Therapeutic Effects of Long-Term Administration of Tranilast in an Animal Model for the Treatment of Fibroids
Abstract
1. Introduction
2. Results
2.1. Tranilast Has No Side Effects and Is Well Tolerated by Mice
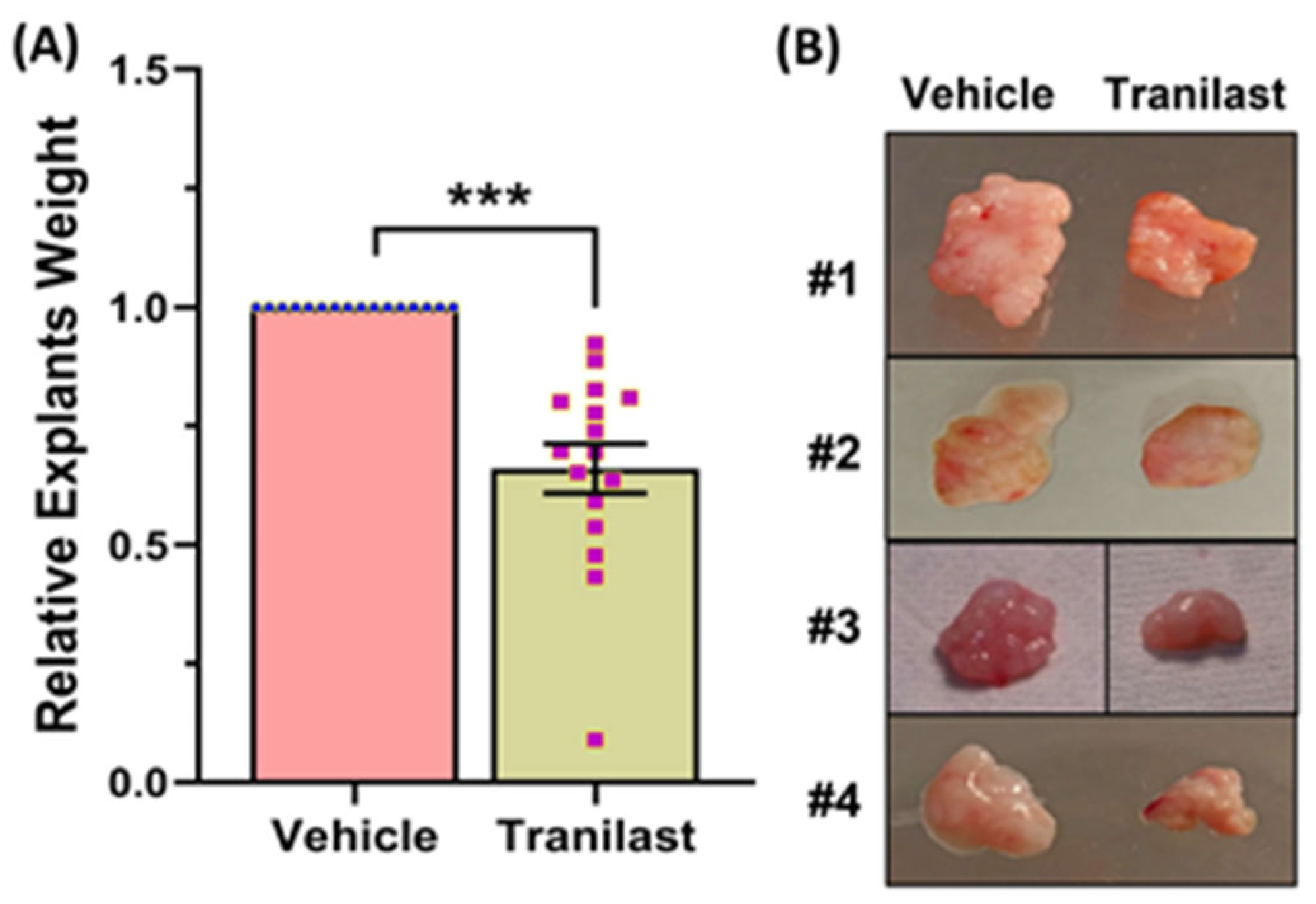
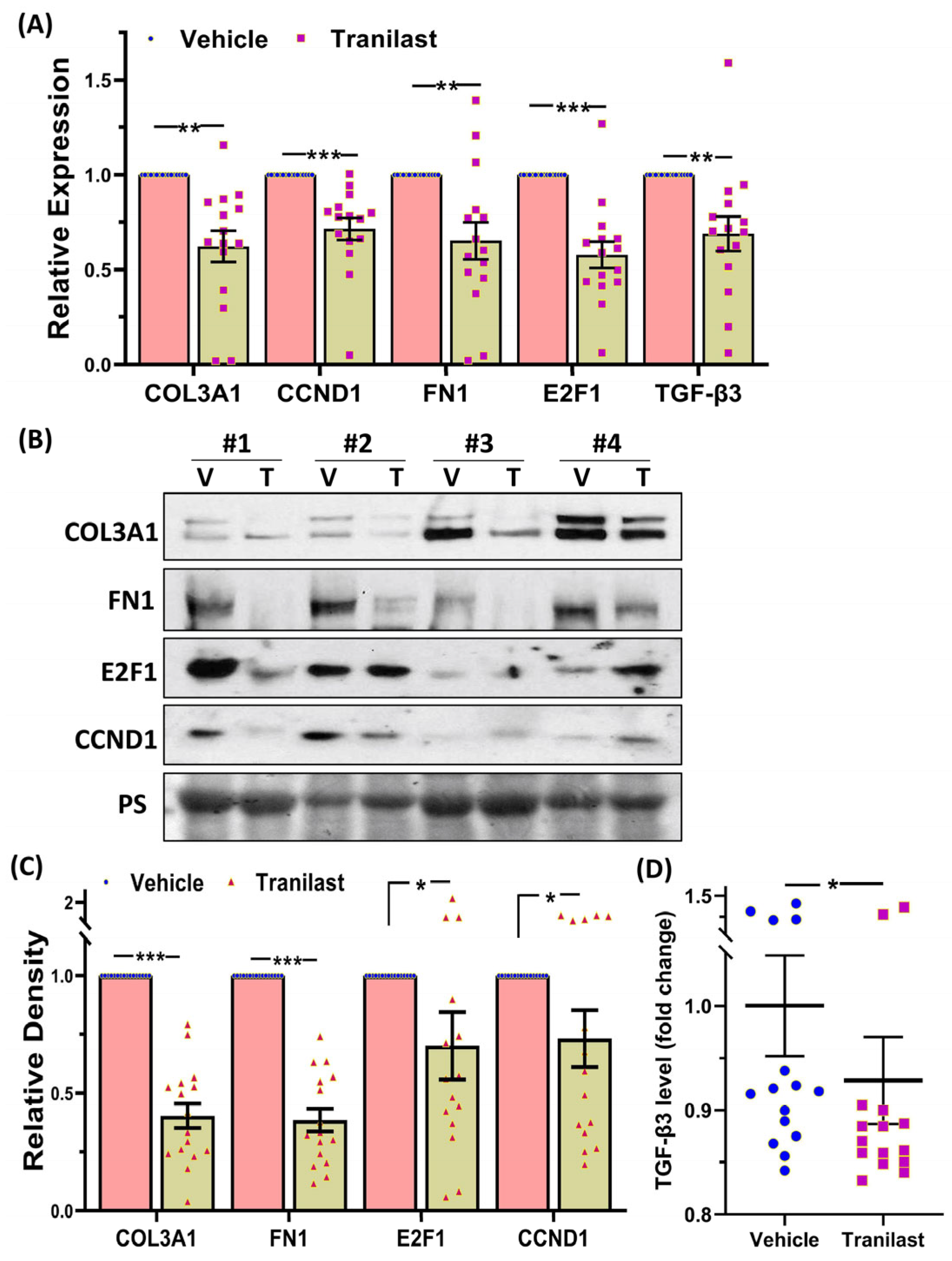
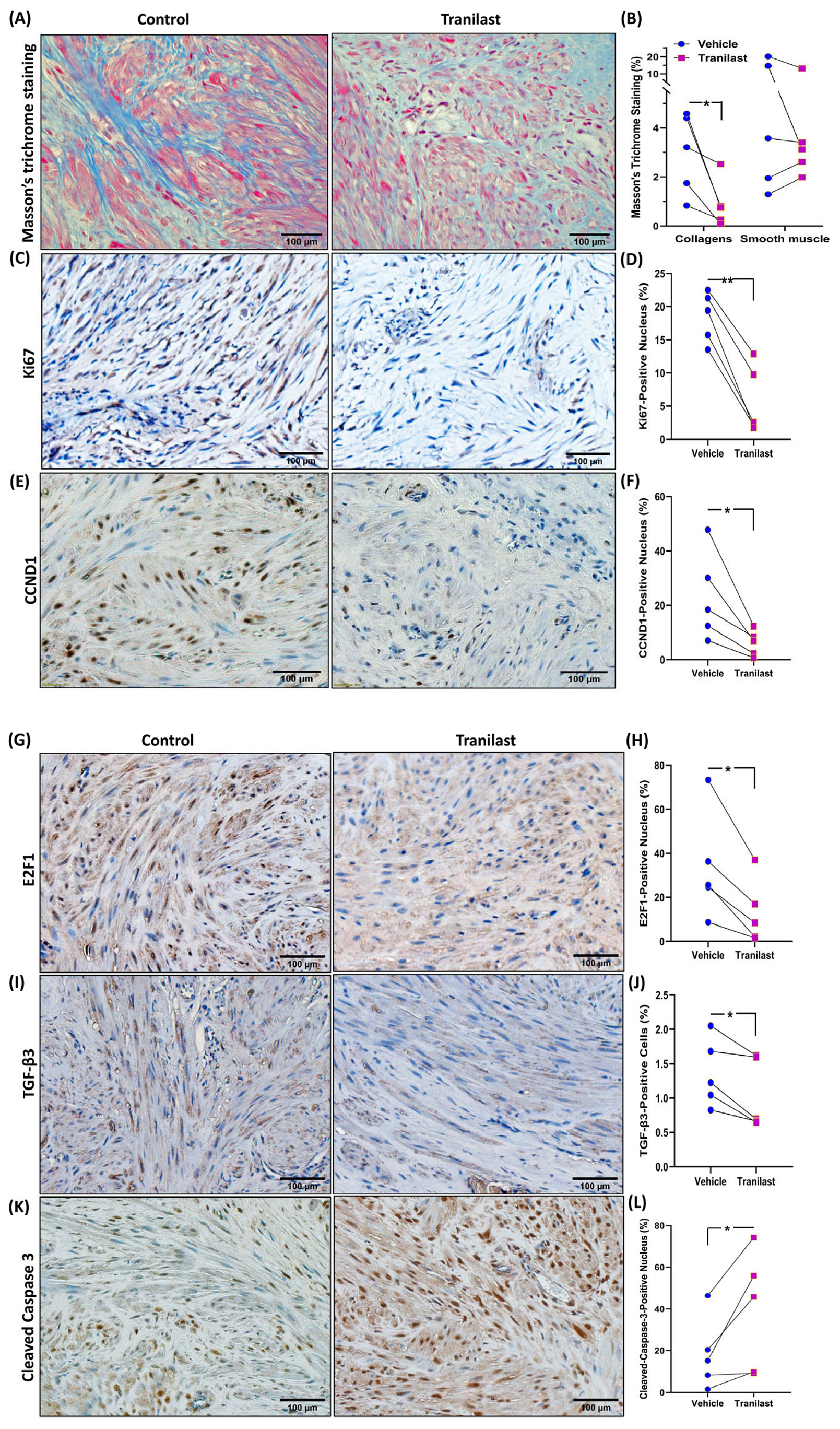
2.2. The Effects of Tranilast on Tumor Size and Expression of Genes Regulating Growth, Apoptosis, and ECM Compsoition
3. Discussion
4. Materials and Methods
4.1. Fibroid Specimens Collection
4.2. Fibroid Animal Model
4.3. Histology
4.4. Safety Test
4.5. Quantitative RT-PCR
4.6. Immunoblotting
4.7. Enzyme-Linked Immunosorbent Assay
4.8. Immunohistochemistry
4.9. Statistics and Power Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Segars, J.H.; Parrott, E.C.; Nagel, J.D.; Guo, X.C.; Gao, X.; Birnbaum, L.S.; Pinn, V.W.; Dixon, D. Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: Comprehensive review, conference summary and future recommendations. Hum. Reprod. Update 2014, 20, 309–333. [Google Scholar] [CrossRef] [PubMed]
- Doherty, L.; Mutlu, L.; Sinclair, D.; Taylor, H. Uterine fibroids: Clinical manifestations and contemporary management. Reprod. Sci. 2014, 21, 1067–1092. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular matrix in uterine leiomyoma pathogenesis: A potential target for future therapeutics. Hum. Reprod. Update 2018, 24, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A.; Cookson, C.L.; Gandolfo, R.A.; Schulze-Rath, R. Epidemiology of uterine fibroids: A systematic review. BJOG 2017, 124, 1501–1512. [Google Scholar] [CrossRef]
- Chegini, N. Proinflammatory and profibrotic mediators: Principal effectors of leiomyoma development as a fibrotic disorder. Semin. Reprod Med. 2010, 28, 180–203. [Google Scholar] [CrossRef]
- Taylor, D.K.; Holthouser, K.; Segars, J.H.; Leppert, P.C. Recent scientific advances in leiomyoma (uterine fibroids) research facilitates better understanding and management. F1000Research 2015, 4, 183. [Google Scholar] [CrossRef]
