HRD Testing of Ovarian Cancer in Routine Practice: What Are We Dealing With?
Abstract
1. Introduction
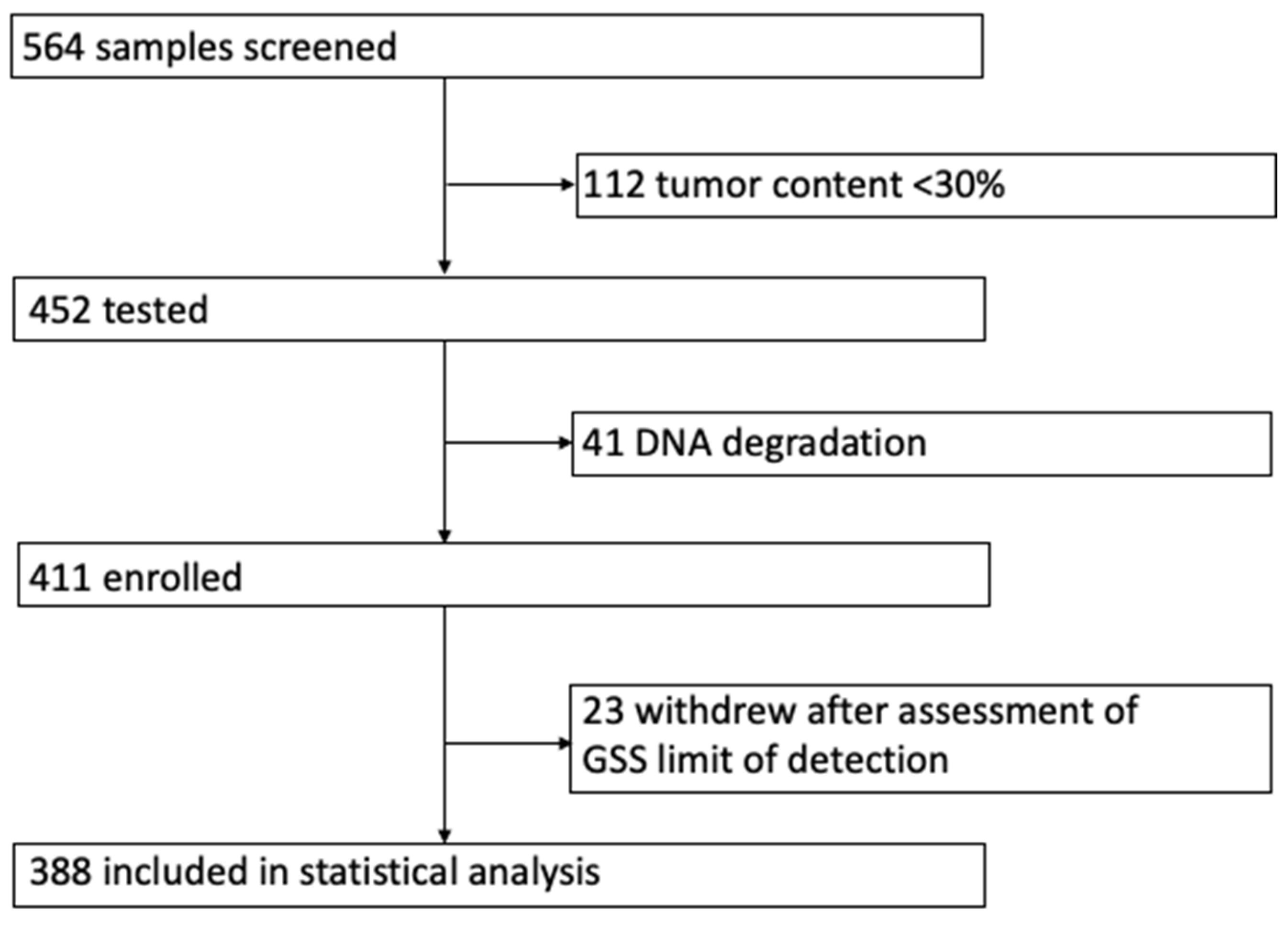
2. Results
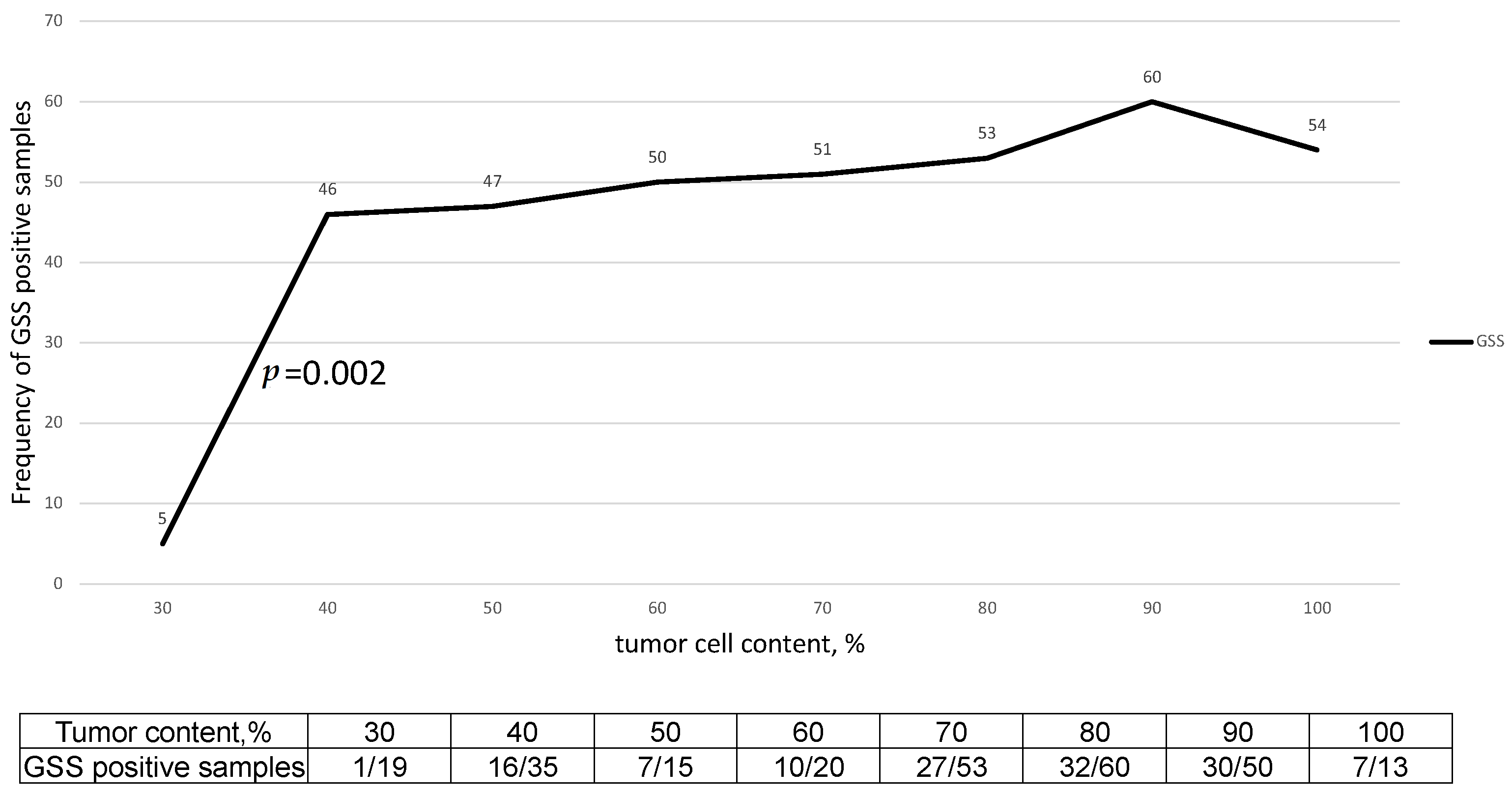
2.1. Impact of Tumor Purity
2.2. HRD and BRCA Rates
2.3. Analysis of Pre- and Post-CT Tumor Samples
2.4. Biallelic versus Monoallelic BRCA1/2 Mutations
3. Discussion
4. Material and Methods
4.1. Study Cohort
4.2. Histopathological Specimens
4.3. Evaluation of Neoadjuvant Chemotherapy
4.4. Sample Preparation and NGS Analysis
4.5. Biallelic versus Monoallelic Alterations for BRCA Mutant
4.6. Variant Classification
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reid, B.; Permuth, J.; Sellers, T. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- D’Oria, O.; Golia D’Auge, T.; Baiocco, E.; Vincenzoni, C.; Mancini, E.; Bruno, V.; Chiofalo, B.; Mancari, R.; Vizza, R.; Cutillo, G.; et al. The role of preoperative frailty assessment in patients affected by gynecological cancer: A narrative review. Ital. J. Gynaecol. Obstet. 2022, 34, 76–83. [Google Scholar] [CrossRef]
- Cortez, A.; Tudrej, P.; Kujawa, K.; Lisowska, K. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38. [Google Scholar] [CrossRef]
- Petrucci, E.; Pasquini, L.; Bernabei, M.; Saulle, E.; Biffoni, M.; Accarpio, F.; Sibio, S.; Giorgio, A.D.; Donato, V.D.; Casorelli, A.; et al. A small molecule SMAC mimic LBW242 potentiates TRAIL- and anticancer drug-mediated cell death of ovarian cancer cells. PLoS ONE 2012, 7, e35073. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Fleming, G.F.; Brady, M.F.; Swisher, E.M.; Steffensen, K.D.; Friedlander, M.; Okamoto, A.; Moore, K.N.; Efrat Ben-Baruch, N.; Werner, T.L.; et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2403–2415. [Google Scholar] [CrossRef]
- Rempel, E.; Kluck, K.; Beck, S.; Ourailidis, I.; Kazdal, D.; Neumann, O.; Volckmar, A.L.; Kirchner, M.; Goldschmid, H.; Pfarr, N.; et al. Pan-cancer analysis of genomic scar patterns caused by homologous repair deficiency (HRD). NPJ Precis. Oncol. 2022, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, N.; Yang, H.; Shin, Y. Homologous Recombination Deficiency in Ovarian, Breast, Colorectal, Pancreatic, Non-Small Cell Lung and Prostate Cancers, and the Mechanisms of Resistance to PARP Inhibitors. Front. Oncol. 2022, 12, 880643. [Google Scholar] [CrossRef]
