Target Identification in Anti-Tuberculosis Drug Discovery
Abstract
1. Introduction
2. Challenges in TB Drug Discovery
3. Emerging Mtb Drug Targets
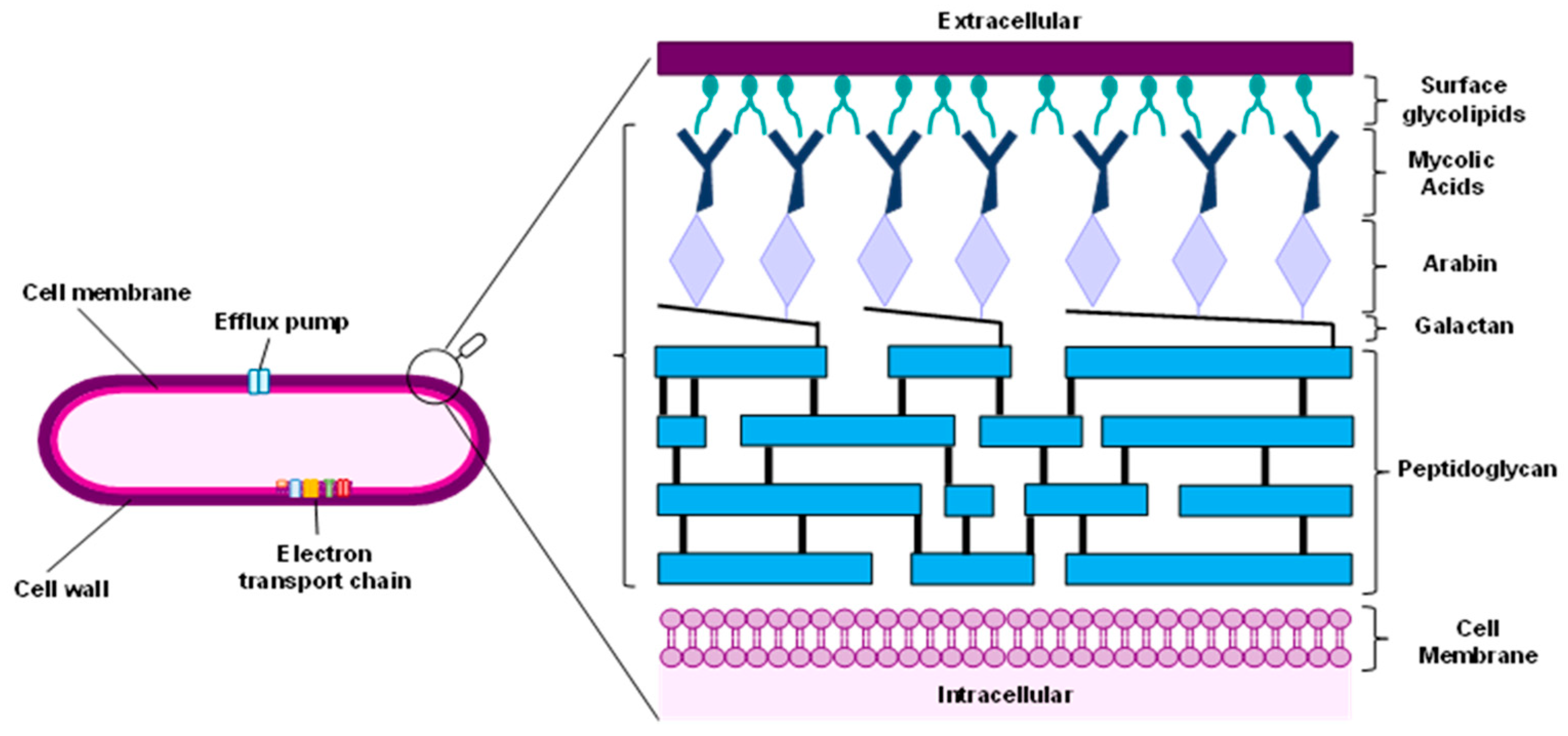
3.1. Cell Wall
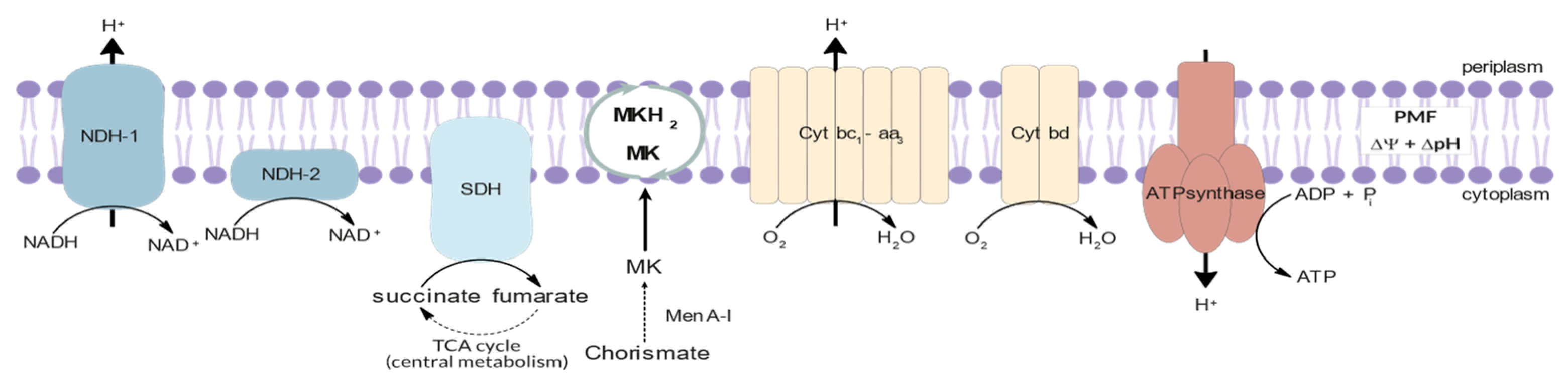
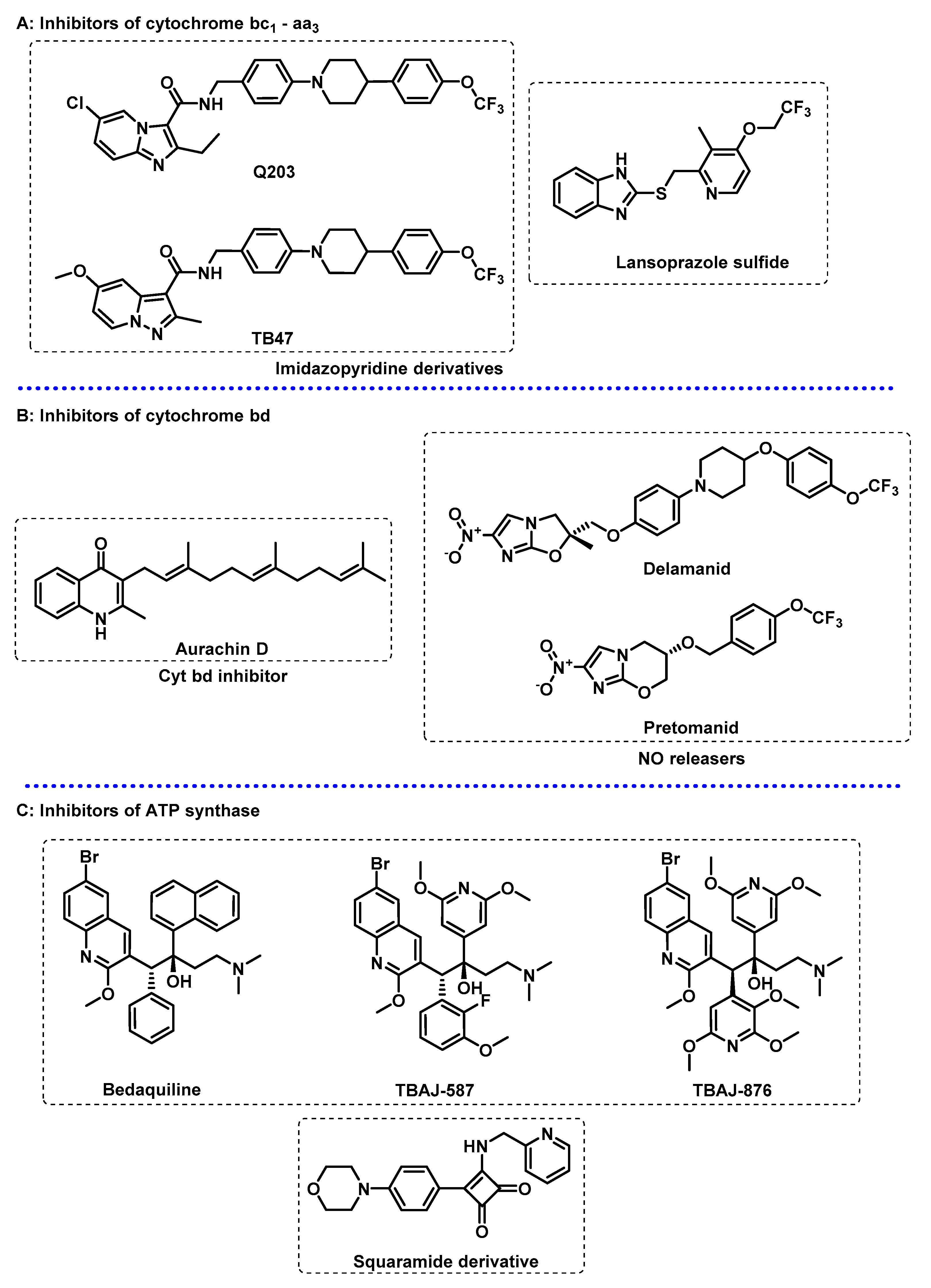
3.2. Energy Metabolism
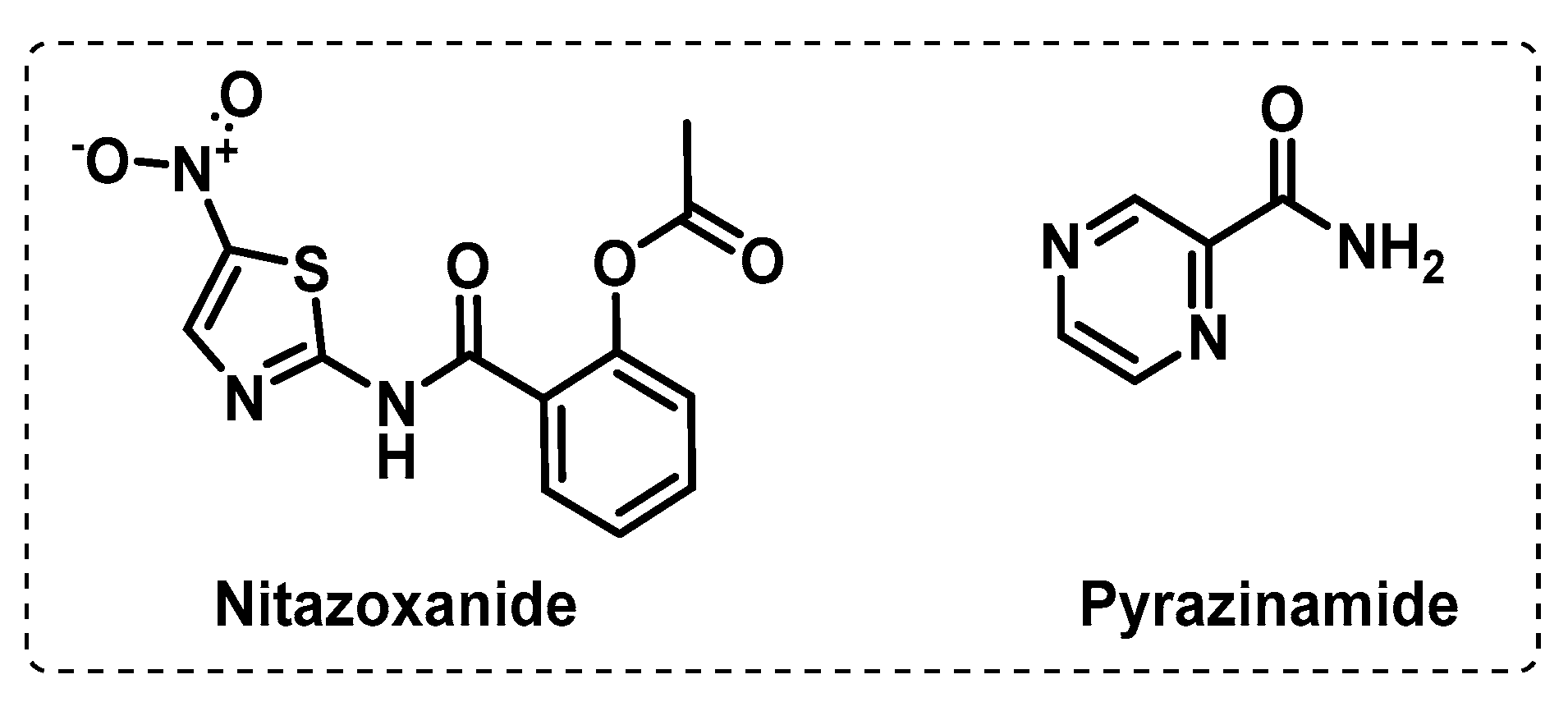
3.3. Other Targets
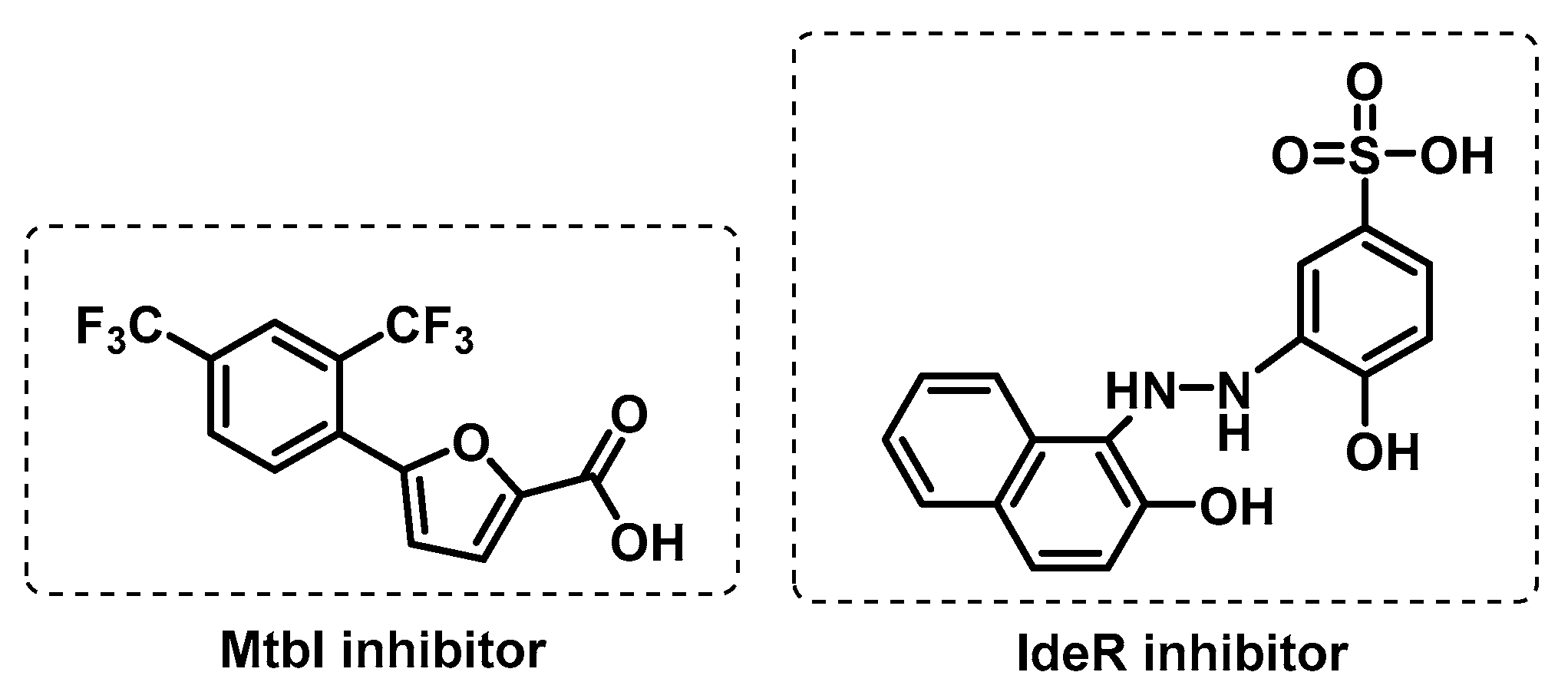
3.3.1. Iron Uptake
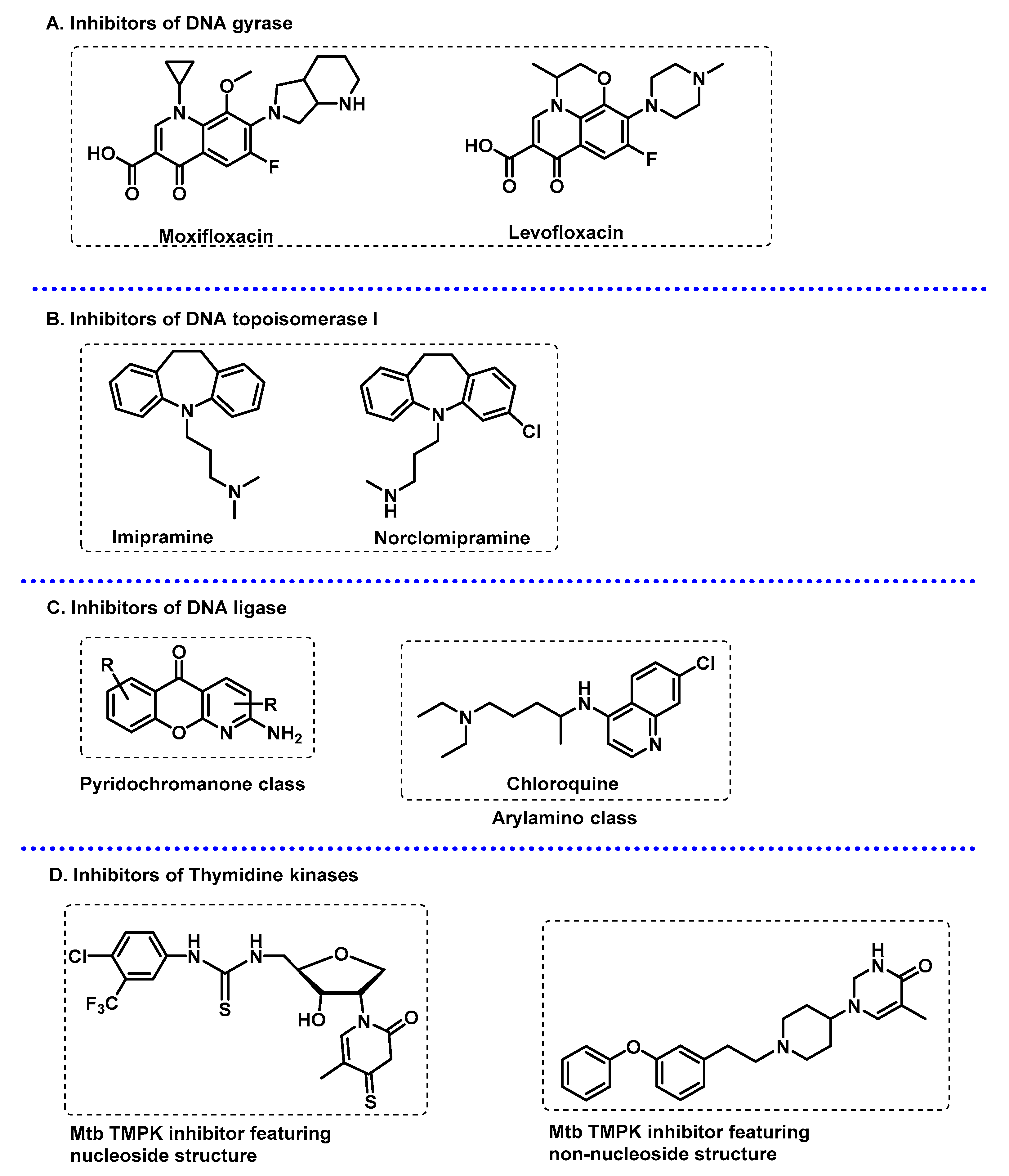
3.3.2. DNA-Related Enzymes
4. Chemical Probes for Target Identification in Mycobacteria
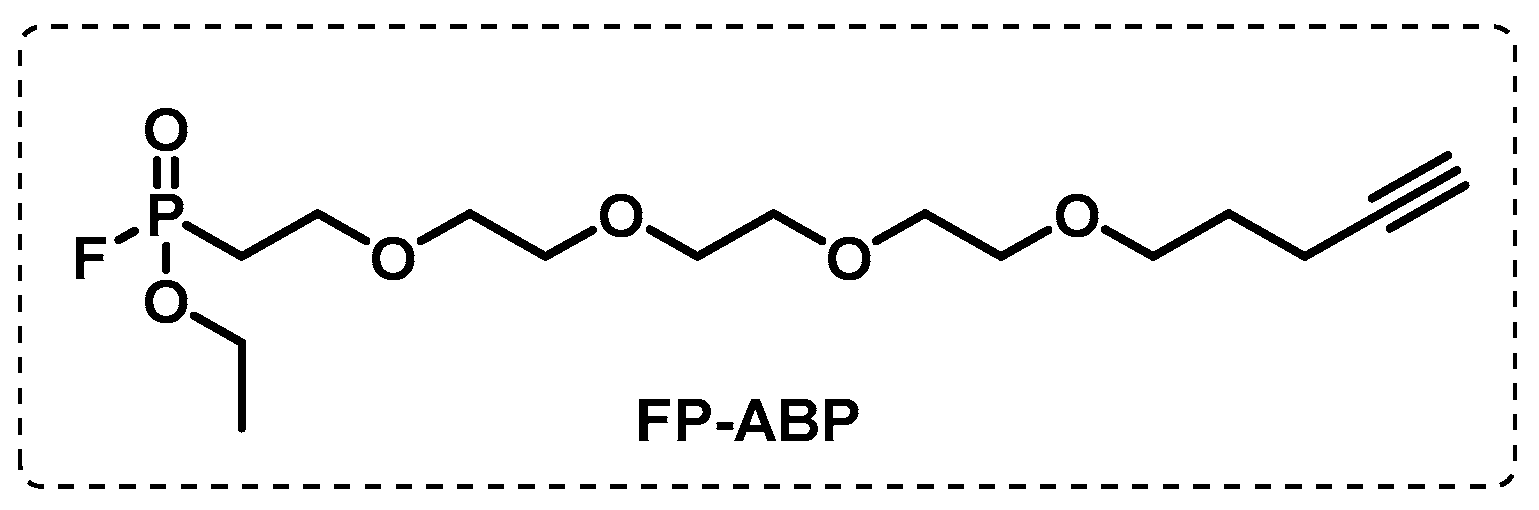
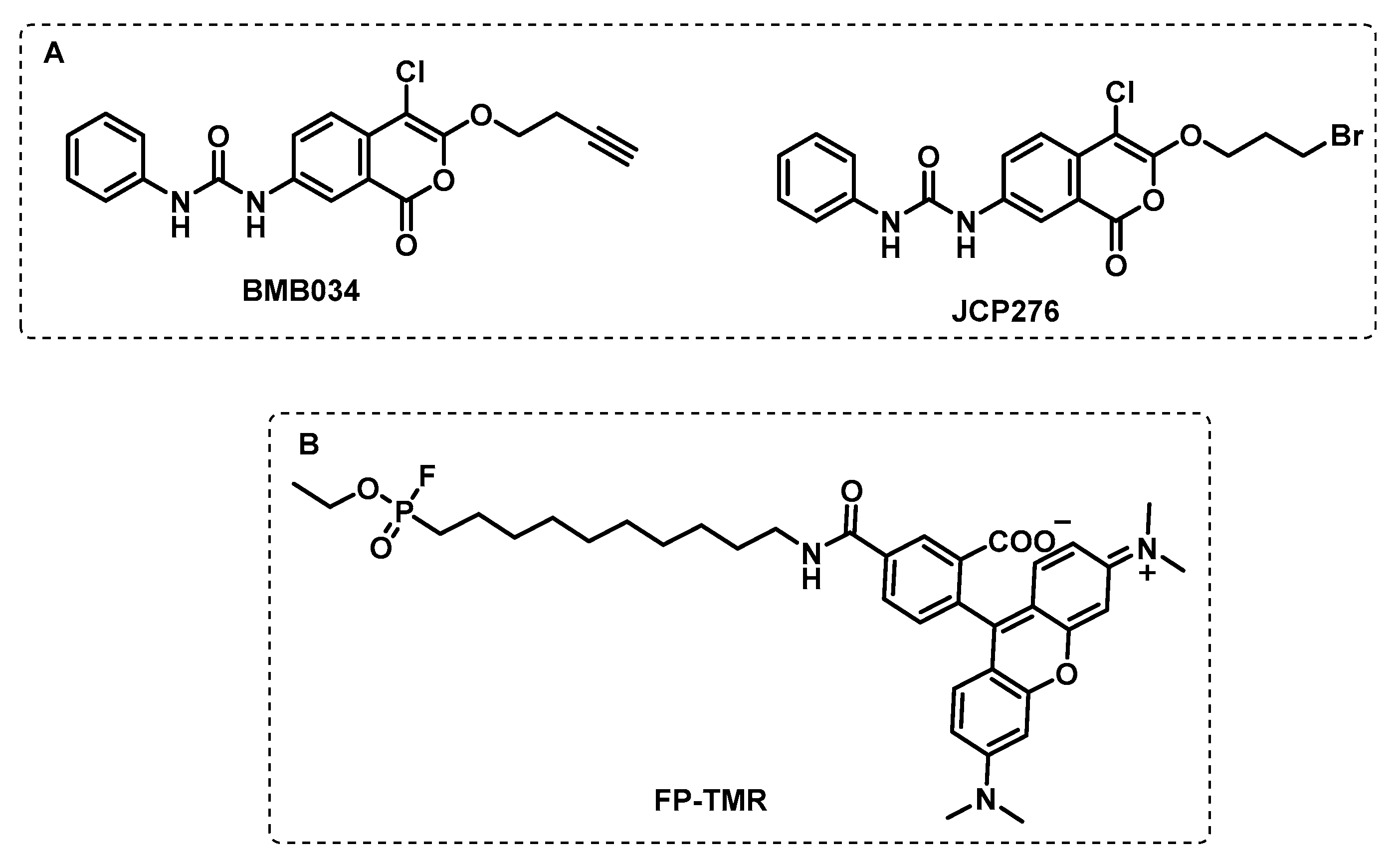
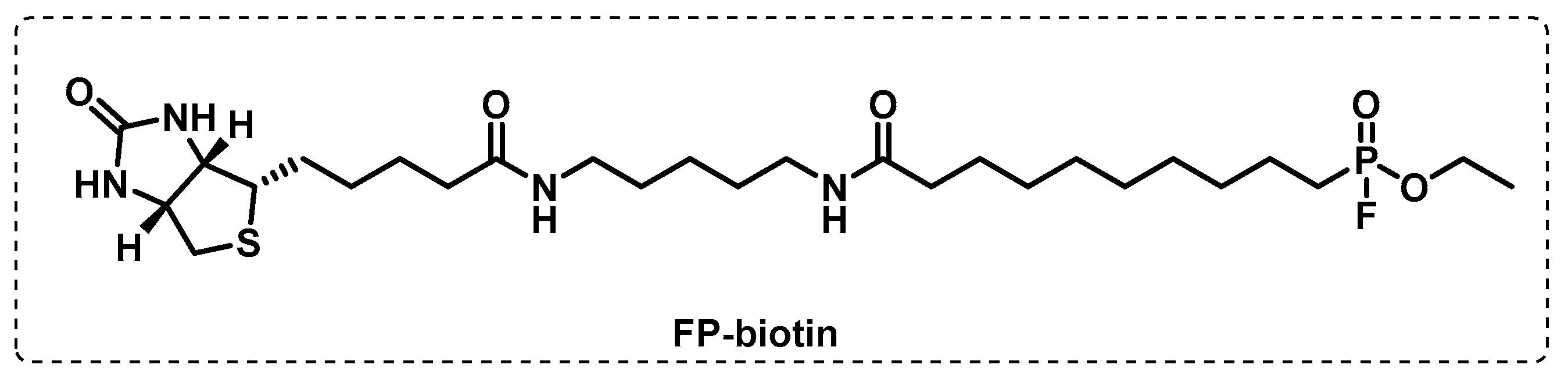
4.1. Activity-Based Protein Profiling (ABPP)
4.1.1. Cytosolic Serine Hydrolases
4.1.2. Membrane Serine and Cysteine Hydrolases
4.1.3. Other Membrane Targets
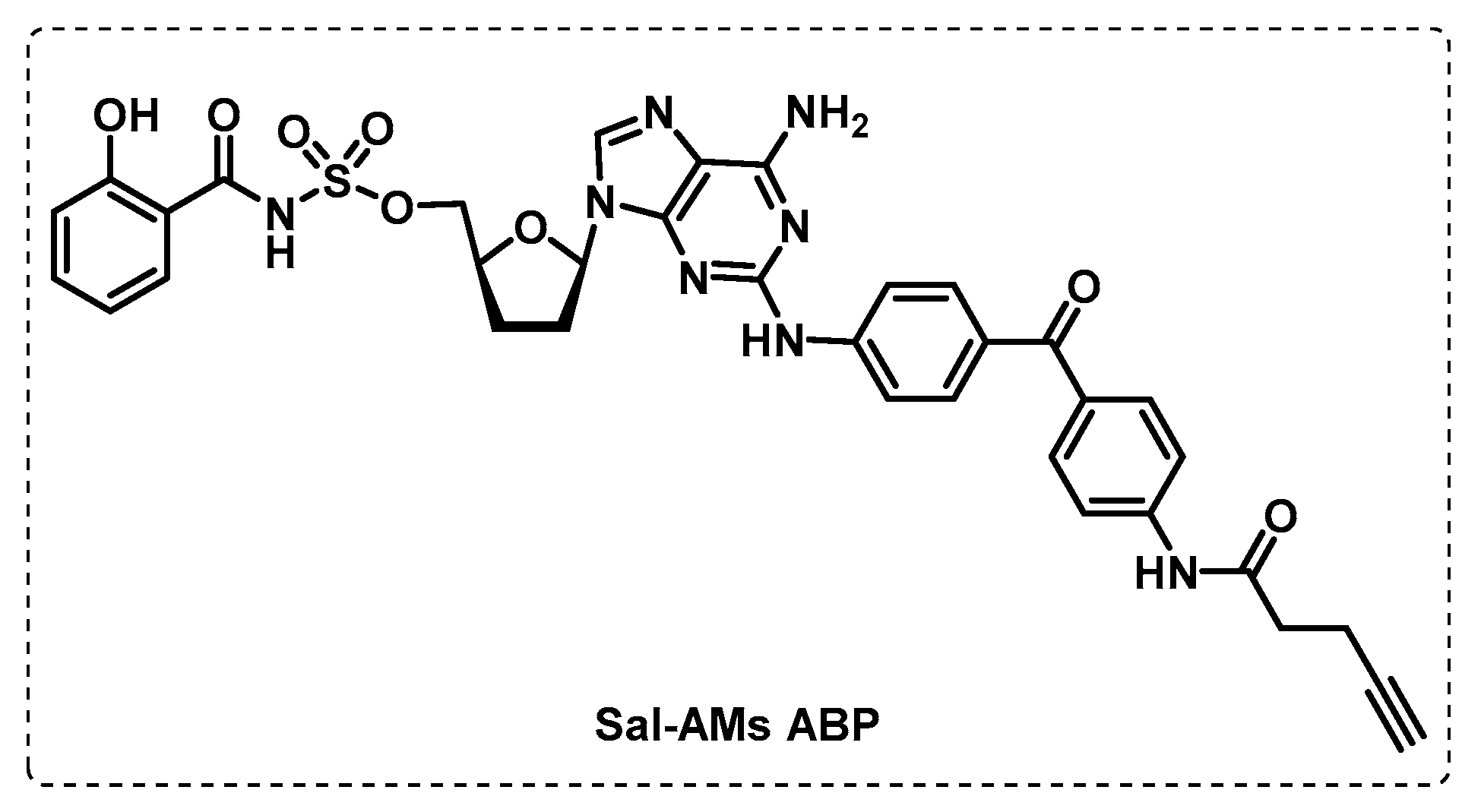
4.1.4. ATP-Binding Enzymes
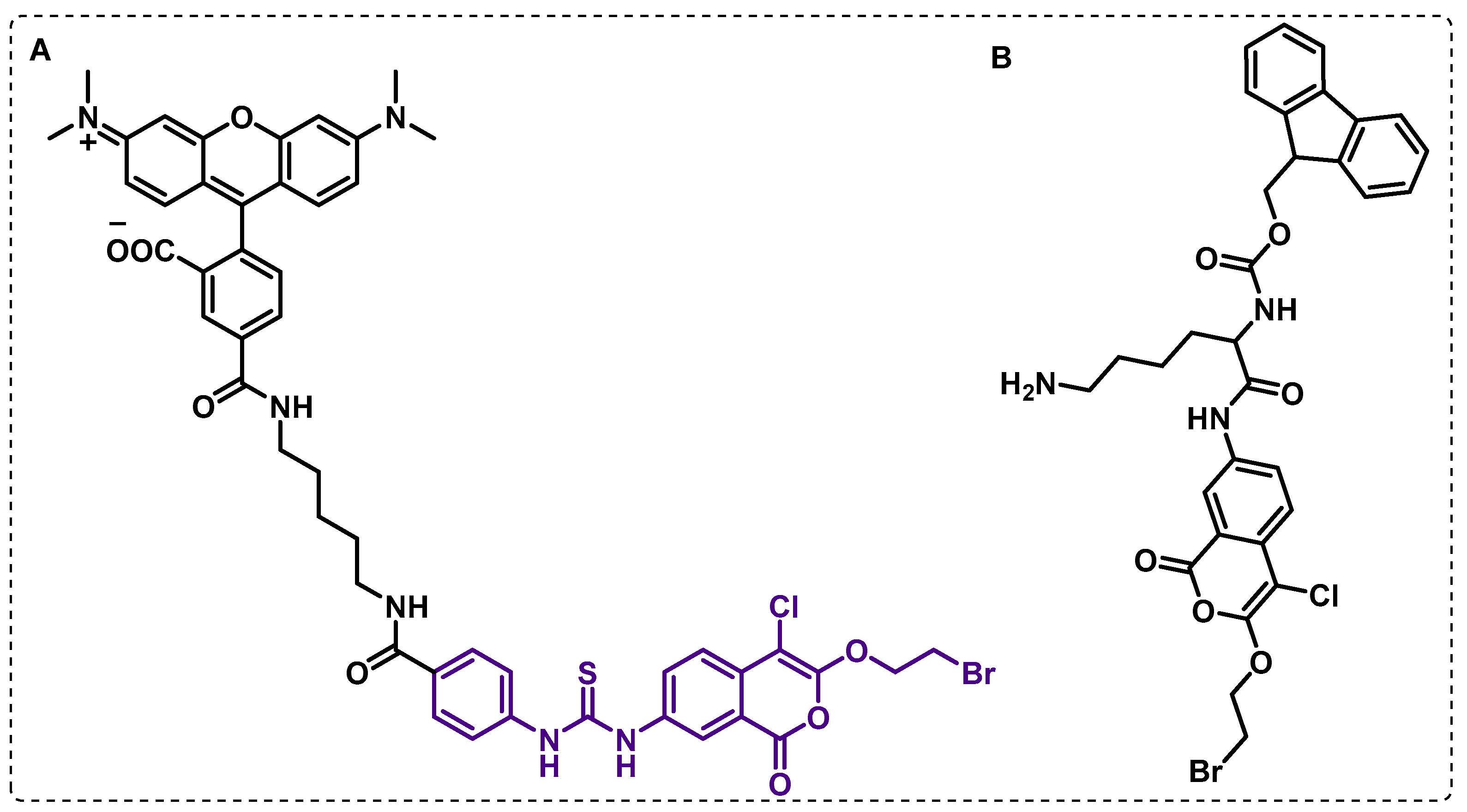
4.2. Affinity-Based Probes (AfBPs)
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gagneux, S. Ecology and evolution of Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2018, 16, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Mashabela Gabriel, T.; de Wet Timothy, J.; Warner Digby, F. Mycobacterium tuberculosis Metabolism. Microbiol. Spectr. 2019, 7, 7-4. [Google Scholar] [CrossRef] [PubMed]
- Woodman, M.; Haeusler, I.L.; Grandjean, L. Tuberculosis Genetic Epidemiology: A Latin American Perspective. Genes 2019, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Kiazyk, S.; Ball, T.B. Latent tuberculosis infection: An overview. Can. Commun. Dis. Rep. 2017, 43, 62–66. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Campaniço, A.; Moreira, R.; Lopes, F. Drug discovery in tuberculosis. New drug targets and antimycobacterial agents. Eur. J. Med. Chem. 2018, 150, 525–545. [Google Scholar] [CrossRef]
- Tiberi, S.; du Plessis, N.; Walzl, G.; Vjecha, M.J.; Rao, M.; Ntoumi, F.; Mfinanga, S.; Kapata, N.; Mwaba, P.; McHugh, T.D.; et al. Tuberculosis: Progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect. Dis. 2018, 18, 183–198. [Google Scholar] [CrossRef]
- Reid, M.J.A.; Arinaminpathy, N.; Bloom, A.; Bloom, B.R.; Boehme, C.; Chaisson, R.; Chin, D.P.; Churchyard, G.; Cox, H.; Ditiu, L.; et al. Building a tuberculosis-free world: The Lancet Commission on tuberculosis. Lancet 2019, 393, 1331–1384. [Google Scholar] [CrossRef]
- Nuermberger Eric, L. Preclinical Efficacy Testing of New Drug Candidates. Microbiol. Spectr. 2017, 5, 1–22. [Google Scholar] [CrossRef]
- Şenol, G. Recent and New Strategies for Extensively Drug-Resistant Tuberculosis. Mediterr. J. Infect. Microbes Antimicrob. 2018, 7, 22. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, M.M.; Singh, D.; Bisht, D.; Sharma, S. Drug targets in dormant Mycobacterium tuberculosis: Can the conquest against tuberculosis become a reality? Infect. Dis. 2018, 50, 81–94. [Google Scholar] [CrossRef]
- Reddy, D.S.; Sinha, A.; Kumar, A.; Saini, V.K. Drug re-engineering and repurposing: A significant and rapid approach to tuberculosis drug discovery. Arch. Der Pharm. 2022, 355, 1–26. [Google Scholar] [CrossRef]
- Macalino, S.J.Y.; Billones, J.B.; Organo, V.G.; Carrillo, M.C.O. In Silico Strategies in Tuberculosis Drug Discovery. Molecules 2020, 25, 665. [Google Scholar] [CrossRef] [PubMed]
- Huszár, S.; Chibale, K.; Singh, V. The quest for the holy grail: New antitubercular chemical entities, targets and strategies. Drug Discov. Today 2020, 25, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Velayati, A.A.; Farnia, P.; Hoffner, S. Drug-resistant Mycobacterium tuberculosis: Epidemiology and role of morphological alterations. J. Glob. Antimicrob. Resist. 2018, 12, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Sarathy, J.; Dartois, V.; Dick, T.; Gengenbacher, M. Reduced Drug Uptake in Phenotypically Resistant Nutrient-Starved Nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2013, 57, 1648–1653. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chettiar, S.; Parish, T. Current challenges in drug discovery for tuberculosis. Expert Opin. Drug Discov. 2017, 12, 1–4. [Google Scholar] [CrossRef]
