produced by Combining of Cryostructuration, Ionotropic Gelation and Ion Replacement Methods. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 34–44. [Google Scholar] [CrossRef]
- Huang, N.; Lin, J.; Li, S.; Deng, Y.; Kong, S.; Hong, P.; Yang, P.; Liao, M.; Hu, Z. Preparation and Evaluation of Squid Ink Polysaccharide-Chitosan as a Wound-Healing Sponge. Mater. Sci. Eng. C 2018, 82, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Buriuli, M.; Kumari, W.G.; Verma, D. Evaluation of Hemostatic Effect of Polyelectrolyte Complex-Based Dressings. J. Biomater. Appl. 2017, 32, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Misgav, M.; Lubetszki, A.; Brutman-Barazani, T.; Martinowitz, U.; Kenet, G. The Hemostatic Efficacy of Chitosan-Pads in Hemodialysis Patients with Significant Bleeding Tendency. J. Vasc. Access 2017, 18, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yan, S.; Wang, Y.; Zhang, C. Preparation and Properties of Electrospun Chitosan/Polybutylenes Succinate Nanofiber Membrane for Wound Hemostatic Dressing. J. Ind. Text. 2022, 52, 15280837221113086. [Google Scholar] [CrossRef]
- Akram, A.M.; Omar, R.A.; Ashfaq, M. Chitosan/Calcium Phosphate-Nanoflakes-Based Biomaterial: A Potential Hemostatic Wound Dressing Material. Polym. Bull. 2022, 80, 5071–5086. [Google Scholar] [CrossRef]
- Wang, Y.W.; Liu, C.C.; Cherng, J.H.; Lin, C.S.; Chang, S.J.; Hong, Z.J.; Liu, C.C.; Chiu, Y.K.; Hsu, S.D.; Chang, H. Biological Effects of Chitosan-Based Dressing on Hemostasis Mechanism. Polymers 2019, 11, 1906. [Google Scholar] [CrossRef]
- Wang, C.H.; Cherng, J.H.; Liu, C.C.; Fang, T.J.; Hong, Z.J.; Chang, S.J.; Fan, G.Y.; Hsu, S. Der Procoagulant and Antimicrobial Effects of Chitosan in Wound Healing. Int. J. Mol. Sci. 2021, 22, 7067. [Google Scholar] [CrossRef]
- Xie, M.; Zeng, Y.; Wu, H.; Wang, S.; Zhao, J. Multifunctional Carboxymethyl Chitosan/Oxidized Dextran/Sodium Alginate Hydrogels as Dressing for Hemostasis and Closure of Infected Wounds. Int. J. Biol. Macromol. 2022, 219, 1337–1350. [Google Scholar] [CrossRef]
- Elangwe, C.N.; Morozkina, S.N.; Olekhnovich, R.O.; Krasichkov, A.; Polyakova, V.O.; Uspenskaya, M.V. A Review on Chitosan and Cellulose Hydrogels for Wound Dressings. Polymers 2022, 14, 5163. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, Z.; Li, S.; Zhang, L.; Lu, S.; Liang, F. Chitosan-Based Thermo-Sensitive Hydrogel Loading Oyster Peptides for Hemostasis Application. Materials 2020, 13, 5038. [Google Scholar] [CrossRef]
- Patil, G.; Pawar, R.; Jadhav, S.; Ghormade, V. A Chitosan Based Multimodal “Soft” Hydrogel for Rapid Hemostasis of Non-Compressible Hemorrhages and Its Mode of Action. Carbohydr. Polym. Technol. Appl. 2022, 4, 100237. [Google Scholar] [CrossRef]
- Lin, X.; Shen, Y.; Wang, L. Multi-Scale Photoacoustic Assessment of Wound Healing Using Chitosan–Graphene Oxide Hemostatic Sponge. Nanomaterials 2021, 11, 2879. [Google Scholar] [CrossRef]
- Kim, K.; Ryu, J.H.; Koh, M.-Y.; Yun, S.P.; Kim, S.; Park, J.P.; Jung, C.-W.; Lee, M.S.; Seo, H.-I.; Kim, J.H.; et al. Coagulopathy-Independent, Bioinspired Hemostatic Materials: A Full Research Story from Preclinical Models to a Human Clinical Trial. Sci. Adv. 2021, 7, eabc9992. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, W.; Deng, W.; Xu, C.; Cai, Y.; Wang, X. Antibacterial and Hemostatic Thiol-Modified Chitosan-Immobilized AgNPs Composite Sponges. ACS Appl. Mater. Interfaces 2020, 12, 20307–20320. [Google Scholar] [CrossRef] [PubMed]
- Deineka, V.; Sulaieva, O.; Pernakov, M.; Korniienko, V.; Husak, Y.; Yanovska, A.; Yusupova, A.; Tkachenko, Y.; Kalinkevich, O.; Zlatska, A.; et al. Hemostatic and Tissue Regeneration Performance of Novel Electrospun Chitosan-Based Materials. Biomedicines 2021, 9, 588. [Google Scholar] [CrossRef] [PubMed]
- Bal-Ozturk, A.; Karal-Yilmaz, O.; Akguner, Z.P.; Aksu, S.; Tas, A.; Olmez, H. Sponge-like Chitosan-Based Nanostructured Antibacterial Material as a Topical Hemostat. J. Appl. Polym. Sci. 2019, 136, 47522. [Google Scholar] [CrossRef]
- Zheng, L.; Gu, B.; Li, S.; Luo, B.; Wen, Y.; Chen, M.; Li, X.; Zha, Z.; Zhang, H.T.; Wang, X. An Antibacterial Hemostatic AuNPs@corn Stalk/Chitin Composite Sponge with Shape Recovery for Promoting Wound Healing. Carbohydr. Polym. 2022, 296, 119924. [Google Scholar] [CrossRef] [PubMed]
| Materials and Trademarks | Hemostatic Mechanism | Characteristics | Ref. |
