Multisensory Stimulation Reverses Memory Impairment in Adrβ3KO Male Mice
Abstract
1. Introduction
2. Results
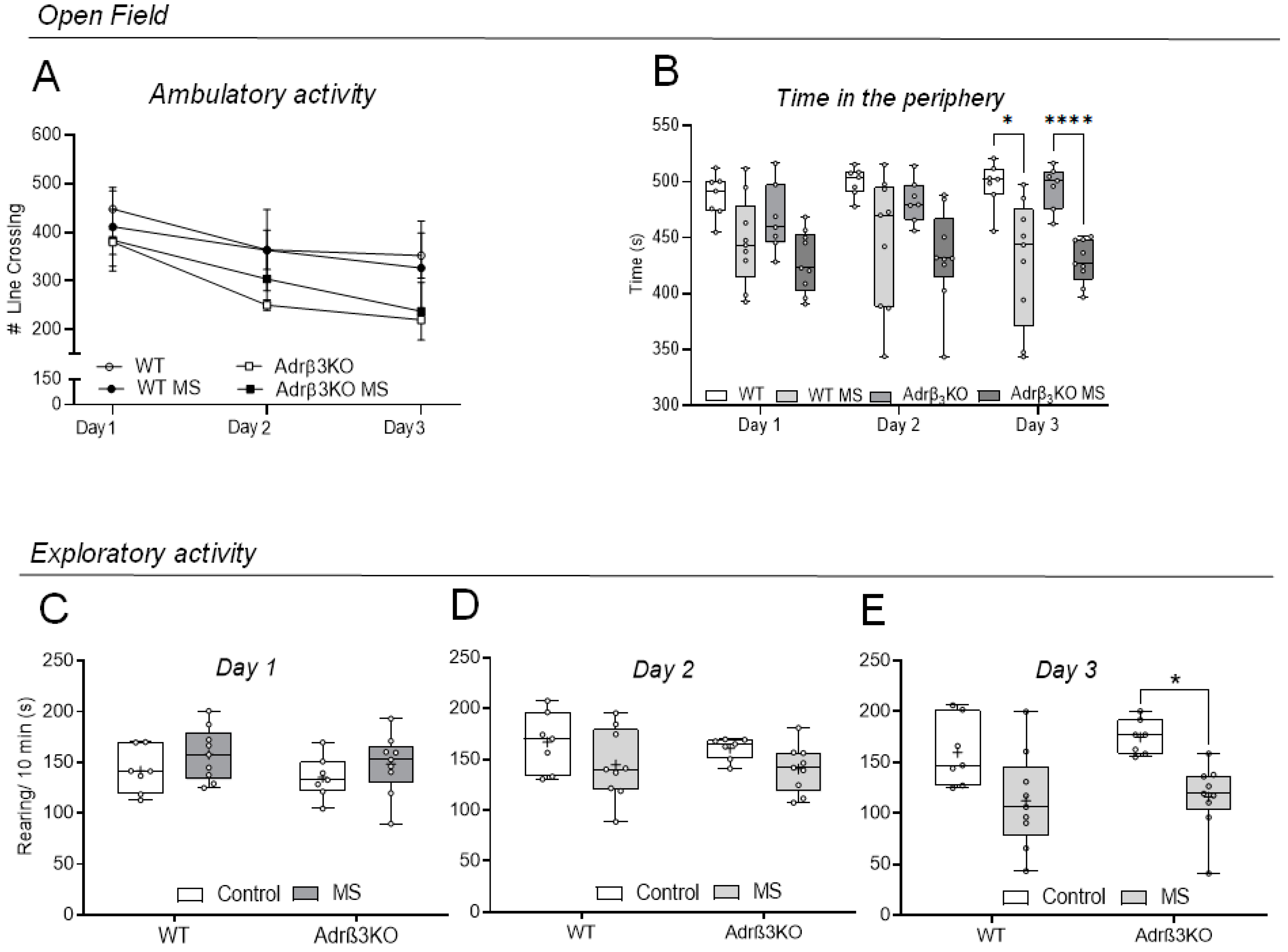
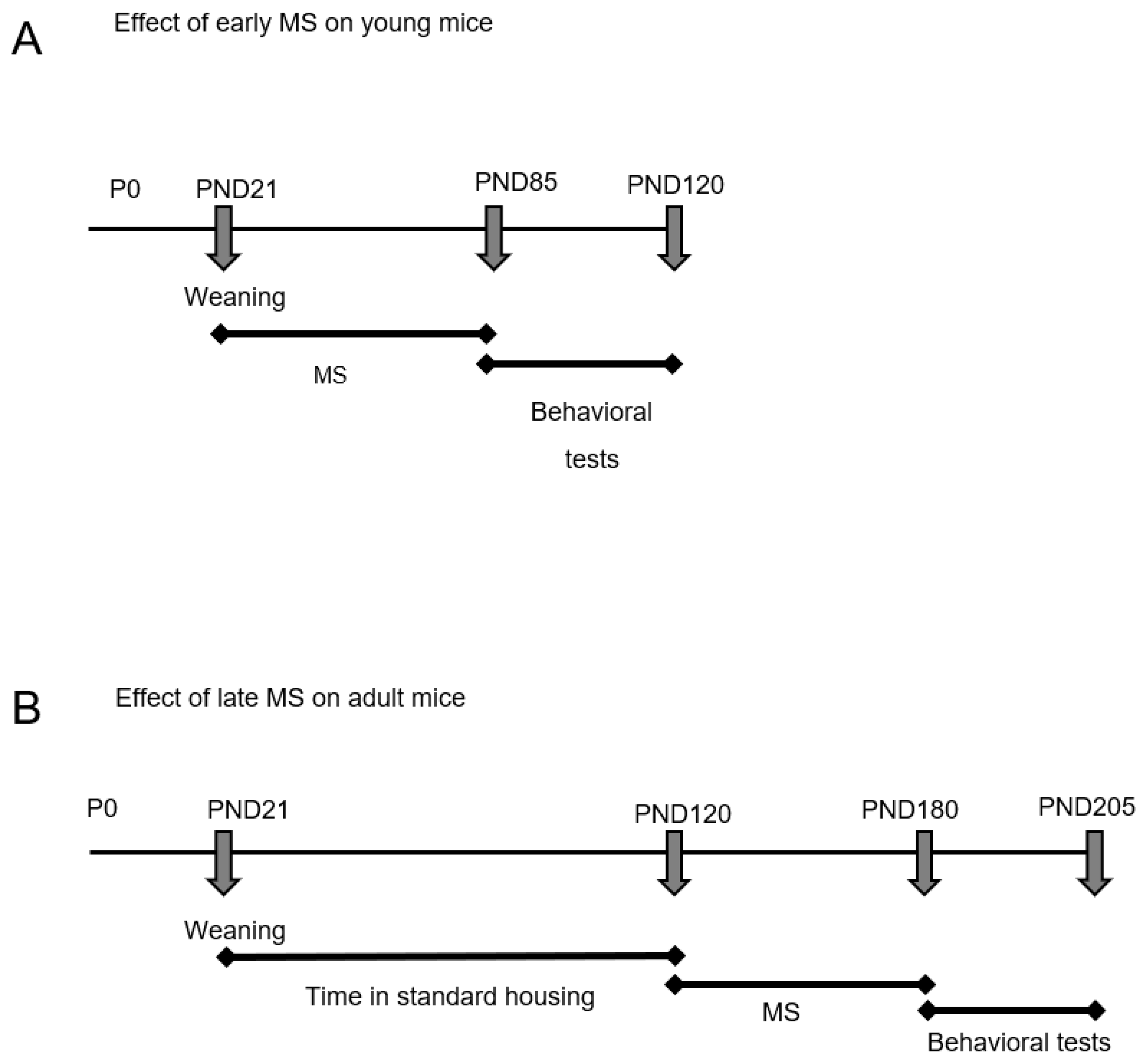
2.1. Ambulatory and Exploratory Activity of Adrβ3KO and WT Mice Exposed to MS Early in Life
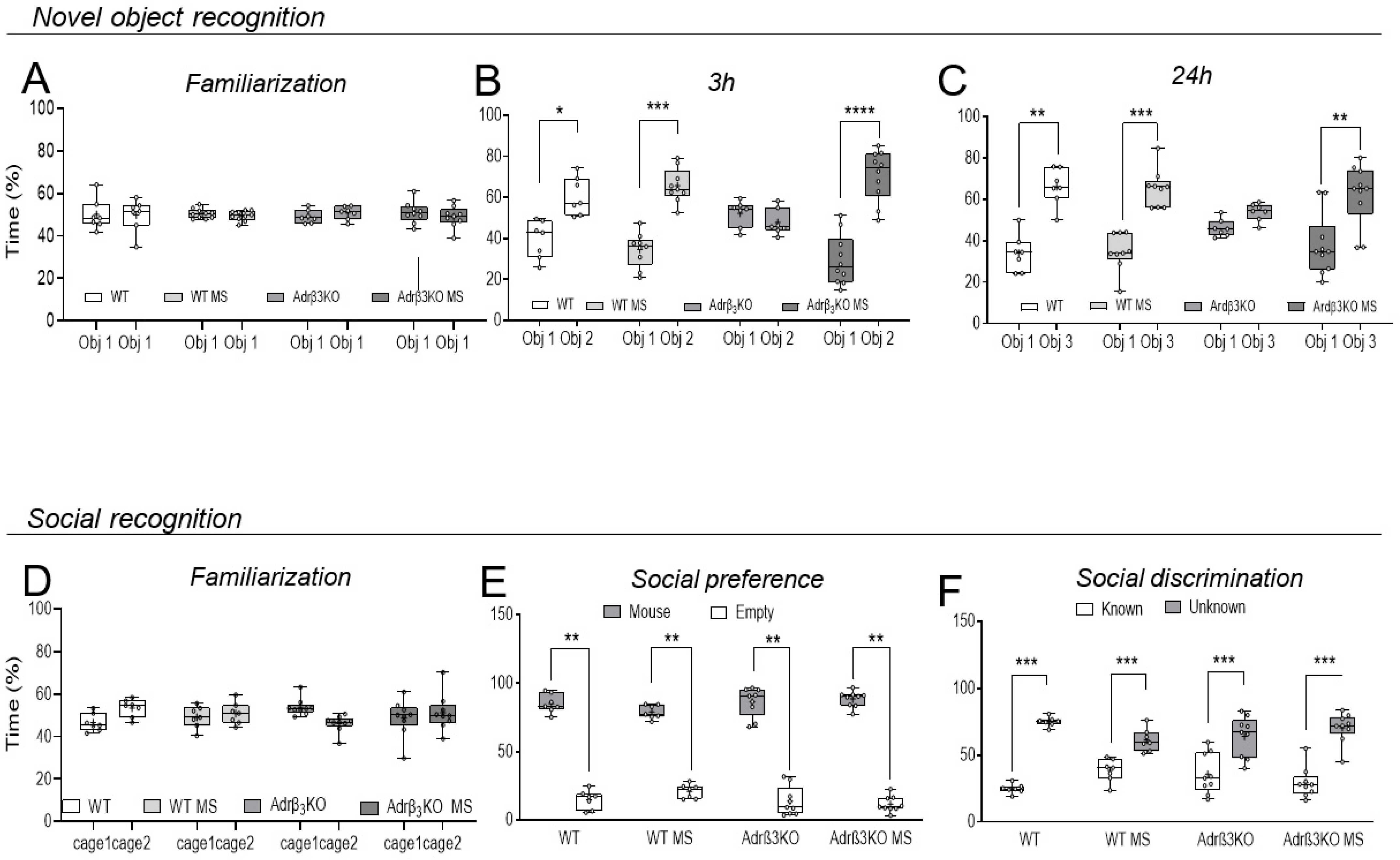
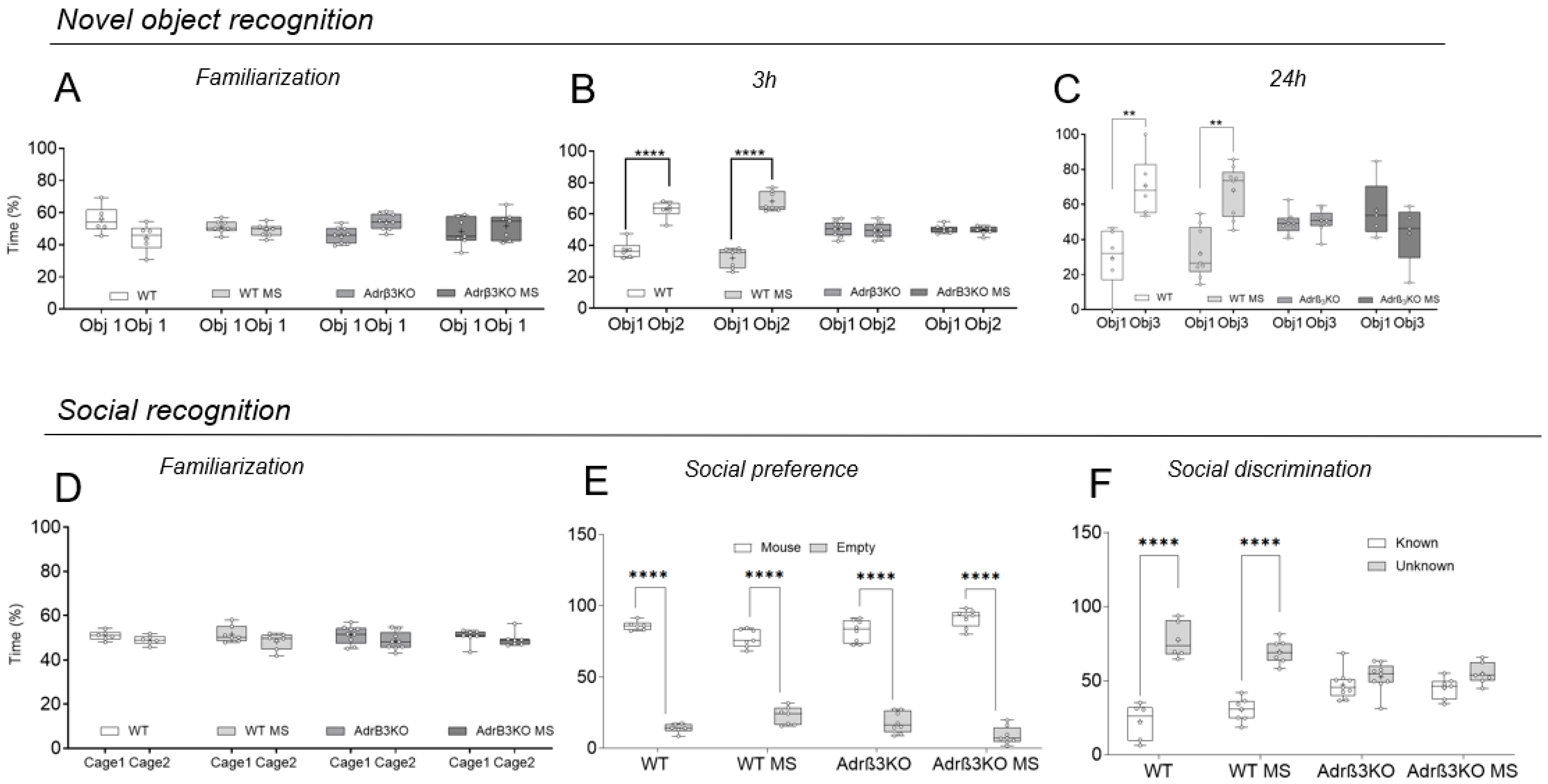
2.2. MS Exposure Early in Life Corrects Cognitive Impairment in Young Adult Adrβ3KO Mice
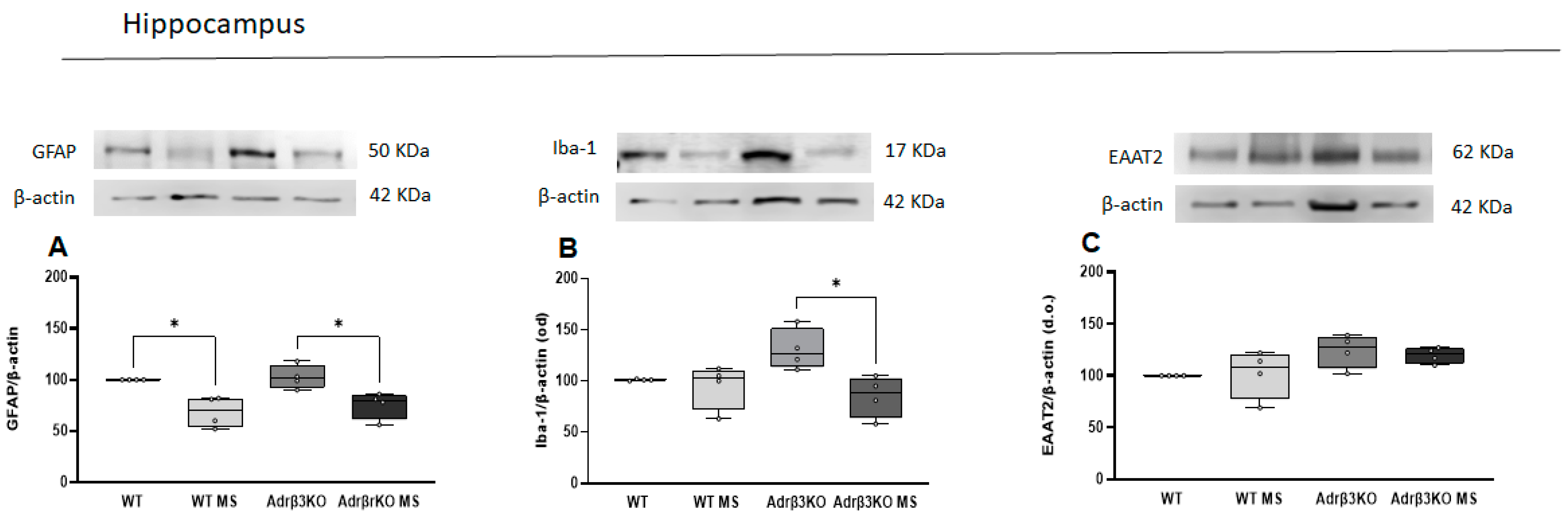
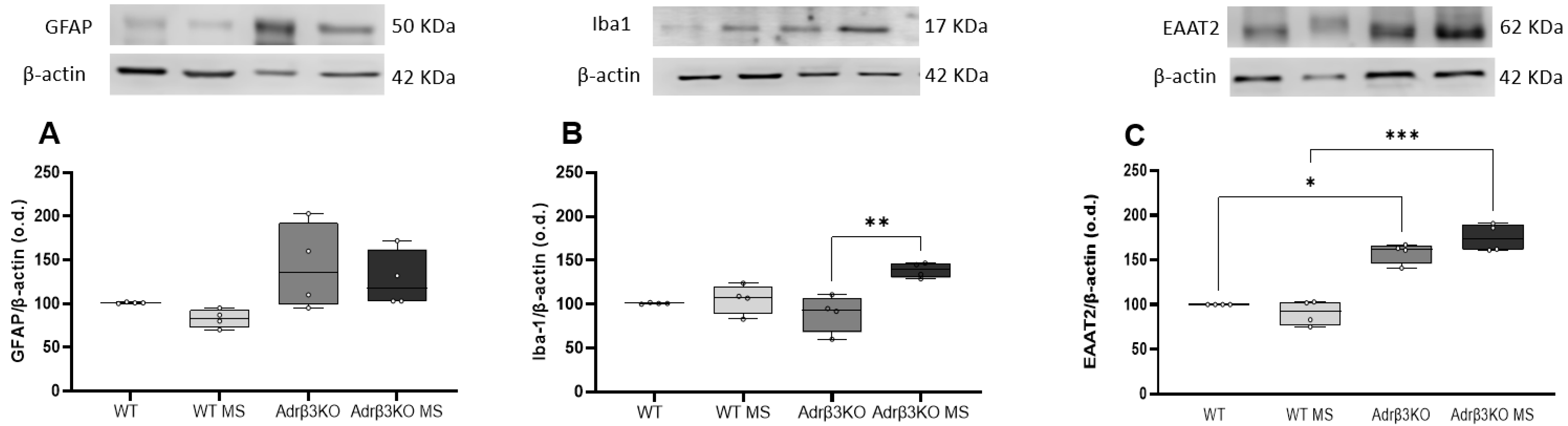
2.3. MS Protocol in Early Life Decreases Glial Cell Activation
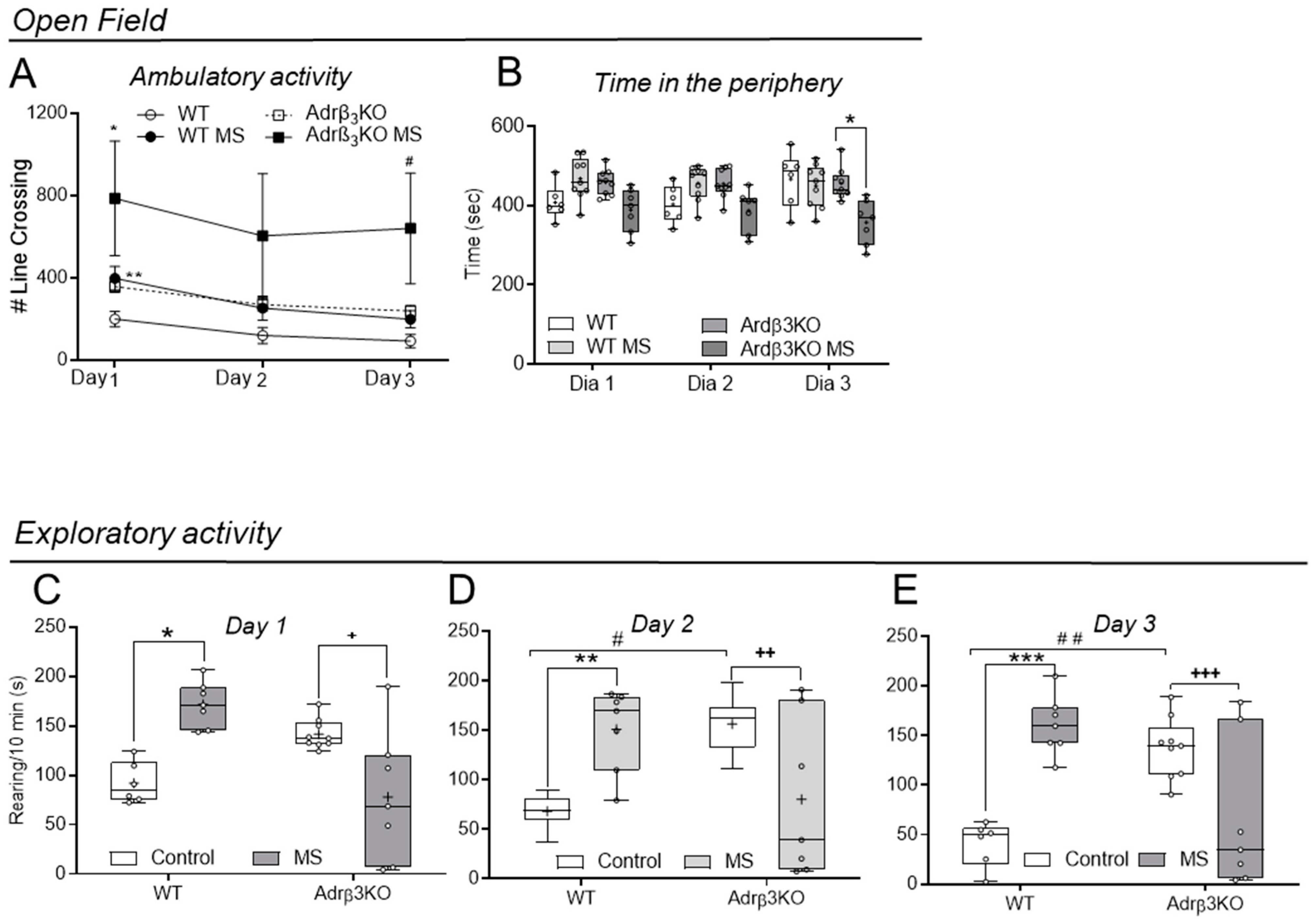
2.4. Ambulatory and Exploratory Activity of Adrβ3KO and WT Mice Exposed to MS Late in Life
2.5. MS Exposure Late in Life Does Not Correct the Worst Cognitive Impairment Observed in Adult Adrβ3KO Mice
2.6. MS Protocol Late in Life Does Not Decreases Glial Cell Activation
3. Discussion
4. Materials and Methods
Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harley, C.W. Norepinephrine and dopamine as learning signals. Neural Plast. 2004, 11, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Sara, S.J. Locus Coeruleus in time with the making of memories. Curr. Opin. Neurobiol. 2015, 35, 87–94. [Google Scholar] [CrossRef] [PubMed]
- McGaugh, J.L. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004, 27, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Pang, P.T.; Woo, N.H. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 2005, 6, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Abbaspour, R.; Nahabedian, D.; Connor, S.A. Norepinephrine, beyond the Synapse: Coordinating Epigenetic Codes for Memory. Int. J. Mol. Sci. 2022, 23, 9916. [Google Scholar] [CrossRef]
- Straube, T.; Korz, V.; Balschun, D.; Uta Frey, J. Requirement of beta-adrenergic receptor activation and protein synthesis for LTP-reinforcement by novelty in rat dentate gyrus. J. Physiol. 2003, 552, 953–960. [Google Scholar] [CrossRef]
- Ji, J.Z.; Wang, X.M.; Li, B.M. Deficit in long-term contextual fear memory induced by blockade of beta-adrenoceptors in hippocampal CA1 region. Eur. J. Neurosci. 2003, 17, 1947–1952. [Google Scholar] [CrossRef]
- Ji, J.Z.; Zhang, X.H.; Li, B.M. Deficient spatial memory induced by blockade of beta-adrenoceptors in the hippocampal CA1 region. Behav. Neurosci. 2003, 117, 1378–1384. [Google Scholar] [CrossRef]
- Gibbs, M.E.; Hutchinson, D.S.; Summers, R.J. Role of beta-adrenoceptors in memory consolidation: Beta3-adrenoceptors act on glucose uptake and beta2-adrenoceptors on glycogenolysis. Neuropsychopharmacology 2008, 33, 2384–2397. [Google Scholar] [CrossRef]