- Rogosnitzky, M.; Danks, R.; Kardash, E. Therapeutic potential of tranilast, an anti-allergy drug, in proliferative disorders. Anticancer Res. 2012, 32, 2471–2478. [Google Scholar]
- Darakhshan, S.; Pour, A.B. Tranilast: A review of its therapeutic applications. Pharmacol. Res. 2015, 91, 15–28. [Google Scholar] [CrossRef]
- Shime, H.; Kariya, M.; Orii, A.; Momma, C.; Kanamori, T.; Fukuhara, K.; Kusakari, T.; Tsuruta, Y.; Takakura, K.; Nikaido, T.; et al. Tranilast inhibits the proliferation of uterine leiomyoma cells in vitro through G1 arrest associated with the induction of p21(waf1) and p53. J. Clin. Endocrinol. Metab. 2002, 87, 5610–5617. [Google Scholar] [CrossRef]
- Chuang, T.D.; Khorram, O. Tranilast Inhibits Genes Functionally Involved in Cell Proliferation, Fibrosis, and Epigenetic Regulation and Epigenetically Induces miR-29c Expression in Leiomyoma Cells. Reprod. Sci. 2017, 24, 1253–1263. [Google Scholar] [CrossRef]
- Chuang, T.D.; Rehan, A.; Khorram, O. Tranilast induces MiR-200c expression through blockade of RelA/p65 activity in leiomyoma smooth muscle cells. Fertil. Steril. 2020, 113, 1308–1318. [Google Scholar] [CrossRef]
- Islam, M.S.; Protic, O.; Ciavattini, A.; Giannubilo, S.R.; Tranquilli, A.L.; Catherino, W.H.; Castellucci, M.; Ciarmela, P. Tranilast, an orally active antiallergic compound, inhibits extracellular matrix production in human uterine leiomyoma and myometrial cells. Fertil. Steril. 2014, 102, 597–606. [Google Scholar] [CrossRef]
- Suzawa, H.; Kikuchi, S.; Ichikawa, K.; Koda, A. Inhibitory action of tranilast, an anti-allergic drug, on the release of cytokines and PGE2 from human monocytes-macrophages. Jpn. J. Pharmacol. 1992, 60, 85–90. [Google Scholar] [CrossRef]