|---|---|---|---|
| Chitosan-based materials ChitoFlex, Chitoseal, Celox, TraumaStat, HemCon. | Positive surface charge enables it to bind with negatively charged blood components, promoting platelet activation and the agglutination of blood proteins to facilitate fibrin clot formation, while also forming a strong physical barrier that adheres to wet tissues and seals wounds. | Biocompatible, biodegradable, antibacterial ability, stimulatory effect on tissue regeneration, hemostatic effect, cost-effective, easy to store, and long shelf-life; suitable for patients with coagulopathy, although it may not be entirely effective for extensively bleeding wounds. | [26,27,28,29,30] |
| Cellulose-based materials BloodSTOP, WoundClot, Surgicel, Suntouch, ActCel. | Absorbs fluids, forms a physical barrier to prevent blood loss, exhibits anti-microbial activity, is bioabsorbable, and aids clotting by binding to calcium ions, initiating the clotting cascade through contact activation and decreasing pH at the wound site, leading to platelet activation and aggregation. | Appropriate for achieving hemostasis in cases of capillary, arteriolar, venous, and bone bleeding, and is also biocompatible, non-immunogenic, and bactericidal, conforming well to the wound site. However, it may not be effective in managing severe bleeding. | [2,31,32,33,34] |
| Starch-based materials PerClot, EndoClot | Absorbs water from the blood, leading to the formation of a gel-like matrix that can adhere to tissue and promote the aggregation of platelets and the activation of clotting factors, ultimately resulting in the formation of a stable fibrin clot that can help to stop bleeding. | Reduces bleeding and the need for transfusions, minimizes the risk of blood infections, and has no known immune or allergic reactions or toxic side effects. Should not be used in blood vessels to avoid the risk of embolism and is suitable for minor injuries. It is easy to use, lightweight, has a long shelf life, and is inexpensive. | [35,36,37,38,39,40] |
| Collagen-based materials Avitene, Helitene, Hemopad, Helistat, Collastat, Instat, CoStasis, D-stat. | Forms a physical matrix, triggers the process of the coagulation cascade, and induces the activation of platelets, leading to the release of clotting factors such as thrombin and fibrinogen. | Promotes tissue regeneration and repair and possesses characteristics such as biocompatibility, cell adhesion, biodegradability, non-toxicity, and low antigenicity. Effective in heparinized patients, not suitable for use in patients with thrombocytopenia. | [2,12,26,41,42] |
| Gelatin-based materials Gelfoam, Surgifoam, Gelfilm, Gelita-spon. | Triggers the activation and aggregation of platelets, expedites the formation of clots, and provides structural support to the clot formed by enhancing thrombin generation and subsequently propagating the coagulation cascade. | Applicable for treating various types of wounds and injuries, but caution must be taken when used in restricted areas or near nerve structures due to the potential risk of compressive complications. It is a cost-effective solution that remains stable at room temperature, non-toxic, and non-antigenic. Furthermore, it has strong adsorption capability, can stick to the wound surface, and can increase its volume up to twice its original size by absorbing fluids. | [14,43,44,45,46] |
| Fibrin-based materials Artiss, Tissel, Evicel, Tissucol, TachoSil. | Major protein component of blood clots, is formed as the final step in the coagulation cascade, serving as a scaffold for tissue repair and providing cues for cell behavior during injury healing. | Exhibits excellent hemostatic and adhesive properties and biocompatibility and can be used for severe bleeding and patients with coagulation disorders, but is not recommended for application on blood vessels. It also aids in tissue regeneration following injury due to its fast polymerization dynamics and ease of tunability. | [47,48,49,50] |
| Polycyanoacrylates-based materials Dermabond, Omnex, Glubran, Histoacryl, GLUture. | Rapidly polymerizes upon contact with fluids to create a mechanical barrier or plug that occludes the bleeding vessel or tissue, resulting in hemostasis. | Presents bactericidal and bacteriostatic effects, non-toxic, non-carcinogenic, and good histocompatibility, with considerable hemostatic ability and can be applied in anastomosis, wound hemostasis, wound adhesion, and tendon repair; however, it may result in vascular embolization and the release of toxic substances. | [45,51,52,53] |