- da Silva, P.E.A.; Machado, D.; Ramos, D.; Couto, I.; Von Groll, A.; Viveiros, M. Efflux Pumps in Mycobacteria: Antimicrobial Resistance, Physiological Functions, and Role in Pathogenicity. In Efflux-Mediated Antimicrobial Resistance in Bacteria: Mechanisms, Regulation and Clinical Implications; Li, X.-Z., Elkins, C.A., Zgurskaya, H.I., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 527–559. [Google Scholar] [CrossRef]
- Eoh, H.; Wang, Z.; Layre, E.; Rath, P.; Morris, R.; Branch Moody, D.; Rhee, K.Y. Metabolic anticipation in Mycobacterium tuberculosis. Nat. Microbiol. 2017, 2, 17084. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, D.; Kalia, N.P. Editorial: Approaches to Address Resistance, Drug Discovery, and Vaccine Development in Mycobacterium tuberculosis: Challenges and Opportunities. Front. Microbiol. 2022, 13, 871464. [Google Scholar] [CrossRef]
- Tomasi, F.G.; Rubin, E.J. Failing upwards: Genetics-based strategies to improve antibiotic discovery and efficacy in Mycobacterium tuberculosis. Front. Cell. Infect. Microbiol. 2022, 12, 932556. [Google Scholar] [CrossRef] [PubMed]
- Mugumbate, G.; Mendes, V.; Blaszczyk, M.; Sabbah, M.; Papadatos, G.; Lelievre, J.; Ballell, L.; Barros, D.; Abell, C.; Blundell, T.L.; et al. Target Identification of Mycobacterium tuberculosis Phenotypic Hits Using a Concerted Chemogenomic, Biophysical, and Structural Approach. Front. Pharmacol. 2017, 8, 00681. [Google Scholar] [CrossRef]
- Lechartier, B.; Rybniker, J.; Zumla, A.; Cole, S.T. Tuberculosis drug discovery in the post-post-genomic era. EMBO Mol. Med. 2014, 6, 158–168. [Google Scholar] [CrossRef]
- Borsari, C.; Ferrari, S.; Venturelli, A.; Costi, M.P. Target-based approaches for the discovery of new antimycobacterial drugs. Drug Discov. Today 2017, 22, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Sang, H.C.; Warit, S.; Wan, B.; Chang, H.H.; Guido, F.P.; Scott, G.F. Low-Oxygen-Recovery Assay for High-Throughput Screening of Compounds against Nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2007, 51, 1380–1385. [Google Scholar] [CrossRef]
- Wayne, L.G.; Hayes, L.G. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 1996, 64, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sala, C.; Hartkoorn Ruben, C.; Dhar, N.; Mendoza-Losana, A.; Cole Stewart, T. Streptomycin-Starved Mycobacterium tuberculosis 18b, a Drug Discovery Tool for Latent Tuberculosis. Antimicrob. Agents Chemother. 2012, 56, 5782–5789. [Google Scholar] [CrossRef]
- Darby, C.M.; Ingólfsson, H.I.; Jiang, X.; Shen, C.; Sun, M.; Zhao, N.; Burns, K.; Liu, G.; Ehrt, S.; Warren, J.D.; et al. Whole Cell Screen for Inhibitors of pH Homeostasis in Mycobacterium tuberculosis. PLoS ONE 2013, 8, e68942. [Google Scholar] [CrossRef]
- Gold, B.; Warrier, T.; Nathan, C. A Multistress Model for High Throughput Screening (HTS) Against Nonreplicating Mycobacterium tuberculosis (M. tuberculosis). In Mycobacteria Protocols; Parish, T., Kumar, A., Eds.; Springer: New York, NY, USA, 2021; pp. 611–635. [Google Scholar] [CrossRef]
- Aguilar-Ayala, D.A.; Cnockaert, M.; Vandamme, P.; Palomino, J.C.; Martin, A.; Gonzalez-Y-Merchand, J. Antimicrobial activity against Mycobacterium tuberculosis under in vitro lipid-rich dormancy conditions. J. Med. Microbiol. 2018, 67, 282–285. [Google Scholar] [CrossRef]
- Wang, F.; Sambandan, D.; Halder, R.; Wang, J.; Batt, S.M.; Weinrick, B.; Ahmad, I.; Yang, P.; Zhang, Y.; Kim, J.; et al. Identification of a small molecule with activity against drug-resistant and persistent tuberculosis. Proc. Natl. Acad. Sci. USA 2013, 110, E2510–E2517. [Google Scholar] [CrossRef]
- Egorova, A.; Salina, E.G.; Makarov, V. Targeting Non-Replicating Mycobacterium tuberculosis and Latent Infection: Alternatives and Perspectives (Mini-Review). Int. J. Mol. Sci. 2021, 22, 13317. [Google Scholar] [CrossRef]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Prim. 2016, 2, 16076. [Google Scholar] [CrossRef]
- Dartois, V.A.; Rubin, E.J. Anti-tuberculosis treatment strategies and drug development: Challenges and priorities. Nat. Rev. Microbiol. 2022, 20, 685–701. [Google Scholar] [CrossRef]