- Souza-Braga, P.; Lorena, F.B.; Nascimento, B.P.P.; Marcelino, C.P.; Ravache, T.T.; Ricci, E.; Bernardi, M.M.; Ribeiro, M.O. Adrenergic receptor beta3 is involved in the memory consolidation process in mice. Braz. J. Med. Biol. Res. 2018, 51, e7564. [Google Scholar] [CrossRef]
- Tournissac, M.; Vu, T.-M.; Vrabic, N.; Hozer, C.; Tremblay, C.; Mélançon, K.; Planel, E.; Pifferi, F.; Calon, F. Repurposing beta-3 adrenergic receptor agonists for Alzheimer’s disease: Beneficial effects in a mouse model. Alzheimers Res. Ther. 2021, 13, 103. [Google Scholar] [CrossRef]
- Batistuzzo, A.; de Almeida, G.G.; Brás, T.S.; Zucato, V.P.; Arnold, A.J.T.; Giannocco, G.; Sato, J.M.; Yamanouchi, L.M.; Dias, E.; Lorena, F.B.; et al. Multisensory Stimulation Improves Cognition and Behavior in Adult Male Rats Born to LT4-treated Thyroidectomized Dams. Endocrinology 2022, 163, bqac105. [Google Scholar] [CrossRef]
- Zendel, B.R.; Willoughby, K.A.; Rovet, J.F. Neuroplastic effects of music lessons on hippocampal volume in children with congenital hypothyroidism. Neuroreport 2013, 24, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Johnson, A.B.; Boespflug-Tanguy, O.; Rodriguez, D.; Goldman, J.E.; Messing, A. Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat. Genet. 2001, 27, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, W.; Mamber, C.; Moeton, M.; Kooijman, L.; Sluijs, J.A.; Jansen, A.H.P.; Verveer, M.; de Groot, L.R.; Smith, V.D.; Rangarajan, S.; et al. GFAP isoforms in adult mouse brain with a focus on neurogenic astrocytes and reactive astrogliosis in mouse models of Alzheimer disease. PLoS ONE 2012, 7, e42823. [Google Scholar] [CrossRef]
- Calcia, M.A.; Bonsall, D.R.; Bloomfield, P.S.; Selvaraj, S.; Barichello, T.; Howes, O.D. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology 2016, 233, 1637–1650. [Google Scholar] [CrossRef]
- Khakh, B.S.; Beaumont, V.; Cachope, R.; Munoz-Sanjuan, I.; Goldman, S.A.; Grantyn, R. Unravelling and exploiting astrocyte dysfunction in Huntington’s disease. Trends Neurosci. 2017, 40, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kazim, S.F.; Larson, C.S.; Ramakrishnan, A.; Gray, J.D.; McEwen, B.S.; Rosenberg, P.A.; Shen, L.; Pereira, A.C. Divergent roles of astrocytic versus neuronal EAAT2 deficiency on cognition and overlap with aging and Alzheimer’s molecular signatures. Proc. Natl. Acad. Sci. USA 2019, 116, 21800–21811. [Google Scholar] [CrossRef] [PubMed]
- Dhapola, R.; Hota, S.S.; Sarma, P.; Bhattacharyya, A.; Medhi, B.; Reddy, D.H. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 2021, 29, 1669–1681. [Google Scholar] [CrossRef]
