A Sequential Micro-Immunotherapy Medicine Increases Collagen Deposition in Human Gingival Fibroblasts and in an Engineered 3D Gingival Model under Inflammatory Conditions
Abstract
1. Introduction
2. Results
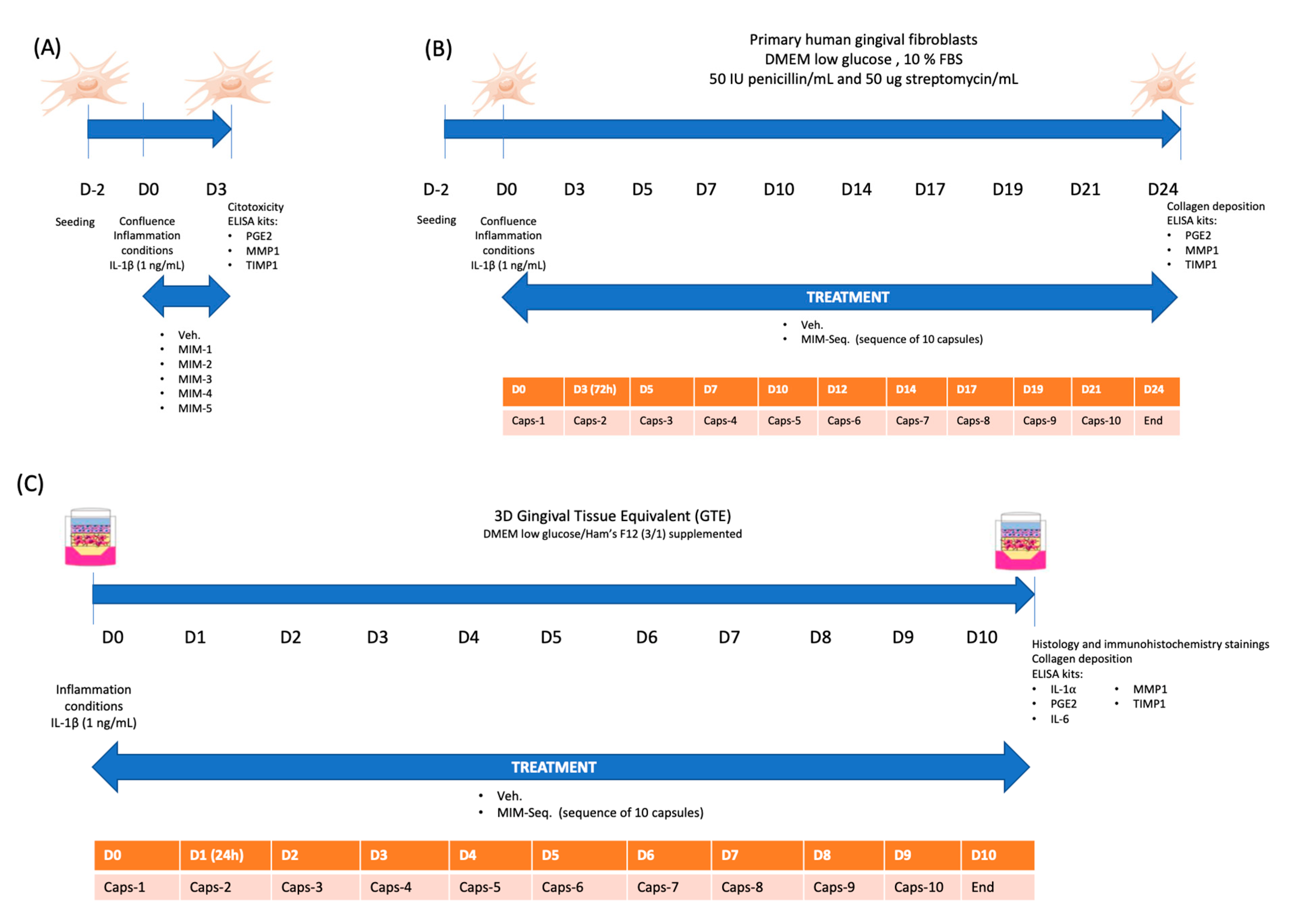
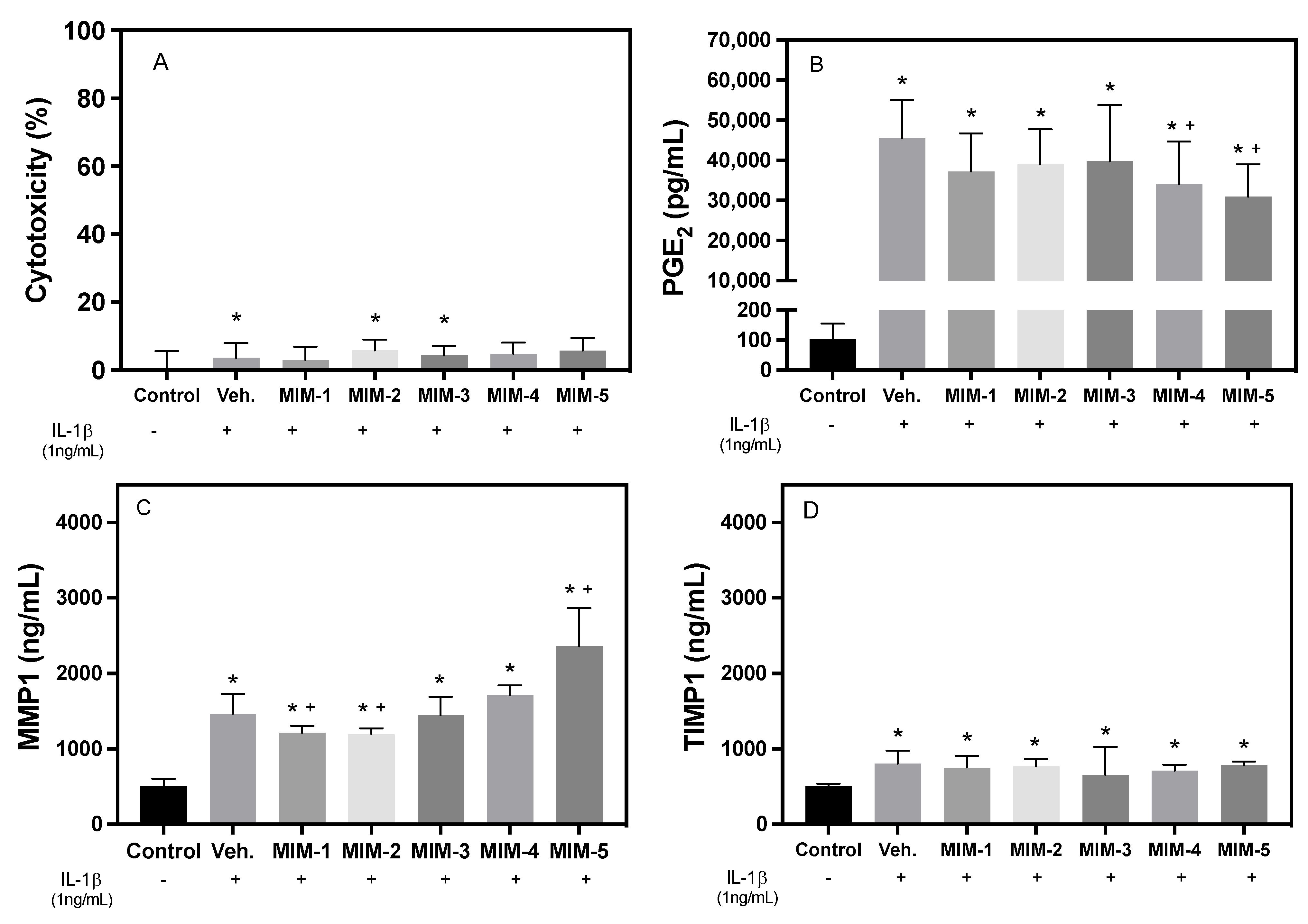
2.1. Short-Time Experiments
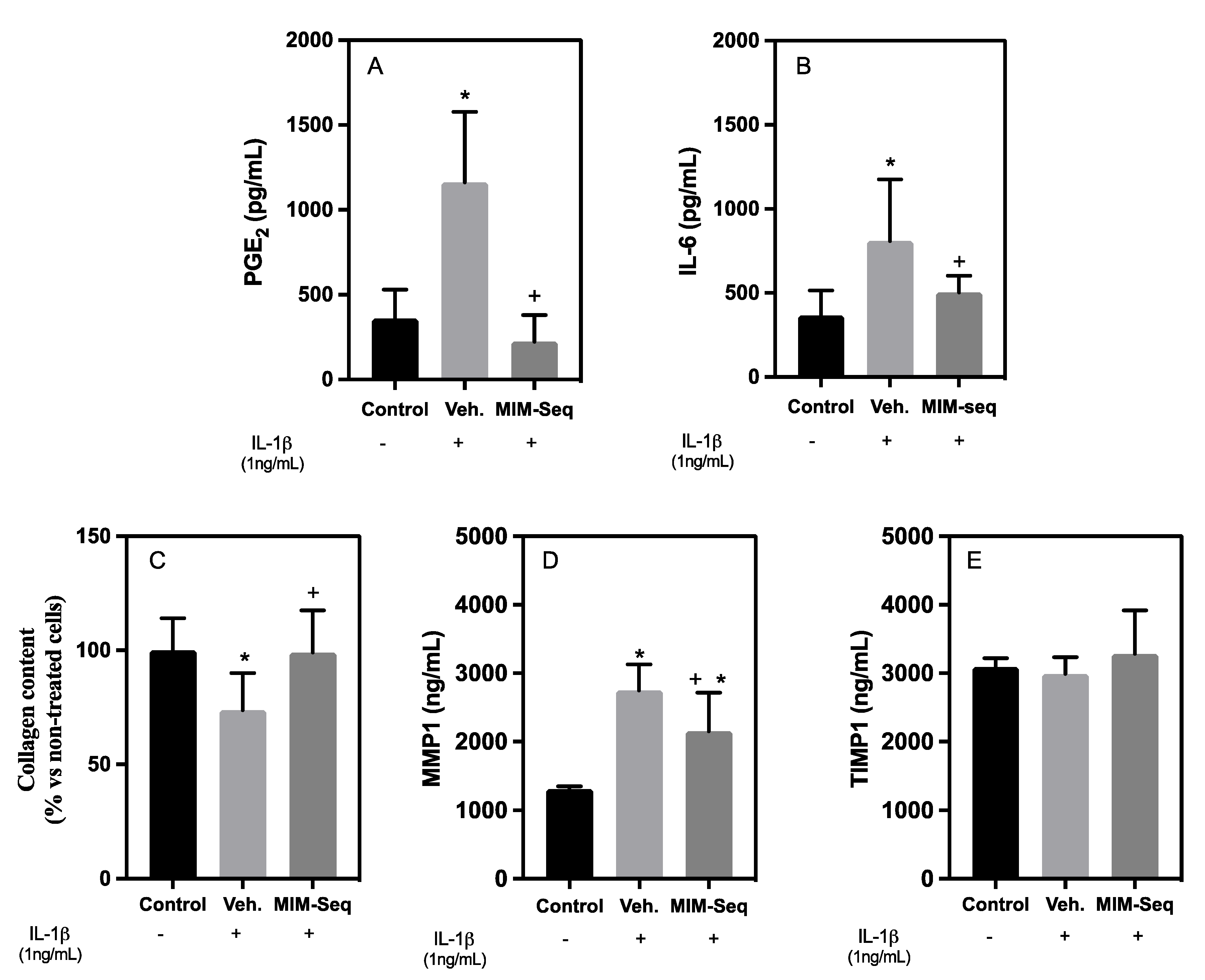
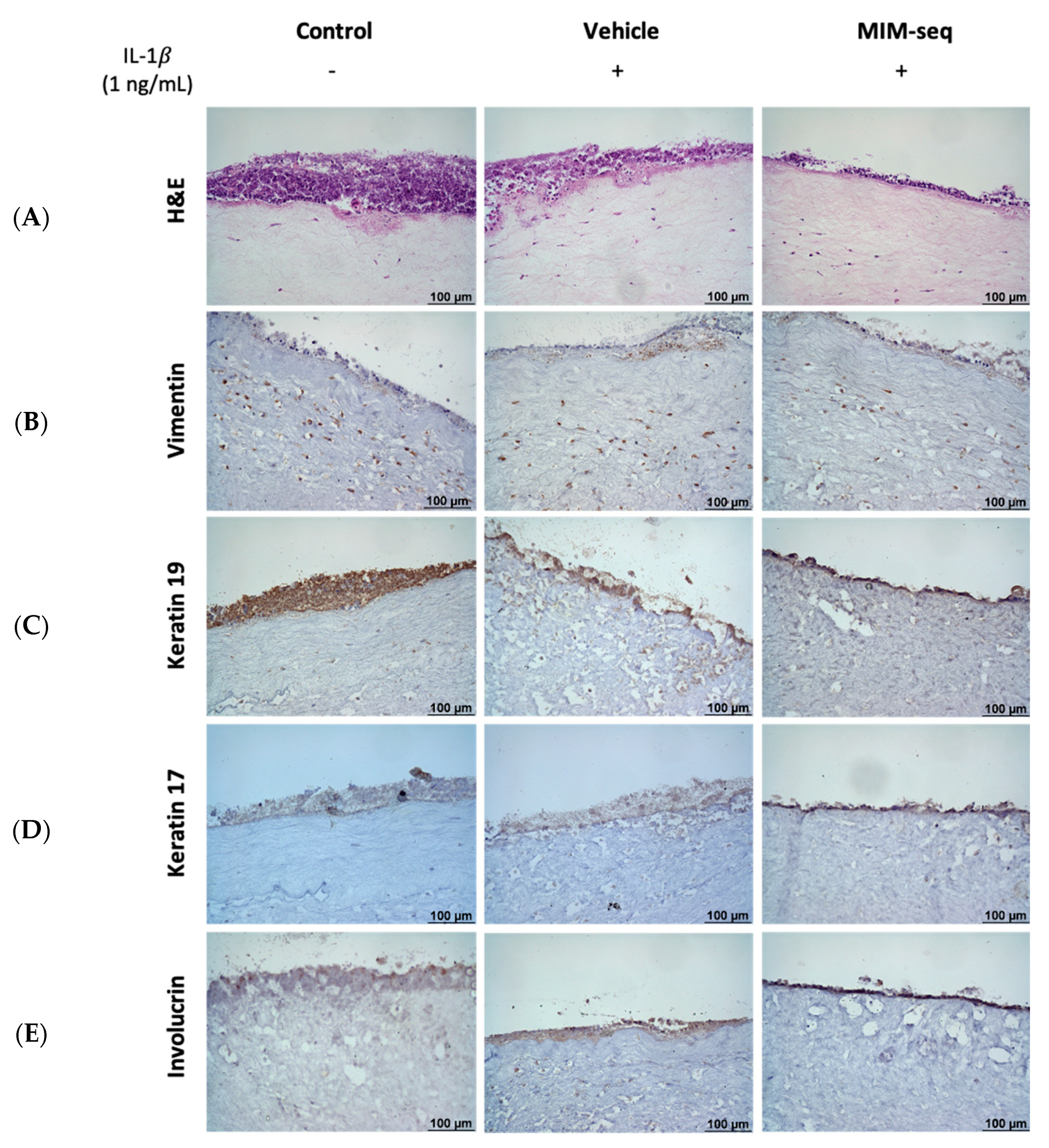
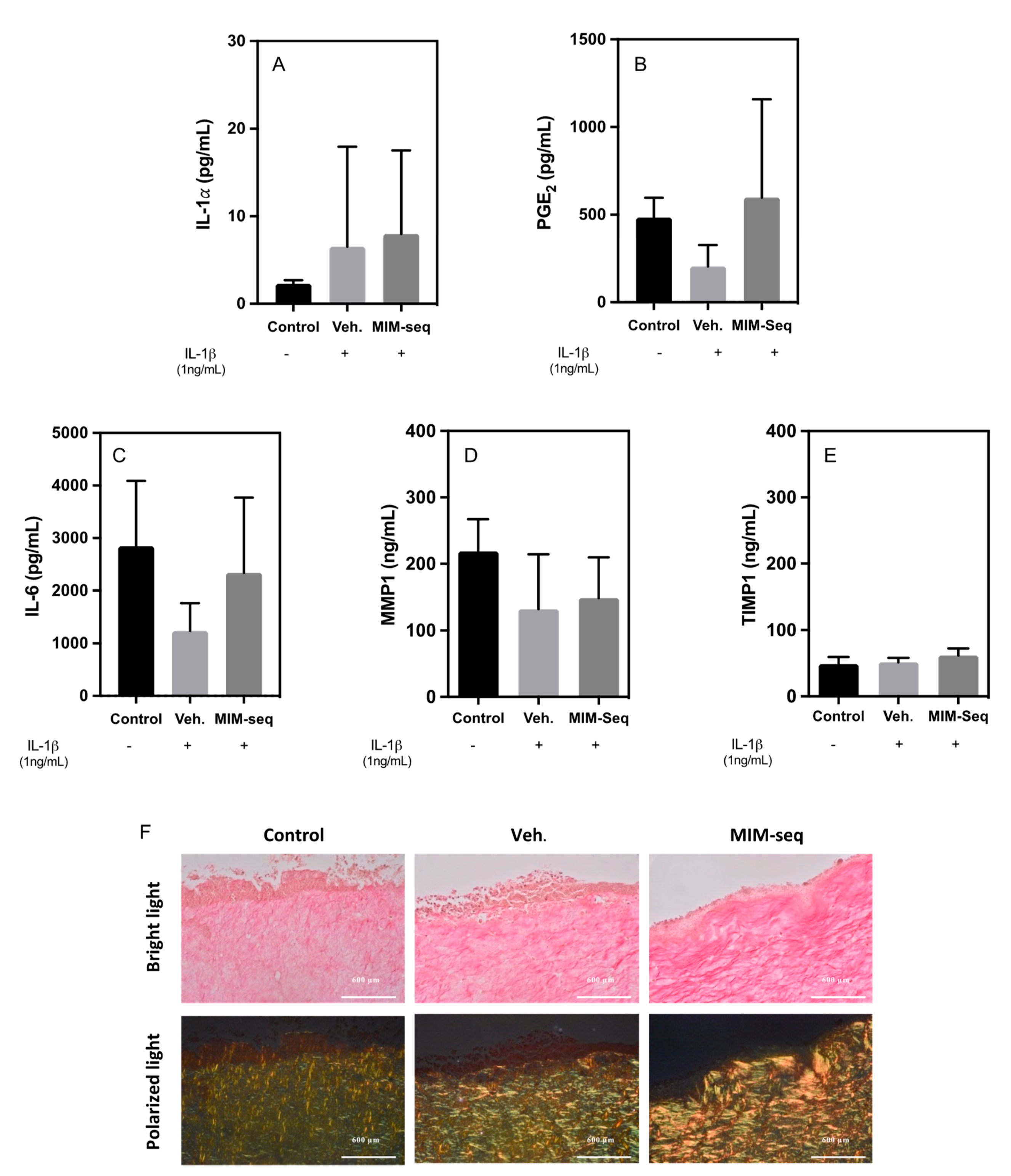
2.2. Long-Time Experiments
3. Discussion
4. Materials and Methods
4.1. Testing the Micro-Immunotherapy Medicine
4.2. hGF Cell Culture
4.3. hGF Short-Time and Long-Time Treatment Experiments
4.4. Tridimensional Model of Gingival Tissue Equivalent (GTE) and Long-Time Treatment Experiment
4.5. Cell Cytotoxicity Assay
4.6. Collagen Deposition Assessment
4.7. Enzyme-Linked Immunosorbent Assays
4.8. Histology and Immunohistochemistry of GTE Tissues
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- De Araújo, A.A.; de Morais, H.B.; de Medeiros, C.A.C.X.; de Castro Brito, G.A.; Guedes, P.M.M.; Hiyari, S.; Pirih, F.Q.; de Araújo Júnior, R.F. Gliclazide Reduced Oxidative Stress, Inflammation, and Bone Loss in an Experimental Periodontal Disease Model. J. Appl. Oral Sci. 2019, 27, e20180211. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Florit, M.; Monjo, M.; Ramis, J.M. Quercitrin for Periodontal Regeneration: Effects on Human Gingival Fibroblasts and Mesenchymal Stem Cells. Sci. Rep. 2015, 5, 16593. [Google Scholar] [CrossRef] [PubMed]
- Shazam, H.; Shaikh, F.; Hussain, Z. Bone Turnover Markers in Chronic Periodontitis: A Literature Review. Cureus 2020, 12, e6699. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.M.; Dörfer, C.E. Animal Models for Periodontal Tissue Engineering: A Knowledge-Generating Process. Tissue Eng. Part C Methods 2017, 23, 900–925. [Google Scholar] [CrossRef]
- Ramadan, D.E.; Hariyani, N.; Indrawati, R.; Ridwan, R.D.; Diyatri, I. Cytokines and Chemokines in Periodontitis. Eur. J. Dent. 2020, 14, 483. [Google Scholar] [CrossRef] [PubMed]
