Telomere Length in Human Spermatogenic Cells as a New Potential Predictor of Clinical Outcomes in ART Treatment with Intracytoplasmic Injection of Testicular Spermatozoa
Abstract
1. Introduction
2. Results
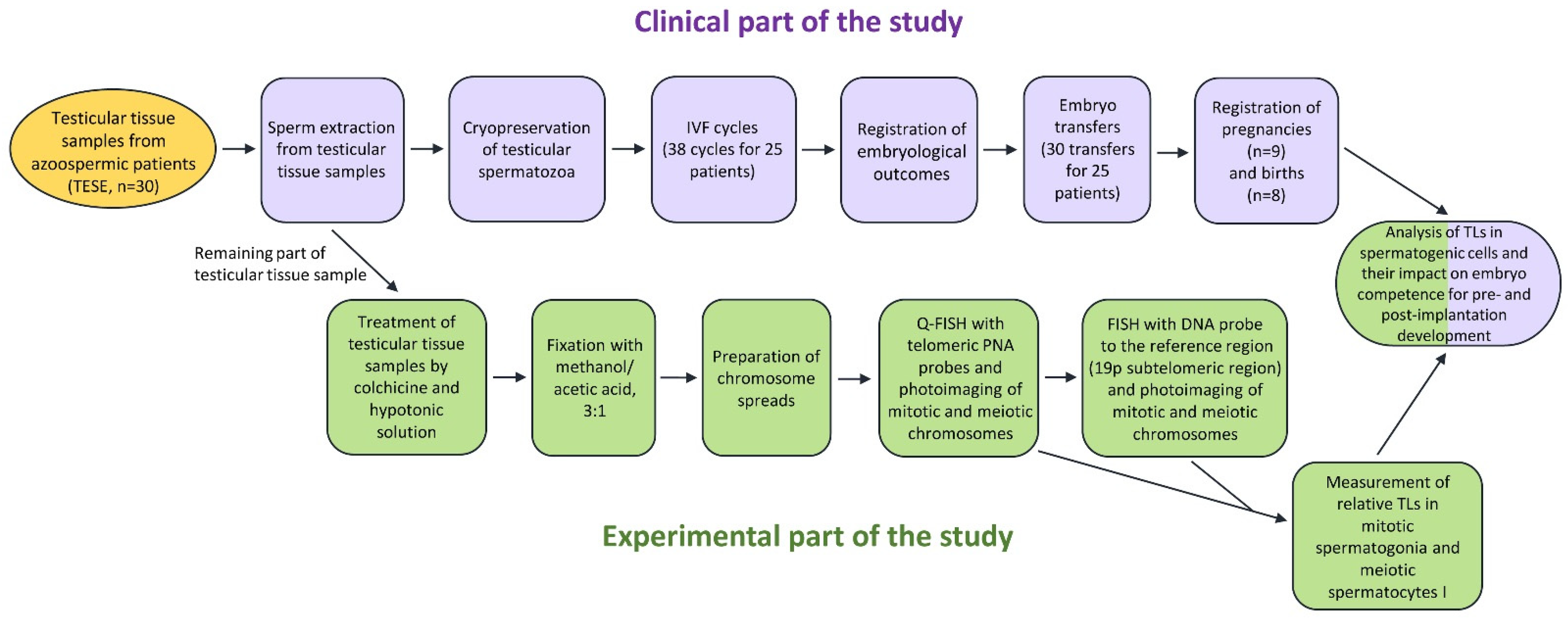
2.1. Patients and Samples
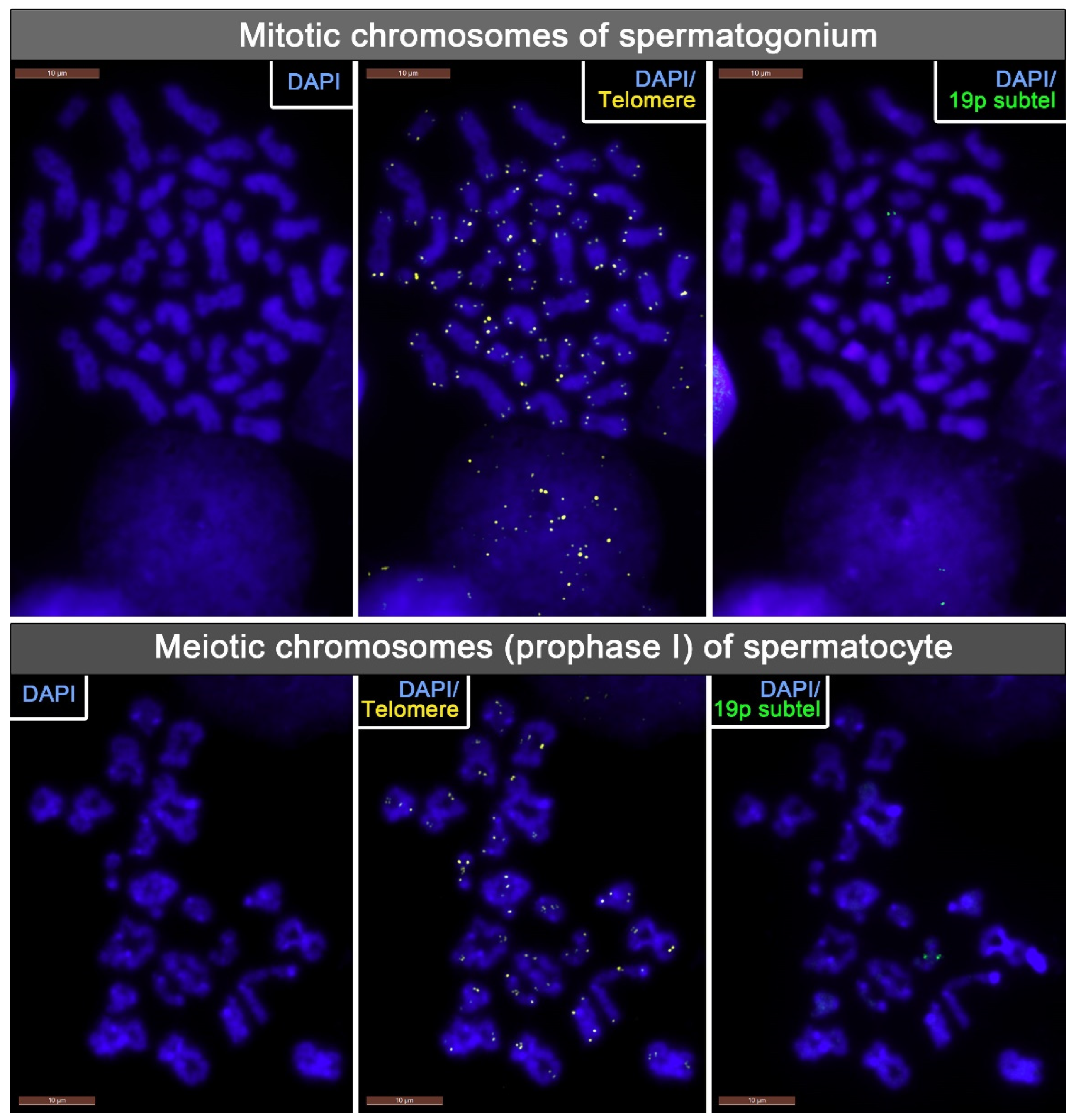
2.2. TL Analysis in Mitotic Chromosomes of Spermatogonia and Meiotic Chromosomes of Spermatocytes I in Azoospermic Patients’ Testicular Tissue Samples
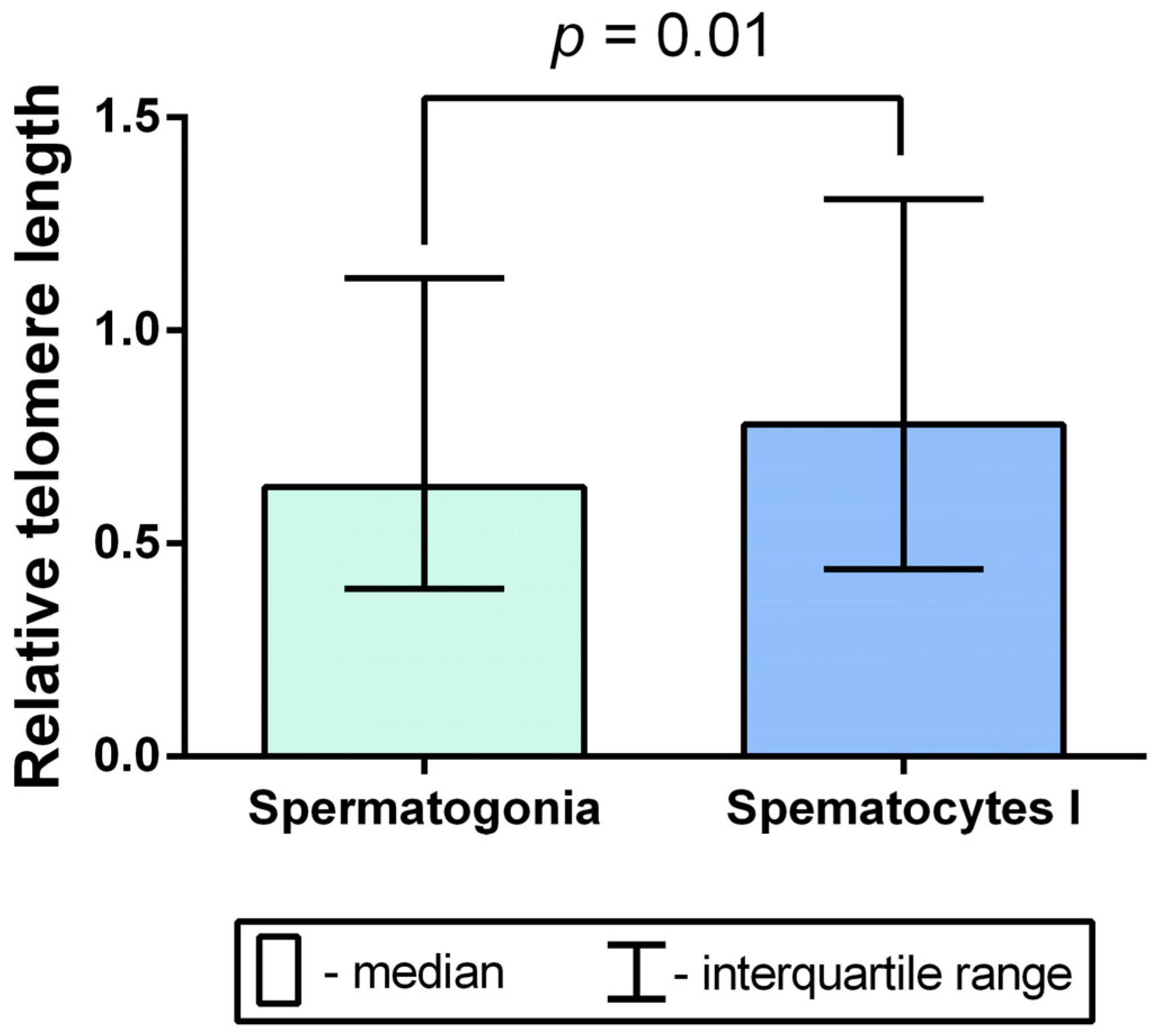
2.3. A Comparison of Intraindividual and Interindividual Relative TL Variability in Spermatogonia and Spermatocytes I
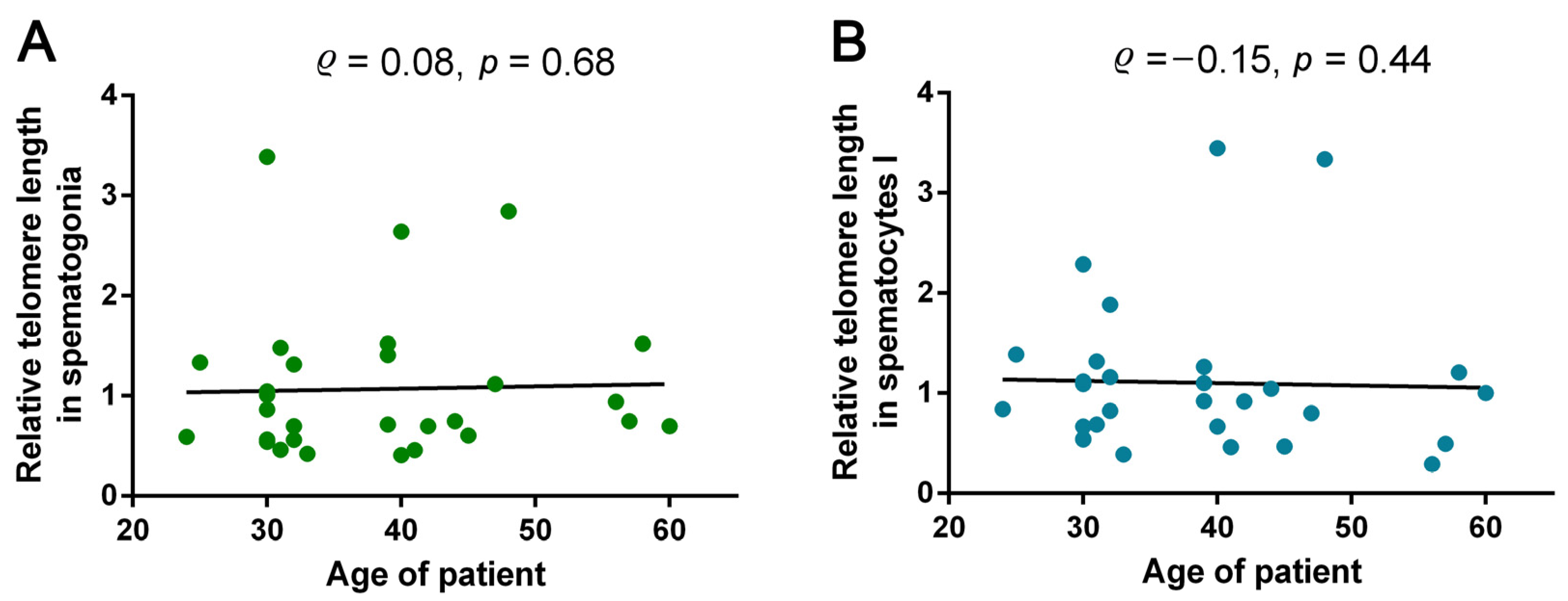
2.4. Analysis of Correlations between Azoospermic Patients’ Age and TL in Spermatogonia and Spermatocytes I
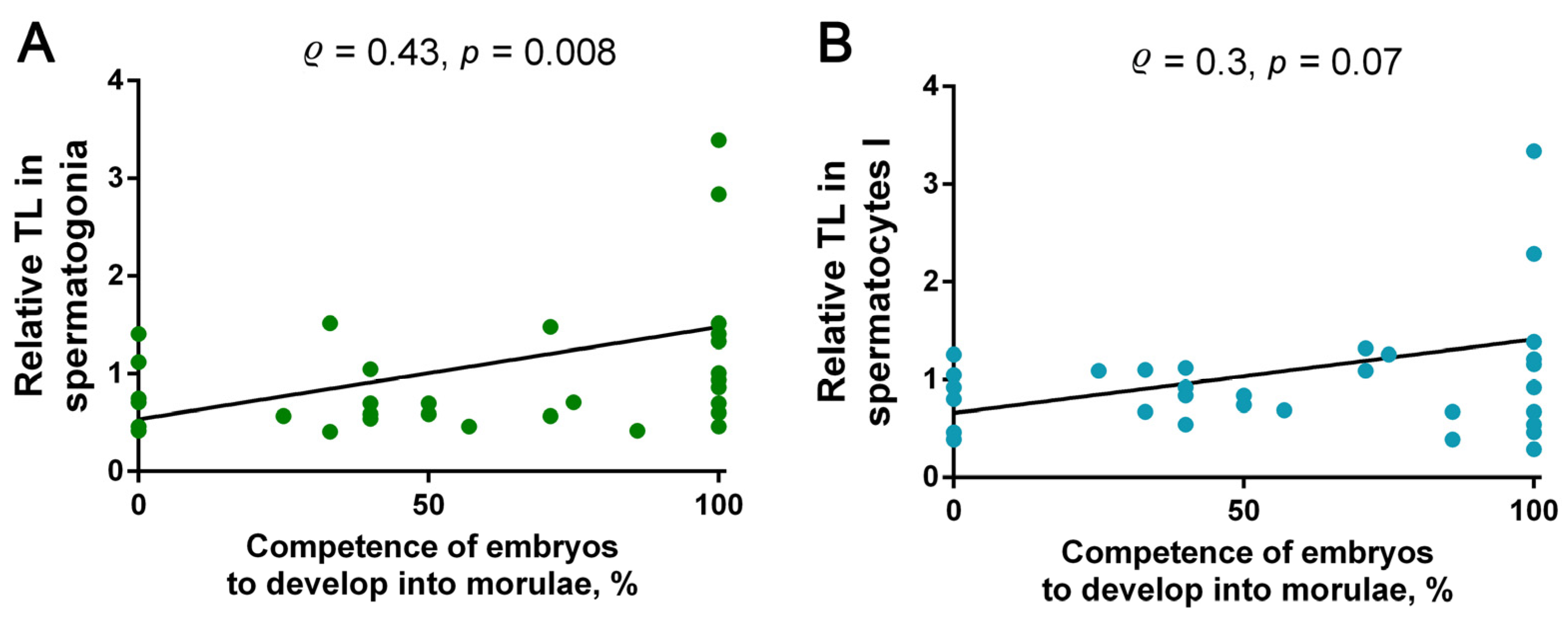
2.5. Analysis of Correlations between TL in Spermatogonia and Spermatocytes I and the Competence of Testicular-Sperm-Derived Embryos for In Vitro Preimplantation Development to the Morulae Stage
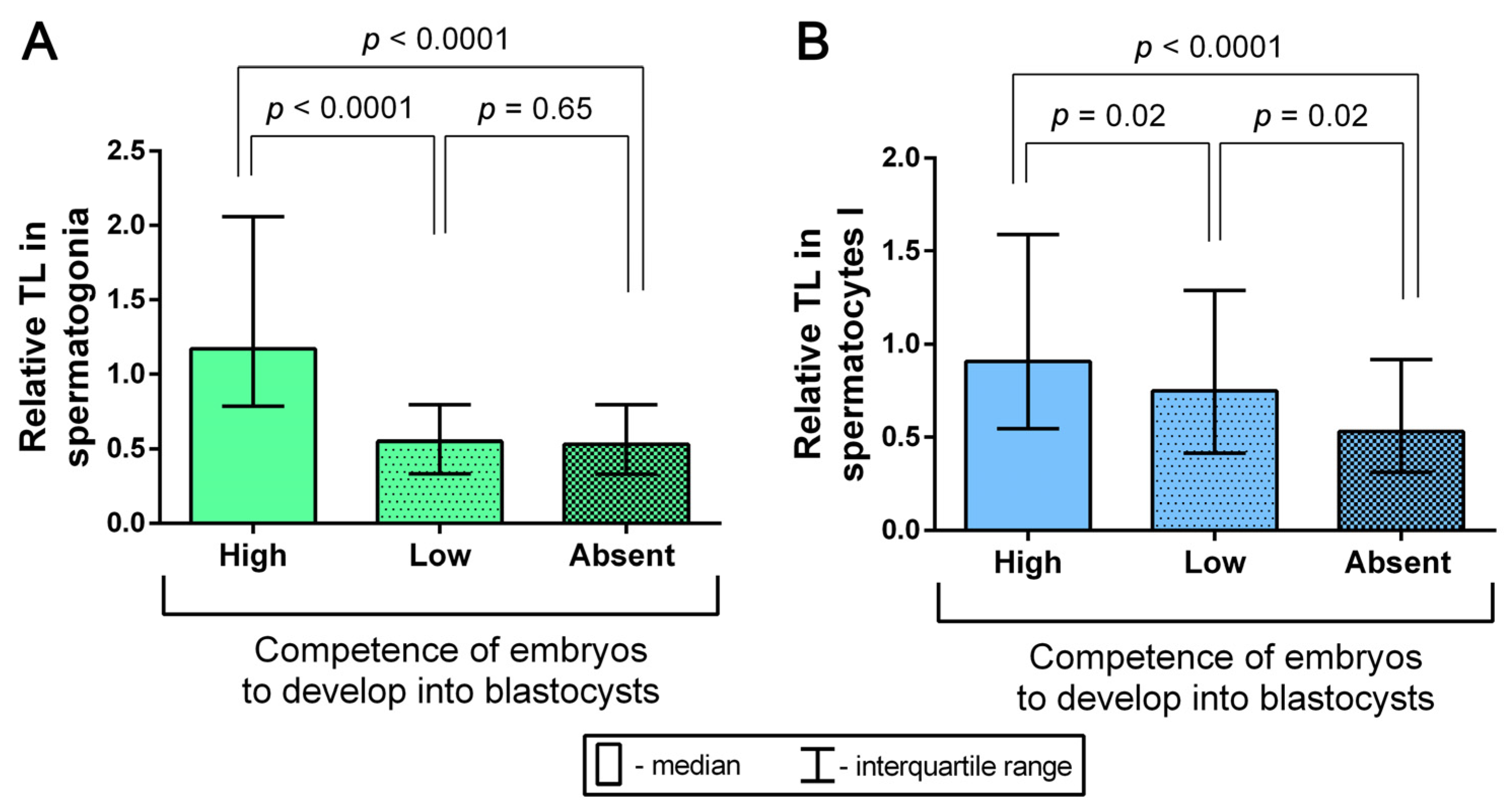
2.6. Assessment of the Competence of Testicular-Sperm-Derived Embryos for In Vitro Development to the Blastocyst Stage Depending on TL in Spermatogonia and Spermatocytes I
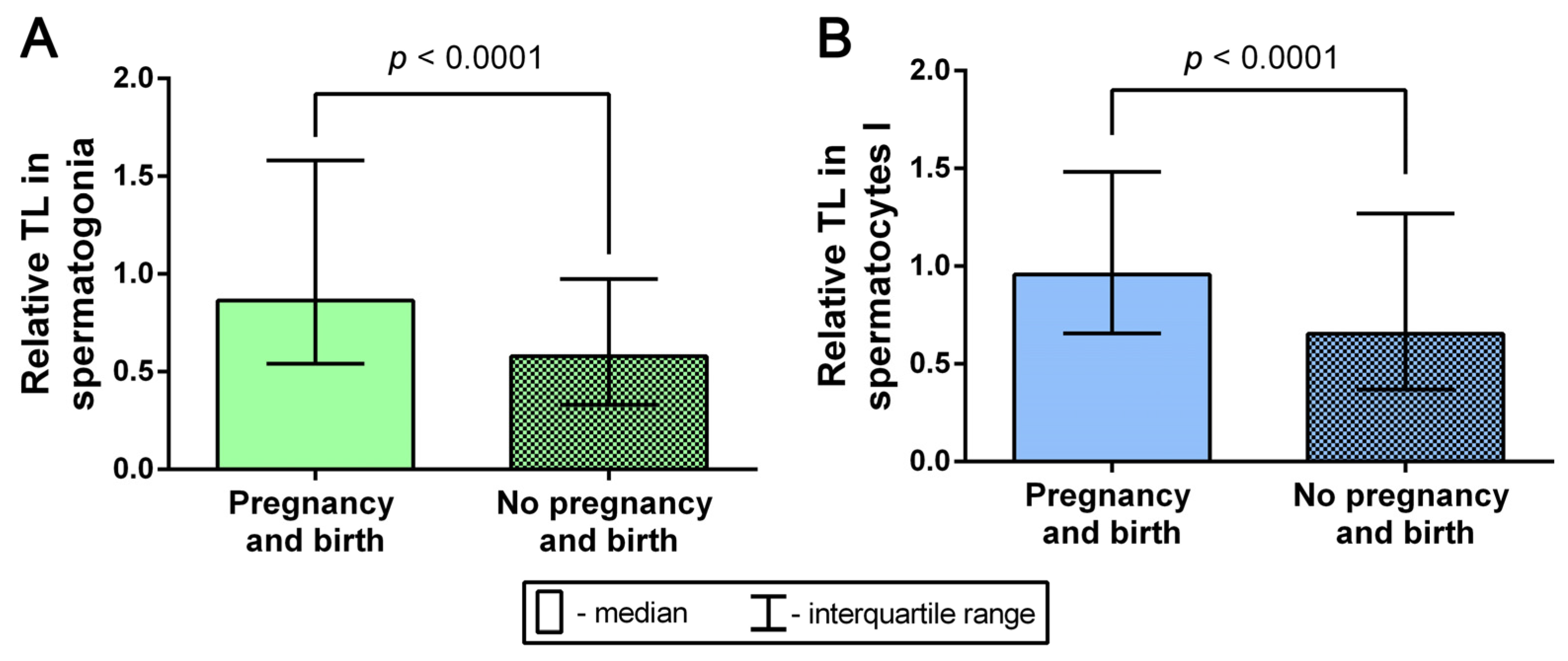
2.7. Analysis of the Relationship between TLs in Spermatogonia and Spermatocytes I and the Outcomes of Pregnancy and Birth after Testicular-Sperm-Derived Embryo Transfer
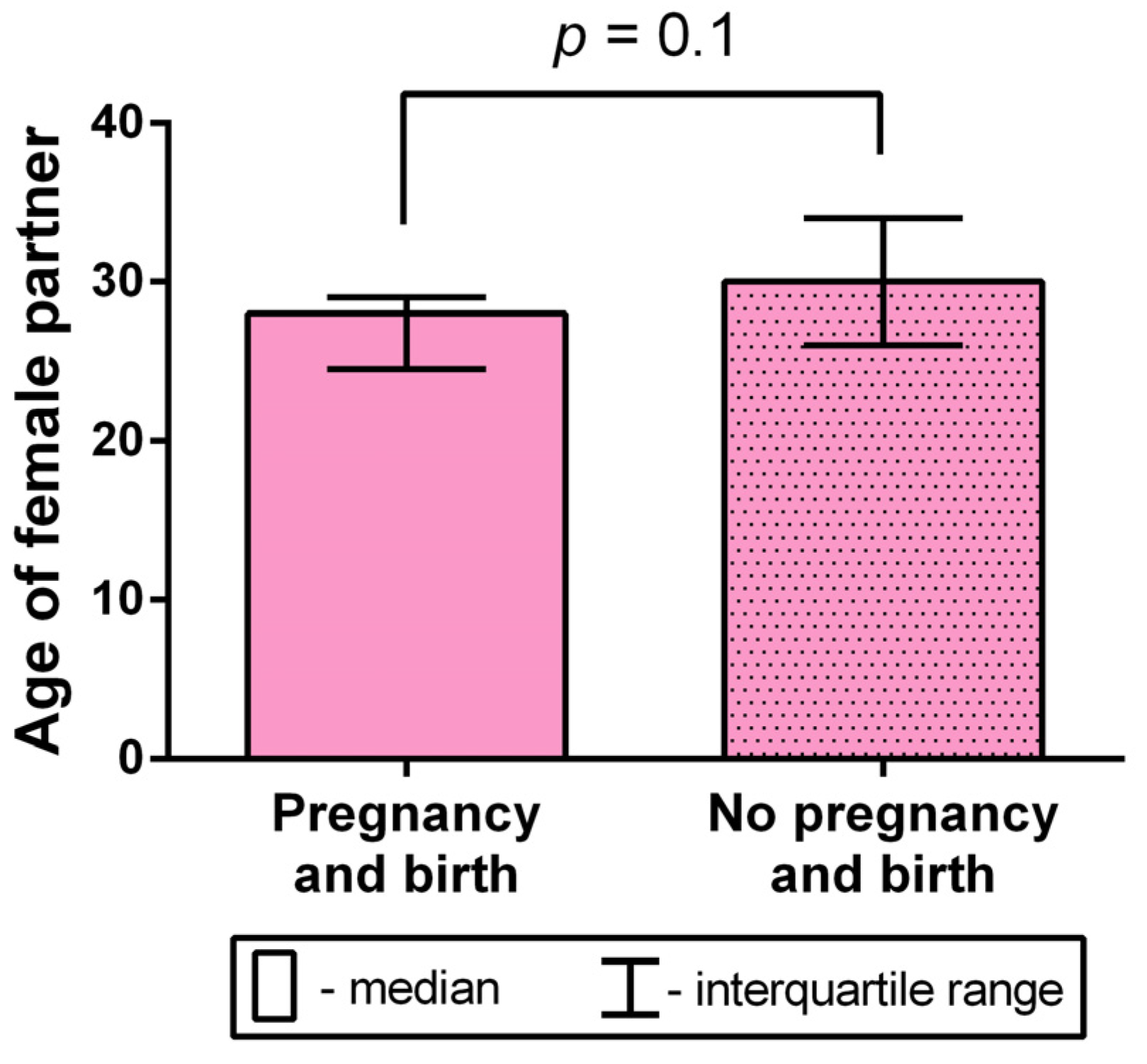
2.8. Analysis of the Female Partner’s Age Impact on the Occurrence of Birth after Testicular-Sperm-Derived Embryo Transfer to the Uterus
3. Discussion
4. Materials and Methods
4.1. Collection of Human Testicular Biopsy Samples
4.2. Chromosome Preparation
4.3. Fluorescence In Situ Hybridization (FISH)
4.4. Image Acquisition and the Evaluation of Telomeric and 19p Subtelomeric FISH Probe Signal Intensity
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, C.C.; Huang, F.J.; Wang, L.J.; Lin, Y.J.; Kung, F.T.; Hsieh, C.H.; Lan, K.C. Clinical outcomes and development of children born after intracytoplasmic spermiInjection (ICSI) using extracted testicular sperm or ejaculated extreme severe oligo-astheno-teratozoospermia sperm: A comparative study. Fertil. Steril. 2011, 9, 567–571. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, C.W.; Cheon, Y.P.; Lim, C.K. Potential of testicular sperm to support embryonic development to the blastocyst stage is comparable to that of ejaculated sperm. J. Assist. Reprod. Genet. 2018, 35, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Karavani, G.; Kan-Tor, Y.; Schachter-Safrai, N.; Levitas, E.; Or, Y.; Ben-Meir, A.; Buxboim, A.; Har-Vardi, I. Does sperm origin-Eeaculated or testicular-affect embryo morphokinetic parameters? Andrology 2021, 9, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Minhas, S.; Giwercman, A.; Bettocchi, C.; Dinkelman-Smit, M.; Dohle, G.; Fusco, F.; Kadioglou, A.; Kliesch, S.; Kopa, Z.; et al. Sperm recovery and ICSI outcomes in men with non-obstructive azoospermia: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 733–757. [Google Scholar] [CrossRef]