| PEG-based materials Coseal | Upon contact with tissue fluids, forms a gel-like matrix, which adheres to the tissue and provides a mechanical barrier to prevent bleeding. | Favorable biocompatibility, minimal cytotoxicity, and excellent hemostatic properties, commonly utilized in surgical settings to reduce bleeding and facilitate wound healing with a low occurrence of unfavorable consequences. | [41,54,55,56,57] |
| Polyurethane-based materials Bioclusive, Opsite Flexigrid, Tegaderm, Allevyn, Tegaderm (3M Science), TissuGlu (Cohera Medical, Inc.), ResQFoam (Arsenal Medical, Inc.), Nanosan-Sorb (SNS Nano Fiber Technology) | Triggers the activation and aggregation of thrombocytes, initiating the coagulation cascade. | Polyurethane dressings can maintain a moist wound environment by allowing the transmission of moisture, oxygen, and air while blocking fluids and bacteria and providing thermal insulation, promoting autolytic debridement; their high absorbency is due to a hydrophilic contact surface, microporous foam, and hydrophobic backing. Maintains its shape and firmness when exposed to blood, | [44,58,59,60] |
| Zeolite and kaolin powder-based materials Quikclot, Woundstat Combat Gauze | Has a hygroscopic action, which allows it to quickly absorb water from blood to concentrate coagulation factors; it can also release Ca++ in blood and activate FXII to trigger the intrinsic coagulation pathway and potentially induce the contact activation of platelets. | Ease of use, stability, no biological toxicity or disease transmission, provide deep tissue access, are inert, and do not elicit an immune response. Not bioabsorbable and are less effective for arterial bleeding or coagulopathic patients. Their success is dependent on the patient’s blood-clotting activity. May cause thrombotic complications if particles enter the bloodstream. | [19,41,61,62,63] |
| Trademark | Mechanism of Action and Characteristics |
|---|---|
| ChitoFlex | Wound dressing specifically engineered to minimize substantial bleeding by securely attaching to tissue surfaces, creating a flexible barrier that effectively seals and stabilizes the wound area. It possesses antibacterial properties and is biocompatible, ensuring compatibility with the body’s natural processes [64,65]. |
| Chitoseal | Enhanced with a cellulose coating, this wound dressing aids in the management of hemorrhage wounds by reducing the time required for compression. It effectively stops bleeding at sites of vascular access, as well as around percutaneous catheters or tubes [64,65]. |
| Celox | Specifically designed to address life-threatening bleeding, demonstrating remarkable effectiveness within just 30 s. Notably, it achieves hemostasis without generating heat and forms a strong plug when it comes into contact with red blood cells. Celox proves to be effective even for individuals taking antiplatelet or anticoagulant medications, as well as those experiencing hypothermia. It is available in both granular and bandage forms, with the powdered version having some limitations in certain clinical conditions [66,67]. |
| TraumaStat | Product composed of freeze-dried chitosan that incorporates highly porous silica (acts as a robust activator of the intrinsic clotting cascade) and polyethylene. This unique composition is specifically designed for external temporary use, aiming to effectively control moderate to severe bleeding [65,67]. |
| HemCon | A type of freeze-dried chitosan acetate salt that is primarily employed in emergency situations to halt blood loss. Its mechanism of action involves the interaction between the protonated amine groups present in chitosan molecules and the negatively charged residues on the membranes of red blood cells. This bandage has been developed in collaboration with the U.S. Army and is primarily utilized on battlefields to effectively control bleeding [65,66]. |
| Forms | Composition | Characteristics | Ref. |
|---|---|---|---|
| Dressing | CS, aluminum chloride | The microporous structure of the dressing is irregular, which allows it to absorb the maximum amount of blood and promote clot formation. | [28] |