- Takahashi, K.; Menju, T.; Nishikawa, S.; Miyata, R.; Tanaka, S.; Yutaka, Y.; Yamada, Y.; Nakajima, D.; Hamaji, M.; Ohsumi, A.; et al. Tranilast Inhibits TGF-β1-induced Epithelial-mesenchymal Transition and Invasion/Metastasis via the Suppression of Smad4 in Human Lung Cancer Cell Lines. Anticancer Res. 2020, 40, 3287–3296. [Google Scholar] [CrossRef]
- Minaguchi, H.; Wong, J.M.; Snabes, M.C. Clinical use of nafarelin in the treatment of leiomyomas. A review of the literature. J. Reprod. Med. 2000, 45, 481–489. [Google Scholar]
- Koda, A.; Kurashina, Y.; Nakazawa, M. The inhibition mechanism of histamine release by N-(3,4-dimethoxycinnamoyl) anthranilic acid. Int. Arch. Allergy Appl. Immunol. 1985, 77, 244–245. [Google Scholar] [CrossRef]
- Okuda, M.; Ishikawa, T.; Saito, Y.; Shimizu, T.; Baba, S. A clinical evaluation of N-5′ with perennial-type allergic rhinitis--a test by the multi-clinic, intergroup, double-blind comparative method. Ann. Allergy 1984, 53, 178–185. [Google Scholar]
- Maluf, H.M.; Gersell, D.J. Uterine leiomyomas with high content of mast cells. Arch. Pathol. Lab Med. 1994, 118, 712–714. [Google Scholar]
- Orii, A.; Mori, A.; Zhai, Y.L.; Toki, T.; Nikaido, T.; Fujii, S. Mast cells in smooth muscle tumors of the uterus. Int. J. Gynecol. Pathol. 1998, 17, 336–342. [Google Scholar] [CrossRef]
- Jiang, L.; Hua, Y.; Shen, Q.; Ding, S.; Jiang, W.; Zhang, W.; Zhu, X. Role of mast cells in gynecological neoplasms. Front. Biosci.-Landmark 2013, 18, 773–781. [Google Scholar]
- Horiuchi, A.; Nikaido, T.; Ya-Li, Z.; Ito, K.; Orii, A.; Fujii, S. Heparin inhibits proliferation of myometrial and leiomyomal smooth muscle cells through the induction of alpha-smooth muscle actin, calponin h1 and p27. Mol. Hum. Reprod. 1999, 5, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Sarrouilhe, D.; Mesnil, M. Serotonin and human cancer: A critical view. Biochimie 2019, 161, 46–50. [Google Scholar] [CrossRef]