- Lan, Y.; Zheng, H.; Fu, X.; Peng, T.; Liao, C.; Liu, J.; Liu, M.; An, G. Clinical outcomes and live birth rate resulted from microdissection testicular sperm extraction with ICSI-IVF in non-obstructive azoospermia: A single-center cohort study. Front. Endocrinol. 2022, 13, 893679. [Google Scholar] [CrossRef] [PubMed]
- Olovnikov, A.M. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J. Theor. Biol. 1973, 41, 181–190. [Google Scholar] [CrossRef] [PubMed]
- DePamphilis, M.L.; Wassarman, P.M. Replication of eukaryotic chromosomes: A close-up of the replication fork. Annu. Rev. Biochem. 1980, 49, 627–666. [Google Scholar] [CrossRef]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef]
- Moyzis, R.K.; Buckingham, J.M.; Cram, L.S.; Dani, M.; Deaven, L.L.; Jones, M.D.; Meyne, J.; Ratliff, R.L.; Wu, J.R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. USA 1988, 85, 6622–6626. [Google Scholar] [CrossRef] [PubMed]
- de Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- Azzalin, C.M.; Reichenbach, P.; Khoriauli, L.; Giulotto, E.; Lingner, J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 2007, 318, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhang, Y.; Liu, D.; Songyang, Z.; Wan, M. Telomeres-structure, function, and regulation. Exp. Cell Res. 2013, 319, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Giardini, M.A.; Segatto, M.; da Silva, M.S.; Nunes, V.S.; Cano, M.I. Telomere and telomerase biology. Prog. Mol. Biol. Transl. Sci. 2014, 125, 1–40. [Google Scholar] [CrossRef]
- Watson, J.D. Origin of concatemeric T7DNA. Nat. New Biol. 1972, 239, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Ohki, R.; Tsurimoto, T.; Ishikawa, F. In vitro reconstitution of the end replication problem. Mol. Cell. Biol. 2001, 21, 5753–5766. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef]
- Oikawa, S.; Kawanishi, S. Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. FEBS Lett. 1999, 453, 365–368. [Google Scholar] [CrossRef]
- Kawanishi, S.; Oikawa, S. Mechanism of telomere shortening by oxidative stress. Ann. N. Y. Acad. Sci. 2004, 1019, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Bryan, T.M.; Englezou, A.; Dalla-Pozza, L.; Dunham, M.A.; Reddel, R.R. Evidence for an alternative mechanism for maintainingtelomere length in human tumors and tumor-derived cell lines. Nat. Med. 1997, 3, 1271–1274. [Google Scholar] [CrossRef]
- Dunham, M.A.; Neumann, A.A.; Fasching, C.L.; Reddel, R.R. Telomere maintenance by recombination in human cells. Nat. Genet. 2000, 26, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Zou, L. Alternative lengthening of telomeres: From molecular mechanisms to therapeutic outlooks. Cell Biosci. 2020, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Pendina, A.A.; Krapivin, M.I.; Efimova, O.A.; Tikhonov, A.V.; Mekina, I.D.; Komarova, E.M.; Koltsova, A.S.; Gzgzyan, A.M.; Kogan, I.Y.; Chiryaeva, O.G.; et al. Telomere length in metaphase chromosomes of human triploid zygotes. Int. J. Mol. Sci. 2021, 22, 5579. [Google Scholar] [CrossRef] [PubMed]