- Perveen, S.; Sharma, R. Screening approaches and therapeutic targets: The two driving wheels of tuberculosis drug discovery. Biochem. Pharmacol. 2022, 197, 114906. [Google Scholar] [CrossRef]
- Abrahams, K.A.; Besra, G.S. Mycobacterial drug discovery. RSC Med. Chem. 2020, 11, 1354–1365. [Google Scholar] [CrossRef]
- Xu, X.; Dong, B.; Peng, L.; Gao, C.; He, Z.; Wang, C.; Zeng, J. Anti-tuberculosis drug development via targeting the cell envelope of Mycobacterium tuberculosis. Front. Microbiol. 2022, 13, 1056608. [Google Scholar] [CrossRef]
- Shetye, G.S.; Franzblau, S.G.; Cho, S. New tuberculosis drug targets, their inhibitors, and potential therapeutic impact. Transl. Res. 2020, 220, 68–97. [Google Scholar] [CrossRef]
- Nataraj, V.; Varela, C.; Javid, A.; Singh, A.; Besra, G.S.; Bhatt, A. Mycolic acids: Deciphering and targeting the Achilles’ heel of the tubercle bacillus. Mol. Microbiol. 2015, 98, 7–16. [Google Scholar] [CrossRef]
- PaweŁczyk, J.; Kremer, L. The Molecular Genetics of Mycolic Acid Biosynthesis. Microbiol. Spectr. 2014, 2, 611–631. [Google Scholar] [CrossRef] [PubMed]
- DeJesus Michael, A.; Gerrick Elias, R.; Xu, W.; Park Sae, W.; Long Jarukit, E.; Boutte Cara, C.; Rubin Eric, J.; Schnappinger, D.; Ehrt, S.; Fortune Sarah, M.; et al. Comprehensive Essentiality Analysis of the Mycobacterium tuberculosis Genome via Saturating Transposon Mutagenesis. mBio 2017, 8, e02133-16. [Google Scholar] [CrossRef] [PubMed]
- Inoyama, D.; Awasthi, D.; Capodagli, G.C.; Tsotetsi, K.; Sukheja, P.; Zimmerman, M.; Li, S.-G.; Jadhav, R.; Russo, R.; Wang, X.; et al. A Preclinical Candidate Targeting Mycobacterium tuberculosis KasA. Cell Chem. Biol. 2020, 27, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, K.A.; Chung, C.-W.; Ghidelli-Disse, S.; Rullas, J.; Rebollo-López, M.J.; Gurcha, S.S.; Cox, J.A.G.; Mendoza, A.; Jiménez-Navarro, E.; Martínez-Martínez, M.S.; et al. Identification of KasA as the cellular target of an anti-tubercular scaffold. Nat. Commun. 2016, 7, 12581. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Capodagli Glenn, C.; Awasthi, D.; Shrestha, R.; Maharaja, K.; Sukheja, P.; Li, S.-G.; Inoyama, D.; Zimmerman, M.; Hsin, P.H.L.; et al. Synergistic Lethality of a Binary Inhibitor of Mycobacterium tuberculosis KasA. mBio 2018, 9, e02101–e02117. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, R.; Shingare, R.D.; Kumar, V.; Anand, A.B.S.; Veeraraghavan, S.; Viswanadha, S.; Ummanni, R.; Gokhale, R.; Srinivasa Reddy, D. Repurposing of a drug scaffold: Identification of novel sila analogues of rimonabant as potent antitubercular agents. Eur. J. Med. Chem. 2016, 122, 723–730. [Google Scholar] [CrossRef]
- Kwofie, S.K.; Hanson, G.; Sasu, H.; Enninful, K.S.; Mensah, F.A.; Nortey, R.T.; Yeboah, O.P.; Agoni, C.; Wilson, M.D. Molecular Modelling and Atomistic Insights into the Binding Mechanism of MmpL3 Mtb. Chem. Biodivers. 2022, 19, e202200160. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, K.A.; Besra, G.S. Mycobacterial cell wall biosynthesis: A multifaceted antibiotic target. Parasitology 2018, 145, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, Y.; Gao, Y.; Wu, L.; Gao, R.; Zhang, Q.; Wang, Y.; Wu, C.; Wu, F.; Gurcha, S.S.; et al. Structures of cell wall arabinosyltransferases with the anti-tuberculosis drug ethambutol. Science 2020, 368, 1211–1219. [Google Scholar] [CrossRef]
- Kastrinsky, D.B.; McBride, N.S.; Backus, K.M.; LeBlanc, J.J.; Barry, C.E. 1.04—Mycolic Acid/Cyclopropane Fatty Acid/Fatty Acid Biosynthesis and Health Relations. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 65–145. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Gao, R.; Li, J.; Yang, X.; Gao, Y.; Zhao, W.; Gurcha, S.S.; Veerapen, N.; Batt, S.M.; et al. Cryo-EM snapshots of mycobacterial arabinosyltransferase complex EmbB2-AcpM2. Protein Cell 2020, 11, 505–517. [Google Scholar] [CrossRef]
- Goude, R.; Amin, A.G.; Chatterjee, D.; Parish, T. The Arabinosyltransferase EmbC Is Inhibited by Ethambutol in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2009, 53, 4138–4146. [Google Scholar] [CrossRef]