- Plemmenos, G.; Evangeliou, E.; Polizogopoulos, N.; Chalazias, A.; Deligianni, M.; Piperi, C. Central Regulatory Role of Cytokines in Periodontitis and Targeting Options. Curr. Med. Chem. 2021, 28, 3032–3058. [Google Scholar] [CrossRef]
- Decambron, A.; Devriendt, N.; Larochette, N.; Manassero, M.; Bourguignon, M.; El-Hafci, H.; Petite, H.; Viateau, V.; Logeart-Avramoglou, D. Effect of the Bone Morphogenetic Protein-2 Doses on the Osteogenic Potential of Human Multipotent Stromal Cells-Containing Tissue Engineered Constructs. Tissue Eng. Part A 2019, 25, 642–651. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284. [Google Scholar] [CrossRef]
- Madhav, N.V.S.; Shakya, A.K.; Shakya, P.; Singh, K. Orotransmucosal Drug Delivery Systems: A Review. J. Control. Release 2009, 140, 2–11. [Google Scholar] [CrossRef]
- Offenbacher, S.; Collins, J.G.; Arnold, R.R. New Clinical Diagnostic Strategies Based on Pathogenesis of Disease. J. Periodontal Res. 1993, 28, 523–535. [Google Scholar] [CrossRef]
- Tariq, M.; Iqbal, Z.; Ali, J.; Baboota, S.; Talegaonkar, S.; Ahmad, Z.; Sahni, J.K. Treatment Modalities and Evaluation Models for Periodontitis. Int. J. Pharm. Investig. 2012, 2, 106. [Google Scholar] [CrossRef] [PubMed]
- Solakoglu, Ö.; Heydecke, G.; Amiri, N.; Anitua, E. The Use of Plasma Rich in Growth Factors (PRGF) in Guided Tissue Regeneration and Guided Bone Regeneration. A Review of Histological, Immunohistochemical, Histomorphometrical, Radiological and Clinical Results in Humans. Ann. Anat. 2020, 231, 151528. [Google Scholar] [CrossRef] [PubMed]
- Benic, G.I.; Hämmerle, C.H.F. Horizontal Bone Augmentation by Means of Guided Bone Regeneration. Periodontol 2000 2014, 66, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.H.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Klatzmann, D.; Abbas, A.K. The Promise of Low-Dose Interleukin-2 Therapy for Autoimmune and Inflammatory Diseases. Nat. Rev. Immunol. 2015, 15, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Murugan, T.; Elavarasu, S. Host Modulation by Therapeutic Agents. J. Pharm. Bioallied. Sci. 2012, 4, 256. [Google Scholar] [CrossRef]