- Susulic, V.S.; Frederich, R.C.; Lawitts, J.; Tozzo, E.; Kahn, B.B.; Harper, M.E.; Himms-Hagen, J.; Flier, J.S.; Lowell, B.B. Targeted disruption of the beta 3-adrenergic receptor gene. J. Biol. Chem. 1995, 270, 29483–29492. [Google Scholar] [CrossRef]
- Simpson, J.; Kelly, J.P. The impact of environmental enrichment in laboratory rats—Behavioural and neurochemical aspects. Behav. Brain Res. 2011, 222, 246–264. [Google Scholar] [CrossRef] [PubMed]
- Huttenrauch, M.; Salinas, G.; Wirths, O. Effects of Long-Term Environmental Enrichment on Anxiety, Memory, Hippocampal Plasticity and overall Brain Gene Expression in C57BL6 Mice. Front. Mol. Neurosci. 2016, 9, 62. [Google Scholar] [CrossRef]
- Duncan, G.E.; Knapp, D.J.; Breese, G.R. Neuroanatomical characterization of Fos induction in rat behavioral models of anxiety. Brain Res. 1996, 713, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Balleine, B.W.; Killcross, S. Parallel incentive processing: An integrated view of amygdala function. Trends Neurosci. 2006, 29, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Sergerie, K.; Chochol, C.; Armony, J.L. The role of the amygdala in emotional processing: A quantitative meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2008, 32, 811–830. [Google Scholar] [CrossRef] [PubMed]
- Murack, M.; Smith, K.B.; Traynor, O.H.; Pirwani, A.F.; Gostlin, S.K.; Mohamed, T.; Tata, D.A.; Messier, C.; Ismail, N. Environmental enrichment alters LPS-induced changes in BDNF and PSD-95 expressions during puberty. Brain Res. 2023, 1806, 148283. [Google Scholar] [CrossRef] [PubMed]
- Loisy, M.; Farah, A.; Fafouri, A.; Fanton, A.; Ahmadi, M.; Therreau, L.; Chevaleyre, V.; Piskorowski, R.A. Environmental enrichment and social isolation modulate inhibitory transmission and plasticity in hippocampal area CA2. Hippocampus 2023, 33, 197–207. [Google Scholar] [CrossRef]
- Woitke, F.; Blank, A.; Fleischer, A.-L.; Zhang, S.; Lehmann, G.-M.; Broesske, J.; Haase, M.; Redecker, C.; Schmeer, C.W.; Keiner, S. Post-Stroke Environmental Enrichment Improves Neurogenesis and Cognitive Function and Reduces the Generation of Aberrant Neurons in the Mouse Hippocampus. Cells 2023, 12, 652. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Gutiérrez-Vera, B.; Ortiz-López, L.; Vega-Rivera, N.M.; Meneses-San Juan, D.; Granados-Juárez, A.; Castro-de Aquino, D.V.; Castro-García, M.; Ramos, M.F. Environmental enrichment: Dissociated effects between physical activity and changing environmental complexity on anxiety and neurogenesis in adult male Balb/C mice. Physiol. Behav. 2022, 254, 113878. [Google Scholar] [CrossRef]