- Meniche, X.; de Sousa-d’Auria, C.; Van-der-Rest, B.; Bhamidi, S.; Huc, E.; Huang, H.; De Paepe, D.; Tropis, M.; McNeil, M.; Daffé, M.; et al. Partial redundancy in the synthesis of the d-arabinose incorporated in the cell wall arabinan of Corynebacterineae. Microbiology 2008, 154, 2315–2326. [Google Scholar] [CrossRef]
- Xu, L.; Qian, L.; Kang, J.; Sha, S.; Xin, Y.; Lu, S.; Ma, Y. Down-regulation of N-acetylglucosamine-1-phosphate transferase (WecA) enhanced the sensitivity of Mycobacterium smegmatis against rifampin. J. Appl. Microbiol. 2016, 121, 966–972. [Google Scholar] [CrossRef]
- Young, E.F.; Durham, P.G.; Perkowski, E.F.; Malik, S.; Hickey, A.J.; Braunstein, M. Efficacy of inhaled CPZEN-45 in treating tuberculosis in the guinea pig. Tuberculosis 2022, 135, 102207. [Google Scholar] [CrossRef]
- Sammartino, J.C.; Morici, M.; Stelitano, G.; Degiacomi, G.; Riccardi, G.; Chiarelli, L.R. Functional investigation of the antitubercular drug target Decaprenylphosphoryl-β-D-ribofuranose-2-epimerase DprE1/DprE2 complex. Biochem. Biophys. Res. Commun. 2022, 607, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.; Kumar, S.; Parkesh, R. Chemical Space Exploration of DprE1 Inhibitors Using Chemoinformatics and Artificial Intelligence. ACS Omega 2021, 6, 14430–14441. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, I.K.; Bajeli, S.; Akela, A.K.; Kumar, A. Bioenergetics of Mycobacterium: An Emerging Landscape for Drug Discovery. Pathogens 2018, 7, 24. [Google Scholar] [CrossRef]
- Wani, M.A.; Dhaked, D.K. Targeting the cytochrome bc1 complex for drug development in M. tuberculosis: Review. Mol. Divers. 2022, 26, 2949–2965. [Google Scholar] [CrossRef] [PubMed]
- Hasenoehrl, E.J.; Wiggins, T.J.; Berney, M. Bioenergetic Inhibitors: Antibiotic Efficacy and Mechanisms of Action in Mycobacterium tuberculosis. Front. Cell. Infect. Microbiol. 2021, 10, 611683. [Google Scholar] [CrossRef]
- Bajeli, S.; Baid, N.; Kaur, M.; Pawar, G.P.; Chaudhari, V.D.; Kumar, A. Terminal Respiratory Oxidases: A Targetables Vulnerability of Mycobacterial Bioenergetics? Front. Cell. Infect. Microbiol. 2020, 10, 589318. [Google Scholar] [CrossRef] [PubMed]
- Foo, C.S.; Pethe, K.; Lupien, A. Oxidative Phosphorylation—An Update on a New, Essential Target Space for Drug Discovery in Mycobacterium tuberculosis. Appl. Sci. 2020, 10, 2339. [Google Scholar] [CrossRef]
- Borisov, V.B.; Forte, E. Bioenergetics and Reactive Nitrogen Species in Bacteria. Int. J. Mol. Sci. 2022, 23, 7321. [Google Scholar] [CrossRef]
- Wiseman, B.; Nitharwal, R.G.; Fedotovskaya, O.; Schäfer, J.; Guo, H.; Kuang, Q.; Benlekbir, S.; Sjöstrand, D.; Ädelroth, P.; Rubinstein, J.L.; et al. Structure of a functional obligate complex III2IV2 respiratory supercomplex from Mycobacterium smegmatis. Nat. Struct. Mol. Biol. 2018, 25, 1128–1136. [Google Scholar] [CrossRef]
- Gregory, A.H.; Anne, E.M.B.; Singh, M.; Jayaraman, K.; Leslie, A.W.; Rachel, L.K.; Janessa, S.A.; Flentie, K.; Miranda, E.S.; Gaggioli, M.; et al. Identification of 4-Amino-Thieno [2,3-d]Pyrimidines as QcrB Inhibitors in Mycobacterium tuberculosis. mSphere 2019, 4, e00606–e00619. [Google Scholar] [CrossRef]
- Lupien, A.; Foo, C.S.-Y.; Savina, S.; Vocat, A.; Piton, J.; Monakhova, N.; Benjak, A.; Lamprecht, D.A.; Steyn, A.J.C.; Pethe, K.; et al. New 2-Ethylthio-4-methylaminoquinazoline derivatives inhibiting two subunits of cytochrome bc1 in Mycobacterium tuberculosis. PLoS Pathog. 2020, 16, e1008270. [Google Scholar] [CrossRef]
- Moraski, G.C.; Deboosère, N.; Marshall, K.L.; Weaver, H.A.; Vandeputte, A.; Hastings, C.; Woolhiser, L.; Lenaerts, A.J.; Brodin, P.; Miller, M.J. Intracellular and in vivo evaluation of imidazo[2,1-b]thiazole-5-carboxamide anti-tuberculosis compounds. PLoS ONE 2020, 15, e0227224. [Google Scholar] [CrossRef]
- Roy, K.K.; Wani, M.A. Emerging opportunities of exploiting mycobacterial electron transport chain pathway for drug-resistant tuberculosis drug discovery. Expert Opin. Drug Discov. 2020, 15, 231–241. [Google Scholar] [CrossRef]
- Thompson, A.M.; Denny, W.A. Chapter Four—Inhibitors of enzymes in the electron transport chain of Mycobacterium tuberculosis. In Annual Reports in Medicinal Chemistry; Chibale, K., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 52, pp. 97–130. [Google Scholar]