- Reig-Viader, R.; Capilla, L.; Vila-Cejudo, M.; Garcia, F.; Anguita, B.; Garcia-Caldés, M.; Ruiz-Herrera, A. Telomere homeostasis is compromised in spermatocytes from patients with idiopathic infertility. Fertil. Steril. 2014, 102, 728–738.e1. [Google Scholar] [CrossRef]
- Stalf, T.; Mehnert, C.; Hajimohammad, A.; Manolopoulos, K.; Shen, Y.; Schuppe, H.-C.; Diemer, T.; Schill, W.-B.; Weidner, W.; Tinneberg, H.-R. Influence of motility and vitality in intracytoplasmic sperm injection with ejaculated and testicular sperm. Andrologia 2005, 37, 125–130. [Google Scholar] [CrossRef]
- Mehta, A.; Bolyakov, A.; Schlegel, P.N.; Paduch, D.A. Higher pregnancy rates using testicular sperm in men with severe oligospermia. Fertil. Steril. 2015, 104, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Cariati, F.; Jaroudi, S.; Alfarawati, S.; Raberi, A.; Alviggi, C.; Pivonello, R.; Wells, D. Investigation of sperm telomere length as a potential marker of paternal genome integrity and semen quality. Reprod. Biomed. Online 2016, 33, 404–411. [Google Scholar] [CrossRef]
- Gentiluomo, M.; Luddi, A.; Cingolani, A.; Fornili, M.; Governini, L.; Lucenteforte, E.; Baglietto, L.; Piomboni, P.; Campa, D. Telomere length and male fertility. Int. J. Mol. Sci. 2021, 22, 3959. [Google Scholar] [CrossRef]
- Yuan, Y.; Tan, Y.; Qiu, X.; Luo, H.; Li, Y.; Li, R.; Yang, X. Sperm telomere length as a novel biomarker of male infertility and embryonic development: A systematic review and meta-analysis. Front. Endocrinol. 2023, 13, 1079966. [Google Scholar] [CrossRef]
- Krapivin, M.I.; Tikhonov, A.V.; Efimova, O.A.; Pendina, A.A.; Smirnova, A.A.; Chiryaeva, O.G.; Talantova, O.E.; Petrova, L.I.; Dudkina, V.S.; Baranov, V.S. Telomere Length in Chromosomally Normal and Abnormal Miscarriages and Ongoing Pregnancies and Its Association with 5-hydroxymethylcytosine Patterns. Int. J. Mol. Sci. 2021, 22, 6622. [Google Scholar] [CrossRef]
- Wright, W.E.; Piatyszek, M.A.; Rainey, W.E.; Byrd, W.; Shay, J.W. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 1996, 18, 173–179. [Google Scholar] [CrossRef]
- Wright, D.L.; Jones, E.L.; Mayer, J.F.; Oehninger, S.; Gibbons, W.E.; Lanzendorf, S.E. Characterization of telomerase activity in the human oocyte and preimplantation embryo. Mol. Hum. Reprod. 2001, 7, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Keefe, D.L. Telomeres and genomic instability during early development. Eur. J. Med. Genet. 2020, 63, 103638. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Cherkas, L.F.; Kato, B.S.; Demissie, S.; Hjelmborg, J.B.; Brimacombe, M.; Cupples, A.; Hunkin, J.L.; Gardner, J.P.; Lu, X.; et al. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008, 4, e37. [Google Scholar] [CrossRef]
- de Frutos, C.; Lopez-Cardona, A.P.; Balvis, N.F.; Laguna-Barraza, R.; Rizos, D.; Gutierrez-Adan, A.; Bermejo-Alvarez, P. Spermatozoa telomeres determine telomere length in early embryos and offspring. Reproduction 2016, 151, 1–7. [Google Scholar] [CrossRef]
- Kohlrausch, F.B.; Wang, F.; Chamani, I.; Keefe, D.L. Telomere Shortening and Fusions: A Link to Aneuploidy in Early Human Embryo Development. Obstet. Gynecol. Surv. 2021, 76, 429–436. [Google Scholar] [CrossRef]