- Leira, Y.; Iglesias-Rey, R.; Gómez-Lado, N.; Aguiar, P.; Campos, F.; D’Aiuto, F.; Castillo, J.; Blanco, J.; Sobrino, T. Porphyromonas Gingivalis Lipopolysaccharide-Induced Periodontitis and Serum Amyloid-Beta Peptides. Arch. Oral Biol. 2019, 99, 120–125. [Google Scholar] [CrossRef]
- Floris, I.; Appel, K.; Rose, T.; Lejeune, B. 2LARTH®, a Micro-Immunotherapy Medicine, Exerts Anti-Inflammatory Effects in Vitro and Reduces TNF-α and IL-1β Secretion. J. Inflamm. Res. 2018, 11, 397–405. [Google Scholar] [CrossRef]
- Floris, I.; García-González, V.; Palomares, B.; Appel, K.; Lejeune, B. The Micro-Immunotherapy Medicine 2LARTH® Reduces Inflammation and Symptoms of Rheumatoid Arthritis In Vivo. Int. J. Rheumatol. 2020, 2020, 1594573. [Google Scholar] [CrossRef]
- Floris, I.; Chenuet, P.; Togbe, D.; Volteau, C.; Lejeune, B. Potential Role of the Micro-Immunotherapy Medicine 2LALERG in the Treatment of Pollen-Induced Allergic Inflammation. Dose-Response 2020, 18, 1–10. [Google Scholar] [CrossRef]
- Floris, I.; Rose, T.; Rojas, J.A.C.; Appel, K.; Roesch, C.; Lejeune, B. Pro-Inflammatory Cytokines at Ultra-Low Dose Exert Anti-Inflammatory Effect In Vitro: A Possible Mode of Action Involving Sub-Micron Particles? Dose-Response 2020, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Floris, I.; Lejeune, B. Ultra-Low Dose Cytokines in Rheumatoid Arthritis, Three Birds with One Stone as the Rationale of the 2LARTH® Micro-Immunotherapy Treatment. Int. J. Mol. Sci. 2021, 22, 6717. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Ferrà-Cañellas, M.; Munar-Bestard, M.; Garcia-Sureda, L.; Lejeune, B.; Ramis, J.M.; Monjo, M. BMP4 Micro-Immunotherapy Increases Collagen Deposition and Reduces PGE2 Release in Human Gingival Fibroblasts and Increases Tissue Viability of Engineered 3D Gingiva under Inflammatory Conditions. J. Periodontol. 