- Lee, B.S.; Kalia, N.P.; Jin, X.E.F.; Hasenoehrl, E.J.; Berney, M.; Pethe, K. Inhibitors of energy metabolism interfere with antibiotic-induced death in mycobacteria. J. Biol. Chem. 2019, 294, 1936–1943. [Google Scholar] [CrossRef]
- Yu, W.; Chiwala, G.; Gao, Y.; Liu, Z.; Sapkota, S.; Lu, Z.; Guo, L.; Khan, S.A.; Zhong, N.; Zhang, T. TB47 and clofazimine form a highly synergistic sterilizing block in a second-line regimen for tuberculosis in mice. Biomed. Pharmacother. 2020, 131, 110782. [Google Scholar] [CrossRef]
- Yu, W.; Yusuf, B.; Wang, S.; Tian, X.; Hameed, H.M.A.; Lu, Z.; Chiwala, G.; Alam Md, S.; Cook Gregory, M.; Maslov Dmitry, A.; et al. Sterilizing Effects of Novel Regimens Containing TB47, Clofazimine, and Linezolid in a Murine Model of Tuberculosis. Antimicrob. Agents Chemother. 2021, 65, e00706–e00721. [Google Scholar] [CrossRef]
- Cai, Y.; Jaecklein, E.; Mackenzie, J.S.; Papavinasasundaram, K.; Olive, A.J.; Chen, X.; Steyn, A.J.C.; Sassetti, C.M. Host immunity increases Mycobacterium tuberculosis reliance on cytochrome bd oxidase. PLoS Pathog. 2021, 17, e1008911. [Google Scholar] [CrossRef]
- Harikishore, A.; Chong, S.S.M.; Ragunathan, P.; Bates, R.W.; Grüber, G. Targeting the menaquinol binding loop of mycobacterial cytochrome bd oxidase. Mol. Divers. 2021, 25, 517–524. [Google Scholar] [CrossRef]
- Friedrich, T.; Wohlwend, D.; Borisov, V.B. Recent Advances in Structural Studies of Cytochrome bd and Its Potential Application as a Drug Target. Int. J. Mol. Sci. 2022, 23, 3166. [Google Scholar] [CrossRef]
- Foo Caroline, S.; Lupien, A.; Kienle, M.; Vocat, A.; Benjak, A.; Sommer, R.; Lamprecht Dirk, A.; Steyn Adrie, J.C.; Pethe, K.; Piton, J.; et al. Arylvinylpiperazine Amides, a New Class of Potent Inhibitors Targeting QcrB of Mycobacterium tuberculosis. mBio 2018, 9, e01276-18. [Google Scholar] [CrossRef]
- Moosa, A.; Lamprecht Dirk, A.; Arora, K.; Barry Clifton, E.; Boshoff Helena, I.M.; Ioerger Thomas, R.; Steyn Adrie, J.C.; Mizrahi, V.; Warner Digby, F. Susceptibility of Mycobacterium tuberculosis Cytochrome bd Oxidase Mutants to Compounds Targeting the Terminal Respiratory Oxidase, Cytochrome c. Antimicrob. Agents Chemother. 2017, 61, e01338-17. [Google Scholar] [CrossRef] [PubMed]
- Sarathy, J.P.; Gruber, G.; Dick, T. Re-Understanding the Mechanisms of Action of the Anti-Mycobacterial Drug Bedaquiline. Antibiotics 2019, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Fatima, Z.; Kumawat, S. Study of the bioenergetics to identify the novel pathways as a drug target against Mycobacterium tuberculosis using Petri net. Biosystems 2021, 209, 104509. [Google Scholar] [CrossRef] [PubMed]
- Jeon, A.B.; Ackart, D.F.; Li, W.; Jackson, M.; Melander, R.J.; Melander, C.; Abramovitch, R.B.; Chicco, A.J.; Basaraba, R.J.; Obregón-Henao, A. 2-aminoimidazoles collapse mycobacterial proton motive force and block the electron transport chain. Sci. Rep. 2019, 9, 1513. [Google Scholar] [CrossRef]
- Odingo, J.; Bailey, M.A.; Files, M.; Early, J.V.; Alling, T.; Dennison, D.; Bowman, J.; Dalai, S.; Kumar, N.; Cramer, J.; et al. In Vitro Evaluation of Novel Nitazoxanide Derivatives against Mycobacterium tuberculosis. ACS Omega 2017, 2, 5873–5890. [Google Scholar] [CrossRef]
- Chao, A.; Sieminski, P.J.; Owens, C.P.; Goulding, C.W. Iron Acquisition in Mycobacterium tuberculosis. Chem. Rev. 2019, 119, 1193–1220. [Google Scholar] [CrossRef]
- Chiarelli, L.R.; Mori, M.; Barlocco, D.; Beretta, G.; Gelain, A.; Pini, E.; Porcino, M.; Mori, G.; Stelitano, G.; Costantino, L.; et al. Discovery and development of novel salicylate synthase (MbtI) furanic inhibitors as antitubercular agents. Eur. J. Med. Chem. 2018, 155, 754–763. [Google Scholar] [CrossRef]
- Chiarelli, L.R.; Mori, M.; Beretta, G.; Gelain, A.; Pini, E.; Sammartino, J.C.; Stelitano, G.; Barlocco, D.; Costantino, L.; Lapillo, M.; et al. New insight into structure-activity of furan-based salicylate synthase (MbtI) inhibitors as potential antitubercular agents. J. Enzym. Inhib. Med. Chem. 2019, 34, 823–828. [Google Scholar] [CrossRef]