- Fujisawa, M.; Tanaka, H.; Tatsumi, N.; Okada, H.; Arakawa, S.; Kamidono, S. Telomerase activity in the testis of infertile patients with selected causes. Hum. Reprod. 1998, 13, 1476–1479. [Google Scholar] [CrossRef]
- Yashima, K.; Maitra, A.; Rogers, B.B.; Timmons, C.F.; Rathi, A.; Pinar, H.; Wright, W.E.; Shay, J.W.; Gazdar, A.F. Expression of the RNA component of telomerase during human development and differentiation. Cell Growth Differ. 1998, 9, 805–813. [Google Scholar]
- Izadyar, F.; Wong, J.; Maki, C.; Pacchiarotti, J.; Ramos, T.; Howerton, K.; Yuen, C.; Greilach, S.; Zhao, H.H.; Chow, M.; et al. Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Hum. Reprod. 2011, 26, 1296–1306. [Google Scholar] [CrossRef]
- Fattet, A.J.; Chaillot, M.; Koscinski, I. Telomere Length, a New Biomarker of Male (in)Fertility? A Systematic Review of the Literature. Genes 2023, 14, 425. [Google Scholar] [CrossRef]
- Wang, Z.; Rhee, D.B.; Lu, J.; Bohr, C.T.; Zhou, F.; Vallabhaneni, H.; de Souza-Pinto, N.C.; Liu, Y. Characterization of oxidative guanine damage and repair in mammalian telomeres. PLoS Genet. 2010, 6, e1000951. [Google Scholar] [CrossRef] [PubMed]
- Fouquerel, E.; Barnes, R.P.; Uttam, S.; Watkins, S.C.; Bruchez, M.P.; Opresko, P.L. Targeted and Persistent 8-Oxoguanine Base Damage at Telomeres Promotes Telomere Loss and Crisis. Mol. Cell 2019, 75, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Wang, S.R.; Wu, L.Y.; Zhang, Y.; Song, Y.; Wei, L.; Tian, T.; Zhou, X. Biochemical insights into the role of guanosine oxidation on RNA G-quadruplex. CCS Chem. 2020, 2, 605–612. [Google Scholar] [CrossRef]
- Antunes, D.M.; Kalmbach, K.H.; Wang, F.; Dracxler, R.C.; Seth-Smith, M.L.; Kramer, Y.; Buldo-Licciardi, J.; Kohlrausch, F.B.; Keefe, D.L. A single-cell assay for telomere DNA content shows increasing telomere length heterogeneity, as well as increasing mean telomere length in human spermatozoa with advancing age. J. Assist. Reprod. Genet. 2015, 32, 1685–1690. [Google Scholar] [CrossRef]
- Torra-Massana, M.; Barragán, M.; Bellu, E.; Oliva, R.; Rodríguez, A.; Vassena, R. Sperm telomere length in donor samples is not related to ICSI outcome. J. Assist. Reprod. Genet. 2018, 35, 649–657. [Google Scholar] [CrossRef]
- Allsopp, R.C.; Vaziri, H.; Patterson, C.; Goldstein, S.; Younglai, E.V.; Futcher, A.B.; Greider, C.W.; Harley, C.B. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA 1992, 89, 10114–10118. [Google Scholar] [CrossRef]
- Baird, D.M.; Britt-Compton, B.; Rowson, J.; Amso, N.N.; Gregory, L.; Kipling, D. Telomere instability in the male germline. Hum. Mol. Genet. 2006, 15, 45–51. [Google Scholar] [CrossRef]
- Aston, K.I.; Hunt, S.C.; Susser, E.; Kimura, M.; Factor-Litvak, P.; Carrell, D.; Aviv, A. Divergence of sperm and leukocyte age-dependent telomere dynamics: Implications for male-driven evolution of telomere length in humans. Mol. Hum. Reprod. 2012, 18, 517–522. [Google Scholar] [CrossRef]
