Ethyl Caffeate Can Inhibit Aryl Hydrocarbon Receptor (AhR) Signaling and AhR-Mediated Potentiation of Mast Cell Activation
Abstract
1. Introduction
2. Results
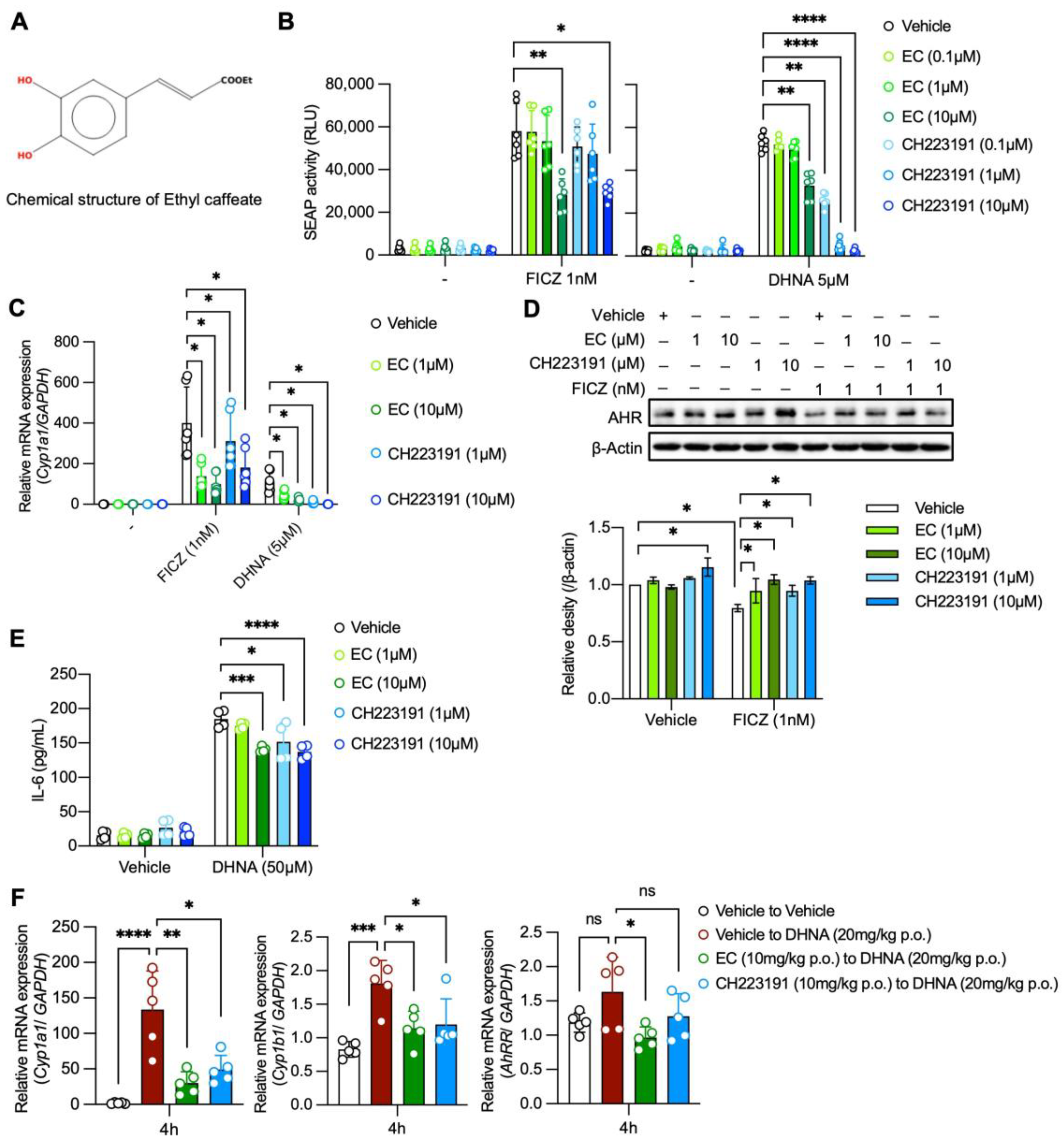
2.1. EC Antagonizes AhR Activation Both In Vitro and In Vivo
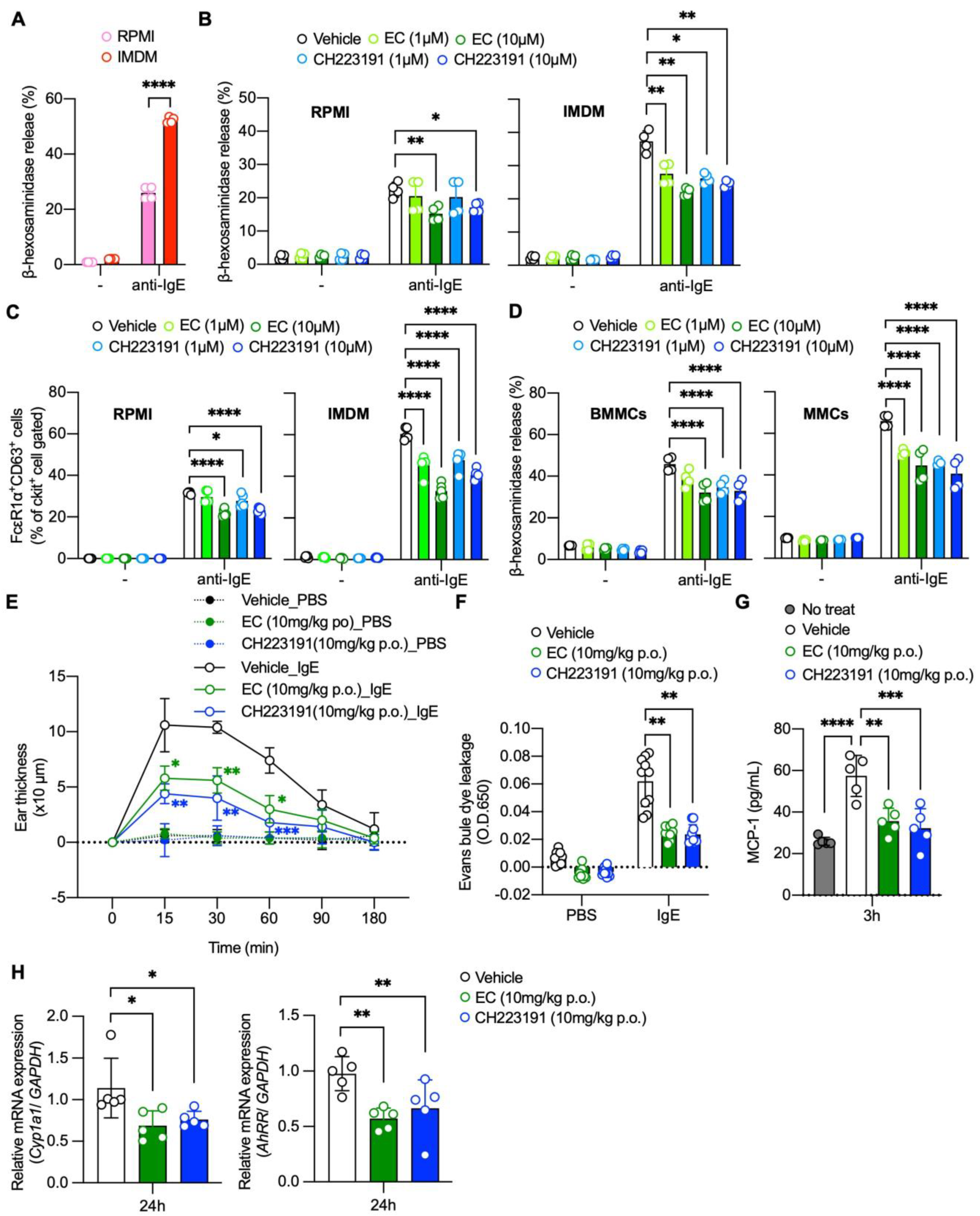
2.2. EC Inhibits AhR-Mediated Potentiation of Mast Cell Activation
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Mice
4.3. SEAP Assay
4.4. Generation of Mouse Bone Marrow-Derived Mast Cells (BMMCs) and Mucosal Mast Cells (MMCs)
4.5. FACS Staining
4.6. Quantitative Real-Time PCR
4.7. Cell Viability
4.8. Western Blot Analysis
4.9. ELISA
4.10. β-Hexosaminidase Release and Surface CD63 Expression
4.11. Passive Cutaneous Anaphylaxis (PCA) Reaction
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiang, Y.-M.; Chuang, D.-Y.; Wang, S.-Y.; Kuo, Y.-H.; Tsai, P.-W.; Shyur, L.-F. Metabolite profiling and chemopreventive bioactivity of plant extracts from Bidens pilosa. J. Ethnopharmacol. 2004, 95, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.M.; Lo, C.P.; Chen, Y.P.; Wang, S.Y.; Yang, N.S.; Kuo, Y.H.; Shyur, L.F. Ethyl caffeate suppresses NF-kappaB activation and its downstream inflammatory mediators, iNOS, COX-2, and PGE2 in vitro or in mouse skin. Br. J. Pharmacol. 2005, 146, 352–363. [Google Scholar] [CrossRef]
- Xu, S.; Zuo, A.; Guo, Z.; Wan, C. Ethyl Caffeate Ameliorates Collagen-Induced Arthritis by Suppressing Th1 Immune Response. J. Immunol. Res. 2017, 2017, 7416792. [Google Scholar] [CrossRef]
- Larigot, L.; Benoit, L.; Koual, M.; Tomkiewicz, C.; Barouki, R.; Coumoul, X. Aryl Hydrocarbon Receptor and Its Diverse Ligands and Functions: An Exposome Receptor. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 383–404. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Maaetoft-Udsen, K.; Shimoda, L.M.N.; Frøkiær, H.; Turner, H. Aryl Hydrocarbon Receptor Ligand Effects in RBL2H3 Cells. J. Immunotoxicol. 