2021, 92, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, M.; Yamaguchi, Y.; Matsumoto, N.; Micke, P.; Takenouchi, Y.; Nishida, T.; Kato, M.; Komiyama, K.; Abiko, Y.; Ito, K.; et al. TGF-β Signaling in Gingival Fibroblast-Epithelial Interaction. J. Dent. Res. 2010, 89, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Jiang, Z.; Pan, Y.; Yang, G.; Wang, Y. Role and Mechanism of BMP4 in Bone, Craniofacial, and Tooth Development. Arch. Oral Biol. 2022, 140, 105465. [Google Scholar] [CrossRef]
- Costello, L.C.; Chellaiah, M.A.; Zou, J.; Reynolds, M.A.; Franklin, R.B. In Vitro BMP2 Stimulation of Osteoblast Citrate Production in Concert with Mineralized Bone Nodule Formation. J. Regen. Med. Tissue Eng. 2015, 4, 2. [Google Scholar] [CrossRef]
- Lean, J.M.; Mackay, A.G.; Chow, J.W.M.; Chambers, T.J. Osteocytic Expression of MRNA for C-Fos and IGF-I: An Immediate Early Gene Response to an Osteogenic Stimulus. Am. J. Physiol. Endocrinol. Metab. 1996, 270, E937–E945. [Google Scholar] [CrossRef]
- Sheng, M.H.C.; Zhou, X.D.; Bonewald, L.F.; Baylink, D.J.; Lau, K.H.W. Disruption of the Insulin-like Growth Factor-1 Gene in Osteocytes Impairs Developmental Bone Growth in Mice. Bone 2013, 52, 133–144. [Google Scholar] [CrossRef]
- Tagliaferri, C.; Wittrant, Y.; Davicco, M.J.; Walrand, S.; Coxam, V. Muscle and Bone, Two Interconnected Tissues. Ageing Res. Rev. 2015, 21, 55–70. [Google Scholar] [CrossRef]
- Lam, R.S.; O’Brien-Simpson, N.M.; Hamilton, J.A.; Lenzo, J.C.; Holden, J.A.; Brammar, G.C.; Orth, R.K.; Tan, Y.; Walsh, K.A.; Fleetwood, A.J.; et al. GM-CSF and UPA Are Required for Porphyromonas Gingivalis-Induced Alveolar Bone Loss in a Mouse Periodontitis Model. Immunol. Cell Biol. 2015, 93, 705–715. [Google Scholar] [CrossRef]
- Jacques, C.; Chatelais, M.; Fekir, K.; Fauconnier, L.; Mellier, M.; Togbe, D.; Floris, I. The Micro-Immunotherapy Medicine 2leid Exhibits an Immunostimulant Effect by Boosting Both Innate and Adaptive Immune Responses. Int. J. Mol. Sci. 2022, 23, 110. [Google Scholar] [CrossRef]