- Zalensky, A.O.; Allen, M.J.; Kobayashi, A.; Zalenskaya, I.A.; Balhórn, R.; Bradbury, E.M. Well-defined genome architecture in the human sperm nucleus. Chromosoma 1995, 103, 577–590. [Google Scholar] [CrossRef]
- Zalensky, A.O.; Tomilin, N.V.; Zalenskaya, I.A.; Teplitz, R.L.; Bradbury, E.M. Telomere-telomere interactions and candidate telomere binding protein(s) in mammalian sperm cells. Exp. Cell Res. 1997, 232, 29–41. [Google Scholar] [CrossRef]
- Zalenskaya, I.A.; Bradbury, E.M.; Zalensky, A.O. Chromatin structure of telomere domain in human sperm. Biochem. Biophys. Res. Commun. 2000, 279, 213–218. [Google Scholar] [CrossRef]
- Zalensky, A.; Zalenskaya, I. Organization of chromosomes in spermatozoa: An additional layer of epigenetic information? Biochem. Soc. Trans. 2007, 35, 609–611. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Garcia-Bonavila, E.; Hidalgo, C.O.; Catalán, J.; Miró, J.; Yeste, M. Species-Specific Differences in Sperm Chromatin Decondensation Between Eutherian Mammals Underlie Distinct Lysis Requirements. Front. Cell Dev. Biol. 2021, 9, 669182. [Google Scholar] [CrossRef] [PubMed]
- Gineitis, A.A.; Zalenskaya, I.A.; Yau, P.M.; Bradbury, E.M.; Zalensky, A.O. Human sperm telomere-binding complex involves histone H2B and secures telomere membrane attachment. J. Cell Biol. 2000, 151, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bailey, S.M.; Okuka, M.; Muñoz, P.; Li, C.; Zhou, L.; Wu, C.; Czerwiec, E.; Sandler, L.; Seyfang, A.; et al. Telomere lengthening early in development. Nat. Cell Biol. 2007, 9, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Kalmbach, K.; Robinson, L.G., Jr.; Wang, F.; Liu, L.; Keefe, D. Telomere length reprogramming in embryos and stem cells. BioMed Res. Int. 2014, 2014, 925121. [Google Scholar] [CrossRef]
- Keefe, D.L.; Liu, L. Telomeres and reproductive aging. Reprod. Fertil. Dev. 2009, 21, 10–14. [Google Scholar] [CrossRef]
- Treff, N.R.; Su, J.; Taylor, D.; Scott, R.T., Jr. Telomere DNA deficiency is associated with development of human embryonic aneuploidy. PLoS Genet. 2011, 7, e1002161. [Google Scholar] [CrossRef]
- Huleyuk, N.; Tkach, I.; Zastavna, D.; Tyrka, M. Can telomere shortening be the main indicator of non-viable fetus elimination? Mol. Cytogenet. 2018, 11, 11. [Google Scholar] [CrossRef]
- Daphnis, D.D.; Delhanty, J.D.; Jerkovic, S.; Geyer, J.; Craft, I.; Harper, J.C. Detailed FISH analysis of day 5 human embryos reveals the mechanisms leading to mosaic aneuploidy. Hum. Reprod. 2005, 20, 129–137. [Google Scholar] [CrossRef]
- Rubio, C.; Rodrigo, L.; Mercader, A.; Mateu, E.; Buendía, P.; Pehlivan, T.; Viloria, T.; De los Santos, M.J.; Simón, C.; Remohí, J.; et al. Impact of chromosomal abnormalities on preimplantation embryo development. Prenat. Diagn. 2007, 27, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Fragouli, E.; Alfarawati, S.; Daphnis, D.D.; Goodall, N.N.; Mania, A.; Griffiths, T.; Gordon, A.; Wells, D. Cytogenetic analysis of human blastocysts with the use of FISH, CGH and aCGH: Scientific data and technical evaluation. Hum. Reprod. 