- Jeffrey, J.J.; Ehlich, L.S.; Roswit, W.T. Serotonin: An inducer of collagenase in myometrial smooth muscle cells. J. Cell. Physiol. 1991, 146, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Dumin, J.; Wilcox, B.D.; Otterness, I.; Melendez, J.A.; Huang, C.; Jeffrey, J.J. Serotonin-mediated production of interstitial collagenase by uterine smooth muscle cells requires interleukin-1alpha, but not interleukin-1beta. J. Biol. Chem. 1998, 273, 25488–25494. [Google Scholar] [CrossRef] [PubMed]
- Gurbuz, N.; Asoglu, M.R.; Ashour, A.A.; Salama, S.; Kilic, G.S.; Ozpolat, B. A selective serotonin 5-HT1B receptor inhibition suppresses cells proliferation and induces apoptosis in human uterine leiomyoma cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.D.; Quintanilla, D.; Boos, D.; Khorram, O. Further characterization of tryptophan metabolism and its dysregulation in fibroids. F S Sci. 2022, 3, 392–400. [Google Scholar] [CrossRef]
- Chuang, T.D.; Quintanilla, D.; Boos, D.; Khorram, O. Tryptophan catabolism is dysregulated in leiomyomas. Fertil. Steril. 2021, 116, 1160–1171. [Google Scholar] [CrossRef]
- Hutchinson, A.P.; Yin, P.; Neale, I.; Coon, J.S.t.; Kujawa, S.A.; Liu, S.; Bulun, S.E. Tryptophan 2,3-Dioxygenase-2 in Uterine Leiomyoma: Dysregulation by MED12 Mutation Status. Reprod. Sci. 2022, 29, 743–749. [Google Scholar] [CrossRef]
- Prud’homme, G.J.; Glinka, Y.; Toulina, A.; Ace, O.; Subramaniam, V.; Jothy, S. Breast cancer stem-like cells are inhibited by a non-toxic aryl hydrocarbon receptor agonist. PLoS ONE 2010, 5, e13831. [Google Scholar] [CrossRef]
- Noakes, R.R. Effects of tranilast on the urinary excretion of kynurenic and quinolinic Acid under conditions of L tryptophan loading. Int. J. Tryptophan. Res. 2013, 6, 67–71. [Google Scholar] [CrossRef]
- Protic, O.; Toti, P.; Islam, M.S.; Occhini, R.; Giannubilo, S.R.; Catherino, W.H.; Cinti, S.; Petraglia, F.; Ciavattini, A.; Castellucci, M.; et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 2016, 364, 415–427. [Google Scholar] [CrossRef]
- AlAshqar, A.; Reschke, L.; Kirschen, G.W.; Borahay, M.A. Role of inflammation in benign gynecologic disorders: From pathogenesis to novel therapies. Biol. Reprod. 2021, 105, 7–31. [Google Scholar] [CrossRef]