2012, 9, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Sibilano, R.; Frossi, B.; Calvaruso, M.; Danelli, L.; Betto, E.; Dall’agnese, A.; Tripodo, C.; Colombo, M.P.; Pucillo, C.E.; Gri, G. The Aryl Hydrocarbon Receptor Modulates Acute and Late Mast Cell Responses. J. Immunol. 2012, 189, 120–127. [Google Scholar] [CrossRef]
- Zhou, Y.; Tung, H.-Y.; Tsai, Y.-M.; Hsu, S.-C.; Chang, H.-W.; Kawasaki, H.; Tseng, H.-C.; Plunkett, B.; Gao, P.; Hung, C.-H.; et al. Aryl hydrocarbon receptor controls murine mast cell homeostasis. Blood 2013, 121, 3195–3204. [Google Scholar] [CrossRef]
- Kawasaki, H.; Chang, H.-W.; Tseng, H.-C.; Hsu, S.-C.; Yang, S.-J.; Hung, C.-H.; Zhou, Y.; Huang, S.-K. A tryptophan metabolite, kynurenine, promotes mast cell activation through aryl hydrocarbon receptor. Allergy 2014, 69, 445–452. [Google Scholar] [CrossRef]
- Veldhoen, M.; Hirota, K.; Christensen, J.; O’Garra, A.; Stockinger, B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J. Exp. Med. 2009, 206, 43–49. [Google Scholar] [CrossRef]
- Ilchmann, A.; Krause, M.; Heilmann, M.; Burgdorf, S.; Vieths, S.; Toda, M. Impact of culture medium on maturation of bone marrow-derived murine dendritic cells via the aryl hydrocarbon receptor. Mol. Immunol. 2012, 51, 42–50. [Google Scholar] [CrossRef]
- Bungsu, I.; Kifli, N.; Ahmad, S.R.; Ghani, H.; Cunningham, A.C. Herbal Plants: The Role of AhR in Mediating Immunomodulation. Front. Immunol. 2021, 12, 697663. [Google Scholar] [CrossRef] [PubMed]
- Kasai, A.; Hiramatsu, N.; Meng, Y.; Yao, J.; Takeda, M.; Maeda, S.; Kitamura, M. DRESSA: Biosensing of dioxin and dioxin-like chemicals using secreted alkaline phosphatase. Anal. Biochem. 2004, 335, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Davarinos, N.A.; Pollenz, R.S. Aryl Hydrocarbon Receptor Imported into the Nucleus following Ligand Binding Is Rapidly Degraded via the Cytosplasmic Proteasome following Nuclear Export. J. Biol. Chem. 1999, 274, 28708–28715. [Google Scholar] [CrossRef] [PubMed]