- Jacques, C.; Chatelais, M.; Fekir, K.; Brulefert, A.; Floris, I. The Unitary Micro-Immunotherapy Medicine Interferon-γ (4 CH) Displays Similar Immunostimulatory and Immunomodulatory Effects than Those of Biologically Active Human Interferon-γ on Various Cell Types. Int. J. Mol. Sci. 2022, 23, 2314. [Google Scholar] [CrossRef]
- Casas, J.W.; Lewerenz, G.M.; Rankin, E.A.; Willoughby, J.A.; Blakeman, L.C.; McKim, J.M.; Coleman, K.P. In Vitro Human Skin Irritation Test for Evaluation of Medical Device Extracts. Toxicol. Vitr. 2013, 27, 2175–2183. [Google Scholar] [CrossRef]
- Mariotti, M.; Castiglioni, S.; Bernardini, D.; Maier, J.A.M. Interleukin 1 Alpha Is a Marker of Endothelial Cellular Senescent. Immun. Ageing 2006, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Idan, C.; Peleg, R.; Elena, V.; Martin, T.; Cicerone, T.; Mareike, W.; Lydia, B.; Marina, F.; Gerhard, M.; Elisa, F.M.; et al. IL-1α Is a DNA Damage Sensor Linking Genotoxic Stress Signaling to Sterile Inflammation and Innate Immunity. Sci. Rep. 2015, 5, 14756. [Google Scholar] [CrossRef]
- Park, J.Y.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 Synthesis and Secretion: The Role of PGE2 Synthases. Clin. Immunol. 2006, 119, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, P.; Wähämaa, H.; Idborg, H.; Jakobsson, P.J.; Harris, H.E.; Korotkova, M. IL-1β/HMGB1 Complexes Promote The PGE2 Biosynthesis Pathway in Synovial Fibroblasts. Scand. J. Immunol. 2013, 77, 350–360. [Google Scholar] [CrossRef]
- Molina-Holgado, E.; Ortiz, S.; Molina-Holgado, F.; Guaza, C. Induction of COX-2 and PGE2 Biosynthesis by IL-1β Is Mediated by PKC and Mitogen-Activated Protein Kinases in Murine Astrocytes. Br. J. Pharmacol. 2000, 131, 152–159. [Google Scholar] [CrossRef]
- Yamamoto, M.; Aoyagi, M.; Fukai, N.; Matsushima, Y.; Yamamoto, K. Increase in Prostaglandin E2 Production by Interleukin-1β in Arterial Smooth Muscle Cells Derived From Patients With Moyamoya Disease. Circ. Res. 1999, 85, 912–918. [Google Scholar] [CrossRef]