- Sah, A.; Rooney, S.; Kharitonova, M.; Sartori, S.B.; Wolf, S.A.; Singewald, N. Enriched Environment Attenuates Enhanced Trait Anxiety in Association with Normalization of Aberrant Neuro-Inflammatory Events. Int. J. Mol. Sci. 2022, 23, 13052. [Google Scholar] [CrossRef]
- Guo, Y.-S.; Yuan, M.; Han, Y.; Shen, X.-Y.; Gao, Z.-K.; Bi, X. Effects of enriched environment on microglia and functional white matter recovery in rats with post stroke cognitive impairment. Neurochem. Int. 2022, 154, 105295. [Google Scholar] [CrossRef]
- Dossi, E.; Vasile, F.; Rouach, N. Human astrocytes in the diseased brain. Brain Res. Bull. 2018, 136, 139–156. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T.; Jalouli, M.; Rahman, M.A.; Jeandet, P.; Behl, T.; Alexiou, A.; Albadrani, G.M.; Abdel-Daim, M.M.; Perveen, A.; et al. Neuroinflammatory signaling in the pathogenesis of Alzheimer’s disease. Curr. Neuropharmacol. 2022, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M. Microglia and astrocytes in Alzheimer’s disease: Implications for therapy. Curr. Neuropharmacol. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Dzamba, D.; Harantova, L.; Butenko, O.; Anderova, M. Glial cells–The key elements of Alzheimer s disease. Curr. Alzheimer Res. 2016, 13, 894–911. [Google Scholar] [CrossRef] [PubMed]
- Furman, J.L.; Sama, D.M.; Gant, J.C.; Beckett, T.L.; Murphy, M.P.; Bachstetter, A.D.; Van Eldik, L.J.; Norris, C.M. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J. Neurosci. 2012, 32, 16129–16140. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Yarishkin, O.; Hwang, Y.J.; Chun, Y.E.; Park, M.; Woo, D.H.; Bae, J.Y.; Kim, T.; Lee, J.; Chun, H.; et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 2014, 20, 886–896. [Google Scholar] [CrossRef]
- Cao, S.; Fisher, D.W.; Rodriguez, G.; Yu, T.; Dong, H. Comparisons of neuroinflammation, microglial activation, and degeneration of the locus coeruleus-norepinephrine system in APP/PS1 and aging mice. J. Neuroinflammation 2021, 18, 10. [Google Scholar] [CrossRef]
- Braun, D.; Madrigal, J.L.; Feinstein, D.L. Noradrenergic regulation of glial activation: Molecular mechanisms and therapeutic implications. Curr. Neuropharmacol. 2014, 12, 342–352. [Google Scholar] [CrossRef]
- Dello Russo, C.; Boullerne, A.I.; Gavrilyuk, V.; Feinstein, D.L. Inhibition of microglial inflammatory responses by norepinephrine: Effects on nitric oxide and interleukin-1β production. J. Neuroinflammation 2004, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.H.; Chen, X.; Cui, M.; Yu, X.; Pang, Q.; Sun, J.P. β2-adrenergic receptor and astrocyte glucose metabolism. J. Mol. Neurosci. 2012, 48, 456–463. [Google Scholar] [CrossRef] [PubMed]