- Harper, P.A.; Riddick, D.S.; Okey, A.B. Regulating the regulator: Factors that control levels and activity of the aryl hydrocarbon receptor. Biochem. Pharmacol. 2006, 72, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Toshimitsu, T.; Matsuoka, S.; Maruyama, A.; Oh-Oka, K.; Takamura, T.; Nakamura, Y.; Ishimaru, K.; Fujii-Kuriyama, Y.; Ikegami, S.; et al. Identification of a probiotic bacteria-derived activator of the aryl hydrocarbon receptor that inhibits colitis. Immunol. Cell Biol. 2014, 92, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Honjo, A.; Nakano, N.; Yamazaki, S.; Hara, M.; Uchida, K.; Kitaura, J.; Nishiyama, C.; Yagita, H.; Ohtsuka, Y.; Ogawa, H.; et al. Pharmacologic inhibition of Notch signaling suppresses food antigen-induced mucosal mast cell hyperplasia. J. Allergy Clin. Immunol. 2017, 139, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Noakes, R. The Aryl Hydrocarbon Receptor: A Review of Its Role in the Physiology and Pathology of the Integument and Its Relationship to the Tryptophan Metabolism. Int. J. Tryptophan Res. 2015, 8, 7–18. [Google Scholar] [CrossRef]
- Fernández-Gallego, N.; Sánchez-Madrid, F.; Cibrian, D. Role of AHR Ligands in Skin Homeostasis and Cutaneous Inflammation. Cells 2021, 10, 3176. [Google Scholar] [CrossRef]
- Wincent, E.; Amini, N.; Luecke, S.; Glatt, H.; Bergman, J.; Crescenzi, C.; Rannug, A.; Rannug, U. The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 sub-strate 6-formylindolo[3,2-β]carbazole is present in humans. J. Biol. Chem. 2009, 284, 2690–2696. [Google Scholar] [CrossRef]
- Haas, K.; Weighardt, H.; Deenen, R.; Köhrer, K.; Clausen, B.; Zahner, S.; Boukamp, P.; Bloch, W.; Krutmann, J.; Esser, C. Aryl Hydrocarbon Receptor in Keratinocytes Is Essential for Murine Skin Barrier Integrity. J. Investig. Dermatol. 2016, 136, 2260–2269. [Google Scholar] [CrossRef] [PubMed]