- Jacques, C.; Floris, I. Special Focus on the Cellular Anti-Inflammatory Effects of Several Micro-Immunotherapy Formulations: Considerations Regarding Intestinal-, Immune-Axis-Related and Neuronal-Inflammation Contexts. J. Inflamm. Res. 2022, in press. [CrossRef]
- Jacques, C.; Marchesi, I.; Fiorentino, F.P.; Chatelais, M.; Lilli, N.L.; Appel, K.; Lejeune, B.; Floris, I. A Micro-Immunotherapy Sequential Medicine MIM-Seq Displays Immunomodulatory Effects on Human Macrophages and Anti-Tumor Properties towards In Vitro 2D and 3D Models of Colon Carcinoma and in an In Vivo Subcutaneous Xenograft Colon Carcinoma Model. Int. J. Mol. Sci. 2022, 23, 6059. [Google Scholar] [CrossRef] [PubMed]
- Beklen, A.; Ainola, M.; Hukkanen, M.; Gürgan, C.; Sorsa, T.; Konttinen, Y.T. MMPs, IL-1, and TNF Are Regulated by IL-17 in Periodontitis. J. Dent. Res. 2016, 86, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.J.; Hembry, R.M.; Meikle, M.C. Connective Tissue Degradation in Health and Periodontal Disease and the Roles of Matrix Metalloproteinases and Their Natural Inhibitors. Adv. Dent. Res. 1994, 8, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Séguier, S.; Gogly, B.; Bodineau, A.; Godeau, G.; Brousse, N. Is Collagen Breakdown During Periodontitis Linked to Inflammatory Cells and Expression of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Human Gingival Tissue? J. Periodontol. 2001, 72, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Bao, K.; Akguel, B.; Bostanci, N. Establishment and Characterization of Immortalized Gingival Epithelial and Fibroblastic Cell Lines for the Development of Organotypic Cultures. Cells Tissues Organs 2014, 199, 228–237. [Google Scholar] [CrossRef]