- De Keyser, J.; Wilczak, N.; Leta, R.; Streetland, C. Astrocytes in multiple sclerosis lack beta-2 adrenergic receptors. Neurology 1999, 53, 1628. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Tanaka, J.; Maeda, N.; Sakanaka, M. Adrenergic agonists suppress the proliferation of microglia through β2-adrenergic receptor. Neurosci. Lett. 1998, 242, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Bronzuoli, M.R.; Facchinetti, R.; Valenza, M.; Cassano, T.; Steardo, L.; Scuderi, C. Astrocyte function is affected by aging and not Alzheimer’s disease: A preliminary investigation in hippocampi of 3xTg-AD mice. Front. Pharmacol. 2019, 10, 644. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, J.D.; Dykes-Hoberg, M.; Pardo, C.A.; Bristol, L.A.; Jin, L.; Kuncl, R.W.; Kanai, Y.; Hediger, M.A.; Wang, Y.; Schielke, J.P.; et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 1996, 16, 675–686. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.-G.; Kegelman, T.P.; Su, Z.-Z.; Das, S.K.; Dash, R.; Dasgupta, S.; Barral, P.M.; Hedvat, M.; Diaz, P.; et al. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: Opportunities for developing novel therapeutics. J. Cell. Physiol. 2011, 226, 2484–2493. [Google Scholar] [CrossRef]
- Sheldon, A.L.; Robinson, M.B. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem. Int. 2007, 51, 333–355. [Google Scholar] [CrossRef]
- Monai, H.; Wang, X.; Yahagi, K.; Lou, N.; Mestre, H.; Xu, Q.; Abe, Y.; Yasui, M.; Iwai, Y.; Nedergaard, M.; et al. Adrenergic receptor antagonism induces neuroprotection and facilitates recovery from acute ischemic stroke. Proc. Natl. Acad. Sci. USA 2019, 116, 11010–11019. [Google Scholar] [CrossRef]
- Rosenblum, L.T.; Trotti, D. EAAT2 and the molecular signature of amyotrophic lateral sclerosis. Glial Amino Acid Transp. 2017, 16, 117–136. [Google Scholar]
- Meng, S.; Wang, B.; Li, W. Serum expression of EAAT2 and ADORA2A in patients with different degrees of Alzheimer’s disease. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11783–11792. [Google Scholar]
- Spangaro, M.; Bosia, M.; Zanoletti, A.; Bechi, M.; Cocchi, F.; Pirovano, A.; Lorenzi, C.; Bramanti, P.; Benedetti, F.; Smeraldi, E.; et al. Cognitive dysfunction and glutamate reuptake: Effect of EAAT2 polymorphism in schizophrenia. Neurosci. Lett. 2012, 522, 151–155. [Google Scholar] [CrossRef]