- Jaikang, C.; Chaiyasut, C.; Narongchai, P.; Niwatananun, K.; Narongchai, S.; Kusirisin, W. Inhibitory Effects of Caffeic Acid Ester Analogues on Free Radicals and Human Liver Microsome CYP1A2 Activities. Med. Chem. 2011, 7, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Alson, S.G.; Jansen, O.; Cieckiewicz, E.; Rakotoarimanana, H.; Rafatro, H.; Degotte, G.; Francotte, P.; Frederich, M. In-vitro and in-vivo antimalarial activity of caffeic acid and some of its derivatives. J. Pharm. Pharmacol. 2018, 70, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Kim, J.K.; Kim, J.H.; Lee, S.J.; Ahn, E.K.; Oh, J.S.; Seo, D.W. A mechanistic study on the anti-cancer activity of ethyl caffeate in human ovarian cancer SKOV-3 cells. Chem. Biol. Interact. 2014, 219, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.A.; Patterson, A.D.; Perdew, G.H. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer 2014, 14, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Van den Bogaard, E.H.; Bergboer, J.G.; Vonk-Bergers, M.; van Vlijmen-Willems, I.M.; Hato, S.V.; van der Valk, P.G.; Schröder, J.M.; Joosten, I.; Zeeuwen, P.L.; Schalkwijk, J. Coal tar induces AHR-dependent skin barrier repair in atopic der-matitis. J. Clin. Investig. 2013, 123, 917–927. [Google Scholar]
- Di Meglio, P.; Duarte, J.H.; Ahlfors, H.; Owens, N.D.; Li, Y.; Villanova, F.; Tosi, I.; Hirota, K.; Nestle, F.O.; Mrowietz, U.; et al. Activation of the Aryl Hydrocarbon Receptor Dampens the Severity of Inflammatory Skin Conditions. Immunity 2014, 40, 989–1001. [Google Scholar] [CrossRef]
- Haarmann-Stemmann, T.; Esser, C.; Krutmann, J. The Janus-Faced Role of Aryl Hydrocarbon Receptor Signaling in the Skin: Consequences for Prevention and Treatment of Skin Disorders. J. Investig. Dermatol. 2015, 135, 2572–2576. [Google Scholar] [CrossRef]