- Rohilla, A.; Khare, G.; Tyagi, A.K. Virtual Screening, pharmacophore development and structure based similarity search to identify inhibitors against IdeR, a transcription factor of Mycobacterium tuberculosis. Sci. Rep. 2017, 7, 4653. [Google Scholar] [CrossRef]
- Yang, L.; Hu, X.; Chai, X.; Ye, Q.; Pang, J.; Li, D.; Hou, T. Opportunities for overcoming tuberculosis: Emerging targets and their inhibitors. Drug Discov. Today 2022, 27, 326–336. [Google Scholar] [CrossRef]
- Khisimuzi, M.; Zhenkun, M. Mycobacterium tuberculosis DNA Gyrase as a Target for Drug Discovery. Infect. Disord. Drug Targets 2007, 7, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Sankhe, K.; Suvarna, V.; Sherje, A.; Patel, K.; Dravyakar, B. DNA gyrase inhibitors: Progress and synthesis of potent compounds as antibacterial agents. Biomed. Pharmacother. 2018, 103, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Godbole Adwait, A.; Ahmed, W.; Bhat Rajeshwari, S.; Bradley Erin, K.; Ekins, S.; Nagaraja, V. Targeting Mycobacterium tuberculosis Topoisomerase I by Small-Molecule Inhibitors. Antimicrob. Agents Chemother. 2015, 59, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, N.; Dube, D.; Pandey, J.; Singh, B.; Kukshal, V.; Ramachandran, R.; Tripathi, R.P. NAD+-Dependent DNA Ligase: A novel target waiting for the right inhibitor. Med. Res. Rev. 2008, 28, 545–568. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Tripathi, R.P.; Ramachandran, R. NAD+-dependent DNA Ligase (Rv3014c) from Mycobacterium tuberculosis: Crystal structure of the adenylation domain and identification of novel inhibitors*. J. Biol. Chem. 2005, 280, 30273–30281. [Google Scholar] [CrossRef]
- Jian, Y.; Risseeuw, M.D.P.; Froeyen, M.; Song, L.; Cappoen, D.; Cos, P.; Munier-Lehmann, H.; van Calenbergh, S. 1-(Piperidin-3-yl)thymine amides as inhibitors of M. tuberculosis thymidylate kinase. J. Enzym. Inhib. Med. Chem. 2019, 34, 1730–1739. [Google Scholar] [CrossRef]
- Fang, H.; Peng, B.; Ong, S.Y.; Wu, Q.; Li, L.; Yao, S.Q. Recent advances in activity-based probes (ABPs) and affinity-based probes (AfBPs) for profiling of enzymes. Chem. Sci. 2021, 12, 8288–8310. [Google Scholar] [CrossRef]
- Conlon, B.P.; Nakayasu, E.S.; Fleck, L.E.; LaFleur, M.D.; Isabella, V.M.; Coleman, K.; Leonard, S.N.; Smith, R.D.; Adkins, J.N.; Lewis, K. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 2013, 503, 365–370. [Google Scholar] [CrossRef]
- Vandal, O.H.; Pierini, L.M.; Schnappinger, D.; Nathan, C.F.; Ehrt, S. A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat. Med. 2008, 14, 849–854. [Google Scholar] [CrossRef]
- Simon, G.M.; Cravatt, B.F. Activity-based Proteomics of Enzyme Superfamilies: Serine Hydrolases as a Case Study. J. Biol. Chem. 2010, 285, 11051–11055. [Google Scholar] [CrossRef]
- Ortega, C.; Lindsey, N.A.; Frando, A.; Natalie, C.S.; Robert, W.B.; Richard, D.S.; Aaron, T.W.; Grundner, C. Systematic Survey of Serine Hydrolase Activity in Mycobacterium tuberculosis Defines Changes Associated with Persistence. Cell Chem. Biol. 2016, 23, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Bachovchin, D.A.; Ji, T.; Li, W.; Simon, G.M.; Blankman, J.L.; Adibekian, A.; Hoover, H.; Niessen, S.; Cravatt, B.F. Superfamily-wide portrait of serine hydrolase inhibition achieved by library-versus-library screening. Proc. Natl. Acad. Sci. USA 2010, 107, 20941–20946. [Google Scholar] [CrossRef] [PubMed]
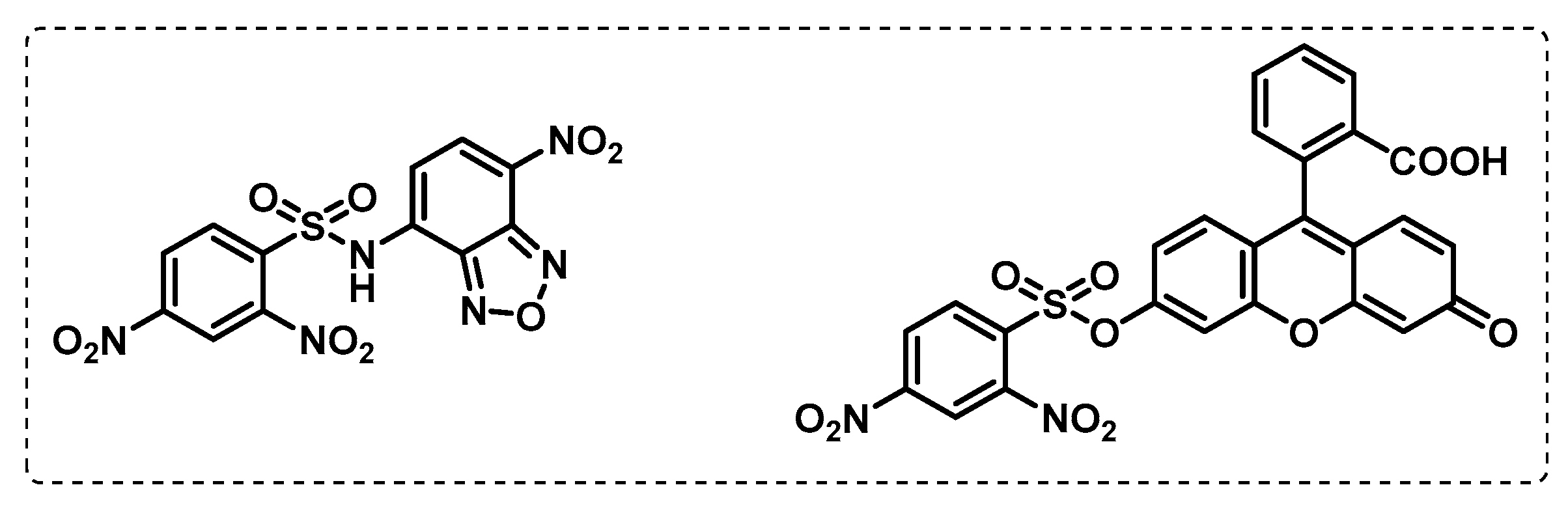
- Lentz, C.S.; Ordonez, A.A.; Kasperkiewicz, P.; La Greca, F.; O’Donoghue, A.J.; Schulze, C.J.; Powers, J.C.; Craik, C.S.; Drag, M.; Jain, S.K.; et al. Design of Selective Substrates and Activity-Based Probes for Hydrolase Important for Pathogenesis 1 (HIP1) from Mycobacterium tuberculosis. ACS Infect. Dis. 2016, 2, 807–815. [Google Scholar] [CrossRef] [PubMed]
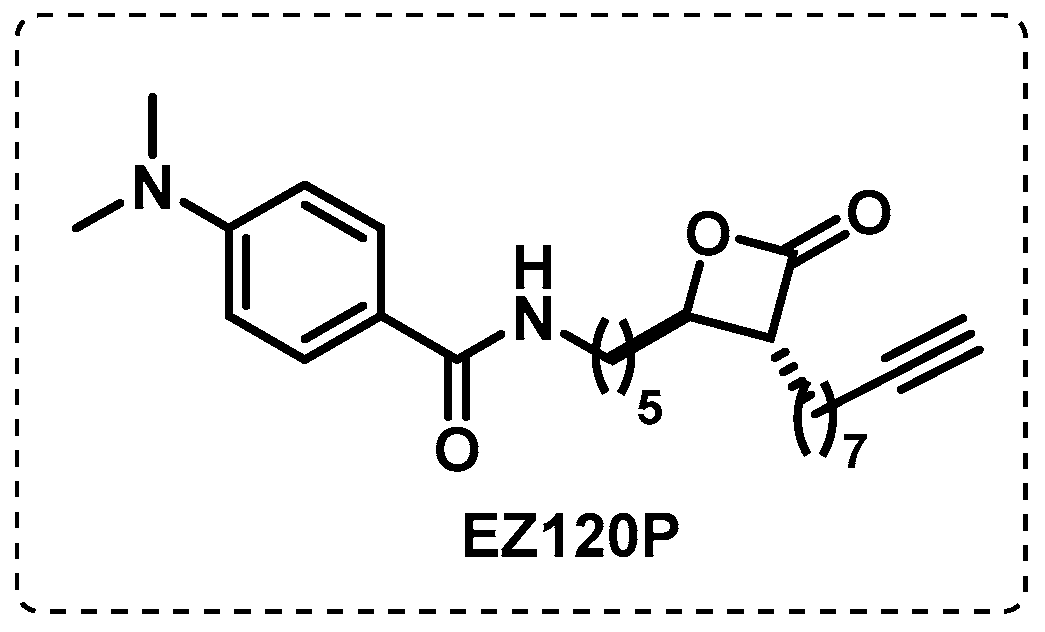
- Babin, B.M.; Keller, L.J.; Pinto, Y.; Li, V.L.; Eneim, A.S.; Vance, S.E.; Terrell, S.M.; Bhatt, A.S.; Long, J.Z.; Bogyo, M. Identification of covalent inhibitors that disrupt M. tuberculosis growth by targeting multiple serine hydrolases involved in lipid metabolism. Cell Chem. Biol. 2022, 29, 897–909.e897. [Google Scholar] [CrossRef]
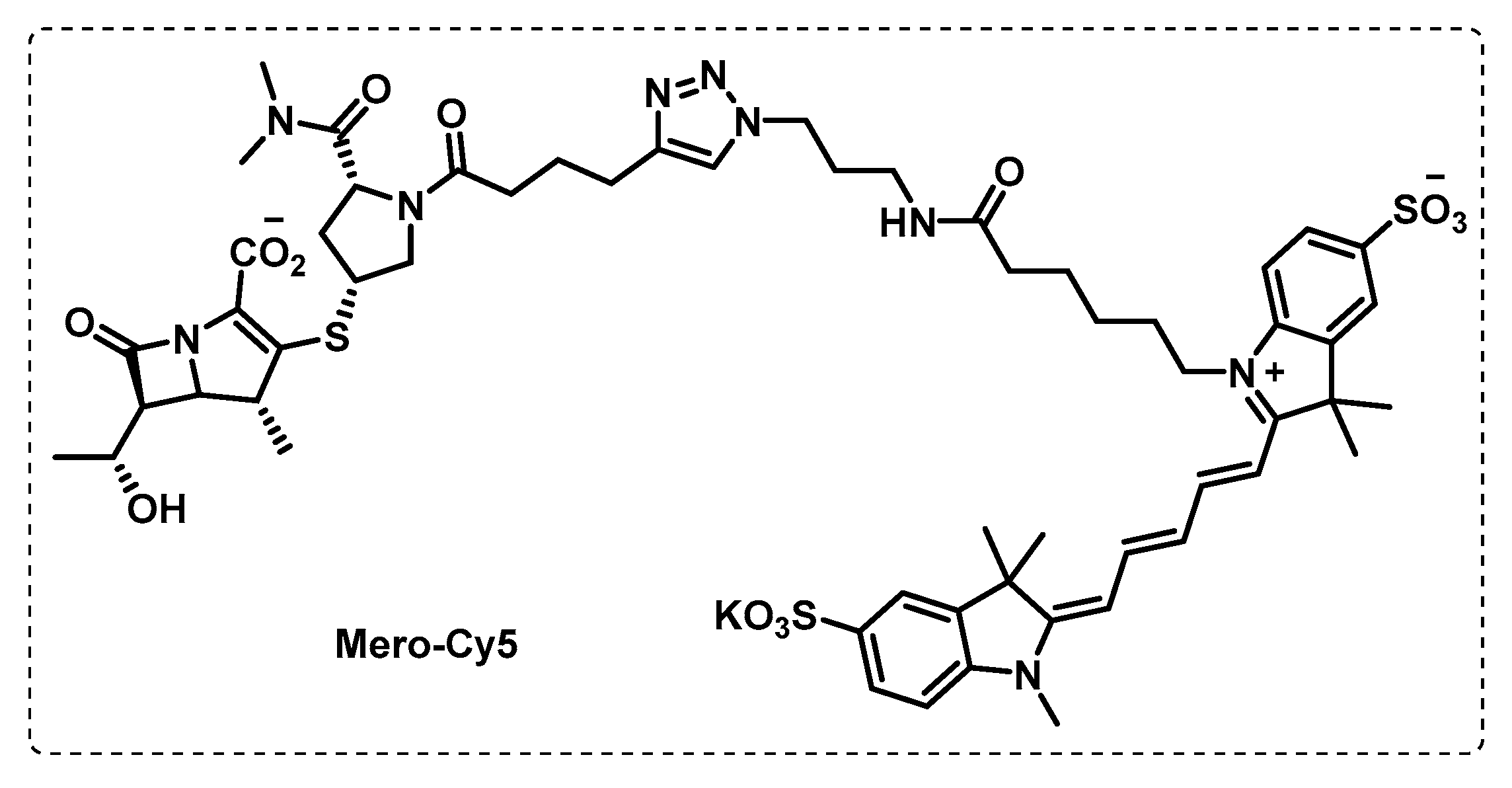