2011, 26, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Harton, G.L.; Cinnioglu, C.; Fiorentino, F. Current experience concerning mosaic embryos diagnosed during preimplantation genetic screening. Fertil. Steril. 2017, 107, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Capalbo, A.; Poli, M.; Rienzi, L.; Girardi, L.; Patassini, C.; Fabiani, M.; Cimadomo, D.; Benini, F.; Farcomeni, A.; Cuzzi, J.; et al. Mosaic human preimplantation embryos and their developmental potential in a prospective, non-selection clinical trial. Am. J. Hum. Genet. 2021, 108, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Poon, S.S.; Martens, U.M.; Ward, R.K.; Lansdorp, P.M. Telomere length measurements using digital fluorescence microscopy. Cytometry 1999, 36, 267–278. [Google Scholar] [CrossRef]
- Perner, S.; Brüderlein, S.; Hasel, C.; Waibel, I.; Holdenried, A.; Ciloglu, N.; Chopurian, H.; Nielsen, K.V.; Plesch, A.; Högel, J.; et al. Quantifying telomere lengths of human individual chromosome arms by centromere-calibrated fluorescence in situ hybridization and digital imaging. Am. J. Pathol. 2003, 163, 1751–1756. [Google Scholar] [CrossRef]
- Cantara, S.; Pisu, M.; Frau, D.V.; Caria, P.; Dettori, T.; Capezzone, M.; Capuano, S.; Vanni, R.; Pacini, F. Telomere abnormalities and chromosome fragility in patients affected by familial papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2012, 97, E1327–E1331. [Google Scholar] [CrossRef]
- Squassina, A.; Pisanu, C.; Congiu, D.; Caria, P.; Frau, D.; Niola, P.; Melis, C.; Baggiani, G.; Lopez, J.P.; Cruceanu, C.; et al. Leukocyte telomere length positively correlates with duration of lithium treatment in bipolar disorder patients. Eur. Neuropsychopharmacol. 2016, 26, 1241–1247. [Google Scholar] [CrossRef]
- Nonaka, K.; Aida, J.; Takubo, K.; Yamazaki, Y.; Takakuma, S.; Kakizaki, M.; Matsuda, Y.; Ishikawa, N.; Ishiwata, T.; Chong, J.M.; et al. Correlation Between Differentiation of Adrenocortical Zones and Telomere Lengths Measured by Q-FISH. J. Clin. Endocrinol. Metab. 2019, 104, 5642–5650. [Google Scholar] [CrossRef]
- Koltsova, A.S.; Efimova, O.A.; Malysheva, O.V.; Osinovskaya, N.S.; Liehr, T.; Al-Rikabi, A.; Shved, N.Y.; Sultanov, I.Y.; Chiryaeva, O.G.; Yarmolinskaya, M.I.; et al. Cytogenomic Profile of Uterine Leiomyoma: In Vivo vs. In Vitro Comparison. Biomedicines 2021, 9, 1777. [Google Scholar] [CrossRef]
- Khavinson, V.K.; Pendina, A.A.; Efimova, O.A.; Tikhonov, A.V.; Koltsova, A.S.; Krapivin, M.I.; Petrovskaia-Kaminskaia, A.V.; Petrova, L.I.; Lin’kova, N.S.; Baranov, V.S. Effect of Peptide AEDG on Telomere Length and Mitotic Index of PHA-Stimulated Human Blood Lymphocytes. Bull. Exp. Biol. Med. 2019, 168, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Efimova, O.A.; Pendina, A.A.; Tikhonov, A.V.; Parfenyev, S.E.; Mekina, I.D.; Komarova, E.M.; Mazilina, M.A.; Daev, E.V.; Chiryaeva, O.G.; Galembo, I.A.; et al. Genome-wide 5-hydroxymethylcytosine patterns in human spermatogenesis are associated with semen quality. Oncotarget 2017, 8, 88294–88307. [Google Scholar] [CrossRef] [PubMed]