- Ngoi, N.; Tan, D. The role of homologous recombination deficiency testing in ovarian cancer and its clinical implications: Do we need it? ESMO Open 2021, 6, 100144. [Google Scholar] [CrossRef]
- Davies, H.; Glodzik, D.; Morganella, S.; Yates, L.R.; Staaf, J.; Zou, X.; Ramakrishna, M.; Martin, S.; Boyault, S.; Sieuwerts, A.M.; et al. HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures. Nat. Med. 2017, 23, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, B.D.; Dougherty, B.V.; Bell, J.S.K.; Kapilivsky, J.; Michuda, J.; Sedgewick, A.J.; Munson, W.A.; Chandra, T.A.; Dry, J.R.; Beaubier, N.; et al. Validation of genomic and transcriptomic models of homologous recombination deficiency in a real-world pan-cancer cohort. BMC Cancer 2022, 22, 587. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Ni, J.; Wen, H.; Shi, W.; Chen, X.; Huang, H.; Zhang, X.; Lu, X.; Zhu, C.; Dong, H.; et al. Genomic Scar Score: A robust model predicting homologous recombination deficiency based on genomic instability. BJOG Int. J. Obstet. Gynaecol. 2022, 129 (Suppl. S2), 14–22. [Google Scholar] [CrossRef]
- Patel, A.; Iyer, P.; Matsuzaki, S.; Matsuo, K.; Sood, A.; Fleming, N. Emerging Trends in Neoadjuvant Chemotherapy for Ovarian Cancer. Cancers 2021, 13, 626. [Google Scholar] [CrossRef]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, I.I.I.A. AJCC. PART III: Digestive Systems. AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010; pp. 103–126. [Google Scholar]
- Smits, A.J.J.; Kummer, J.A.; de Bruin, P.C.; Bol, M.; Tweel, J.G.V.D.; Seldenrijk, K.A.; Willems, S.M.; Offerhaus, G.J.A.; de Weger, R.A.; van Diest, P.J.; et al. The estimation of tumor cell percentage for molecular testing by pathologists is not accurate. Mod. Pathol. 2014, 27, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Tyulyandina, A.; Gorbunova, V.; Khokhlova, S.; Kolomiets, L.; Filipenko, M.; Imyanitov, E.; Demidova, I.; Moliaka, Y.; Cherdyntseva, N.; Vodolajskiy, D.; et al. Profile of BRCA1/BRCA2 mutations in Russian ovarian cancer population detected by NGS and MLPA analysis: Interim results of OVATAR study. Cancer Res. 2018, 78 (Suppl. S13), 1241. [Google Scholar] [CrossRef]