- Mierke, C.T. Physical and Biological Advances in Endothelial Cell-Based Engineered Co-Culture Model Systems. Semin. Cell Dev. Biol. 2023, 147, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Chen, C.S. Deconstructing the Third Dimension: How 3D Culture Microenvironments Alter Cellular Cues. J. Cell. Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Donos, N. The Periodontal Pocket. Periodontol 2000 2018, 76, 7–15. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius Red Staining: A Useful Tool to Appraise Collagen Networks in Normal and Pathological Tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef]
- López De Padilla, C.M.; Coenen, M.J.; Tovar, A.; De la Vega, R.E.; Evans, C.H.; Müller, S.A. Picrosirius Red Staining: Revisiting Its Application to the Qualitative and Quantitative Assessment of Collagen Type I and Type III in Tendon. J. Histochem. Cytochem. 2021, 69, 633–643. [Google Scholar] [CrossRef]
- Higuchi, T.; Nishikawa, J.; Inoue, H. Sucrose Induces Vesicle Accumulation and Autophagy. J. Cell. Biochem. 2015, 116, 609–617. [Google Scholar] [CrossRef] [PubMed]
- DeCourcy, K.; Storrie, B. Osmotic Swelling of Endocytic Compartments Induced by Internalized Sucrose Is Restricted to Mature Lysosomes in Cultured Mammalian Cells. Exp. Cell Res. 1991, 192, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, M.; Li, L.; Xu, S.; Huang, D.; Ju, M.; Huang, J.; Chen, K.; Gu, H. Trehalose, Sucrose and Raffinose Are Novel Activators of Autophagy in Human Keratinocytes through an MTOR-Independent Pathway. Sci. Rep. 2016, 6, 28423. [Google Scholar] [CrossRef] [PubMed]
- Munar-Bestard, M.; Llopis-Grimalt, M.A.; Ramis, J.M.; Monjo, M. Comparative In Vitro Evaluation of Commercial Periodontal Gels on Antibacterial, Biocompatibility and Wound Healing Ability. Pharmaceutics 2021, 13, 1502. [Google Scholar] [CrossRef]
| MIM-seq | MIM-1 | MIM-2 | MIM-3 | MIM-4 | MIM-5 |
|---|---|---|---|---|---|
| IL-1β (17 CH) | IL-1β (17 CH) | IL-1β (17 CH) | |||
| IL-6 (17 CH) | IL-6 (17 CH) | IL-6 (17 CH) | IL-6 (17 CH) | ||
| IL-11 (15 CH) | IL-11 (15 CH) | IL-11 (15 CH) | IL-11 (15 CH) | ||
| BMP-2 (5 CH) | BMP-2 (5 CH) | BMP-2 (5 CH) | BMP-2 (5 CH) | BMP-2 (5 CH) | BMP-2 (5 CH) |
| BMP-4 (5 CH) | BMP-4 (5 CH) | BMP-4 (5 CH) | BMP-4 (5 CH) | BMP-4 (5 CH) | BMP-4 (5 CH) |