- Chuang, T.D.; Khorram, O. miR-200c Regulates IL8 Expression by Targeting IKBKB: A Potential Mediator of Inflammation in Leiomyoma Pathogenesis. PLoS ONE 2014, 9, e95370. [Google Scholar] [CrossRef]
- Chuang, T.D.; Khorram, O. Mechanisms underlying aberrant expression of miR-29c in uterine leiomyoma. Fertil. Steril. 2016, 105, 236–245.e1. [Google Scholar] [CrossRef]
- Spiecker, M.; Lorenz, I.; Marx, N.; Darius, H. Tranilast inhibits cytokine-induced nuclear factor kappaB activation in vascular endothelial cells. Mol. Pharmacol 2002, 62, 856–863. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhuo, J. Tranilast Treatment Attenuates Cerebral Ischemia-Reperfusion Injury in Rats through the Inhibition of Inflammatory Responses Mediated by NF-κB and PPARs. Clin. Transl. Sci. 2019, 12, 196–202. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Savage, M.; LaBlanche, J.M.; Grip, L.; Serruys, P.W.; Fitzgerald, P.; Fischman, D.; Goldberg, S.; Brinker, J.A.; Zeiher, A.M.; et al. Results of Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 2002, 106, 1243–1250. [Google Scholar] [CrossRef]
- Chuang, T.D.; Rehan, A.; Khorram, O. Functional role of the long noncoding RNA X-inactive specific transcript in leiomyoma pathogenesis. Fertil. Steril. 2021, 115, 238–247. [Google Scholar] [CrossRef]
- Chuang, T.D.; Khorram, O. Regulation of Cell Cycle Regulatory Proteins by MicroRNAs in Uterine Leiomyoma. Reprod. Sci. 2019, 26, 250–258. [Google Scholar] [CrossRef]
- Chuang, T.D.; Luo, X.; Panda, H.; Chegini, N. miR-93/106b and their host gene, MCM7, are differentially expressed in leiomyomas and functionally target F3 and IL-8. Mol. Endocrinol. 2012, 26, 1028–1042. [Google Scholar] [CrossRef]