- Kechin, A.; Boyarskikh, U.; Barinov, A.; Tanas, A.; Kazakova, S.; Zhevlova, A.; Khrapov, E.; Subbotin, S.; Mishukova, O.; Kekeeva, T.; et al. A spectrum of BRCA1 and BRCA2 germline deleterious variants in ovarian cancer in Russia. Breast Cancer Res. Treat. 2023, 197, 387–3955. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, J.; Li, X.; Westin, S.; Malpica, A.; Lawson, B.; Lee, S.; Fellman, B.M.; Coleman, R.L.; Sood, A.K.; et al. A Modified 2 Tier Chemotherapy Response Score (CRS) and Other Histopathologic Features for Predicting Outcomes of Patients with Advanced Extrauterine High-Grade Serous Carcinoma after Neoadjuvant Chemotherapy. Cancers 2021, 13, 704. [Google Scholar] [CrossRef]
- Arend, R.; Londoño, A.; Montgomery, A.; Smith, H.; Dobbin, Z.; Katre, A.; Martinez, A.; Yang, E.S.; Alvarez, R.D.; Huh, W.K.; et al. Molecular Response to Neoadjuvant Chemotherapy in High-Grade Serous Ovarian Carcinoma. Mol. Cancer Res. 2018, 16, 813–824. [Google Scholar] [CrossRef]
- Wijngaart, H.; Hoes, L.; JHenegouwen, M.; Velden, D.; Zeverijn, L.; Roepman, P.; Van Werkhoven, E.; De Leng, W.W.; Jansen, A.M.; Mehra, N.; et al. Patients with Biallelic BRCA1/2 Inactivation Respond to Olaparib Treatment Across Histologic Tumor Types. Clin. Cancer Res. 2021, 27, 6106–6114. [Google Scholar] [CrossRef] [PubMed]
- Weichert, W.; Lukashchuk, N.; Yarunin, A.; Riva, L.; Easter, A.; Bannister, H.; Qiu, P.; French, T. An evaluation of the performance of molecular assays to identify homologous recombination deficiency-positive tumours in ovarian cancer. Int. J. Gynecol. Cancer 2021, 31, A1–A395. [Google Scholar]
- Magliacane, G.; Brunetto, E.; Calzavara, S.; Bergamini, A.; Pipitone, G.B.; Marra, G.; Redegalli, M.; Grassini, G.; Rabaiotti, E.; Taccagni, G.; et al. Locally Performed HRD Testing for Ovarian Cancer? Yes, We Can! Cancers 2022, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, C.; Betella, I.; Ranghiero, A.; Guerini-Rocco, E.; Bonaldo, G.; Rappa, A.; Vacirca, D.; Colombo, N.; Barberis, M. In-house testing for homologous recombination repair deficiency (HRD) testing in ovarian carcinoma: A feasibility study comparing AmoyDx HRD Focus panel with Myriad myChoiceCDx assay. Pathologica 2022, 114, 288–294. [Google Scholar] [CrossRef]