| DNA (5 CH) | ADN (5 CH) | ADN (5 CH) | |||
| IGF-1 (9 CH) | IGF-1 (9 CH) | ||||
| GMCSF (15 CH) | GMCSF (15 CH) | GMCSF 1(5 CH) | |||
| Na2SiF6 (3CH) | Na2SiF6 (3 CH) | ||||
| RNA (5 CH) | RNA (5 CH) | RNA (5 CH) | RNA (5 CH) | ||
| TGF-β (5 CH) | TGF-β (5 CH) | ||||
| TNF-a (17 CH) | TNF-a (17 CH) | TNF-a (17 CH) | |||
| SNA-OSTEOa-02 (18 CH) | SNA-OSTEOa-02 (18 CH) | ||||
| SNA-OSTEOb-02 (18 CH) | SNA-OSTEOb-02 (18 CH) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrà-Cañellas, M.d.M.; Munar-Bestard, M.; Floris, I.; Ramis, J.M.; Monjo, M.; Garcia-Sureda, L. A Sequential Micro-Immunotherapy Medicine Increases Collagen Deposition in Human Gingival Fibroblasts and in an Engineered 3D Gingival Model under Inflammatory Conditions. Int. J. Mol. Sci. 2023, 24, 10484. https://doi.org/10.3390/ijms241310484
Ferrà-Cañellas MdM, Munar-Bestard M, Floris I, Ramis JM, Monjo M, Garcia-Sureda L. A Sequential Micro-Immunotherapy Medicine Increases Collagen Deposition in Human Gingival Fibroblasts and in an Engineered 3D Gingival Model under Inflammatory Conditions. International Journal of Molecular Sciences. 2023; 24(13):10484. https://doi.org/10.3390/ijms241310484
Chicago/Turabian StyleFerrà-Cañellas, Maria del Mar, Marta Munar-Bestard, Ilaria Floris, Joana Maria Ramis, Marta Monjo, and Laura Garcia-Sureda. 2023. "A Sequential Micro-Immunotherapy Medicine Increases Collagen Deposition in Human Gingival Fibroblasts and in an Engineered 3D Gingival Model under Inflammatory Conditions" International Journal of Molecular Sciences 24, no. 13: 10484. https://doi.org/10.3390/ijms241310484
APA StyleFerrà-Cañellas, M. d. M., Munar-Bestard, M., Floris, I., Ramis, J. M., Monjo, M., & Garcia-Sureda, L. (2023). A Sequential Micro-Immunotherapy Medicine Increases Collagen Deposition in Human Gingival Fibroblasts and in an Engineered 3D Gingival Model under Inflammatory Conditions. International Journal of Molecular Sciences, 24(13), 10484. https://doi.org/10.3390/ijms241310484