- Chuang, T.D.; Panda, H.; Luo, X.; Chegini, N. miR-200c is aberrantly expressed in leiomyomas in an ethnic-dependent manner and targets ZEBs, VEGFA, TIMP2, and FBLN5. Endocr. Relat. Cancer 2012, 19, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Ishi, K.; Serna, V.A.; Kakazu, R.; Bulun, S.E.; Kurita, T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 2010, 151, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Yin, P.; Navarro, A.; Moravek, M.B.; Coon, J.S.t.; Druschitz, S.A.; Serna, V.A.; Qiang, W.; Brooks, D.C.; Malpani, S.S.; et al. Paracrine activation of WNT/beta-catenin pathway in uterine leiomyoma stem cells promotes tumor growth. Proc. Natl. Acad. Sci. USA 2013, 110, 17053–17058. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, J.W.; Kim, Y.H.; Lee, S.H.; Yoon, H.K.; Kim, C.H.; Ahn, J.H.; Lee, J.M.; Kim, J.S.; Kim, S.C.; et al. Effects of tranilast and pentoxifylline in a mouse model of chronic asthma using house dust mite antigen. J. Asthma 2009, 46, 884–894. [Google Scholar] [CrossRef]
- Chuang, T.-D.; Khorram, O. Cross-talk between miR-29c and transforming growth factor-β3 is mediated by an epigenetic mechanism in leiomyoma. Fertil. Steril. 2019, 112, 1180–1189. [Google Scholar] [CrossRef]
- Chuang, T.D.; Quintanilla, D.; Boos, D.; Khorram, O. Long Noncoding RNA MIAT Modulates the Extracellular Matrix Deposition in Leiomyomas by Sponging MiR-29 Family. Endocrinology 2021, 162, bqab186. [Google Scholar] [CrossRef]
- Chuang, T.D.; Gao, J.; Quintanilla, D.; McSwiggin, H.; Boos, D.; Yan, W.; Khorram, O. Differential Expression of MED12-Associated Coding RNA Transcripts in Uterine Leiomyomas. Int. J. Mol. Sci. 2023, 24, 3742. [Google Scholar] [CrossRef]
- Almeida, T.A.; Quispe-Ricalde, A.; Montes de Oca, F.; Foronda, P.; Hernandez, M.M. A high-throughput open-array qPCR gene panel to identify housekeeping genes suitable for myometrium and leiomyoma expression analysis. Gynecol. Oncol. 2014, 134, 138–143. [Google Scholar] [CrossRef]

| Chemistry Panel Marker | Vehicle | Tranilast | p-Value |
|---|---|---|---|
| (Mean ± SEM) | (Mean ± SEM) | ||
| General metabolism | |||