- Kim, S.H.; Henry, E.C.; Kim, D.K.; Kim, Y.H.; Shin, K.J.; Han, M.S.; Lee, T.G.; Kang, J.K.; Gasiewicz, T.A.; Ryu, S.H.; et al. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydro-carbon receptor. Mol. Pharmacol. 2006, 69, 1871–1878. [Google Scholar] [CrossRef]
- Giménez-Rivera, V.A.; Metz, M.; Siebenhaar, F. Mast Cell-Mediated Reactions In Vivo. Methods Mol. Biol. 2014, 1192, 239–247. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, P.-T.; Nakamura, Y.; Tran, N.Q.V.; Ishimaru, K.; Nguyen, T.-A.; Kobayashi, Y.; Watanabe-Saito, F.; Okuda, T.; Nakano, N.; Nakao, A. Ethyl Caffeate Can Inhibit Aryl Hydrocarbon Receptor (AhR) Signaling and AhR-Mediated Potentiation of Mast Cell Activation. Int. J. Mol. Sci. 2023, 24, 9997. https://doi.org/10.3390/ijms24129997
Nguyen P-T, Nakamura Y, Tran NQV, Ishimaru K, Nguyen T-A, Kobayashi Y, Watanabe-Saito F, Okuda T, Nakano N, Nakao A. Ethyl Caffeate Can Inhibit Aryl Hydrocarbon Receptor (AhR) Signaling and AhR-Mediated Potentiation of Mast Cell Activation. International Journal of Molecular Sciences. 2023; 24(12):9997. https://doi.org/10.3390/ijms24129997
Chicago/Turabian StyleNguyen, Phuc-Tan, Yuki Nakamura, Nguyen Quoc Vuong Tran, Kayoko Ishimaru, Thuy-An Nguyen, Yoshiaki Kobayashi, Fumie Watanabe-Saito, Tohru Okuda, Nobuhiro Nakano, and Atsuhito Nakao. 2023. "Ethyl Caffeate Can Inhibit Aryl Hydrocarbon Receptor (AhR) Signaling and AhR-Mediated Potentiation of Mast Cell Activation" International Journal of Molecular Sciences 24, no. 12: 9997. https://doi.org/10.3390/ijms24129997
APA StyleNguyen, P.-T., Nakamura, Y., Tran, N. Q. V., Ishimaru, K., Nguyen, T.-A., Kobayashi, Y., Watanabe-Saito, F., Okuda, T., Nakano, N., & Nakao, A. (2023). Ethyl Caffeate Can Inhibit Aryl Hydrocarbon Receptor (AhR) Signaling and AhR-Mediated Potentiation of Mast Cell Activation. International Journal of Molecular Sciences, 24(12), 9997. https://doi.org/10.3390/ijms24129997