- Katagiri, H.; Tanaka, K.; Manabe, T. Requirement of appropriate glutamate concentrations in the synaptic cleft for hippocampal LTP induction. Eur. J. Neurosci. 2001, 14, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Lauriat, T.; McInnes, L. EAAT2 regulation and splicing: Relevance to psychiatric and neurological disorders. Mol. Psychiatry 2007, 12, 1065–1078. [Google Scholar] [CrossRef]
- McMillan, P.J.; White, S.S.; Franklin, A.; Greenup, J.L.; Leverenz, J.B.; Raskind, M.A.; Szot, P. Differential response of the central noradrenergic nervous system to the loss of locus coeruleus neurons in Parkinson’s disease and Alzheimer’s disease. Brain Res. 2011, 1373, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Weinshenker, D. Long Road to Ruin: Noradrenergic Dysfunction in Neurodegenerative Disease. Trends Neurosci. 2018, 41, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Pamphlett, R. Uptake of environmental toxicants by the locus ceruleus: A potential trigger for neurodegenerative, demyelinating and psychiatric disorders. Med. Hypotheses 2014, 82, 97–104. [Google Scholar] [CrossRef]
- von Coelln, R.; Thomas, B.; Savitt, J.M.; Lim, K.L.; Sasaki, M.; Hess, E.J.; Dawson, V.L.; Dawson, T.M. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proc. Natl. Acad. Sci. USA 2004, 101, 10744–10749. [Google Scholar] [CrossRef]
- Betts, M.J.; Kirilina, E.; Otaduy, M.C.G.; Ivanov, D.; Acosta-Cabronero, J.; Callaghan, M.F.; Lambert, C.; Cardenas-Blanco, A.; Pine, K.; Passamonti, L.; et al. Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain 2019, 142, 2558–2571. [Google Scholar] [CrossRef]
- Xu, W.; Yu, J.-T.; Tan, M.-S.; Tan, L. Cognitive reserve and Alzheimer’s disease. Mol. Neurobiol. 2015, 51, 187–208. [Google Scholar] [CrossRef]
- Shirokawa, T.; Ishida, Y.; Isobe, K.I. Age-dependent changes in axonal branching of single locus coeruleus neurons projecting to two different terminal fields. J. Neurophysiol. 2000, 84, 1120–1122. [Google Scholar] [CrossRef]
- Ishida, Y.; Shirokawa, T.; Miyaishi, O.; Komatsu, Y.; Isobe, K. Age-dependent changes in projections from locus coeruleus to hippocampus dentate gyrus and frontal cortex. Eur. J. Neurosci. 2000, 12, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Haberman, R.P.; Colantuoni, C.; Stocker, A.M.; Schmidt, A.C.; Pedersen, J.T.; Gallagher, M. Prominent hippocampal CA3 gene expression profile in neurocognitive aging. Neurobiol Aging 2011, 32, 1678–1692. [Google Scholar] [CrossRef] [PubMed]
- Almaguer-Melian, W.; Cruz-Aguado, R.; de la Riva, C.; Kendrick, K.; Frey, J.; Bergado, J. Effect of LTP-reinforcing paradigms on neurotransmitter release in the dentate gyrus of young and aged rats. Biochem. Biophys. Res. Commun. 2005, 327, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.; Che, P.; Chen, Y.; Jiao, K.; Roberson, E.D.; Wang, Q. Noradrenergic dysfunction in Alzheimer’s disease. Front. Neurosci. 2015, 9, 220. [Google Scholar] [CrossRef]