- Efimova, O.A.; Pendina, A.A.; Krapivin, M.I.; Kopat, V.V.; Tikhonov, A.V.; Petrovskaia-Kaminskaia, A.V.; Navodnikova, P.M.; Talantova, O.E.; Glotov, O.S.; Baranov, V.S. Inter-Cell and Inter-Chromosome Variability of 5-Hydroxymethylcytosine Patterns in Noncultured Human Embryonic and Extraembryonic Cells. Cytogenet. Genome Res. 2018, 156, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Pendina, A.A.; Efimova, O.A.; Chiryaeva, O.G.; Tikhonov, A.V.; Petrova, L.I.; Dudkina, V.S.; Sadik, N.A.; Fedorova, I.D.; Galembo, I.A.; Kuznetzova, T.V.; et al. A comparative cytogenetic study of miscarriages after IVF and natural conception in women aged under and over 35 years. J. Assist. Reprod. Genet. 2014, 31, 149–155. [Google Scholar] [CrossRef] [PubMed]

| # Couple | Azoospermic Patient’s Age | Number of Sperm in TESE Sample Field of View | Relative TL | Female Partner’s Age | # ICSI Protocol | Embryological Outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In Spermatogonia | In Spermatocytes I | 2p Zygotes | 4-Cell Embryos on Day 2 | 8-Cell Embryos on Day 3 | Morulae on Day 4 | Blastocysts on Day 5 | Embryo Competence for Development into Blastocyst | Embryo Transfer to the Uterus | Pregnancy Occurrence | Birth Occurrence | |||||
| 1 | 30 | 5–10 | 1.009 | 0.544 | 24 | I | 3 | 3 | 3 | 3 | 3 | High | Yes | No | No |
| 2 | 30 | 3–5 | 3.387 | 2.287 | 27 | I | 4 | 4 | 4 | 4 | 1 | High | Yes | No | No |
| 29 | II | 6 | 6 | 6 | 6 | 6 | Yes | No | No | ||||||
| 3 | 58 | >10 | 1.520 | 1.207 | 25 | I | 2 | 1 | 1 | 1 | 1 | High | Yes | Yes | Yes |
| 4 | 39 | 3–5 | 1.407 | 0.923 | 28 | I | 0 | 0 | 0 | 0 | 0 | High | No | No | No |
| 29 | II | 6 | 6 | 6 | 6 | 3 | Yes | Yes | Yes | ||||||
| 5 | 25 | 5–7 | 1.334 | 1.390 | 24 | I | 2 | 2 | 2 | 2 | 2 | High | Yes | No | No |
| 24 | II | 2 | 2 | 2 | 2 | 0 | * Yes | No | No | ||||||
| 6 | 30 | 4–5 | 0.863 | 0.669 | 28 | I | 5 | 4 | 4 | 4 | 4 | High | Yes | Yes | Yes |
| 7 | 31 | 2–3 | 1.482 | 1.318 | 23 | I | 7 | 7 | 5 | 5 | 5 | High | Yes | Yes | Yes |
| 8 | 24 | 1–2 | 0.592 | 0.842 | 25 | I | 7 | 2 | 1 | 1 | 1 | Low | Yes | No | No |
| 26 | II | 10 | 5 | 3 | 2 | 2 | Yes | Yes | Yes | ||||||
| 9 | 30 | 2–3 | 0.565 | 1.090 | 33 | I | 6 | 4 | 1 | 1 | 0 | Low | No | No | No |
| 35 | II | 7 | 7 | 5 | 5 | 4 | Yes | No | No | ||||||
| 10 | 30 | 2 | 1.045 | 1.119 | 28 | I | 7 | 5 | 5 | 2 | 2 | Low | Yes | Yes | Yes |
| 11 | 31 | 3–5 | 0.463 | 0.688 | 26 | I | 10 | 7 | 7 | 4 | 2 | Low | Yes | No | No |
| 12 | 33 | 5–10 | 0.423 | 0.390 | 31 | I | 7 | 7 | 6 | 6 | 5 | Low | Yes | Yes, ectopic | No |
| 29 | II | 3 | 0 | 0 | 0 | 0 | No | No | No | ||||||
| 13 | 42 | 8 | 0.698 | 0.917 | 29 | I | 5 | 5 | 2 | 2 | 2 | Low | Yes | No | No |
| 14 | 39 | 8–10 | 0.714 | 1.264 | 32 | I | 4 | 4 | 3 | 3 | 3 | Low | Yes | No | No |
| 33 | II | 1 | 0 | 0 | 0 | 0 | * Yes | Yes | Yes | ||||||
| 15 | 40 | 3–5 | 0.408 | 0.667 | 30 | I | 9 | 6 | 2 | 2 | 0 | Low | * Yes | No | No |
| 30 | II | 8 | 7 | 7 | 6 | 1 | Yes | No | No | ||||||
| 16 | 39 | 2–3 | 1.520 | 1.101 | 36 | I | 7 | 3 | 3 | 1 | 1 | Low | Yes | No | No |
| 17 | 45 | >10 | 0.603 | 0.469 | 33 | I | 3 | 3 | 3 | 3 | 1 | Low | Yes | No | No |
| 37 | II | 0 | 0 | 0 | 0 | 0 | No | No | No | ||||||