- Knudson, A. Two genetic hits (more or less) to cancer. Nat. Rev. Cancer 2001, 1, 157–162. [Google Scholar] [CrossRef]

| Age, years, median | 57 (31–85) |
| Diagnosis | 388 |
| Epithelial ovarian cancer | 365 (94%) |
| Fallopian tube cancer | 13 (3%) |
| Primary peritoneal cancer | 10 (3%) |
| Tumor obtained before CT treatment | 327 (84%) |
| Tumor obtained after CT treatment | 53 (14%) |
| No information about CT | 8 (2%) |
| Samples tested | 452 |
| Undetectable HRD status | 64 |
| tBRCA mutant | 114/388 (29%) |
| tBRCA1 mutant | 72 (18.6%) |
| tBRCA2 mutant | 40 (10.3%) |
| Both genes | 2 (0.5%) |
| tBRCA mutant GSS high | 101/114 |
| tBRCA mutant GSS low | 13/114 |
| tBRCA wild type | |
| GSS high | 138 (36%) |
| GSS low | 136 (35%) |
| HRD high | 252/388 (65%) |
| Gene | Variant Name | Occurrence |
|---|---|---|
| BRCA1 | NM_007294.4:exon20:c.5266dup:p.(Q1756Pfs*74) | 34 (8.8%) |
| BRCA2 | NM_000059.3:exon11:c.5286T>G:p.(Y1762*) | 7 (1.8%) |
| BRCA1 | NM_007294.4:exon11:c.1961del:p.(K654Sfs*47) | 3 (0.8%) |
| BRCA2 | NM_000059.3:exon11:c.3847_3848del:p.(V1283Kfs*2) | 3 (0.8%) |
| BRCA1 | NM_007294.4:intron18:c.5152+1G>T | 3 (0.8%) |
| BRCA1 | NM_007294.4:exon11:c.1687C>T:p.(Q563*) | 2 (0.5%) |
| BRCA2 | NM_000059.3:exon11:c.2808_2811del:p.(A938Pfs*21) | 2 (0.5%) |
| BRCA1 | NM_007294.4:exon20:c.5251C>T:p.(R1751*) | 2 (0.5%) |
| Case Number | Pretreatment | Tumor Cell Content, % | GSS Score | BRCA Variant | VAF, % | Biallelic Inactivation |
|---|---|---|---|---|---|---|
| 1314 | yes | 60 | 11 | NM_007294.4:exon20:c.5266dup:p.(Q1756Pfs*74) | 44 | no |
| 423 | yes | 90 | 45 | NM_007294.4:exon11:c.1961del:p.(K654Sfs*47) | 49 | no |
| 1322 | no | 70 | 46 | NM_007294.4:exon20:c.5266dup:p.(Q1756Pfs*74) | 46 | no |
| 266 | no | 80 | 23 | NM_007294.4:exon11:c.2157dup:p.(E720Rfs*6) | 18 | no |
| 1518 | no | 45 | 9 | NM_007294.4:exon20:c.5266dup:p.(Q1756Pfs*74) | 51 | NA |