- Kazlauckas, V.; Pagnussat, N.; Mioranzza, S.; Kalinine, E.; Nunes, F.; Pettenuzzo, L.O.; Souza, D.; Portela, L.V.; Porciúncula, L.O.; Lara, D.R. Enriched environment effects on behavior, memory and BDNF in low and high exploratory mice. Physiol. Behav. 2011, 102, 475–480. [Google Scholar] [CrossRef]
- Amaral, O.B.; Vargas, R.S.; Hansel, G.; Izquierdo, I.; Souza, D.O. Duration of environmental enrichment influences the magnitude and persistence of its behavioral effects on mice. Physiol. Behav. 2008, 93, 388–394. [Google Scholar] [CrossRef]
- Doreste-Mendez, R.; Ríos-Ruiz, E.J.; Rivera-López, L.L.; Gutierrez, A.; Torres-Reveron, A. Effects of Environmental Enrichment in Maternally Separated Rats: Age and Sex-Specific Outcomes. Front. Behav Neurosci. 2019, 13, 198. [Google Scholar] [CrossRef]
- Cosgrove, J.A.; Kelly, L.K.; Kiffmeyer, E.A.; Kloth, A.D. Sex-dependent influence of postweaning environmental enrichment in Angelman syndrome model mice. Brain Behav. 2022, 12, e2468. [Google Scholar] [CrossRef]
- Warren, S.G.; Juraska, J.M. Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci. 1997, 111, 259–266. [Google Scholar] [CrossRef]
- Marcondes, F.K.; Miguel, K.J.; Melo, L.L.; Spadari-Bratfisch, R.C. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 2001, 74, 435–440. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015, 96, e52434. [Google Scholar]
- Hall, C.; Ballachey, E.L. A study of the Rat’s Behavior in a Field. A Contribution to Method in Comparative Psychology. University of California Publications in Psychology: Berkeley, CA, USA, 1932; Volume 6, pp. 1–12. [Google Scholar]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Moy, S.S.; Nadler, J.J.; Young, N.B.; Nonneman, R.J.; Segall, S.K.; Andrade, G.M.; Crawley, J.N.; Magnuson, T.R. Social approach and repetitive behavior in eleven inbred mouse strains. Behav. Brain Res. 2008, 191, 118–129. [Google Scholar] [CrossRef]
- Crawley, J.N. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007, 17, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Novaes, G.F.; Amado, D.; Scorza, F.A.; Cysneiros, R.M. Social behavior impairment in offspring exposed to maternal seizures in utero. J. Neural Transm. 2012, 119, 639–644. [Google Scholar] [CrossRef]
- Kaur, H.; Patro, I.; Tikoo, K.; Sandhir, R. Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem. Int. 2015, 89, 40–50. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| Weeks | First Intervention | Second Intervention |