| Carrageenan, CS | The composite dressing’s greater swelling, larger surface area, and mesoporous structure result in superior hemostatic activity by promoting the increased adhesion of blood cells and platelets. | [109] | |
| CS, calcium alginate | Biocompatibility, antibacterial, moisture retention, healing promotion, and noncytotoxicity characteristics make chitosan–calcium alginate dressing a superior option for wound care. | [110] | |
| Hydrogel | CS, PEG | The combination of biodegradability, self-adhesiveness, self-healing ability, stretchability, antibacterial properties, and biocompatibility makes it a promising material for emergency hemostasis, particularly for joint and limb injuries. The hydrogel showed strong adhesion to various substrates (PTFE, pigskin, and glass tubes) and provided long-term stability when applied to bleeding wounds in both static and dynamic humid environments. | [111] |
| Hydroxybutyl-functionalized CS | The material possesses thermosensitive characteristics, strong adhesion ability, effective hemostasis, appropriate mechanical properties, self-healing capability, easy removal as needed, antioxidant properties, as well as photothermal and intrinsic antibacterial activity. | [112] | |
| FCMCS, PDA, PAM | The hydrogel exhibited a variety of functions including tissue adhesion, biocompatibility, self-healing, and antibacterial properties. It also maintained its mechanical characteristics while offering broad-spectrum antibacterial activity. | [113] | |
| CMCS, OHA | The material exhibits favorable biodegradability and biosafety profiles, and possesses strong hemostatic and sealing capabilities, making it a promising candidate for clinical hemostatic sealant applications. | [114] | |
| Sponge | Cs, AgNPs | The chitosan/Ag nanocomposite sponges demonstrated outstanding antibacterial activity against Staphylococcus aureus and E. coli in the antibacterial test. They also displayed good mechanical properties and noncytotoxicity, with cell viability values exceeding 90%. | [115] |
| CS, cellulose | The sponge demonstrates favorable biocompatibility and hemostatic capability, making it a promising option for prompt hemostasis in cases of severe bleeding. | [116] | |
| CS/PVA-PD-FeO NPs | The sponge demonstrated high porosity and water absorption properties, as well as significant antibacterial activity. It facilitated gaseous exchange, absorbed wound exudate, and inhibited microbial growth in diabetic wounds. Therefore, it can be inferred that the chitosan composite sponge’s antioxidant, antidiabetic, and antibacterial properties can contribute to the healing of diabetic wounds. | [117] | |
| CS/AgNPs/alginate | The material demonstrated notable absorbency and a significant antimicrobial impact, particularly in assays involving Bacillus cereus and Staphylococcus aureus. | [118] | |
| CS, SIP | The material exhibits a strong ability to absorb fluids, as well as significant procoagulant effects, making it effective in promoting wound healing. | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghiță, D.; Moldovan, H.; Robu, A.; Bița, A.-I.; Grosu, E.; Antoniac, A.; Corneschi, I.; Antoniac, I.; Bodog, A.D.; Băcilă, C.I. Chitosan-Based Biomaterials for Hemostatic Applications: A Review of Recent Advances. Int. J. Mol. Sci. 2023, 24, 10540. https://doi.org/10.3390/ijms241310540
Gheorghiță D, Moldovan H, Robu A, Bița A-I, Grosu E, Antoniac A, Corneschi I, Antoniac I, Bodog AD, Băcilă CI. Chitosan-Based Biomaterials for Hemostatic Applications: A Review of Recent Advances. International Journal of Molecular Sciences. 2023; 24(13):10540. https://doi.org/10.3390/ijms241310540
Chicago/Turabian StyleGheorghiță, Daniela, Horațiu Moldovan, Alina Robu, Ana-Iulia Bița, Elena Grosu, Aurora Antoniac, Iuliana Corneschi, Iulian Antoniac, Alin Dănuț Bodog, and Ciprian Ionuț Băcilă. 2023. "Chitosan-Based Biomaterials for Hemostatic Applications: A Review of Recent Advances" International Journal of Molecular Sciences 24, no. 13: 10540. https://doi.org/10.3390/ijms241310540
APA StyleGheorghiță, D., Moldovan, H., Robu, A., Bița, A.-I., Grosu, E., Antoniac, A., Corneschi, I., Antoniac, I., Bodog, A. D., & Băcilă, C. I. (2023). Chitosan-Based Biomaterials for Hemostatic Applications: A Review of Recent Advances. International Journal of Molecular Sciences, 24(13), 10540. https://doi.org/10.3390/ijms241310540