- Li, M.; Patel, H.V.; Cognetta, A.B., III; Smith, T.C., II; Mallick, I.; Cavalier, J.-F.; Previti, M.L.; Canaan, S.; Aldridge, B.B.; Cravatt, B.F.; et al. Identification of cell wall synthesis inhibitors active against Mycobacterium tuberculosis by competitive activity-based protein profiling. Cell Chem. Biol. 2022, 29, 883–896.e885. [Google Scholar] [CrossRef] [PubMed]
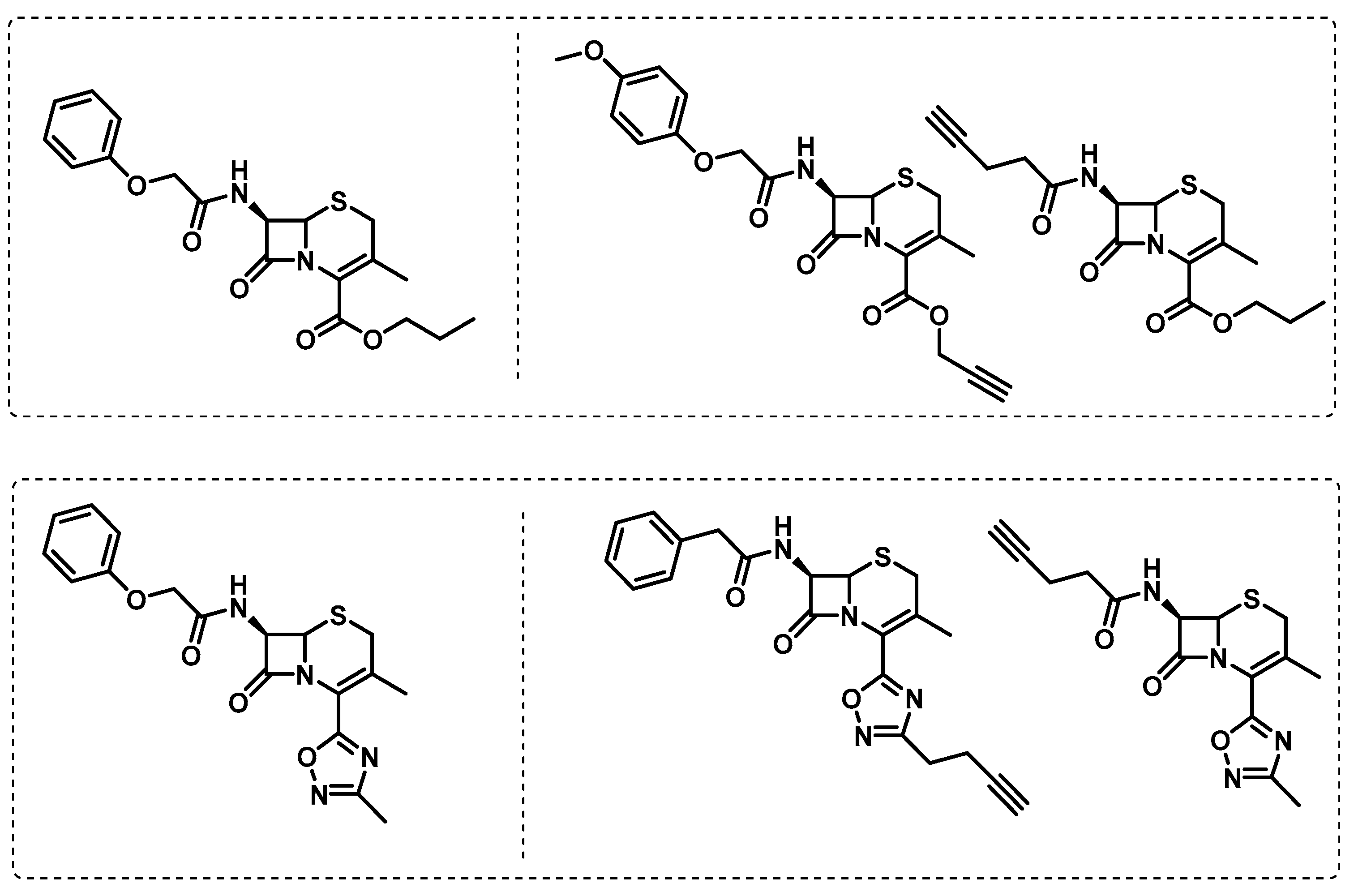
- Gun, M.A.; Bozdogan, B.; Coban, A.Y. Tuberculosis and beta-lactam antibiotics. Future Microbiol. 2020, 15, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Muraoka, A.; Bedenbaugh, M.; Childress, B.; Pernot, L.; Wiencek, M.; Peterson, Y.K. The Chemical Relationship Among Beta-Lactam Antibiotics and Potential Impacts on Reactivity and Decomposition. Front. Microbiol. 2022, 13, 807955. [Google Scholar] [CrossRef]
- Lopez Quezada, L.; Smith, R.; Lupoli, T.J.; Edoo, Z.; Li, X.; Gold, B.; Roberts, J.; Ling, Y.; Park, S.W.; Nguyen, Q.; et al. Activity-Based Protein Profiling Reveals That Cephalosporins Selectively Active on Non-replicating Mycobacterium tuberculosis Bind Multiple Protein Families and Spare Peptidoglycan Transpeptidases. Front. Microbiol. 2020, 11, 1248. [Google Scholar] [CrossRef]
- de Munnik, M.; Lohans, C.T.; Langley, G.W.; Bon, C.; Brem, J.; Schofield, C.J. A Fluorescence-Based Assay for Screening β-Lactams Targeting the Mycobacterium tuberculosis Transpeptidase LdtMt2. ChemBioChem 2020, 21, 368–372. [Google Scholar] [CrossRef]
- Levine, S.R.; Beatty, K.E. Investigating β-Lactam Drug Targets in Mycobacterium tuberculosis Using Chemical Probes. ACS Infect. Dis. 2021, 7, 461–470. [Google Scholar] [CrossRef]
- Lehmann, J.; Cheng, T.-Y.; Aggarwal, A.; Park, A.S.; Zeiler, E.; Raju, R.M.; Akopian, T.; Kandror, O.; Sacchettini, J.C.; Moody, D.B.; et al. An Antibacterial β-Lactone Kills Mycobacterium tuberculosis by Disrupting Mycolic Acid Biosynthesis. Angew. Chem. Int. Ed. 2018, 57, 348–353. [Google Scholar] [CrossRef] [PubMed]
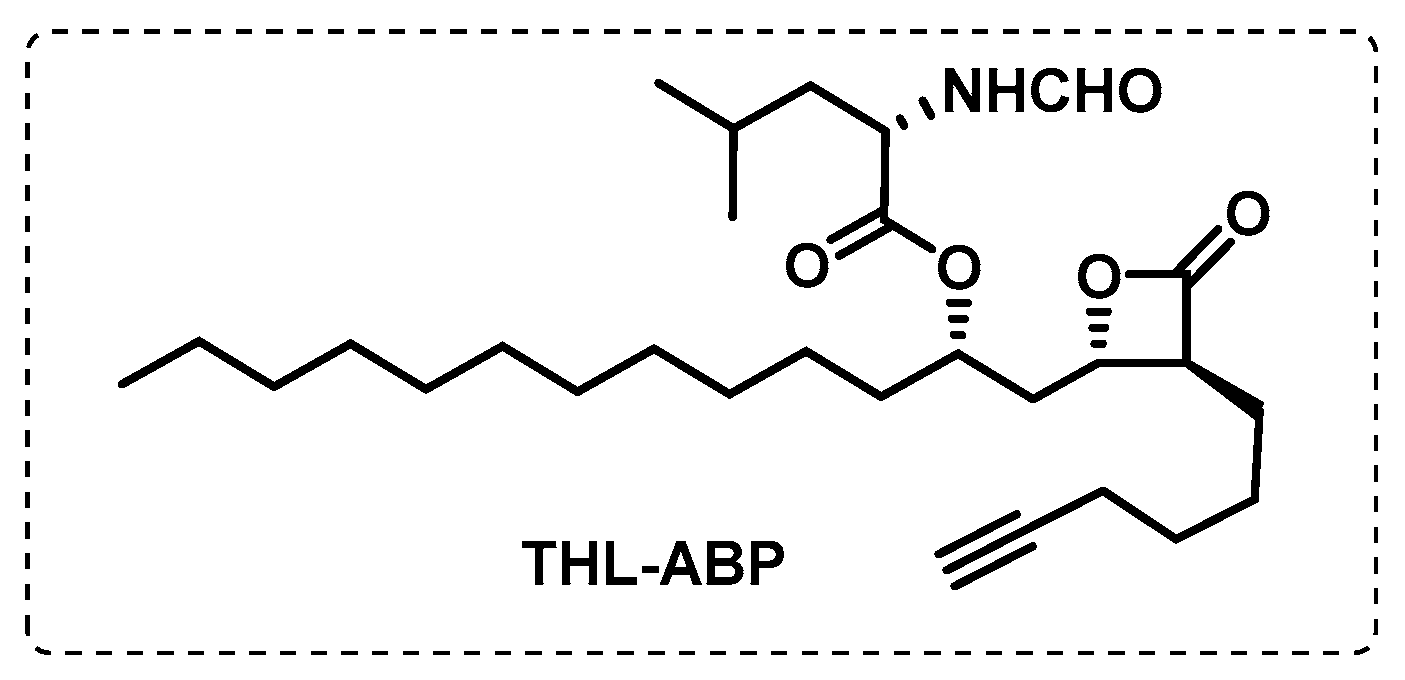
- Ravindran, M.S.; Rao, S.P.S.; Cheng, X.; Shukla, A.; Cazenave-Gassiot, A.; Yao, S.Q.; Wenk, M.R. Targeting Lipid Esterases in Mycobacteria Grown Under Different Physiological Conditions Using Activity-based Profiling with Tetrahydrolipstatin (THL). Mol. Cell. Proteom. 2014, 13, 435–448. [Google Scholar] [CrossRef] [PubMed]
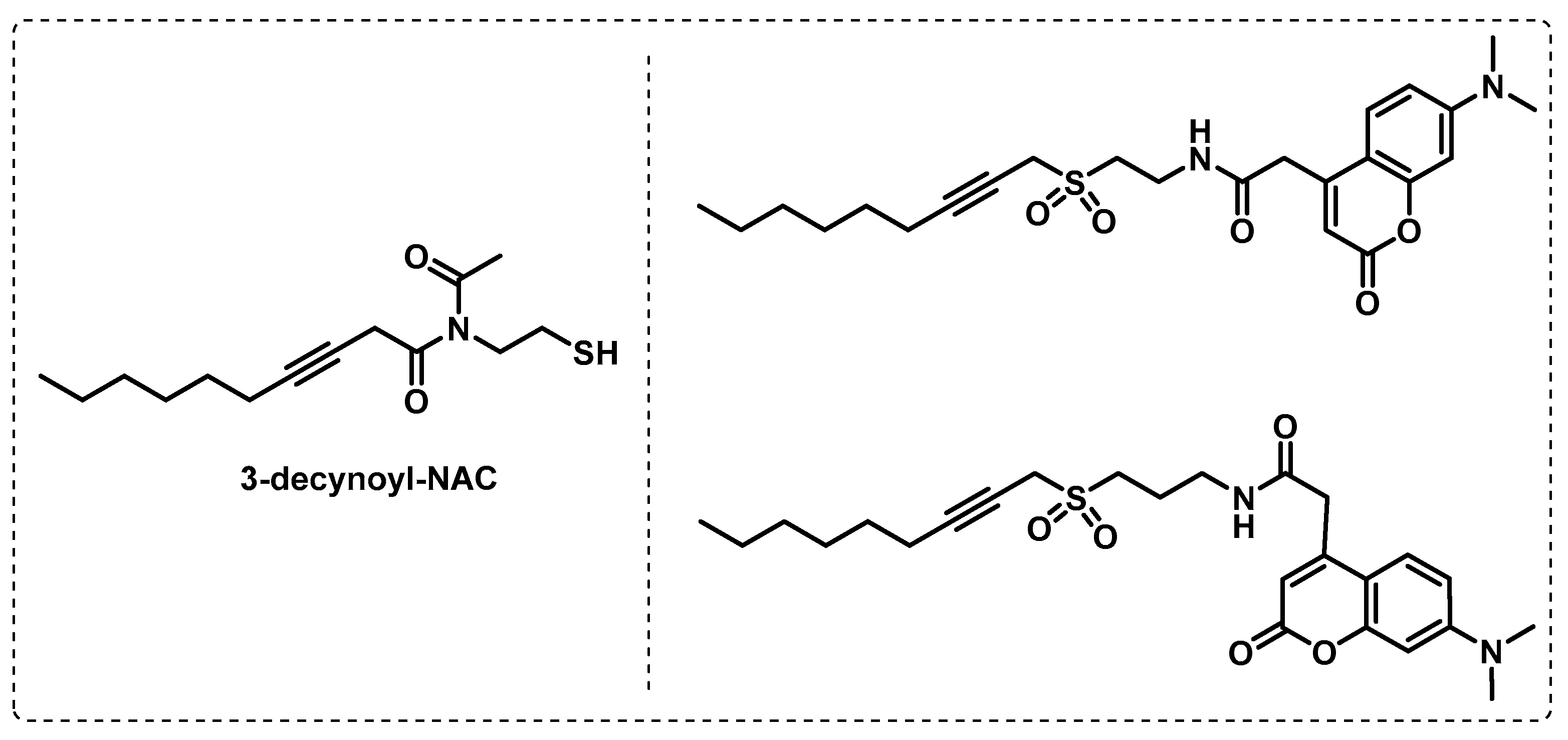
- Tallman, K.R.; Beatty, K.E. Far-red fluorogenic probes for esterase and lipase detection. Chembiochem A Eur. J. Chem. Biol. 2015, 16, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Tallman, K.R.; Levine, S.R.; Beatty, K.E. Small-Molecule Probes Reveal Esterases with Persistent Activity in Dormant and Reactivating Mycobacterium tuberculosis. ACS Infect. Dis. 2016, 2, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Tallman, K.R.; Levine, S.R.; Beatty, K.E. Profiling Esterases in Mycobacterium tuberculosis Using Far-Red Fluorogenic Substrates. ACS Chem. Biol. 2016, 11, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- Touchette Megan, H.; Holsclaw Cynthia, M.; Previti Mary, L.; Solomon Viven, C.; Leary Julie, A.; Bertozzi Carolyn, R.; Seeliger Jessica, C. The rv1184c Locus Encodes Chp2, an Acyltransferase in Mycobacterium tuberculosis Polyacyltrehalose Lipid Biosynthesis. J. Bacteriol. 2015, 197, 201–210. [Google Scholar] [CrossRef]
- Belardinelli, J.M.; Larrouy-Maumus, G.; Jones, V.; de Carvalho, L.P.S.; McNeil, M.R.; Jackson, M. Biosynthesis and Translocation of Unsulfated Acyltrehaloses in Mycobacterium tuberculosis. J. Biol. Chem. 2014, 289, 27952–27965. [Google Scholar] [CrossRef]
- Seeliger, J.C.; Holsclaw, C.M.; Schelle, M.W.; Botyanszki, Z.; Gilmore, S.A.; Tully, S.E.; Niederweis, M.; Cravatt, B.F.; Leary, J.A.; Bertozzi, C.R. Elucidation and Chemical Modulation of Sulfolipid-1 Biosynthesis in Mycobacterium tuberculosis*. J. Biol. Chem. 2012, 287, 7990–8000. [Google Scholar] [CrossRef]
- Ishikawa, F.; Tanabe, G.; Kakeya, H. Activity-Based Protein Profiling of Non-ribosomal Peptide Synthetases. In Activity-Based Protein Profiling; Cravatt, B.F., Hsu, K.-L., Weerapana, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 321–349. [Google Scholar] [CrossRef]