|---|---|---|
| 1st | Familiarization with the new environment | Banana (100 g), apple (50 g), grape (25 g) for 5–6 h |
| 2nd | Exposure to cotton balls of different sizes for 4–5 h | Hiding fruit under the bedding for 4–5 h |
| 3rd | Exposure to ice with and without water for 1 h | Exposure to newspaper sheets for 5–6 h |
| 4th | Exposure to carrots (100 g) in different sizes for 4–5 h | Exposure to plastic balls in a box filled with bedding for 3–4 h |
| 5th | Exposure to trail with seasonings (oregano, lemongrass and chamomile) for 2 h | Exposure to cooked rice (50 g) for 3–4 h |
| 6th | Exposure to two extra burrows made from cardboard | A banana (100 g) hanging from a thread attached to the roof of the housing for 2–3 h |
| 7th | Exposure to mirrors for 15 min | Exposure to neutral jelly with raisins (8 units) inside for 3 h |
| 8th | Exposure to bedding from a rat housing placed in four different locations in the housing for 1 h | Exposure to bowls containing water with frozen peas, carrots and corn for 2–3 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravache, T.T.; Batistuzzo, A.; Nunes, G.G.; Gomez, T.G.B.; Lorena, F.B.; Do Nascimento, B.P.P.; Bernardi, M.M.; Lima, E.R.R.; Martins, D.O.; Campos, A.C.P.; et al. Multisensory Stimulation Reverses Memory Impairment in Adrβ3KO Male Mice. Int. J. Mol. Sci. 2023, 24, 10522. https://doi.org/10.3390/ijms241310522
Ravache TT, Batistuzzo A, Nunes GG, Gomez TGB, Lorena FB, Do Nascimento BPP, Bernardi MM, Lima ERR, Martins DO, Campos ACP, et al. Multisensory Stimulation Reverses Memory Impairment in Adrβ3KO Male Mice. International Journal of Molecular Sciences. 2023; 24(13):10522. https://doi.org/10.3390/ijms241310522
Chicago/Turabian StyleRavache, Thaís T., Alice Batistuzzo, Gabriela G. Nunes, Thiago G. B. Gomez, Fernanda B. Lorena, Bruna P. P. Do Nascimento, Maria Martha Bernardi, Eduarda R. R. Lima, Daniel O. Martins, Ana Carolina P. Campos, and et al. 2023. "Multisensory Stimulation Reverses Memory Impairment in Adrβ3KO Male Mice" International Journal of Molecular Sciences 24, no. 13: 10522. https://doi.org/10.3390/ijms241310522
APA StyleRavache, T. T., Batistuzzo, A., Nunes, G. G., Gomez, T. G. B., Lorena, F. B., Do Nascimento, B. P. P., Bernardi, M. M., Lima, E. R. R., Martins, D. O., Campos, A. C. P., Pagano, R. L., & Ribeiro, M. O. (2023). Multisensory Stimulation Reverses Memory Impairment in Adrβ3KO Male Mice. International Journal of Molecular Sciences, 24(13), 10522. https://doi.org/10.3390/ijms241310522