| 335 | no | 40 | 43 | NM_007294.4:intron18:c.5152+1G>T | 55 | no |
| 1621 | no | 85 | 29 | NM_000059.3:exon11:c.3637G>T:p.(E1213*) | 56 | no |
| 3319 | no | 80 | 7 | NM_000059.3:exon11:c.6082_6086del:p.(E2028Kfs*19) | 44 | no |
| 135 | yes | 40 | 41 | NM_007294.3:exon2:c.53T>C:p.(M18T) | 62 | NA |
| 292 | yes | 50 | 23 | NM_007294.4:exon20:c.5266dup:p.(Q1756Pfs*74) | 50 | NA |
| 332 | yes | 60 | 46 | NM_000059.3:exon11:c.6591_6592del:p.(E2198Nfs*4) | 64 | NA |
| 478 | yes | 50 | 36 | NM_007294.4:exon20:c.5266dup:p.(Q1756Pfs*74) | 48 | NA |
| 425 | no | 50 | 1 | NM_007294.4:exon2:c.66dup:p.(E23Rfs*18) | 52 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kekeeva, T.; Andreeva, Y.; Tanas, A.; Kalinkin, A.; Khokhlova, S.; Tikhomirova, T.; Tyulyandina, A.; Popov, A.; Kuzmenko, M.; Volkonsky, M.; et al. HRD Testing of Ovarian Cancer in Routine Practice: What Are We Dealing With? Int. J. Mol. Sci. 2023, 24, 10497. https://doi.org/10.3390/ijms241310497
Kekeeva T, Andreeva Y, Tanas A, Kalinkin A, Khokhlova S, Tikhomirova T, Tyulyandina A, Popov A, Kuzmenko M, Volkonsky M, et al. HRD Testing of Ovarian Cancer in Routine Practice: What Are We Dealing With? International Journal of Molecular Sciences. 2023; 24(13):10497. https://doi.org/10.3390/ijms241310497
Chicago/Turabian StyleKekeeva, Tatiana, Yulia Andreeva, Alexander Tanas, Alexey Kalinkin, Svetlana Khokhlova, Tatiana Tikhomirova, Alexandra Tyulyandina, Anatoly Popov, Maria Kuzmenko, Mikhail Volkonsky, and et al. 2023. "HRD Testing of Ovarian Cancer in Routine Practice: What Are We Dealing With?" International Journal of Molecular Sciences 24, no. 13: 10497. https://doi.org/10.3390/ijms241310497
APA StyleKekeeva, T., Andreeva, Y., Tanas, A., Kalinkin, A., Khokhlova, S., Tikhomirova, T., Tyulyandina, A., Popov, A., Kuzmenko, M., Volkonsky, M., Chernorubashkina, N., Saevets, V., Dmitriev, V., Nechushkina, V., Vedrova, O., Andreev, S., Kutsev, S., & Strelnikov, V. (2023). HRD Testing of Ovarian Cancer in Routine Practice: What Are We Dealing With? International Journal of Molecular Sciences, 24(13), 10497. https://doi.org/10.3390/ijms241310497