| Glucose (mg/dL) | 228.8 ± 13.12 | 215.4 ± 13.08 | 0.47 |
| Kidney function | |||
| BUN (mg/dL) | 23.5 ± 2.40 | 23.7 ± 2.54 | 0.18 |
| Creatinine (mg/dL) | 0.2 ± 0.01 | 0.2 ± 0.01 | 0.43 |
| Electrolytes | |||
| Sodium (mEq/L) | 153.9 ± 0.72 | 155.5 ± 1.43 | 0.33 |
| Phosphorus (mg/dL) | 8.6 ± 0.49 | 8.7 ± 0.58 | 0.72 |
| Liver function | |||
| Alkaline phosphatase (U/L) | 90 ± 13.49 | 94 ± 13.53 | 0.68 |
| Albumin (g/dL) | 3.7 ± 0.12 | 3.5 ± 0.16 | 0.3 |
| SGPT (ALT) (U/L) | 54.2 ± 10.18 | 112.7 ± 50.87 | 0.27 |
| Total protein (g/dL) | 4.9 ± 0.41 | 4.5 ± 0.11 | 0.26 |
| Globulin (g/dL) | 1.2 ± 0.34 | 1.0 ± 0.12 | 0.48 |
| Total bilirubin (mg/dL) | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.72 |
| Pancreas function | |||
| Amylase (U/L) | 692.0 ± 22.47 | 725.0 ± 90 | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, T.-D.; Munoz, L.; Quintanilla, D.; Boos, D.; Khorram, O. Therapeutic Effects of Long-Term Administration of Tranilast in an Animal Model for the Treatment of Fibroids. Int. J. Mol. Sci. 2023, 24, 10465. https://doi.org/10.3390/ijms241310465
Chuang T-D, Munoz L, Quintanilla D, Boos D, Khorram O. Therapeutic Effects of Long-Term Administration of Tranilast in an Animal Model for the Treatment of Fibroids. International Journal of Molecular Sciences. 2023; 24(13):10465. https://doi.org/10.3390/ijms241310465
Chicago/Turabian StyleChuang, Tsai-Der, Leslie Munoz, Derek Quintanilla, Drake Boos, and Omid Khorram. 2023. "Therapeutic Effects of Long-Term Administration of Tranilast in an Animal Model for the Treatment of Fibroids" International Journal of Molecular Sciences 24, no. 13: 10465. https://doi.org/10.3390/ijms241310465
APA StyleChuang, T.-D., Munoz, L., Quintanilla, D., Boos, D., & Khorram, O. (2023). Therapeutic Effects of Long-Term Administration of Tranilast in an Animal Model for the Treatment of Fibroids. International Journal of Molecular Sciences, 24(13), 10465. https://doi.org/10.3390/ijms241310465