| 18 | 30 | 10 | 0.542 | 0.539 | 35 | I | 5 | 5 | 2 | 2 | 2 | Low | No | No | No |
| 19 | 48 | 5–7 | 2.844 | 3.339 | 29 | I | 4 | 4 | 4 | 4 | 0 | Low | No | No | No |
| 29 | II | 4 | 1 | 1 | 1 | 0 | No | No | No | ||||||
| 29 | III | 4 | 1 | 1 | 1 | 1 | Yes | Yes | Yes | ||||||
| 20 | 41 | 2–3 | 0.459 | 0.462 | 21 | I | 1 | 0 | 0 | 0 | 0 | Absent | *** Yes | No | No |
| 37 | II | 2 | 0 | 0 | 0 | 0 | * Yes | No | No | ||||||
| N/A | III | 5 | 2 | 2 | 2 | 0 | * Yes | No | No | ||||||
| 21 | 44 | 4 | 0.748 | 1.046 | 38 | I | 2 | 1 | 0 | 0 | 0 | Absent | No | No | No |
| 22 | 32 | 2–3 | 0.698 | 1.161 | 30 | I | 12 | 10 | 10 | 10 | 0 | Absent | * Yes | No | No |
| 23 | 47 | 10 | 1.118 | 0.800 | 41 | I | 1 | 0 | 0 | 0 | 0 | Absent | ** Yes | No | No |
| 24 | 56 | 1 | 0.941 | 0.291 | 32 | I | 7 | 6 | 6 | 6 | 0 | Absent | No | No | No |
| 25 | 60 | 3–4 | 0.700 | 0.741 | 37 | I | 2 | 2 | 1 | 1 | 0 | Absent | * Yes | No | No |
| 26 | 57 | >10 | 0.748 | 0.496 | - | - | - | - | - | - | - | - | - | - | - |
| 27 | 42 | 5–7 | 0.681 | 0.919 | - | - | - | - | - | - | - | - | - | - | - |
| 28 | 32 | 0 | 0.561 | 0.824 | - | - | - | - | - | - | - | - | - | - | - |
| 29 | 32 | 1 | 1.315 | 1.884 | - | - | - | - | - | - | - | - | - | - | - |
| 30 | 40 | 1–2 | 2.643 | 3.447 | - | - | - | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pendina, A.A.; Krapivin, M.I.; Sagurova, Y.M.; Mekina, I.D.; Komarova, E.M.; Tikhonov, A.V.; Golubeva, A.V.; Gzgzyan, A.M.; Kogan, I.Y.; Efimova, O.A. Telomere Length in Human Spermatogenic Cells as a New Potential Predictor of Clinical Outcomes in ART Treatment with Intracytoplasmic Injection of Testicular Spermatozoa. Int. J. Mol. Sci. 2023, 24, 10427. https://doi.org/10.3390/ijms241310427
Pendina AA, Krapivin MI, Sagurova YM, Mekina ID, Komarova EM, Tikhonov AV, Golubeva AV, Gzgzyan AM, Kogan IY, Efimova OA. Telomere Length in Human Spermatogenic Cells as a New Potential Predictor of Clinical Outcomes in ART Treatment with Intracytoplasmic Injection of Testicular Spermatozoa. International Journal of Molecular Sciences. 2023; 24(13):10427. https://doi.org/10.3390/ijms241310427
Chicago/Turabian StylePendina, Anna A., Mikhail I. Krapivin, Yanina M. Sagurova, Irina D. Mekina, Evgeniia M. Komarova, Andrei V. Tikhonov, Arina V. Golubeva, Alexander M. Gzgzyan, Igor Yu. Kogan, and Olga A. Efimova. 2023. "Telomere Length in Human Spermatogenic Cells as a New Potential Predictor of Clinical Outcomes in ART Treatment with Intracytoplasmic Injection of Testicular Spermatozoa" International Journal of Molecular Sciences 24, no. 13: 10427. https://doi.org/10.3390/ijms241310427
APA StylePendina, A. A., Krapivin, M. I., Sagurova, Y. M., Mekina, I. D., Komarova, E. M., Tikhonov, A. V., Golubeva, A. V., Gzgzyan, A. M., Kogan, I. Y., & Efimova, O. A. (2023). Telomere Length in Human Spermatogenic Cells as a New Potential Predictor of Clinical Outcomes in ART Treatment with Intracytoplasmic Injection of Testicular Spermatozoa. International Journal of Molecular Sciences, 24(13), 10427. https://doi.org/10.3390/ijms241310427