- Li, W.; Deng, G.; Li, M.; Liu, X.; Wang, Y. Roles of Mucosal Immunity against Mycobacterium tuberculosis Infection. Tuberc. Res. Treat. 2012, 2012, 791728. [Google Scholar] [CrossRef]
- Derrien, M.; van Passel, M.W.J.; van de Bovenkamp, J.H.B.; Schipper, R.; de Vos, W.; Dekker, J. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes 2010, 1, 254–268. [Google Scholar] [CrossRef]
- Mougous, J.D.; Leavell, M.D.; Senaratne, R.H.; Leigh, C.D.; Williams, S.J.; Riley, L.W.; Leary, J.A.; Bertozzi, C.R. Discovery of sulfated metabolites in mycobacteria with a genetic and mass spectrometric approach. Proc. Natl. Acad. Sci. USA 2002, 99, 17037–17042. [Google Scholar] [CrossRef] [PubMed]
- Mougous, J.D.; Lee, D.H.; Hubbard, S.C.; Schelle, M.W.; Vocadlo, D.J.; Berger, J.M.; Bertozzi, C.R. Molecular Basis for G Protein Control of the Prokaryotic ATP Sulfurylase. Mol. Cell 2006, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Kim, H.J.; Jang, S.; Hong, J.-I. Detection of bacterial sulfatase activity through liquid- and solid-phase colony-based assays. AMB Express 2017, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, B.P.; Wilson, D.J.; Nelson, K.M.; Boshoff, H.I.; Barry, C.E., III; Aldrich, C.C. Development of a Selective Activity-Based Probe for Adenylating Enzymes: Profiling MbtA Involved in Siderophore Biosynthesis from Mycobacterium tuberculosis. ACS Chem. Biol. 2012, 7, 1653–1658. [Google Scholar] [CrossRef]
- Wolfe, L.M.; Veeraraghavan, U.; Idicula-Thomas, S.; Schürer, S.; Wennerberg, K.; Reynolds, R.; Besra, G.S.; Dobos, K.M. A chemical proteomics approach to profiling the ATP-binding proteome of Mycobacterium tuberculosis. Mol. Cell. Proteom. 2013, 12, 1644–1660. [Google Scholar] [CrossRef]
- Batt, S.M.; Minnikin, D.E.; Besra, G.S. The thick waxy coat of mycobacteria, a protective layer against antibiotics and the host’s immune system. Biochem. J. 2020, 477, 1983–2006. [Google Scholar] [CrossRef]
- Vilchèze, C. Mycobacterial Cell Wall: A Source of Successful Targets for Old and New Drugs. Appl. Sci. 2020, 10, 2278. [Google Scholar] [CrossRef]
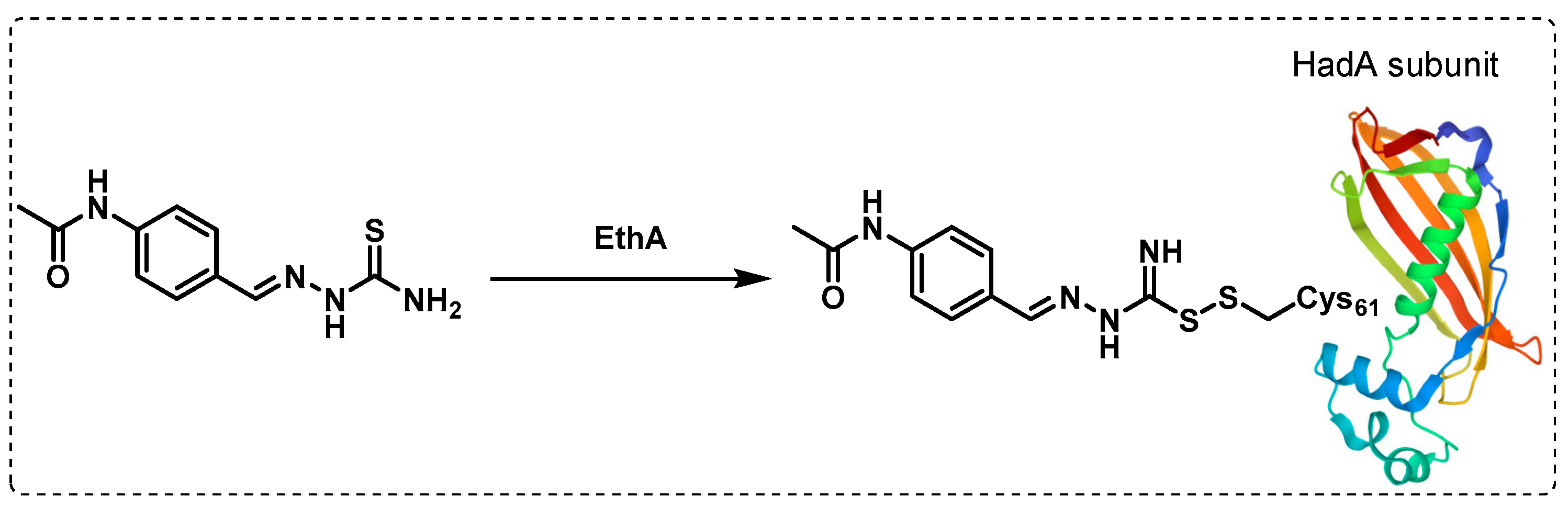
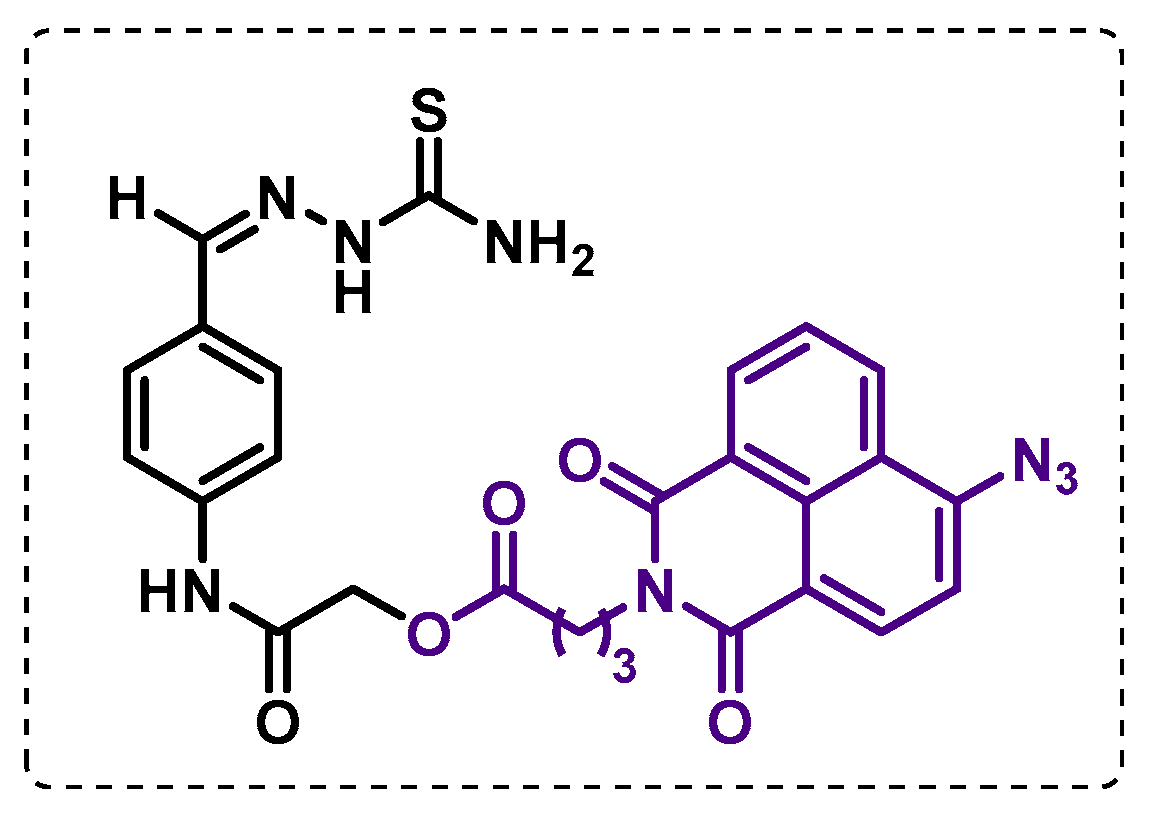
- Grzegorzewicz, A.E.; Eynard, N.; Quémard, A.; North, E.J.; Margolis, A.; Lindenberger, J.J.; Jones, V.; Korduláková, J.; Brennan, P.J.; Lee, R.E.; et al. Covalent Modification of the Mycobacterium tuberculosis FAS-II Dehydratase by Isoxyl and Thiacetazone. ACS Infect. Dis. 2015, 1, 91–97. [Google Scholar] [CrossRef]
- Singh, B.K.; Singha, M.; Basak, S.; Biswas, R.; Das, A.K.; Basak, A. Fluorescently labelled thioacetazone for detecting the interaction with Mycobacterium dehydratases HadAB and HadBC. Org. Biomol. Chem. 2022, 20, 1444–1452. [Google Scholar] [CrossRef]
- Rahlwes, K.C.; Dias, B.R.S.; Campos, P.C.; Alvarez-Arguedas, S.; Shiloh, M.U. Pathogenicity and virulence of Mycobacterium tuberculosis. Virulence 2023, 14, 2150449. [Google Scholar] [CrossRef]
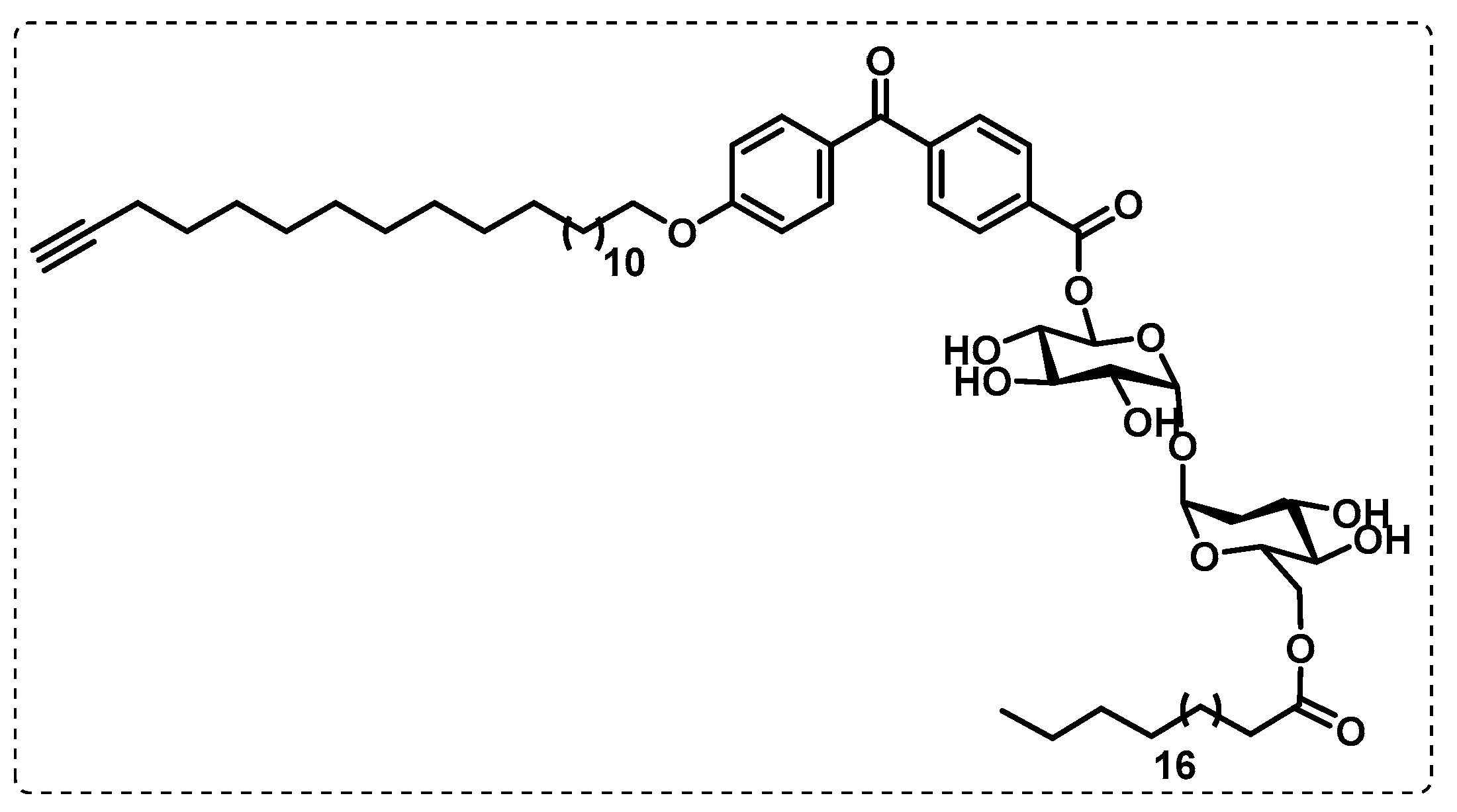
- Khan, A.A.; Kamena, F.; Timmer, M.S.M.; Stocker, B.L. Development of a benzophenone and alkyne functionalised trehalose probe to study trehalose dimycolate binding proteins. Org. Biomol. Chem. 2013, 11, 881–885. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capela, R.; Félix, R.; Clariano, M.; Nunes, D.; Perry, M.d.J.; Lopes, F. Target Identification in Anti-Tuberculosis Drug Discovery. Int. J. Mol. Sci. 2023, 24, 10482. https://doi.org/10.3390/ijms241310482
Capela R, Félix R, Clariano M, Nunes D, Perry MdJ, Lopes F. Target Identification in Anti-Tuberculosis Drug Discovery. International Journal of Molecular Sciences. 2023; 24(13):10482. https://doi.org/10.3390/ijms241310482
Chicago/Turabian StyleCapela, Rita, Rita Félix, Marta Clariano, Diogo Nunes, Maria de Jesus Perry, and Francisca Lopes. 2023. "Target Identification in Anti-Tuberculosis Drug Discovery" International Journal of Molecular Sciences 24, no. 13: 10482. https://doi.org/10.3390/ijms241310482
APA StyleCapela, R., Félix, R., Clariano, M., Nunes, D., Perry, M. d. J., & Lopes, F. (2023). Target Identification in Anti-Tuberculosis Drug Discovery. International Journal of Molecular Sciences, 24(13), 10482. https://doi.org/10.3390/ijms241310482





